ABSTRACT
Influenza A virus (IAV) causes respiratory disease in swine and humans. Vaccines are used to prevent influenza illness in both populations but must be frequently updated due to rapidly evolving strains. Mismatch between the circulating strains and the strains contained in vaccines may cause loss of efficacy. Whole inactivated virus (WIV) vaccines with adjuvant, utilized by the swine industry, are effective against antigenically similar viruses; however, vaccine-associated enhanced respiratory disease (VAERD) may happen when the WIV is antigenically mismatched with the infecting virus. VAERD is a repeatable model in pigs, but had yet to be experimentally demonstrated in other mammalian species. We recapitulated VAERD in ferrets, a standard benchmark animal model for studying human influenza infection, in a direct comparison to VAERD in pigs. Both species were vaccinated with WIV with oil-in-water adjuvant containing a δ-1 H1N2 (1B.2.2) derived from the pre-2009 human seasonal lineage, then challenged with a 2009 pandemic H1N1 (H1N1pdm09, 1A.3.3.2) 5 weeks after vaccination. Nonvaccinated and challenged groups showed typical signs of influenza disease, but the mismatched vaccinated and challenged pigs and ferrets showed elevated clinical signs, despite similar viral loads. VAERD-affected pigs exhibited a 2-fold increase in lung lesions, while VAERD-affected ferrets showed a 4-fold increase. Similar to pigs, antibodies from VAERD-affected ferrets preferentially bound to the HA2 domain of the H1N1pdm09 challenge strain. These results indicate that VAERD is not limited to pigs, as demonstrated here in ferrets, and the need to consider VAERD when evaluating new vaccine platforms and strategies.
IMPORTANCE We demonstrated the susceptibility of ferrets, a laboratory model species for human influenza A virus research, to vaccine-associated enhanced respiratory disease (VAERD) using an experimental model previously demonstrated in pigs. Ferrets developed clinical characteristics of VAERD very similar to that in pigs. The hemagglutinin (HA) stalk is a potential vaccine target to develop more efficacious, broadly reactive influenza vaccine platforms and strategies. However, non-neutralizing antibodies directed toward a conserved epitope on the HA stalk induced by an oil-in-water, adjuvanted, whole influenza virus vaccine were previously shown in VAERD-affected pigs and were also identified here in VAERD-affected ferrets. The induction of VAERD in ferrets highlights the potential risk of mismatched influenza vaccines for humans and the need to consider VAERD when designing and evaluating vaccine strategies.
KEYWORDS: influenza, swine, VAERD, adjuvant, human, adjuvants, vaccines
INTRODUCTION
Influenza A virus (IAV) is a major respiratory pathogen of both human and swine populations globally. IAV in swine places a substantial annual economic burden on the pork industry, and 3 distinct HA/NA subtypes are endemic in commercial US pig populations (1). Genetic diversity is high within each subtype; hemagglutinin (HA) gene segments from eight H1 clades and nine H3 clades have been isolated and sustained in swine in the United States since 2016 (1, 2). This diversity is driven by reassortment, genetic drift, and the occasional introduction of human seasonal IAV viruses into the swine population (3, 4). The broad IAV diversity endemic in US swine poses substantial challenges for effective control of the virus. In addition to the financial and animal health burdens, swine IAV impacts human health as yearly zoonotic infections spark concerns of future pandemics (5–7).
Adjuvanted, whole inactivated vaccines (WIV) are commonly utilized tools to control swine IAV. WIVs are highly effective against homologous challenge but offer limited cross-protection against strains with significant antigenic distance (8–10). Additionally, vaccine-associated enhanced respiratory disease (VAERD) can be induced when WIV-vaccinated pigs are challenged with a homosubtypic, antigenically heterologous challenge virus (11–14). VAERD in pigs is characterized by a prolonged fever, an increase in the severity and distribution of pneumonic lung lesions, peribronchiolar lymphocytic cuffing, and necrotizing bronchiolitis compared to unvaccinated, challenged swine (15). While the mechanisms of VAERD are not fully understood, non-neutralizing antibodies against a conserved region of HA2 promoted fusion and increased viral infectivity (16). Increases in pro-inflammatory and cell-mediated immunity-modulating cytokines were associated with neutrophil infiltration and severe lung pathology in VAERD affected pigs (17). Furthermore, neuraminidase immunity and adjuvant type affected the severity of VAERD, while timing between vaccination and challenge and animal age had no effects (18–20).
Non-adjuvanted, split-virion vaccines are typically utilized to control IAV in humans, however, multiple adjuvanted WIV vaccines are licensed (21). While seasonal human influenza may lack the diversity to elicit VAERD, human seasonal IAVs are periodically introduced and become endemic in swine. These viruses become antigenically distinct while evolving in swine, and many swine lineages have subsequently infected humans as zoonotic “variant” strains. Non-human host specific influenza viruses, such as those that are endemic in swine, may pose a risk of inducing VAERD in humans under the right circumstances due to antigenic mismatch within the same subtype. Should one of these viruses generate a human pandemic, the impact could be substantial. Indeed, multiple studies have found correlation between pre-existing, non-neutralizing anti-H1 antibodies, including those induced by the seasonal H1 vaccine, and severity of disease in the early months of the 2009 pandemic (22–24). Here, we evaluated the susceptibility of ferrets, a standard model for human influenza pathogenesis and vaccine efficacy studies, to VAERD in a direct comparison to the swine model.
RESULTS
Vaccinated ferrets displayed increased clinical signs of disease after heterologous challenge.
All challenged pigs produced a similar febrile response (1.3 to 1.4°C increase) at 1 day postinfection (dpi). Body temperatures began returning to normal by 2 dpi in non-vaccinated/challenged (NV/C) pigs, but remained high until 4 dpi in vaccinated/challenge (V/C) pigs (Fig. 1A). NV/C pigs exhibited mild lethargy for 2 to 3 dpi while V/C pigs displayed more severe lethargy until 4 to 5 dpi. Additionally, anorexia and coughing were observed on 4 to 5 dpi in V/C pigs but were absent in other groups.
FIG 1.
Clinical signs of disease in ferrets and pigs are typical of VAERD. Pig (A) and ferret (B) body temperatures were monitored daily from 0 dpi through 6 dpi. Deviation from the baseline body temperature (average of 0 dpi reading) was calculated for each individual and averaged for each group. Error bars represent standard error of the mean. (C) Additionally, ferret body weight was measured daily from 0 dpi through 14 dpi. Data are presented as percentage changes from the 0 dpi average for each group. Black circles represent naive control groups, blue squares are NV/C groups, and red triangle are V/C groups. Error bars represent standard error of the means.
NV/C ferrets had mild fever that peaked on 2 dpi and returned to baseline by 3 dpi (Fig. 1B) and displayed mild lethargy and anorexia from 1 to 3 dpi. NV/C ferrets exhibited weight loss that peaked at −7.7% at 5 dpi (Fig. 1C). V/C ferrets had a similar febrile response at 1 dpi, but their body temperature fell below baseline from 3 to 7 dpi (Fig. 1B). Body weight loss peaked at −16.1% at 7 dpi before the V/C ferrets began regaining weight at 9 dpi (Fig. 1C).
