Abstract
Background:
Femoroacetabular impingement (FAI) has been proposed as an etiologic factor in up to 50% of hips with osteoarthritis (OA). Inflammation is thought to be one of the main initiators of OA, yet little is known about the origin of intra-articular inflammation in FAI hips.
Hypothesis:
Articular cartilage from the impingement zone of patients with FAI has high levels of inflammation, reflecting initial inflammatory process in the hip.
Study Design:
Controlled laboratory study.
Methods:
Head-neck cartilage samples were obtained from patients with cam FAI (cam FAI, early FAI; n = 15), advanced OA secondary to cam FAI (FAI OA, late FAI; n = 15), and advanced OA secondary to developmental dysplasia of the hip (DDH OA, no impingement; n = 15). Cartilage procured from young adult donors (n = 7) served as control. Safranin O–stained sections were assessed for cartilage abnormality. Tissue viability was detected by TUNEL assay. Immunostaining of interleukin 1β (IL-1β), catabolic markers (matrix metalloproteinase 13 [MMP-13], a disintegrin and metalloproteinase with thrombospondin motif 4 [ADAMTS-4], aggrecan antibody to C-terminal neoepitope [NITEGE]), and an anabolic marker (type II collagen [COL2]) was performed to evaluate molecular inflammation and metabolic activity. The average percentage of immunopositive cells from the total cell count was calculated. Kruskal-Wallis test followed by Steel-Dwass post hoc test was used for multiple comparisons.
Results:
Microscopic osteoarthritic changes were more prevalent in cartilage of cam FAI and FAI OA groups compared with DDH OA and control groups. Cartilage in cam FAI and FAI OA groups, versus the DDH group, had higher expression of inflammatory molecules IL-1β (69.7% ± 18.1% and 72.5% ± 13.2% vs 32.7% ± 14.4%, respectively), MMP-13 (79.6% ± 12.6% and 71.4% ± 18.8% vs 38. 5% ± 13.3%), ADAMTS-4 (83.9% ± 12.2% and 82.6% ± 12.5% vs 45.7% ± 15.5%), and COL2 (93.6% ± 3.9% and 92.5% ± 5.8% vs 53.3% ± 21.0%) (P < .001). Expression of NITEGE was similar among groups (cam FAI, 89.7% ± 7.7%; FAI OA, 95.7% ± 4.7%; DDH OA, 93.9% ± 5.2%; P = .0742). The control group had minimal expression of inflammatory markers. Inflammatory markers were expressed in all cartilage zones of early and late FAI but only in the superficial zone of the no impingement group.
Conclusion:
Cartilage from the impingement zone in FAI is associated with a high expression of inflammatory markers, extending throughout all cartilage zones.
Clinical Relevance:
Inflammation associated with FAI likely has a deleterious effect on joint homeostasis. Further clinical and translational studies are warranted to assess whether and how surgical treatment of FAI reduces molecular inflammation.
Keywords: femoroacetabular impingement, osteoarthritis, inflammation, cartilage degeneration
Prevalence of symptomatic hip osteoarthritis (OA) increases with age, affecting an estimated 21% of the population between 45 and 54 years old, increasing to 42% of the population over 75 years of age.22 More than 300,000 individuals in the United States undergo primary total hip replacement (THR) annually, and the rate of THR is projected to double by 2030.21 Causes of hip OA include femoroacetabular impingement (FAI) due to overcoverage of the femoral head and/or abnormal bone deformity in the femoral head-neck junction, named cam deformity, and developmental dysplasia of the hip (DDH) due to undercoverage of the femoral head without hip impingement.15,29 FAI may play an etiologic role in up to 50% of hip OA cases.33,42,43 Since FAI was established by Ganz et al16 as an important etiologic factor for hip OA, various studies on the pathogenesis and pathologic process of hip OA have been conducted.9,20,36,39 Prevalence of cartilage damage in patients with symptomatic FAI is high, with delamination cartilage lesions observed commonly in the superolateral aspect of the acetabulum (impingement zone) and early degenerative malacia lesions observed in the posterior peripheral rim.6,27,30 However, the molecular mechanisms by which hip FAI leads to progression of disease resulting in hip OA are not well-understood.
A number of molecular mediators of inflammation, such as cytokines, chemokines, and metalloproteinases, have been shown to be present during the process of joint degeneration leading to OA.32 Specifically, interleukin 1β (IL-1β) is a key cytokine involved in the pathogenesis of early and late stage OA, as it induces inflammation and cartilage catabolism.17 IL-1β causes articular cartilage destruction by stimulating the production of catabolic molecules such as matrix metalloproteinase 13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondin motif 4 (ADAMTS-4).2,24 Aggrecan (ACAN) and type II collagen (COL2) are major structural component of the extracellular matrix of articular cartilage. Upregulation and increased production of ACAN and COL2 by chondrocytes are normally observed as a response to the loss of joint homeostasis in OA.32 An inflammatory cascade has been identified in cartilage samples from the impingement zone of the hip in patients undergoing surgical treatment for symptomatic FAI, suggesting that the impingement area is metabolically active and a potential structural precursor to hip OA.18 The head-neck impingement zone could be a focalized metabolically active area initiating loss of joint homeostasis and development of OA. The etiopathogenesis that leads to hip OA from FAI has not been completely elucidated. Study of intra-articular inflammation in hip FAI has been limited,10,18 and the location of these molecules and their expression during different stages of disease (early and late FAI) have not been previously investigated.
The goal of this study is to demonstrate the levels and microscopic patterns of inflammatory markers in cartilage from the head-neck junction in patients with symptomatic cam FAI. We hypothesized that articular cartilage from the impingement zone of patients with FAI exhibits tissue degeneration and has high expression of molecular markers of inflammation. Identification of early changes in the hip resulting from FAI could improve our understanding of how treatment of FAI affects the joint and, in the long term, could potentially lead to the development of novel therapies to delay or prevent the initiation and progression of OA.