Challenge groups had similar viral loads.
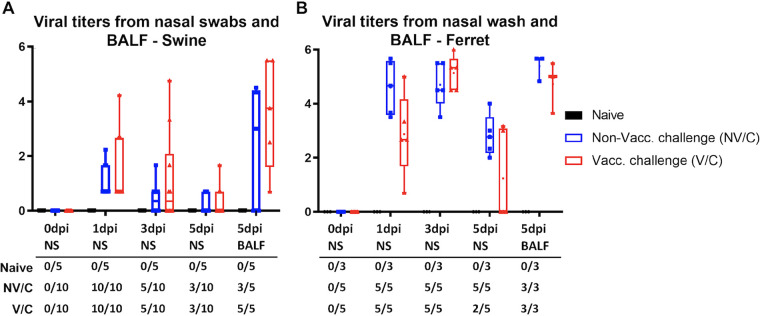
Viral titration of nasal samples or bronchoalveolar lavage fluid (BALF) revealed no statistical difference (P > 0.05) between viral titers of NV/C and V/C animals at any time point (Fig. 2). All challenged pigs shed virus in the nasal swabs at 1 dpi. Both challenge groups saw identical reduction in shedding animals at 3 dpi (5/10 pigs) and 5 dpi (3/10 pigs). All five V/C pigs had recoverable virus in the 5 dpi BALF, compared to only 60% of NV/C pigs. All challenged ferrets shed virus in the nasal wash at both 1 dpi and 3 dpi. All NV/C ferrets had recoverable virus in nasal washes at each time point as well as from BALF. All V/C ferrets were positive for nasal wash virus at 1 and 3 dpi as well as at 5 dpi in BALF, but only 40% had shed virus in the 5-dpi nasal wash. Differences in viral titers between pigs and ferrets are likely due to species differences and differences in sampling methods. Bacteriological screening of BALF to rule out other causes of pneumonia recovered minimal bacterial growth from pigs and ferrets.
FIG 2.
Vaccine status had no effect on viral shedding of heterologous challenge virus. Pigs (A) were nasal swabbed on 0, 1, 3, and 5 dpi, while ferrets (B) were sampled by nasal wash at the same time points. BALF was collected at 5 dpi during necropsy. Samples were serial diluted and incubated on MDCK-London cells for 72 h. Presence of virus was determined by hemagglutinin assay. Median log10 TCID50 titers are represented by box and whisker plots for each group. Boxes indicate 25–75 percentile, and bars mark minimum and maximum values. The table indicates the number of animals that were positive for each group at each time point.
Vaccinated ferrets and pigs displayed enhanced pathology after heterologous challenge.
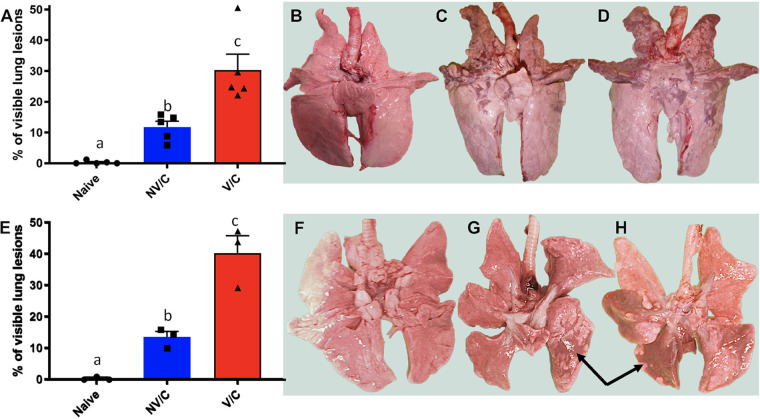
Lungs were evaluated at 5 and 21 dpi to determine the surface area of the lung with visible plum-colored consolidation (Fig. 3). NV/C pigs displayed between 5.9% and 15.8% of the lung surface affected, with an average of 11.8% at 5 dpi. In contrast, V/C pigs had between 22.1% and 50.5% of the surface area affected with an average of 30.3% at 5 dpi (Fig. 3A), consistent with previously described VAERD (18). The percentage of lung surface with consolidation resolved to 2.7% for NV/C pigs and to 10.4% for V/C pigs by 21 dpi (Table 1). NV/C ferrets had 10 to 15.9% macroscopic lung lesions, with an average of 13.4% at 5 dpi. At the same time point, the percentage of macroscopic lung lesions in V/C ferrets ranged from 29.5% to 47.3% and averaged 40.2% (Fig. 3E), indicating that VAERD occurred in ferrets. By 21 dpi, NV/C ferret lung lesion percentages resolved to 5.6%; however, V/C ferret percentages, although reduced, remained significantly higher than NV/C at 23.9%.
FIG 3.
Vaccinated animals had increased macroscopic lung lesions compared to nonvaccinated animals after heterologous challenge. Lungs of pigs (panels A to D) and ferrets (panels E to H) were visually evaluated, and percentages of the total surface area with plum-colored consolidated lesions were estimated for each animal (arrows G and H) and averaged for each treatment group with standard error of the means (panels A and E). Lowercase letters indicate values with significant differences (P < 0.05). Images shown for the lungs of the pig or ferret that most closely represented the average score for naive (panels B and F), NV/C (panels C and G) and V/C (panels D and H) treatment groups.
TABLE 1.
Vaccinated animals exhibit higher pathology scores following heterologous challenge compared to non-vaccinated animals
| Animal | Macroscopic lesionsa (%) |
Microscopic score |
||
|---|---|---|---|---|
| 5 dpi | 21 dpi | 5 dpi: lung (0–22) | 5 dpi: trachea (0–22) | |
| Pigs | ||||
| NV/NC | 0.3 ± 0.2 | - | 0 ± 0 | 0.4 ± 0.4c |
| NV/C | 12 ± 2 | 3 ± 1a | 9 ± 1b | 0 ± 0c |
| V/C | 30 ± 5 | 10 ± 5a | 12 ± 2b | 5.3 ± 0.3 |
| Ferrets | ||||
| NV/NC | 0.3 ± 0.3 | - | 0 ± 0 | 0 ± 0d |
| NV/C | 13 ± 2 | 6 ± 3 | 9 ± 1 | 0.7 ± 0.6d |
| V/C | 40 ± 5 | 24 ± 7 | 18.3 ± 0.8 | 2.8 ± 0.1d |
Average percentage of the lung with macroscopic consolidation or the average composite microscopic lesion score ± SEM. Values within species and pathology columns were statistically different (P < 0.05) except those marked by matching superscript letters.
Microscopic lesions from sections of pig and ferret lungs were evaluated at 5 dpi and scored on a 22-point scale as previously described (25). NV/C pigs had an average microscopic lung lesion score of 9 ± 1, while the mean score for V/C pigs was 12 ± 2. Microscopic trachea lesions were not observed in NV/C pigs at 5 dpi, in contrast to V/C pigs that had an average microscopic trachea lesion score of 5.3 ± 0.3 (Table 1). These results were consistent with VAERD microscopic lung and trachea lesion scores reported in swine from previous studies (15, 18). The NV/C ferret lungs had an average microscopic lung lesion score of 9 ± 1, while V/C ferrets averaged 18.3 ± 0.8. Microscopic trachea lesions were not as pronounced compared to those of the pigs, with scores of 0.7 ± 0.6 in NV/C ferrets and 2.8 ± 0.1 in V/C ferrets (Table 1).
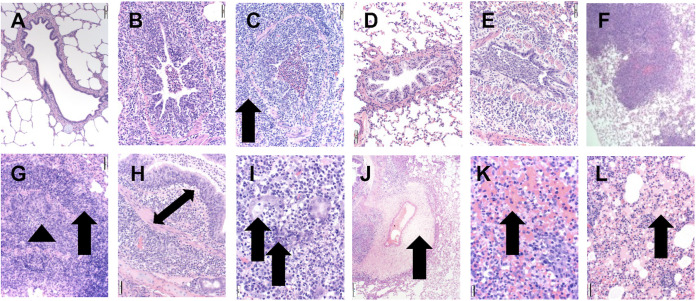
Microscopic lesions were not observed in the lungs or trachea from the NV/NC control groups of either species at 5 dpi (Fig. 4A and D). The NV/C swine (Fig. 4B) and ferrets (Fig. 4E) demonstrated similar microscopic lung lesions that were consistent with uncomplicated influenza A virus infection. The lesions consisted of multifocal bronchitis and bronchiolitis with attenuation or necrosis of the airway epithelium, mild peribronchiolar lymphocytic cuffing, interstitial pneumonia, and low numbers of neutrophils in the airway lumen. Mild to moderate perivascular and peribronchiolar edema was observed in the lungs of NV/C ferrets, whereas the alveoli of NV/C pigs contained sero-proteinaceous fluid.
FIG 4.
Ferrets displayed histological lesions typical of VAERD. Photomicrographs displayed from hematoxylin and eosin (HE)-stained lung tissue sections from non-vaccinated/non-challenged (NV/NC, panels A and D), non-vaccinated/challenged (NV/C, panels B and E), and vaccinated/challenged (V/C, panels C and F) swine (panels A to C) and ferrets (panels D to F). Microscopic lesions were not observed in the lungs from NV/NC swine (panel A) or ferrets (panel D). Moderate bronchi and bronchiolar epithelial necrosis and mild to moderate peribronchiolar lymphocytic cuffing with low numbers of neutrophils in the airway lumen were observed at 200× in NV/C swine (B) and ferrets (E). At low ×40 magnification, ferrets from the V/C group with VAERD demonstrated severe inflammation that exhibited a lobular (F) to diffuse distribution. Severe bronchi and bronchiolar epithelial necrosis or hyperplasia with severe, broad, and densely cellular peribronchiolar lymphocytic cuffing (arrow) and abundant neutrophils expanding the airway lumen (arrowhead) were consistent with VAERD microscopic lesions in swine (C) and ferrets (G, 200×). In VAERD-affected ferrets, a mixed inflammatory infiltrate of neutrophils, macrophages and lymphocytes infiltrated the propria submucosa (double arrow) (H, 200×) and adjacent submucosal glands were effaced by severe necrosis, abundant neutrophils and macrophages (arrows) (I, 400×). Severe perivascular (arrow) and peribronchiolar edema and perivasculitis (J, 100×) was observed in VAERD-affected ferrets with extensive alveolar hemorrhage (arrow) (K) and abundant sero-proteinaceous fluid (arrow) observed in alveolar spaces (L, 200×).
Microscopic lung lesions observed at 5 dpi in the V/C ferrets and the swine exhibiting VAERD were notably similar between the species and more severe compared to those of the NV/C groups. All ferrets in the V/C group had severely affected lung lesion profiles, ranging from a multifocal lobular distribution (Fig. 4F) to locally extensive regions of the pulmonary parenchyma. The V/C ferret microscopic lung lesions were characterized by marked bronchi and bronchiolar epithelial necrosis, dysplasia, or hyperplasia with large numbers of neutrophils occluding the airway lumen (Fig. 4G), which were similar in appearance, frequency, and severity to those of V/C pigs with VAERD (Fig. 4C). An abundant, mixed inflammatory infiltrate consisting of neutrophils, macrophages, and lymphocytes was observed in the bronchiolar propria submucosa (Fig. 4H), extending into the peribronchiolar stroma in ferrets (Fig. 4G) and swine (Fig. 4C). In contrast to swine, V/C ferrets demonstrated submucosal glands adjacent to the affected bronchi which were infiltrated with an abundant mixed inflammatory infiltrate and variable amounts of neutrophilic inflammation inside the glandular lumen (Fig. 4I). Occasional submucosal glands were necrotic and effaced by foci of inflammatory debris. Expansive peribronchiolar cuffs of densely packed lymphocytes, macrophages, and neutrophils were observed in VAERD-affected lungs from ferrets (Fig. 4G) and swine (Fig. 4C). Multifocal, prominent perivascular edema with mixed inflammatory infiltrates was observed expanding the stroma (perivasculitis), which was more severe in V/C ferrets compared to NV/C ferrets (Fig. 4J). Alveolar lumina of V/C ferrets contained large numbers of neutrophils and macrophages that were often admixed with marked hemorrhage (Fig. 4K) and sero-proteinaceous fluid (Fig. 4L), which was not observed in NV/C ferrets. Alveoli in the V/C swine also exhibited similar lesions that included extensive hemorrhage and edema (sero-proteinaceous fluid). Microscopic lesions in the trachea from V/C ferrets and swine consisted of moderate to severe epithelial flattening and necrosis and marked inflammation of the submucosa with large numbers of lymphocytes, macrophages, and neutrophils, which was often mild or absent in the NV/C ferrets and swine.
Vaccine-derived, non-neutralizing, binding antibodies associated with disease state.
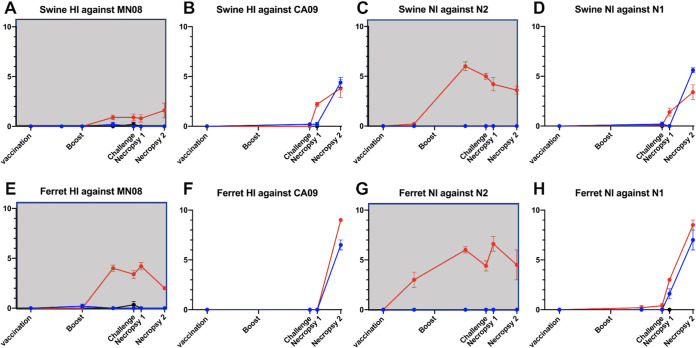
Serum antibody responses to vaccine and challenge virus were measured by HI and NI assays in pigs and ferrets at multiple time points throughout the experiment. All swine and ferret serum samples collected prior to virus or vaccine exposure tested negative by ELISA, HI, and NI. Vaccinated pigs produced minimal homologous HI or NI antibody responses after a single dose of the WIV vaccine against the MN08 HA or NA, but titers increased following the second dose, typical of WIV vaccination (Fig. 5A and C). Vaccinated ferrets had a similar response after the initial vaccination, but produced high titers of inhibitory antibodies against homologous vaccine virus following the second vaccination (Fig. 5E and G). Sera collected from vaccinated pigs and ferrets before challenge were HI- and NI-negative against the heterologous challenge virus CA09 (Fig. 5B, D and F, and H). Although V/C ferrets had no HI or NI response to the heterologous challenge virus at 21 days post-boost or at 0 dpi, antibody binding assays showed that sera collected at these time points had high antibody binding to the HA2 domain of CA09 HA, but not to the HA1 domain (Fig. 6), similar to results shown previously for VAERD-affected pigs (16).