METHODS
Patients and Cartilage Samples
This study was approved by the institutional review board (IRB No. 201703054), and informed consent was obtained from all patients. Full-thickness cartilage samples from the anterolateral head-neck junction of 45 patients (45 hips) undergoing hip surgery were obtained. Of these, 15 patients underwent hip surgery for the treatment of symptomatic cam FAI (cam FAI, early FAI; n = 15) and 15 patients underwent THR for the treatment of advanced OA secondary to cam FAI (FAI OA, late FAI; n = 15). For the comparison group, full-thickness cartilage samples from patients with advanced OA secondary to DDH were used (DDH OA, no impingement; n = 15). None of the patients in the DDH OA group had any evidence of FAI as determined by clinical examination and an alpha angle of less than 55°.12 The diagnosis of cam FAI or OA was determined by the treating surgeons (C.P-G. and J.C.C.) using the minimum basic criteria of pain in the affected hip for a period of more than 3 months, hip range of motion, radiographic findings, and intraoperative findings. The Tönnis classification38 was used to define the severity of hip OA, and OA was diagnosed in all patients with a Tönnis grade of 2 or higher. A cam deformity was defined by an alpha angle greater than 55° on the preoperative anteroposterior (AP) pelvic, frog-leg lateral, and/or 45° Dunn radiographs.12 Acetabular dysplasia was defined by the lateral center-edge angle (LCEA) less than 20° on the preoperative AP pelvic view. All radiographs were performed with a standardized protocol including a supine AP pelvic view and frog-leg lateral view. Interobserver and intraobserver reliability of the radiographic analysis of FAI was previously performed by our group.28
All surgical procedures were performed by 2 experienced surgeons (C.P-G. and J.C.C.) between May 2017 and December 2018. Exclusion criteria included previous surgery, pincer morphology, infection, idiopathic osteonecrosis of the femoral head, psoriasis, and rheumatologic conditions. Patients were asked to stop taking nonsteroidal anti-inflammatory drugs (NSAIDs) a week before surgery. A flowchart of the study population selection including patient enrollment and exclusion criteria is shown in Figure 1. All patients in this study had persistent hip pain that was refractory to at least 3 months of nonoperative treatment (activity modification, NSAIDs, and physical therapy). As a control, cartilage samples were harvested from the anterolateral head-neck area of femoral head fresh allografts from young adult donors (control; n = 7). Allografts were fresh, obtained within 24 hours of donor death, and specifically requested for research reasons (JRF Ortho).
Figure 1.
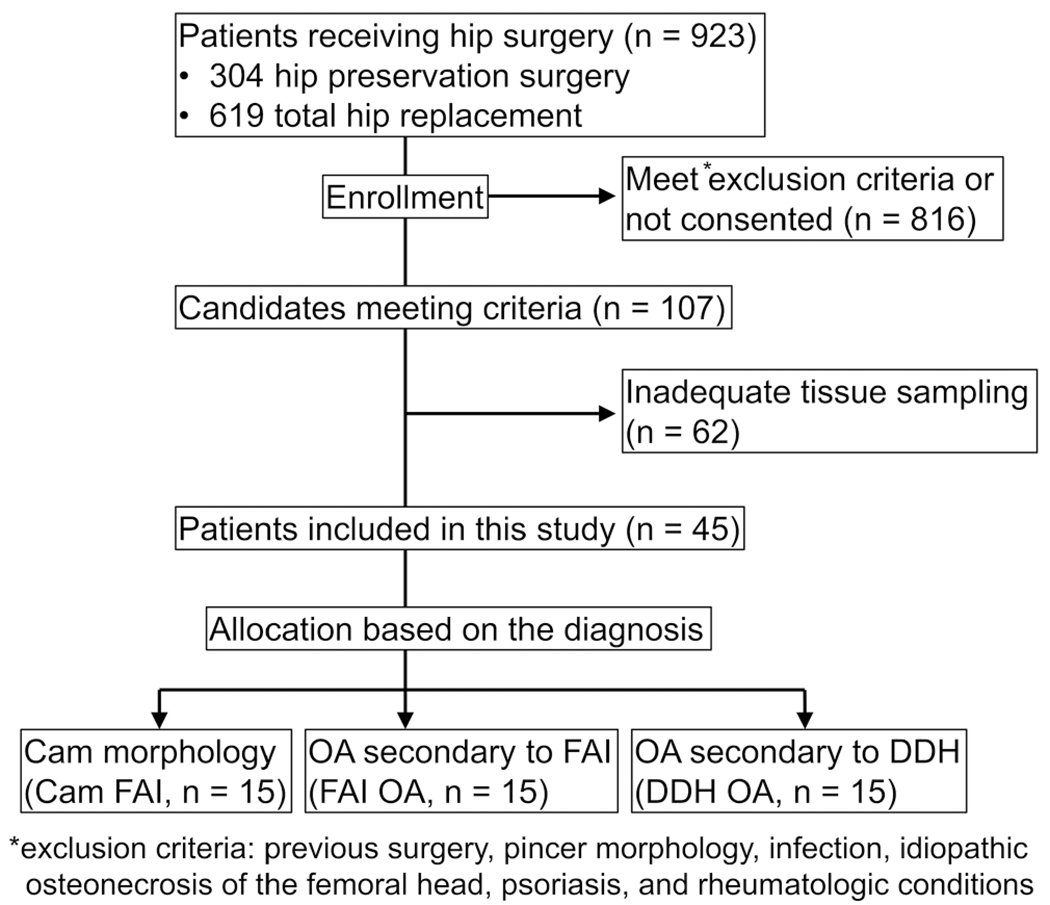
A flowchart of the patients’ enrollment and exclusion criteria. DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; OA, osteoarthritis.
Cartilage Sample Collection
During surgery, full-thickness cartilage samples were obtained through use of an arthroscopic biter in arthroscopic procedures or a half-inch osteotome in patients undergoing open surgical dislocation or THR. All samples were obtained from the anterolateral aspect of the head-neck junction (impingement area) of the proximal femur. Care was taken to harvest cartilage tissue from the most affected areas (visible chondromalacia and discoloration) and localized between the 12- and 4-o’clock positions. The study design is shown in Figure 2. Cartilage samples were immediately transferred to the laboratory in 10% neutral buffered formalin. Femoral head allografts were delivered to the laboratory in medium at 4 °C within 24 hours after donor death.
Figure 2.