FIG 5.
Antibody response to vaccination did not alter antibody response to heterologous challenge virus in pigs or ferrets. Vaccine derived antibodies did not cross-react with heterologous challenge antigens. Exposure to challenge antigen caused transient increases in vaccine HA and NA specific titers that were more apparent in ferrets. Swine (A-D) and ferret (E-H) sera collected throughout the experiment were evaluated by HI (A, B, E, and F) and NI (C, D, G, and H) against the vaccine antigens (gray background A, C, E and G) and challenge antigens (B, D, F, and H). Data points indicate geometric mean log2 transformed titers for vaccinated (V/C, red) and unvaccinated (NV/C, blue) animals. Error bars represent standard error of the mean.
FIG 6.
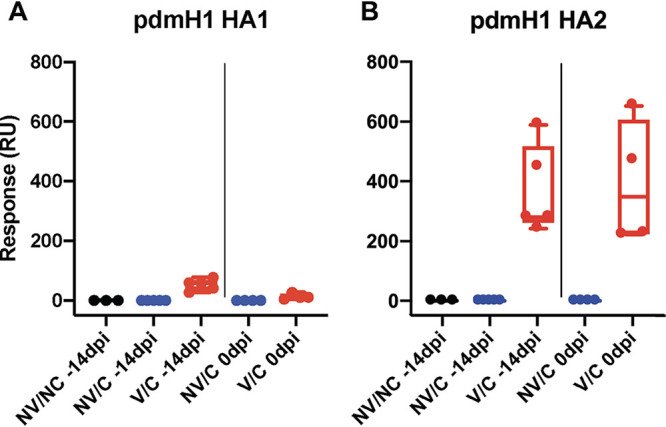
Ferrets vaccinated with a MN08 H1 vaccine produced antibodies that bound H1N1pdm09 HA2, but not HA1. Binding of ferret sera to H1pdm09 (pdmH1) HA1 (panel A) or HA2 (panel B) was measured by surface plasmon resonance. Individual serum was analyzed and displayed by experimental group (NV/NC: black, NV/C: blue, and V/C: red) and time point (−14 or 0 days postinfection). Median values are represented by box and whisker plots for each group. Boxes indicate 25-to-75 percentile and bars mark minimum and maximum values.
Heterologous challenge had minimal effects on the HI and NI responses of pigs against the vaccine virus (Fig. 5A and C). In ferrets, heterologous challenge resulted in slight increases to vaccine-specific HI, from 3.4 at 0 dpi up to 4.2 at 5 dpi; and to NI, from 4.4 to 6.6 (Fig. 5E and G). As described in previous studies (18), vaccinated pigs produced HI and NI antibodies to CA09 after challenge at comparable levels to NV/C pigs, indicating that original antigenic sin is not a sequela to VAERD. In fact, V/C pigs trended toward an earlier HI and NI antibody response to the challenge virus than NV/C pigs (Fig. 5B and D). V/C ferrets had a similar response to the challenge virus which tended to be earlier and higher in titer than that of NV/C ferrets (Fig. 5F and H).
DISCUSSION
In this study, we recapitulated VAERD in ferrets using a previously established experimental model described in pigs, where whole inactivated virus vaccine with an oil-in-water adjuvant was administered followed by challenge with an antigenically mismatched, homosubtypic virus, which resulted in enhanced respiratory disease. Prolonged fever, increased pneumonia, severe, intensive histopathology lesions, and tissue damage in this report were comparable to previous studies of VAERD in swine (15–20). Here, we demonstrated VAERD is not a phenomenon unique to pigs. Ferrets, a standard human IAV model, vaccinated and challenged using the same experimental model, experienced enhanced respiratory disease and lung lesions that were consistent with the VAERD swine model.
Non-vaccinated and challenged (NV/C) swine with uncomplicated influenza-like illness demonstrate microscopic lung lesions which are typically mild to moderate compared to those of their VAERD-affected cohorts, and which often lack the severe suppurative inflammation, edema, and hemorrhage typical of VAERD lesions. In addition to the influenza lesions observed in NV/C swine, NV/C ferrets with uncomplicated influenza infection often demonstrate mild to moderate peribronchiolar and perivascular edema and inflamed submucosal glands, in addition to necrotizing bronchiolitis, peribronchiolar cuffing, and interstitial pneumonia (26).
However, vaccinated, challenged (V/C) ferrets had weight loss and hypothermia consistent with a severe influenza infection (27), while NV/C ferrets displayed signs of disease more typical of a mild to moderate influenza infection. V/C ferrets displayed a 3-fold increase in percentage of macroscopic pneumonia lesions compared to NV/C ferrets and a 2-fold increase in microscopic histological scores. The signs of disease in V/C ferrets tended to be more severe than those measured in V/C pigs. In addition, microscopic lung lesions were remarkably similar between the V/C pigs and ferrets (Fig. 4), albeit more pronounced in ferret lung tissues with severe necrotizing bronchiolitis, prominent peribronchiolar lymphocytic cuffing (Fig. 4C and G), expansive propria submucosa inflammatory response that appeared to occlude the airway lumen (Fig. 4G and H), and extensive alveolar neutrophils, edema, and hemorrhage (Fig. 4K and L). In addition, V/C ferrets in the current study demonstrated marked, prominent perivascular (Fig. 4J) and peribronchiolar edema, perivasculitis, and severe necrotizing lesions of the submucosal glands (Fig. 4I), which were not observed in the V/C swine. The minor species-specific differences may be a result of the slight modification in experiment setup due to different species requirements (infection route, dose, animal age, etc.). However, all microscopic lesions associated with VAERD were more severe in both species compared to those of their NV/C cohorts. The marked vascular changes described in the ferret lungs, as well as the alveolar edema, hemorrhage, and extensive inflammatory response, needs further analysis to discern the mechanisms involved in the VAERD phenomenon. The reproducible VAERD outcome in two primary host species for IAV research provides important animal models to further investigate mechanisms and treatment interventions.