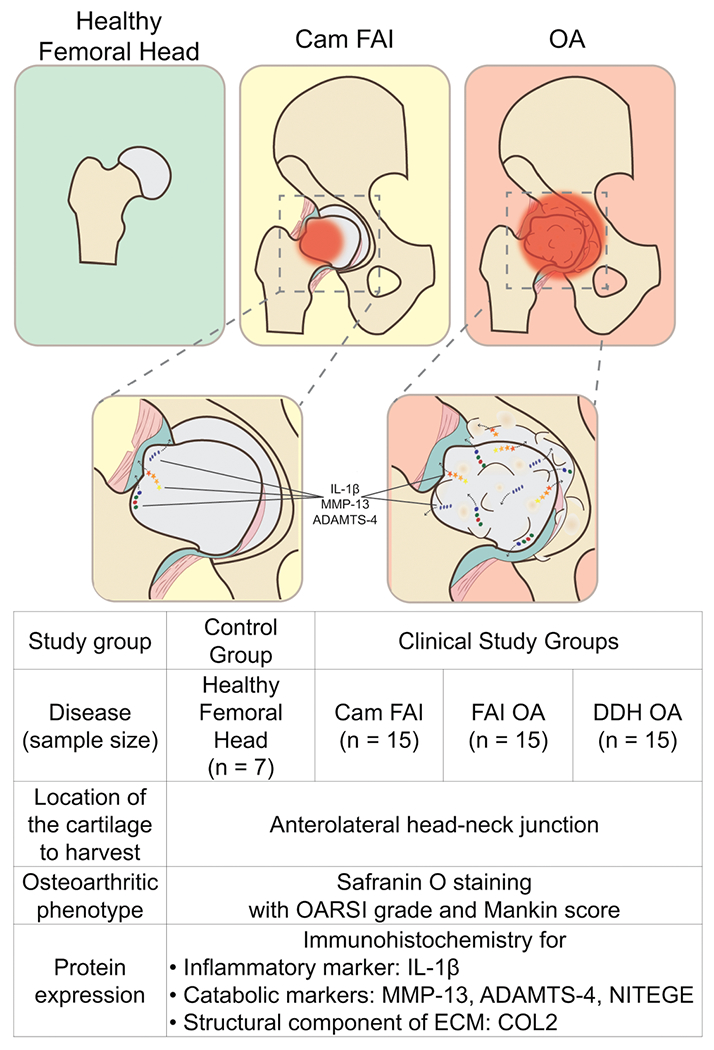
Study design and schema of the structural changes. ADAMTS-4, a disintegrin and metalloproteinase with thrombospondin motif 4; COL2, type II collagen; DDH, developmental dysplasia of the hip; ECM, extracellular matrix; FAI, femoroacetabular impingement; IL-1β, interleukin 1β; MMP-13, matrix metalloproteinase 13; NITEGE, aggrecan antibody to C-terminal neoepitope; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International.
Histological Evaluation
Cartilage specimens were fixed in 10% neutral buffered formalin for 24 to 48 hours, decalcified in Immunocal (Stat-Lab) for 3 days, dehydrated in graded ethanol, embedded in paraffin wax, and sectioned (5 μm) by standard protocol. Sections were stained with safranin O and fast green for histological analysis and to estimate the content and distribution of proteoglycan of articular cartilage. Cartilage degeneration was graded based on the Osteoarthritis Research Society International (OARSI) grade (Table 1)31 and Mankin score (Table 2)23 in a blinded fashion by 3 investigators (M.H., Lei Cai, and C.P-G.). The Mankin score consists of 4 items: structure, cells, safranin O staining, and tidemark integrity.
TABLE 1.
Osteoarthritis Research Society International (OARSI) Grading System
| Grade | Subgrade | Associated Criteria |
|---|---|---|
| 0: Surface intact, cartilage intact | None | Intact, uninvolved cartilage |
| 1: Surface intact | 1.0: Cells intact 1.5: Cell death |
Matrix: superficial zone intact, edema and/or fibrillation Cells: proliferation (clusters), hypertrophy Reaction must be more than fibrillation only |
| 2: Surface discontinuity | 2.0: Fibrillation through superficial zone 2.5: Surface abrasion with matrix loss within superficial zone |
Features for grade 1: + discontinuity at superficial zone, ± safranin O or toluidine blue depletion on upper one-third of cartilage (mid zone), ± chondron column disorientation |
| 3: Vertical fissures | 3.0: Simple fissures 3.5: Branched/complex fissures |
Features for grade 2: ± safranin O or toluidine blue on upper two-thirds of cartilage (deep zone), ± neocartilage formation (polarized light microscopy, picrosirius red stain) |
| 4: Erosion | 4.0: Superficial zone delamination 4.5: Midzone excavation |
Cartilage matrix loss, cyst formation within matrix |
| 5: Denudation | 5.0: Bone surface intact 5.5: Reparative tissue surface present |
Surface is sclerotic bone or reparative tissue including fibrocartilage |
| 6: Deformation | 6.0: Joint margin osteophytes 6.5: Joint margin central osteophyte |
Bone remodeling; deformation of articular surface contour (more than osteophyte formation only); includes microfracture and repair |
TABLE 2.