Antibody-dependent enhancement (ADE) was first proposed in 1964 and has been shown to occur during infections from several viruses, most notably in dengue infections (28–31). ADE of influenza has been shown in multiple natural and experimental host species (13, 15, 22–24, 32–34). Early in vitro studies pointed toward non-neutralizing HA antibodies increasing the uptake of virus into Fc receptor (FcR)-bearing cells as a potential mechanism (35–38). Subsequent in vitro studies disputed this, as enhanced infection in FcR deficient cell lines still occurred (16). Additionally, mice treated with ADE-inducing monoclonal antibodies did not have an increase in infected macrophages (39). Studies done in mice, pigs, and ferrets suggested multiple other mechanisms, including a bias toward a Th2 response, an increase in fusion kinetics, and an infection deeper in the respiratory tract (15, 16, 32, 33, 39, 40). Swine vaccinated with WIV MN08 from the 1B.2.2 clade of human pre-2009 seasonal origin produced antibodies that reacted to the HA2 of CA09 HA in the absence of HI activity (16). These high-affinity, non-neutralizing, cross-reactive antibodies increased infectivity via more efficient membrane fusion and represent a possible mechanism of the VAERD phenomenon. Subsequent studies using ADE-inducing monoclonal antibodies in mice demonstrated that these antibodies destabilized the HA stem and allowed faster membrane fusion as well as an expanded infection from the bronchiole to the alveolar cells (39). Further studies are warranted to determine if one or more of these mechanisms are the driving factor behind VAERD in the ferret model. Results from ferrets, reported here, and our previous swine data indicate that non-neutralizing, cross-reactive HA stalk antibodies derived from an adjuvanted vaccine were implicated in the development of VAERD in pigs and ferrets (16, 39).
While current US seasonal influenza vaccines are not adjuvated WIVs like the ones used in the VAERD models described by our laboratory, adjuvanted WIVs are pre-licensed for use during a potential pandemic (21). Additionally, clinical trials have been initiated to evaluate adjuvanted seasonal influenza vaccines and a universal influenza vaccine candidate that targets HA2 stalk domains (41–43). Swine studies have demonstrated that oil-in-water adjuvated WIVs induced the most reproducible and severe VAERD, but other vaccine and adjuvant types have resulted in similar levels of microscopic lung lesions as WIVs with oil-in-water adjuvants (20, 40). Specifically, although a squalene-based adjuvant similar to MF59 induced lung lesions that were not statistically different from those of the NV/C control group, the microscopic and macroscopic lesions had higher group means than those of the NV/C and also were not statistically different from those of the VAERD V/C groups with oil-in-water adjuvants (20). Furthermore, an HA subunit vaccine with oil-in water adjuvant induced VAERD in pigs, pointing to VAERD-associated immunity being targeted to the HA protein (40). That same study included a live attenuated influenza vaccine, which was not associated with VAERD. The variation in ability to induce VAERD between different adjuvants and vaccine platforms highlights the need to understand the mechanisms of VAERD to better assess the risk of VAERD with current and potential future vaccine strategies.
Humans have complicated serological histories due to prior exposures and vaccinations, and VAERD has thus far only been established in naive lab animals. As such, direct comparisons between immune human populations and naive animal models can be problematic. Evidence of enhanced disease in humans with seasonal IAV is scarce and the human seasonal vaccine has proven to be highly safe. However, the current understanding of VAERD suggests that infection with novel IAV within the same subtype that are antigenically distinct may have the potential for enhanced disease in humans with prior mismatched antibody immunity, especially in the absence of HA1 targeting neutralizing antibodies against the infecting mismatched strain. Epidemiological evidence in humans from the 2009 pandemic indicated that more severe disease following infection with H1N1pdm09 was associated with receipt of the 2008-to-2009 trivalent seasonal vaccine (a non-adjuvanted, split vaccine) or the presence of pre-existing, high avidity, non-neutralizing, binding antibodies (22–24, 34). Indeed, in a follow-up study, ferrets vaccinated with the 2008/09 human vaccine and then challenged with a H1N1pdm09 virus developed more severe disease than placebo-vaccinated ferrets, although there was no statistical difference between the groups (33). This indicates that, while the human seasonal vaccine may not have as great a risk of inducing VAERD as the WIV vaccine presented here, it was associated with enhanced disease in ferrets under the described conditions. Ferrets are a standard model for studying human influenza immunity and disease, but a naive ferret may not adequately recapitulate a human with prior IAV immunity. Understanding the effects of preexisting immunity on VAERD susceptibility is vital to gauge the vulnerability of humans to VAERD, thus requiring more complex animal models to be developed and routinely used.
Antigenic diversity in human seasonal influenza is unlikely to elicit VAERD since homosubtypic seasonal viruses rarely drift from one season to the next to the degree of antigenic heterogeneity needed. However, there are annual human cases of infection with swine influenza that are homosubtypic and antigenically heterologous to components of the seasonal vaccine across the globe. First lifetime exposure and antigenic imprinting may also have immunologic impacts with the potential to reduce heterologous cross-neutralizing antibody activity below a threshold (44). These complex interactions between subsequent exposure or vaccination and potential for VAERD requires further investigation. Previous work has shown that vaccine type, inclusion of adjuvant, and adjuvant type can affect the induction and severity of VAERD. Taken together, the results of this study highlight the need to further explore whether VAERD in humans and other influenza hosts is of concern, particularly when designing and implementing future vaccine strategies against antigenically distinct IAVs of the same subtype.
MATERIALS AND METHODS
Vaccines and challenge virus.
A plasmid-based reverse-genetics was used to construct a 7 + 1 recombinant virus combining the HA of a 1B.2.2 (δ-1) cluster virus, A/swine/MN/02011/2008 (MN08), on a A/turkey/Ohio/313053/2004 (OH04) backbone as previously described (45). Virus stock was UV-inactivated and mixed 4:1 vol:vol with an oil-in-water adjuvant (Emulsigen D, MVP Adjuvants, Omaha, NE) to generate the vaccine as previously described (46). Each vaccine dose contained 128 hemagglutination units determined with turkey red blood cells. A reverse-genetics-derived virus combining the HA and NA of a 1A.3.3.2 (pdm09) virus, A/California/04/2009 (CA09), with the 6 internal gene segments of OH04 was used as the challenge virus. All reverse-engineered viruses were kindly provided by Daniel Perez at the University of Georgia and reported previously (18).
Animals.
Twenty-five 3-week-old cross-bred male and female pigs were obtained from an IAV- and PRRSV-free herd. They were randomly divided into two groups of 10 (vaccinated/challenge and non-vaccinated/challenge) and one group of 5 (nonvaccinated/non-challenge). Upon arrival, all pigs were housed in ABSL2 isolation rooms and prophylactically treated with Baytril and Excede. Thirteen 4-to-6-month-old female and neutered male ferrets were obtained from Triple F Farms (Sayer, PA). Upon arrival, ferrets were divided into two groups of 5 (vaccinated and nonvaccinated challenge) and one group of 3 (non-vaccinated, nonchallenge). They were housed in HEPA-filtered, negative pressure isolators in a BSL3 facility. All animals were implanted with a subcutaneous microchip for temperature monitoring and identification purposes (pigs: Destron Fearing, Dallas, TX; ferrets: Biomedic Data Systems Inc., Seaford, DE). All animals were confirmed to be influenza seronegative by a commercial ELISA kit (Swine Influenza Virus Ab Test, IDEXX, Westbrook, ME) prior to the start of the study, and were cared for in compliance with the Institutional Animal Care and Use Committee of the National Animal Disease Center.
Vaccination and challenge.