Mankin Scoring System
| Grade | |
|---|---|
| Structure | |
| Normal | 0 |
| Surface irregularities | 1 |
| Pannus and surface irregularities | 2 |
| Clefts to transitional zone | 3 |
| Clefts to radial zone | 4 |
| Clefts to calcified zone | 5 |
| Complete disorganization | 6 |
| Cells | |
| Normal | 0 |
| Diffuse hypercellularity | 1 |
| Cloning | 2 |
| Hypocellularity | 3 |
| Safranin O staining | |
| Normal | 0 |
| Slight reduction | 1 |
| Moderate reduction | 2 |
| Severe reduction | 3 |
| No dye noted | 4 |
| Tidemark integrity | |
| Intact | 0 |
| Crossed by blood vessels | 1 |
| Maximum score | 14 |
Assessment of Chondrocyte Apoptosis
Before the immunohistochemistry, the viability of the chondrocytes was confirmed by in situ detection of chondrocyte apoptosis on the sections through use of the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (In situ Cell Death Detection Kit; Roche). Images were taken with a Dmi8 confocal microscope (Leica). The apoptotic chondrocytes in the cartilage were characterized by TUNEL-positive (green) staining. Viability percentage of the chondrocytes was assessed as described previously11 via ImageJ software (National Institutes of Health).34
Immunohistochemistry
Slides were set for baking at 60°C for 15 minutes followed by deparaffinization in xylene twice for 10 minutes. Slides were rehydrated in descending concentrations of ethanol with final washes with distilled water and phosphate-buffered saline (PBS). For antigen retrieval, slides were digested with 10 μg/mL proteinase K (MilliporeSigma) at 37 °C for 20 minutes in a humidifying chamber. After washing with PBS, slides were treated with PeroxAbolish (Biocare Medical) to block endogenous peroxidase activity at room temperature for 20 minutes. After washing with PBS, slides were blocked in 10% normal goat serum in PBS at room temperature for 1 hour. After the blocking buffer was drained off, slides were incubated with the following primary antibodies procured from Abcam for respective proteins at 1:100 dilutions using 2% goat serum in PBS at 4°C overnight: rabbit anti-IL-1β (ab2105), rabbit anti–MMP-13 (ab39012), rabbit anti-ADAMTS-4 (ab185722), rabbit anti-COL2 (ab34712), and rabbit anti-NITEGE (aggrecan antibody to C-terminal neoepitope). Anti-neoepitope antibodies can be used to demonstrate the effects of increased MMP and ADAMTS activities in articular cartilage. Anti-NITEGE antibody is one of the neoepitopes of ACAN that can recognize C-terminal cleavage sites of aggrecan. The C-terminal NITEGE fragment is one of the G1 fragments produced when aggrecan is cleaved with aggrecanases.14 Negative control was performed for all samples by use of 2% goat serum in PBS without primary antibody. After 3 washes with PBS, slides were incubated with secondary anti-rabbit antibody (ab6721; Abcam) at 1:1000 dilutions at room temperature for 2 hours. The signal was developed as brown-reaction products using a peroxidase substrate 3,3 notion product (Betazoid DAB Chromogen Kit; Biocare Medical). Hematoxylin was used as a counterstain. Finally, the slides were rinsed with distilled water and dehydrated before being sealed with mounting medium. Sections were analyzed via NanoZoomer Digital Pathology System (Hamamatsu Photonics). The average percentage of immunopositive cells from the total cell count was calculated by ImageJ software34 in a blinded fashion by 3 observers (M.H., Lei Cai, and C.P-G.) using a slightly modified method previously reported.26 Positive cells superior to the tidemark were included in the assessment.
Statistical Analyses
The Kruskal-Wallis test followed by the Steel-Dwass post hoc test was used for multiple comparisons. A P value of < .05 indicated statistically significant differences. All values are presented as mean ± standard deviation. Data analysis was performed by use of the Bell Curve for Excel software (Social Survey Research Information Co).
RESULTS
Patients’ Characteristics
Characteristics of study participants are presented in Table 3. The average alpha angle and LCEA were higher in the cam FAI and FAI OA groups versus the DDH OA group (alpha angle, 70.1° ± 11.6° and 84.2° ± 12.1° vs 43.1° ± 6.4°, respectively, P < .001; LCEA, 28.0° ± 6.0° and 28.4° ± 8.8° vs 12.5° ± 9.7°, P < .001).
TABLE 3.
Characteristics of Study Participantsa
| Study Group | Control | Cam FAI | FAI OA | DDH OA |
|---|---|---|---|---|
| No. | 7 | 15 | 15 | 15 |
| Age, y | 25.1 ± 7.1 | 32.5 ± 9.0 | 57.1 ± 7.5 | 49.3 ± 7.9 |
| Sex, n | 2 males | 11 males | 13 males | 15 females |
| 5 females | 4 females | 2 females | ||
| BMI, kg/m2 | NA | 28.1 ± 4.7 | 31.4 ± 6.5 | 28.1 ± 5.8 |
| Tönnis classification | NA | 0.5 ± 0.5 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Alpha angle, deg | NA | 70.1 ± 11.6 | 84.2 ± 12.1 | 43.1 ± 6.4 |
| LCEA, deg | NA | 28.0 ± 6.0 | 28.4 ± 8.8 | 12.5 ± 9.7 |
| Tissue source, procedure | Femoral head allograft from young adults | 11 hip arthroscopy, 4 surgical dislocation | 15 THR | 15 THR |
Values are expressed as mean ± SD unless otherwise noted.
BMI, body mass index; DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; LCEA, lateral center-edge angle; NA, not applicable; OA, osteoarthritis; THR, total hip replacement.
Histological Evaluation
Cartilage samples from cam FAI (early FAI) and FAI OA (late FAI) showed worse degenerative changes (osteoarthritic phenotype) than DDH OA. Control cartilage samples showed normal hyaline cartilage. Histological pictures for each item are shown in Figure 3. The average OARSI grade was higher in the cam FAI and FAI OA groups versus the DDH OA group (4.3 ± 0.8 and 3.6 ± 0.9 vs 2.4 ± 0.7, respectively; P < .01). No difference was observed in the OARSI grade between cam FAI and FAI OA (P = .3956). The average OARSI grade in all 3 groups was higher (worse) than control (P < .01). For each item of the Mankin score, cam FAI and FAI OA showed higher (worse) score for structure than DDH OA (P < .05). Safranin O staining in cam FAI and FAI OA was markedly decreased or absent compared with DDH OA and control, suggesting a reduction or absence of proteoglycan content (P < .01). Overall, the average Mankin score (summation of each score) was higher in the cam FAI and FAI OA versus DDH OA (8.3 ± 2.1 and 7.1 ± 1.9 vs 4.5 ± 1.1; P < .01). The average Mankin score in all 3 groups with hip abnormality (cam FAI, FAI OA, and DDH OA) was higher than control (P < .01). No difference was observed in the Mankin score between cam FAI and FAI OA (P = .5402) (Figure 4). All groups showed similar viability in the TUNEL assay (control, 93.5% ± 2.7%; cam FAI, 96.2% ± 4.0%; FAI OA, 93.8% ± 4.4%; DDH OA, 96.4% ± 2.0%; P = .5462) (Figures 3 and 4).
Figure 3.