Animals were held for 1 week to acclimate prior to the start of the study. Vaccine groups were administered one dose (2 mL for swine, 0.5 mL for ferrets) of WIV at −70 days postinfection (dpi) and a second dose at −35 dpi via intramuscular route. All challenge groups were inoculated with 1 × 106 TCID50/mL of challenge virus. Pigs were inoculated with 3 mL, split into 1 mL delivered intranasally and 2 mL intratracheally. Ferrets were inoculated with 1 mL via the intranasal route. Animals were bled for serological analysis at −70, −49, −35, −14, 0, 5, and 21 dpi. Nasal swabs (pigs) or nasal washes (ferret) were collected at 0, 1, 3, and 5 dpi. Temperature (pig and ferret) and body weight (ferret) were taken at −2 through 14 dpi. After humane euthanasia with pentobarbital (pig: FatalPlus, Vortech, Dearborn, MI) or by exsanguination under deep sedation (ferret: 20 mg ketamine/2 mg xylazine per kg ferret), necropsies were performed on 5 pigs and 3 ferrets from each group at 5 dpi. Necropsies were performed on all remaining animals at 21 dpi.
Pathological examination of the respiratory tract.
At necropsy, lungs and trachea were removed for gross pathological examination, broncho-alveolar lavage was performed with 1× Dulbecco’s Modified Eagle Medium with 1% bovine serum albumin and collected fluid (BALF) was cultured on blood agar and NAD-enriched brain heart infusion agar plates to rule out confounding bacterial infections. The total percentage of the surface area affected was determined by visually estimating the affected area of each lobe and then calculated using the weighted proportions of each lobe to the total lung volume (47, 48). Tissue samples of trachea and affected lung were stored in buffered formalin, sectioned, and stained with hematoxylin and eosin. A representative section of lung lesion was evaluated in toto for histologic changes by a veterinarian pathologist blinded to treatment groups. Lung sections were scored on a 22-point scale and trachea sections on an 8-point scale, as previously described (25).
Viral isolation and titration.
Nasal samples and BALF were processed as previously described (13, 49). Briefly, virus isolation was done by incubating 200 mL of sample media on confluent MDCK cells in a 24-well plate with 300 mL of serum-free media for 72 h. Positive samples were identified by the ability of supernatant to agglutinate an equal volume of 0.5% turkey red blood cells. Positive samples were then titrated by standard TCID50. Ten-fold diluted samples were incubated on confluent MDCK-London cells on a 96-well plate. Influenza-positive wells were identified using a hemagglutination assay with 0.5% turkey red blood cells at 72 h postinfection. TCID50 was determined using the Reed-Muench method (50).
Antibody detection assays.
Serum samples were used in hemagglutination inhibition (HI) assays as previously described (51). HI assays were performed against both vaccine and challenge viruses. Swine sera were kaolin-treated and serially diluted 2-fold, and HI titers of turkey red blood cells were measured (51). Following a comparison between receptor-destroying enzyme and kaolin treatment to ensure the effective removal of nonspecific inhibitors, ferret sera were similarly treated with kaolin and serially diluted 2-fold, but HI titers were measured using guinea pig red blood cells. Reciprocal titers were divided by 10 and log2-transformed for statistical analysis. Reported values are geometric means.
An enzyme-linked lectin assay (ELLA) was used to assess neuraminidase-inhibiting antibodies as previously described (52, 53). Briefly, 2-fold serial dilutions of antisera were incubated with H9N2 reassortant viruses containing the NA of A/swine/Nebraska/A01492399/2014 or A/swine/New York/A01104005/2011 on fetuin-coated plates for 18 to 20 h at 37°C. The plates were stained with HRP-conjugated peanut agglutinin (Sigma-Aldrich, St. Louis, MO) and detected with TMB substrate (KPL Laboratories, Gaithersburg, MD). Plates were read at 650 nm and OD650 values were used to calculate percent inhibition. The inverse serum dilution that showed a 50% inhibition of NA activity was considered the NI titer.
Hemagglutinin-binding surface plasmon resonance assay.
Steady-state equilibrium binding of individual post-vaccination sera was monitored at 25°C using a ProteOn surface plasmon resonance (SPR) biosensor (Bio-Rad) as previously described (16, 54–56). The rHA1 and rHA2 domains from the H1N1 A/California/7/2009 HA was coupled to a GLC sensor chip with amine coupling with 500 resonance units (RU) in the test flow cells (16, 56). Samples of 60 μL freshly prepared sera at 10- and 100-fold dilutions were injected at a flow rate of 50 μL/min (120-sec contact time) for association, and dissociation was performed over a 1,200-s interval (at a flow rate of 50 μL/min). Responses from the protein surface were corrected for the response from a mock surface and for responses from a separate, buffer-only injection. MAb 2D7 (anti-CCR5) was used as a negative control in these experiments. Total antibody binding was determined directly from the serum sample interaction with rHA1 and rHA2 protein, which were determined from two independent SPR runs using Bio-Rad ProteOn Manager software (version 3.0.1).
Statistical analysis.
Comparative statistics were performed on the macroscopic and microscopic lesion data sets (Fig. 4 and Table 1). Normality was assessed using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. Analysis of variance (ANOVA) was used to determine significance at P < 0.05 (Prism software, GraphPad, La Jolla, CA). Variables with significant effects by treatment group were subjected to pairwise mean comparison using a Tukey test.
ACKNOWLEDGMENTS
We thank Michelle Harland and Jordyn Zoul for technical assistance and Brett Ashburn, Siu-Yin Virella, Randy Leon, Justin Miller, Jason Heugel, and Jean Kaptur for assistance with animal studies. We thank Hongjun Chen and Daniel Perez for providing reverse-engineered viruses.
This study was supported by USDA-ARS, USDA-APHIS, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services contracts HHSN272201400008C and 75N93021C00015. J.B.K., M.W.B., and B.S.K. were supported by an appointment to the USDA-ARS Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the USDA under contract no. DE-AC05-06OR23100.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services or the FDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Contributor Information
Amy L. Vincent, Email: amy.vincent@usda.gov.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital.