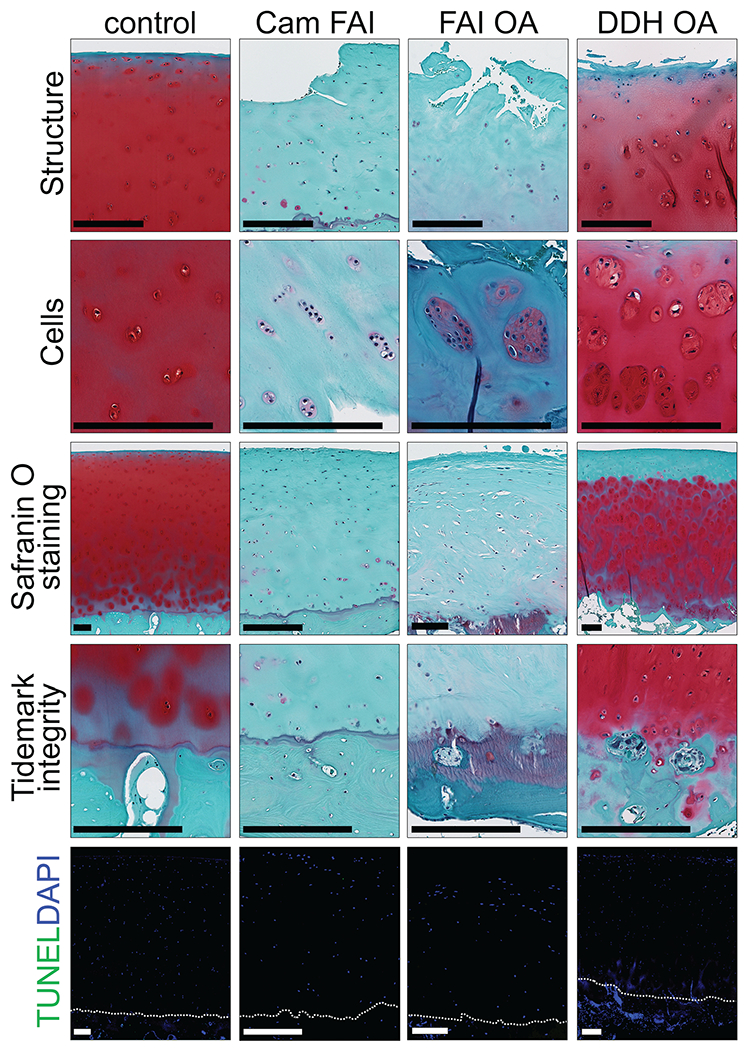
Safranin O staining and TUNEL assay of the cartilage from the head-neck area in all 4 groups. Structure, cells, safranin O staining, and tidemark integrity are shown on each panel. The dotted line shows the tidemark. Scale bar = 250 μm. DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; OA, osteoarthritis.
Figure 4.
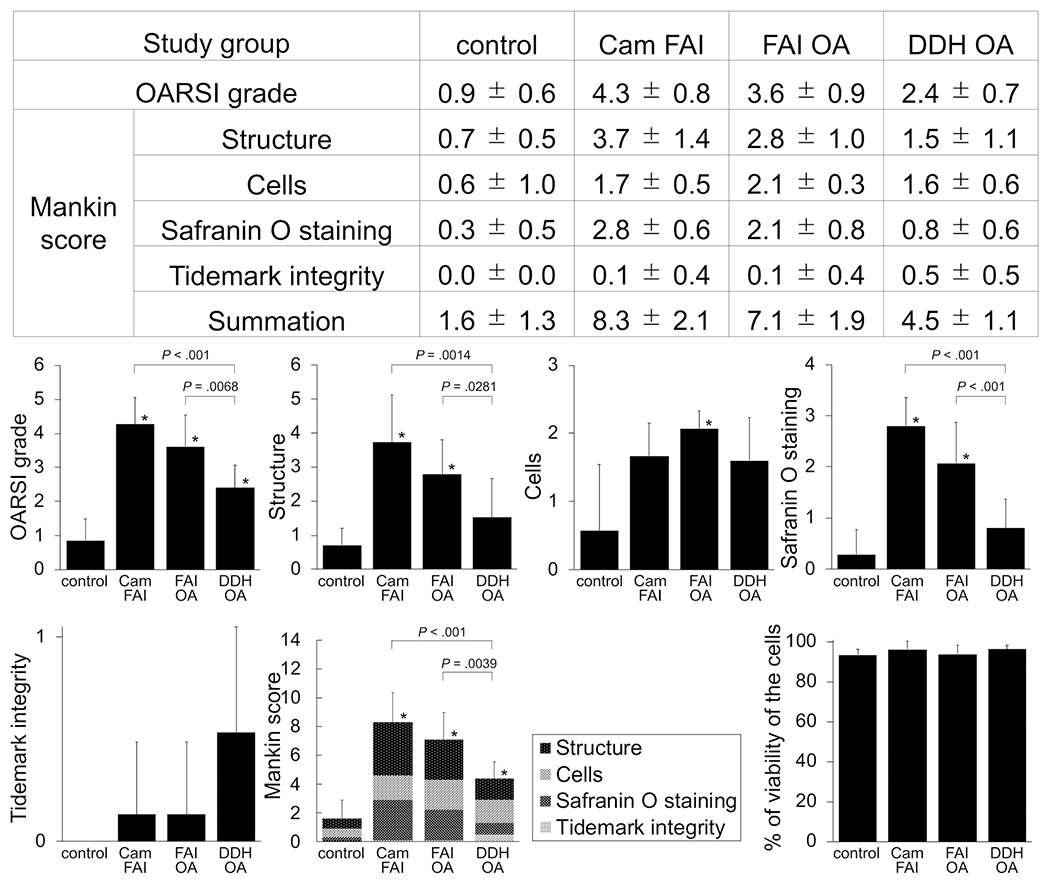
Assessment of cartilage degeneration and chondrocyte viability of the cartilage from the head-neck area in corresponding groups. Bar graphs represent OARSI grade, each item of the Mankin score, summation of the Mankin score, and percentage of viability of the cells across groups. Statistical differences were observed among groups. *Significant difference compared with the control, P < .05. DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; OA, osteoarthritis; OARSI, Osteoarthritis Research Society International.
Levels and Pattern of Inflammatory Markers
Although inflammatory and catabolic proteins (IL-1β, MMP-13, ADAMTS-4) were present in cartilage samples from all groups (cam FAI, FAI OA, DDH OA), their levels were higher in FAI (cam FAI and FAI OA) than DDH OA (P < .001). Minimal expression was observed in the control group. The percentage of immunopositive cells for IL-1β was different across all 4 groups and was higher in the cam FAI and FAI OA groups than the DDH OA group (69.7% ± 18.1% and 72.5% ± 13.2% vs 32.7% ± 14.4%; P < .001). IL-1β was expressed throughout all zones in cam FAI and FAI OA but mainly in the superficial zone in DDH OA. A similar distribution was observed for MMP-13 (cam FAI, 79.6% ± 12.6%; FAI OA, 71.4% ± 18.8%; DDH OA, 38.5% ± 13.3%; P < .001) and ADAMTS-4 (cam FAI, 83.9% ± 12.2%; FAI OA, 82.6% ± 12.5%; DDH OA, 45.7% ± 15.5%; P < .001). Controls had lower (minimal) expression of IL-1β, MMP-13, ADAMTS-4, and NITEGE (20.2% ± 4.9%, 25.3% ± 9.5%, 24.3% ± 11.1%, and 39.8% ± 20.5%; P < .05). The expression of COL2 in controls was similar to that in cam FAI and FAI OA (95.4% ± 6.4%). The NITEGE immunopositive cells were present through all cartilage zones in all study groups (cam FAI, 89.7% ± 7.7%; FAI OA, 95.7% ± 4.7%; DDH OA, 93.9% ± 5.2%; P = .0742) (Figure 5). Areas with weak safranin O staining tended to have strong immunostaining for anti-NITEGE antibody (Figures 3 and 5A).
Figure 5.
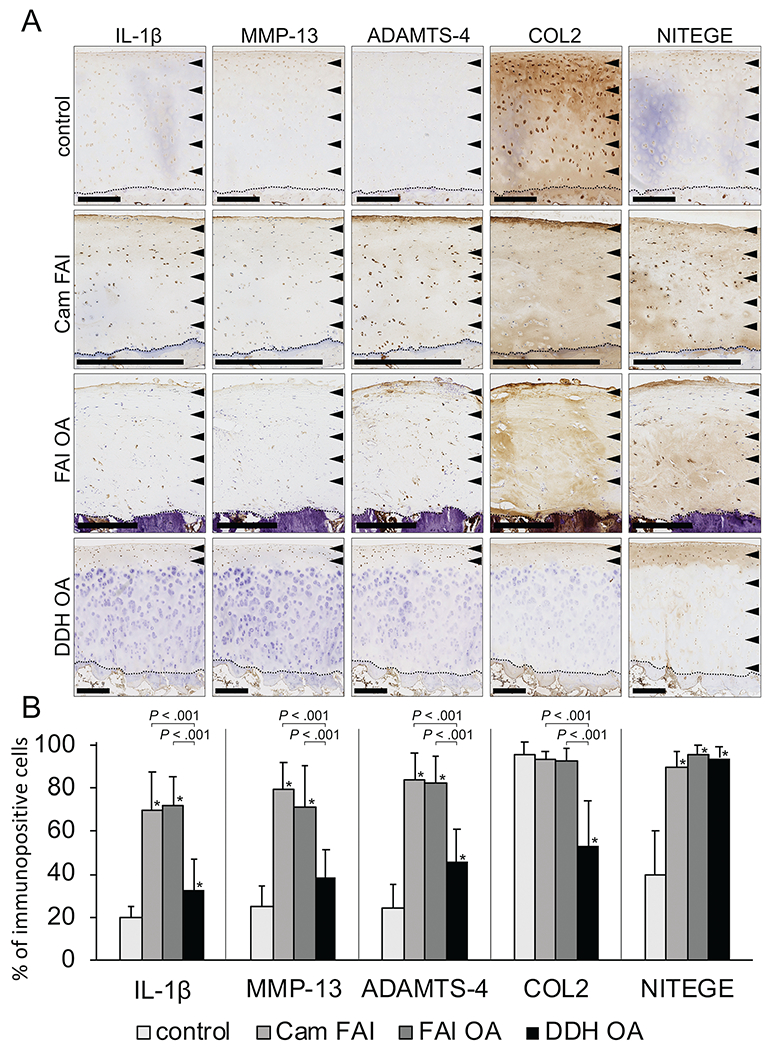
(A) Immunohistochemistry of the cartilage from the head-neck area in corresponding groups. The arrowheads show the zone of immunopositive cells, and the dotted line shows the tidemark on each panel. Scale bar = 500 μm. (B) Bar graphs are represent number of IL-1β, MMP-13, ADAMTS-4, COL2, and NITEGE positive cells across groups. Statistical differences were observed among groups. *Significant difference compared with the control, P < .05. ADAMTS-4, a disintegrin and metalloproteinase with thrombospondin motif 4; COL2, type II collagen; DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; IL-1β, interleukin 1β; MMP-13, matrix metalloproteinase 13; NITEGE, aggrecan antibody to C-terminal neoepitope; OA, osteoarthritis.
DISCUSSION
Cartilage from the impingement zone (anterolateral head-neck junction) of hips with FAI (early and late stage) expressed microscopic degenerative changes (osteoarthritic phenotype) and markedly elevated levels of selected inflammatory proteins compared with cartilage from patients with no impingement (DDH OA) and healthy controls. Increased inflammation in patients with impingement suggest that the chondrocytes are metabolically active in the impingement zone.
The microscopic degenerative osteoarthritic changes we observed in the impingement zone are similar to those reported in previous studies. Wagner et al39 reported that cartilage samples from the impingement zone in 22 patients with hip FAI showed early osteoarthritic changes. In addition, the authors compared these samples with advanced OA cartilage samples obtained from the central weightbearing zone of the femoral head from patients undergoing THR. OA samples showed fissures, clefts, and a severe reduction of safranin O staining in the vast majority, with higher Mankin score (8.6 ± 1.0) than the hips with FAI. In contrast to our study, Wagner et al used cartilage samples from patients with advanced OA, although they did not indicate whether the OA was secondary to FAI or DDH. Their cartilage samples were harvested from the central weightbearing zone of the femoral head and not from the impingement zone. Speirs et al36 evaluated histological changes in cartilage from the head-neck area of the patients with hip cam FAI. Similar to us, they reported that proteoglycan content was substantially depleted and the cartilage exhibited severe degeneration. They also found lower compressive stiffness and higher permeability in their cartilage samples, consistent with degenerative changes associated with OA. Speir et al did not investigate OA cartilage.
A novel aspect of our study was the inclusion of cartilage samples from the head-neck junction and impingement area from patients in all study groups. Similar to previous authors,36,39 we observed microscopic degenerative osteoarthritic changes (disorganization, cluster formation, and loss of proteoglycan) in cartilage samples from patients with FAI in both early and late stages. These findings suggest that early OA changes have already occurred in the head-neck cartilage area in patients with symptomatic hip impingement who undergo surgical treatment.
MMP-13 and ADAMTS-4 have pivotal roles in both normal and pathological turnover of the extracellular matrix in cartilage tissues. Although metalloproteinases such as MMP-13 and ADAMTS-4 can easily be detected by immunohistochemistry, the determination of whether a metalloproteinase is active and acting on a particular substrate can be questioned. Therefore, we performed immunohistochemistry for the anti-NITEGE, an ACAN neoepitope antibody, to detect C-terminal site of cleaved ACAN on the cartilage sections.