REFERENCES
- 1.Zeller MA, Anderson TK, Walia RW, Vincent AL, Gauger PC. 2018. ISU FLUture: a veterinary diagnostic laboratory web-based platform to monitor the temporal genetic patterns of influenza A virus in swine. BMC Bioinformatics 19:397. 10.1186/s12859-018-2408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walia RR, Anderson TK, Vincent AL. 2019. Regional patterns of genetic diversity in swine influenza A viruses in the United States from 2010 to 2016. Influenza Other Respir Viruses 13:262–273. 10.1111/irv.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, Bielejec F, Burke DF, Dudas G, Fonville JM, Fouchier RA, Kellam P, Koel BF, Lemey P, Nguyen T, Nuansrichy B, Peiris JM, Saito T, Simon G, Skepner E, Takemae N, Consortium E, Webby RJ, Van Reeth K, Brookes SM, Larsen L, Watson SJ, Brown IH, Vincent AL, ESNIP3 consortium. 2016. The global antigenic diversity of swine influenza A viruses. Elife 5:e12217. 10.7554/eLife.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson MI, Stratton J, Killian ML, Janas-Martindale A, Vincent AL. 2015. Continual reintroduction of human pandemic H1N1 influenza A viruses into swine in the united states, 2009 to 2014. J Virol 89:6218–6226. 10.1128/JVI.00459-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epperson S, Jhung M, Richards S, Quinlisk P, Ball L, Moll M, Boulton R, Haddy L, Biggerstaff M, Brammer L, Trock S, Burns E, Gomez T, Wong KK, Katz J, Lindstrom S, Klimov A, Bresee JS, Jernigan DB, Cox N, Finelli L, Influenza AvVIT. 2013. Human infections with influenza A(H3N2) variant virus in the United States, 2011–2012. Clin Infect Dis 57 Suppl 1:S4–S11. 10.1093/cid/cit272. [DOI] [PubMed] [Google Scholar]
- 6.Jhung MA, Epperson S, Biggerstaff M, Allen D, Balish A, Barnes N, Beaudoin A, Berman L, Bidol S, Blanton L, Blythe D, Brammer L, D'Mello T, Danila R, Davis W, de Fijter S, Diorio M, Durand LO, Emery S, Fowler B, Garten R, Grant Y, Greenbaum A, Gubareva L, Havers F, Haupt T, House J, Ibrahim S, Jiang V, Jain S, Jernigan D, Kazmierczak J, Klimov A, Lindstrom S, Longenberger A, Lucas P, Lynfield R, McMorrow M, Moll M, Morin C, Ostroff S, Page SL, Park SY, Peters S, Quinn C, Reed C, Richards S, Scheftel J, Simwale O, Shu B, et al. 2013. Outbreak of variant influenza A(H3N2) virus in the United States. Clin Infect Dis 57:1703–1712. 10.1093/cid/cit649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong KK, Gambhir M, Finelli L, Swerdlow DL, Ostroff S, Reed C. 2013. Transmissibility of variant influenza from swine to humans: a modeling approach. Clin Infect Dis 57:S16–S22. 10.1093/cid/cit303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent AL, Ciacci-Zanella JR, Lorusso A, Gauger PC, Zanella EL, Kehrli ME, Jr, Janke BH, Lager KM. 2010. Efficacy of inactivated swine influenza virus vaccines against the 2009 A/H1N1 influenza virus in pigs. Vaccine 28:2782–2787. 10.1016/j.vaccine.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Loving CL, Lager KM, Vincent AL, Brockmeier SL, Gauger PC, Anderson TK, Kitikoon P, Perez DR, Kehrli ME, Jr. 2013. Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011–2012 H3N2v. J Virol 87:9895–9903. 10.1128/JVI.01038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bikour MH, Cornaglia E, Elazhary Y. 1996. Evaluation of a protective immunity induced by an inactivated influenza H3N2 vaccine after an intratracheal challenge of pigs. Can J Vet Res 60:312–314. [PMC free article] [PubMed] [Google Scholar]
- 11.Gauger PC, Vincent AL, Loving CL, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. 2011. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 29:2712–2719. 10.1016/j.vaccine.2011.01.082. [DOI] [PubMed] [Google Scholar]
- 12.Heinen PP, Rijsewijk FA, de Boer-Luijtze EA, Bianchi AT. 2002. Vaccination of pigs with a DNA construct expressing an influenza virus M2-nucleoprotein fusion protein exacerbates disease after challenge with influenza A virus. J Gen Virol 83:1851–1859. 10.1099/0022-1317-83-8-1851. [DOI] [PubMed] [Google Scholar]
- 13.Vincent AL, Lager KM, Janke BH, Gramer MR, Richt JA. 2008. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet Microbiol 126:310–323. 10.1016/j.vetmic.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Kitikoon P, Vincent AL, Janke BH, Erickson B, Strait EL, Yu S, Gramer MR, Thacker EL. 2009. Swine influenza matrix 2 (M2) protein contributes to protection against infection with different H1 swine influenza virus (SIV) isolates. Vaccine 28:523–531. 10.1016/j.vaccine.2009.09.130. [DOI] [PubMed] [Google Scholar]
- 15.Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. 2012. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol 49:900–912. 10.1177/0300985812439724. [DOI] [PubMed] [Google Scholar]
- 16.Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, Vincent AL, Golding H. 2013. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 5:200ra114. 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 17.Gauger PC, Loving CL, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA, Vincent AL. 2013. Vaccine-associated enhanced respiratory disease does not interfere with the adaptive immune response following challenge with pandemic A/H1N1 2009. Viral Immunol 26:314–321. 10.1089/vim.2013.0018. [DOI] [PubMed] [Google Scholar]
- 18.Rajao DS, Chen H, Perez DR, Sandbulte MR, Gauger PC, Loving CL, Shanks GD, Vincent A. 2016. Vaccine-associated enhanced respiratory disease is influenced by haemagglutinin and neuraminidase in whole inactivated influenza virus vaccines. J Gen Virol 97:1489–1499. 10.1099/jgv.0.000468. [DOI] [PubMed] [Google Scholar]
- 19.Souza CK, Rajao DS, Loving CL, Gauger PC, Perez DR, Vincent AL. 2016. Age at vaccination and timing of infection do not alter vaccine-associated enhanced respiratory disease in influenza A virus-infected pigs. Clin Vaccine Immunol 23:470–482. 10.1128/CVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza CK, Rajao DS, Sandbulte MR, Lopes S, Lewis NS, Loving CL, Gauger PC, Vincent AL. 2018. The type of adjuvant in whole inactivated influenza A virus vaccines impacts vaccine-associated enhanced respiratory disease. Vaccine 36:6103–6110. 10.1016/j.vaccine.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 21.Tregoning JS, Russell RF, Kinnear E. 2018. Adjuvanted influenza vaccines. Hum Vaccin Immunother 14:550–564. 10.1080/21645515.2017.1415684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.To KK, Zhang AJ, Hung IF, Xu T, Ip WC, Wong RT, Ng JC, Chan JF, Chan KH, Yuen KY. 2012. High titer and avidity of nonneutralizing antibodies against influenza vaccine antigen are associated with severe influenza. Clin Vaccine Immunol 19:1012–1018. 10.1128/CVI.00081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, Rosella LC, Dickinson JA, Gilca R, Sethi P, Ouhoummane N, Willison DJ, Rouleau I, Petric M, Fonseca K, Drews SJ, Rebbapragada A, Charest H, Hamelin ME, Boivin G, Gardy JL, Li Y, Kwindt TL, Patrick DM, Brunham RC, Canadian ST, for the Canadian SAVOIR Team. 2010. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring–Summer 2009: four observational studies from Canada. PLoS Med 7:e1000258. 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Aguilar L, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE, Jr, Williams JV, Melendi GA, Polack FP. 2011. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 17:195–199. 