Hashimoto et al18 reported that cartilage samples from the impingement zone of hips with FAI express higher gene levels of ADAMTS-4 compared with samples that have OA secondary to FAI. Our histological findings are also in line with those from Chinzei et al,10 who reported that cartilage from the impingement zone shows high inflammation as measured by the mRNA expression of IL-1β, MMP-13, and ADAMTS-4. Unlike Chinzei et al, who reported that the expression of COL2A1 in cartilage samples was higher in hips with OA compared with FAI, we found higher protein expression of COL2 in the FAI groups.
IL-1β, MMP-13, ADAMTS-4, and COL2 were expressed in all zones of the head-neck cartilage in patients with FAI, whereas their expression was primarily limited to the superficial zone of cartilage in patients without FAI. Chondrocytes in the superficial zone are unique in function and phenotypes, containing dense collagen fibrils to resist shear stress.7 Shear stress would be expected to induce inflammatory changes isolated to the superficial zone in the early stages of FAI. This may be more isolated in DDH, where hip instability is the primary problem. In hip impingement, repetitive crush injury between the acetabulum and anterolateral head-neck junction may induce inflammatory changes to all cartilage zones. Also, chondrocytes from the superficial zone show greater vulnerability to the harmful effects of IL-1 than cells in the deeper zone.19 Different phenotypes have been described in articular cartilage zones, with chondrocytes from superficial zones accepted to have specific characteristics of adhesion and protein synthesis.1,3,4,35,44 We believe that inflammation in all cartilage zones may result from chronic shear stress6 to articular cartilage, whereas in patients without impingement, the inflammation is focalized only in superficial zones secondary to a primary process of disease.19,40 Additionally, we confirmed good chondrocyte viability in all groups, noting that the level of the expression of anabolic and catabolic markers was not related to loss of chondrocyte viability but mainly was related to gain or loss of chondrocyte activity. Similarly, previous studies have shown that chondrocytes undergo metabolic alterations and shift from a resting regulatory state to a highly metabolically active state in OA.8,25,41
These observations raise the possibility that surgical correction of the cam deformity in patients with symptomatic hip cam FAI could reduce molecular inflammation and thus improve or even restore joint homeostasis. This theory is consistent with findings described by Beaule et al,5 who reported that surgical correction of a cam deformity in patients with symptomatic cam FAI not only improved clinical function but was also associated with decreases in T1ρ values and acetabular bone mineral density. We did not investigate the response of cartilage to the osteochondroplasty or removal of impingement, an area that deserves further study in the future.
Limitations of the current study included, first, the fact that cartilage samples were harvested only from symptomatic FAI patients. Inflammation may be present, perhaps at a lower level, in patients with FAI with few or no symptoms. Second, we did not match for age and sex between FAI and DDH groups. Although this would have been ideal, matching these 2 populations is a significant undertaking since DDH is primarily observed in females (80%).13,37 Additionally, previous studies have reported no differences in the expression of inflammatory, catabolic, and anabolic molecules (IL-1β, MMP-13, ADAMTS-4, and COL2A1) when comparing cartilage samples from the head-neck junction in younger (<30 years) and older (≥30 years) patients.10 Furthermore, there was no difference in age between the control and cam FAI groups in this study. Third, late FAI (FAI OA) was defined as an alpha angle greater than 55° and Tönnis grade of 2 or higher. None of these patients had a pre-OA radiograph, raising the concern that an increased alpha angle may have been secondary to an osteophyte in the head-neck area secondary to OA. To minimize this potential confounder, we excluded patients with severe deformities or excessive osteophytes where the increased alpha angle may not have been due to preexisting cam lesion. Fourth, to have a true spatial representation of intra-articular joint inflammation, several samples from different intra-articular locations would be needed to represent the status of the whole joint and not only the affected head-neck area. However, this approach would not be ethically appropriate. In the future, animal models of FAI could be used to more precisely define the joint specific location and timing relative to the disease process of this inflammation.
Despite these limitations, the current study suggests that the involvement of molecular inflammation in the cartilage from the head-neck junction is a mechanism by which impingement may be a structural precursor to hip OA. Further studies are needed to better characterize the role of inflammation in this disease process and to determine whether surgical treatment (decompression) of FAI may arrest or reduce the inflammatory cascade and its effect on the hip joint.
ACKNOWLEDGMENT
This study was supported by the OREF/Goldberg Research Grant in Arthritis. The authors thank Lei Cai, Crystal Idle-burg, and Samantha Coleman for their technical assistance and Gail E. Pashos, Sean M. Akers, Karla J. Crook, and Caroline Drain for their support of research coordination. The authors also acknowledge JRF Ortho for providing allografts and Dr Amanda Forsang for providing rabbit anti-NITEGE373 antibody. Figure 2 was provided by Amanda Dicks and Anushree Seth in association with InPrint at Washington University in StLouis, Missouri. The Curing Hip Disease Fund and Jackie and Randy Baker Research Funds provided partial salary support for research personnel.
One or more of the authors has declared the following potential conflict of interest or source of funding:
This work was supported in part by the Washington University Musculoskeletal Research Center (NIH P30 AR057235), the OREF/Goldberg Research Grant in Arthritis, the Curing Hip Disease Fund (J.C.C.), and the Jacqueline & W. Randolph Baker Fund (J.C.C.). JRF Ortho is acknowledged for providing allografts. NanoZoomer was supported by a National Institutes of Health Shared Instrumentation Grant (S10 RR0227552). C.P-G. has received grants from AOSSM/Sanofi, Arthrex, OREF, and Zimmer outside the submitted work. J.C.C. has received grants from Zimmer Biomet and the Department of Defense-USAMRAA, product royalties from Microport Orthopaedics, and publication royalties from Wolters Kluwer Health outside the submitted work, and is a consultant for Zimmer Biomet and Microport Orthopaedics. R.H.B. has received personal fees from Magellan and Arthrex outside the submitted work. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
REFERENCES
- 1.Archer CW, McDowell J, Bayliss MT, Stephens MD, Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990;97(pt 2):361–371. [DOI] [PubMed] [Google Scholar]
- 2.Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase: a soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999;274(10): 6594–6601. [DOI] [PubMed] [Google Scholar]
- 3.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between subpopulations of cultured bovine articular chondrocytes, II: proteoglycan metabolism. Connect Tissue Res. 1988;18(3):223–234. [DOI] [PubMed] [Google Scholar]
- 4.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes, I: morphology and cartilage matrix production. Connect Tissue Res. 1988;18(3):205–222. [DOI] [PubMed] [Google Scholar]
- 5.Beaule PE, Speirs AD, Anwander H, et al. Surgical correction of cam deformity in association with femoroacetabular impingement and its impact on the degenerative process within the hip joint. J Bone Joint Surg Am. 2017;99(16):1373–1381. [DOI] [PubMed] [Google Scholar]
- 6.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012–1018. [DOI] [PubMed] [Google Scholar]
- 7.Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. [DOI] [PubMed] [Google Scholar]
- 8.Blasioli DJ, Kaplan DL. The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev. 2014;20(4):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretschneider H, Stiehler M, Hartmann A, et al. Characterization of primary chondrocytes harvested from hips with femoroacetabular impingement. Osteoarthritis Cartilage. 2016;24(9):1622–1628. [DOI] [PubMed] [Google Scholar]
- 10.Chinzei N, Hashimoto S, Fujishiro T, et al. Inflammation and degeneration in cartilage samples from patients with femoroacetabular impingement. J Bone Joint Surg Am. 2016;98(2):135–141. [DOI] [PubMed] [Google Scholar]
- 11.D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712–719. [DOI] [PubMed] [Google Scholar]
- 12.Domayer SE, Ziebarth K, Chan J, Bixby S, Mamisch TC, Kim YJ. Femoroacetabular cam-type impingement: diagnostic sensitivity and specificity of radiographic views compared to radial MRI. Eur J Radiol. 2011;80(3):805–810. [DOI] [PubMed] [Google Scholar]
- 13.Engesaeter IO, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesaeter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fosang AJ, Last K, Stanton H, et al. Neoepitope antibodies against MMP-cleaved and aggrecanase-cleaved aggrecan. Methods Mol Biol. 2010;622:312–347. [DOI] [PubMed] [Google Scholar]
- 15.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed] [Google Scholar]
- 16.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. [DOI] [PubMed] [Google Scholar]
- 17.Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1–11. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Rai MF, Gill CS, Zhang Z, Sandell LJ, Clohisy JC. Molecular characterization of articular cartilage from young adults with femoroacetabular impingement. J Bone Joint Surg Am. 2013; 95(16):1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauselmann HJ, Flechtenmacher J, Michal L, et al. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis Rheum. 1996;39(3):478–488. [DOI] [PubMed] [Google Scholar]
- 20.Ho CP, Surowiec RK, Frisbie DD, et al. Prospective in vivo comparison of damaged and healthy-appearing articular cartilage specimens in patients with femoroacetabular impingement: comparison of T2 mapping, histologic endpoints, and arthroscopic grading. Arthroscopy. 2016;32(8):1601–1611. [DOI] [PubMed] [Google Scholar]
- 21.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. [DOI] [PubMed] [Google Scholar]
- 22.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36(4):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips, II: correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–537. [PubMed] [Google Scholar]
- 24.Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13(5):302–311. [DOI] [PubMed] [Google Scholar]
- 26.Moldovan F, Pelletier JP, Hambor J, Cloutier JM, Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997; 40(9):1653–1661. [DOI] [PubMed] [Google Scholar]
- 27.Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296–303. [DOI] [PubMed] [Google Scholar]
- 28.Nepple JJ, Martell JM, Kim YJ, et al. Interobserver and intraobserver reliability of the radiographic analysis of femoroacetabular impingement and dysplasia using computer-assisted measurements. Am J Sports Med. 2014;42(10):2393–2401. [DOI] [PubMed] [Google Scholar]
- 29.Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561–570. [DOI] [PubMed] [Google Scholar]
- 30.Pascual-Garrido C, Li DJ, Grammatopoulos G, Yanik EL, Clohisy JC. The pattern of acetabular cartilage wear is hip morphology-dependent and patient demographic-dependent. Clin Orthop Relat Res. 2019;477(5):1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. [DOI] [PubMed] [Google Scholar]
- 32.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3(2): 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankar WN, Nevitt M, Parvizi J, Felson DT, Agricola R, Leunig M. Femoroacetabular impingement: defining the condition and its role in the pathophysiology of osteoarthritis. J Am Acad Orthop Surg. 2013;21(suppl 1):S7–S15. [DOI] [PubMed] [Google Scholar]
- 34.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siczkowski M, Watt FM. Subpopulations of chondrocytes from different zones of pig articular cartilage: isolation, growth and proteoglycan synthesis in culture. J Cell Sci. 1990;97(pt 2):349–360. [DOI] [PubMed] [Google Scholar]
- 36.Speirs AD, Beaule PE, Huang A, Frei H. Properties of the cartilage layer from the cam-type hip impingement deformity. J Biomech. 2017;55:78–84. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson DA, Mineau G, Kerber RA, Viskochil DH, Schaefer C, Roach JW. Familial predisposition to developmental dysplasia of the hip. J Pediatr Orthop. 2009;29(5):463–466. [DOI] [PubMed] [Google Scholar]
- 38.Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81(12): 1747–1770. [DOI] [PubMed] [Google Scholar]
- 39.Wagner S, Hofstetter W, Chiquet M, et al. Early osteoarthritic changes of human femoral head cartilage subsequent to femoro-acetabular impingement. Osteoarthritis Cartilage. 2003;11(7):508–518. [DOI] [PubMed] [Google Scholar]
- 40.Walter H, Kawashima A, Nebelung W, Neumann W, Roessner A. Immunohistochemical analysis of several proteolytic enzymes as parameters of cartilage degradation. Pathol Res Pract. 1998;194(2): 73–81. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Silverman RM, Shen J, O’Keefe RJ. Distinct metabolic programs induced by TGF-beta1 and BMP2 in human articular chondrocytes with osteoarthritis. J Orthop Translat. 2018;12:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyles CC, Heidenreich MJ, Jeng J, Larson DR, Trousdale RT, Sierra RJ. The John Charnley Award: redefining the natural history of osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Relat Res. 2017;475(2):336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036–3043. [DOI] [PubMed] [Google Scholar]
- 44.Zanetti M, Ratcliffe A, Watt FM. Two subpopulations of differentiated chondrocytes identified with a monoclonal antibody to keratan sulfate. J Cell Biol. 1985;101(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