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauger PC, Loving CL, Khurana S, Lorusso A, Perez DR, Kehrli ME, Jr, Roth JA, Golding H, Vincent AL. 2014. Live attenuated influenza A virus vaccine protects against A(H1N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology 471-473:93–104. 10.1016/j.virol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Moore IN, Lamirande EW, Paskel M, Donahue D, Kenney H, Qin J, Subbarao K. 2014. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. J Virol 88:13879–13891. 10.1128/JVI.02341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron CM, Cameron MJ, Bermejo-Martin JF, Ran L, Xu L, Turner PV, Ran R, Danesh A, Fang Y, Chan PK, Mytle N, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Rowe T, Kelvin DJ. 2008. Gene expression analysis of host innate immune responses during lethal H5N1 infection in ferrets. J Virol 82:11308–11317. 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkes RA. 1964. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci 42:465–482. 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- 29.Smatti MK, Al Thani AA, Yassine HM. 2018. Viral-induced enhanced disease illness. Front Microbiol 9:2991. 10.3389/fmicb.2018.02991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halstead SB, Chow JS, Marchette NJ. 1973. Immunological enhancement of dengue virus replication. Nat New Biol 243:24–26. [PubMed] [Google Scholar]
- 31.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 32.Kobinger GP, Meunier I, Patel A, Pillet S, Gren J, Stebner S, Leung A, Neufeld JL, Kobasa D, von Messling V. 2010. Assessment of the efficacy of commercially available and candidate vaccines against a pandemic H1N1 2009 virus. J Infect Dis 201:1000–1006. 10.1086/651171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skowronski DM, Hamelin ME, De Serres G, Janjua NZ, Li G, Sabaiduc S, Bouhy X, Couture C, Leung A, Kobasa D, Embury-Hyatt C, de Bruin E, Balshaw R, Lavigne S, Petric M, Koopmans M, Boivin G. 2014. Randomized controlled ferret study to assess the direct impact of 2008-09 trivalent inactivated influenza vaccine on A(H1N1)pdm09 disease risk. PLoS One 9:e86555. 10.1371/journal.pone.0086555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchihashi Y, Sunagawa T, Yahata Y, Takahashi H, Toyokawa T, Odaira F, Ohyama T, Taniguchi K, Okabe N. 2012. Association between seasonal influenza vaccination in 2008–2009 and pandemic influenza A (H1N1) 2009 infection among school students from Kobe, Japan, April-June 2009. Clin Infect Dis 54:381–383. 10.1093/cid/cir787. [DOI] [PubMed] [Google Scholar]
- 35.Ochiai H, Kurokawa M, Hayashi K, Niwayama S. 1988. Antibody-mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J Virol 62:20–26. 10.1128/JVI.62.1.20-26.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochiai H, Kurokawa M, Kuroki Y, Niwayama S. 1990. Infection enhancement of influenza A H1 subtype viruses in macrophage-like P388D1 cells by cross-reactive antibodies. J Med Virol 30:258–265. 10.1002/jmv.1890300406. [DOI] [PubMed] [Google Scholar]
- 37.Ochiai H, Kurokawa M, Matsui S, Yamamoto T, Kuroki Y, Kishimoto C, Shiraki K. 1992. Infection enhancement of influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J Med Virol 36:217–221. 10.1002/jmv.1890360312. [DOI] [PubMed] [Google Scholar]
- 38.Gotoff R, Tamura M, Janus J, Thompson J, Wright P, Ennis FA. 1994. Primary influenza A virus infection induces cross-reactive antibodies that enhance uptake of virus into Fc receptor-bearing cells. J Infect Dis 169:200–203. 10.1093/infdis/169.1.200. [DOI] [PubMed] [Google Scholar]
- 39.Winarski KL, Tang J, Klenow L, Lee J, Coyle EM, Manischewitz J, Turner HL, Takeda K, Ward AB, Golding H, Khurana S. 2019. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc Natl Acad Sci USA 116:15194–15199. 10.1073/pnas.1821317116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajao DS, Loving CL, Gauger PC, Kitikoon P, Vincent AL. 2014. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine 32:5170–5176. 10.1016/j.vaccine.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 41.NIH. 2019. Flublok or fluzone with Advax-CpG55.2 or AF03. https://clinicaltrials.gov/ct2/show/NCT03945825. Accessed 9/28/2021.
- 42.NIH. 2019. Dose, safety, tolerability and immunogenicity of an influenza H1 stabilized stem ferritin vaccine, VRCFLUNPF099-00-VP, in healthy adults. https://clinicaltrials.gov/ct2/show/NCT03814720. Accessed 4/12/2020.
- 43.Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, Berlanda-Scorza F, Stadlbauer D, Wilson PC, Aydillo T, Behzadi MA, Bhavsar D, Bliss C, Capuano C, Carreno JM, Chromikova V, Claeys C, Coughlan L, Freyn AW, Gast C, Javier A, Jiang K, Mariottini C, McMahon M, McNeal M, Solorzano A, Strohmeier S, Sun W, Van der Wielen M, Innis BL, Garcia-Sastre A, Palese P, Krammer F. 2021. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med 27:106–114. 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- 44.Kelvin AA, Zambon M. 2019. Influenza imprinting in childhood and the influence on vaccine response later in life. Euro Surveill 24:1900720. 10.2807/1560-7917.ES.2019.24.48.1900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. 2002. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20:3165–3170. 10.1016/S0264-410X(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 46.Vincent AL, Ma W, Lager KM, Richt JA, Janke BH, Sandbulte MR, Gauger PC, Loving CL, Webby RJ, Garcia-Sastre A. 2012. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol 86:10597–10605. 10.1128/JVI.01439-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol 32:648–660. 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan BS, Kimble JB, Chang J, Anderson TK, Gauger PC, Janas-Martindale A, Killian ML, Bowman AS, Vincent AL. 2020. Aerosol transmission from infected swine to ferrets of an H3N2 virus collected from an agricultural fair and associated with human variant infections. J Virol 94:e01009-20. 10.1128/JVI.01009-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent AL, Lager KM, Ma W, Lekcharoensuk P, Gramer MR, Loiacono C, Richt JA. 2006. Evaluation of hemagglutinin subtype 1 swine influenza viruses from the United States. Vet Microbiol 118:212–222. 10.1016/j.vetmic.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Reed L, Muench M. 1938. Simple method of estimating fifty percent endpoints. The American J Hygiene 27:493–497. 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 51.Kitikoon P, Gauger PC, Vincent AL. 2014. Hemagglutinin inhibition assay with swine sera. Methods Mol Biol 1161:295–301. 10.1007/978-1-4939-0758-8_24. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan BS, Vincent AL. 2020. Detection and titration of influenza A virus neuraminidase inhibiting (NAI) antibodies using an enzyme-linked lectin assay (ELLA). Methods Mol Biol 2123:335–344. 10.1007/978-1-0716-0346-8_24. [DOI] [PubMed] [Google Scholar]
- 53.Couzens L, Gao J, Westgeest K, Sandbulte M, Lugovtsev V, Fouchier R, Eichelberger M. 2014. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J Virol Methods 210:7–14. 10.1016/j.jviromet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 3:85ra48. 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Jr, Beigel JH. 2016. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 22:1439–1447. 10.1038/nm.4201. [DOI] [PubMed] [Google Scholar]
- 56.Khurana S, Hahn M, Coyle EM, King LR, Lin TL, Treanor J, Sant A, Golding H. 2019. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat Commun 10:3338. 10.1038/s41467-019-11296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]