Abstract
PURPOSE
To explore trial-level and patient-level associations between response (complete remission [CR] and CR + CR with incomplete hematologic [CRi] or platelet [CRp] recovery), event-free survival (EFS), and overall survival (OS) in newly diagnosed acute myeloid leukemia (AML) trials of intensive chemotherapy.
METHODS
We identified data from eight randomized, active-controlled trials of intensive chemotherapy submitted to the US Food and Drug Administration for treatment of newly diagnosed AML (N = 4,482). Associations between trial-level odds ratios (ORs) for CR and CR + CRi or CRp, and hazard ratios (HRs) for EFS and OS were analyzed using weighted linear regression models. We performed patient-level responder analyses to compare OS by response using pooled data from all studies.
RESULTS
In trial-level analyses, association between HR for OS and OR for CR was moderate (R2 = 0.49; 95% CI, 0.05 to 0.86), as was the association with OR for CR + CRi or CRp (R2 = 0.48; 95% CI, 0.05 to 0.99). For OS versus EFS, a strong association was observed (R2 = 0.87; 95% CI, 0.47 to 0.98) when EFS definitions were harmonized across trials using raw data. In the patient-level responder analyses, patients who achieved CR had better OS compared with CRi or CRp responders (0.73; 95% CI, 0.64 to 0.84) and nonresponders (HR, 0.33; 95% CI, 0.31 to 0.37).
CONCLUSION
On a trial level, there is a moderate association between OS and CR rate. A strong association between EFS and OS was observed. However, CIs were wide, and results became moderate using alternative definitions for EFS. Patient-level analyses showed CR responders have better OS compared with CRi or CRp responders and nonresponders. A therapy in newly diagnosed AML with benefit in EFS or substantial benefit in CR rate would be likely to have an OS effect.
INTRODUCTION
Acute myeloid leukemia (AML) is a life-threatening hematopoietic neoplasm, with approximately 20,240 new cases and 11,400 deaths in the United States estimated in 2021.1 New therapies are needed to increase cure rates, extend survival, prevent relapse, and improve patient quality of life.
CONTEXT
Key Objective
Patients with acute myeloid leukemia (AML) are in need of more effective therapies. Using trial-level and patient-level data from eight randomized, controlled trials of intensive chemotherapy in patients with newly diagnosed AML, we examined the relationship between complete remission (CR) rate and event-free survival (EFS) with overall survival (OS).
Knowledge Generated
Trial-level analyses demonstrated a moderate association between odds ratios for CR rate and hazard ratios for OS and a strong association between hazard ratios for EFS and OS using a harmonized definition of EFS. Patient-level responder analyses demonstrated that patients who achieved a CR had better OS compared with CR without full count recovery and nonresponse.
Relevance
Use of CR rate and EFS as clinical trial end points could allow earlier evaluation of novel therapies for patients with newly diagnosed AML, and may correlate with OS. Thus, both end points would be important to study in trials of novel therapeutics in patients with newly diagnosed AML.
Since 2017, nine new drug approvals have been granted by the US Food and Drug Administration (FDA) for the treatment of AML. Three new drugs, midostaurin, gemtuzumab ozogamicin (GO), and daunorubicin and cytarabine liposome injection (also known as CPX-351), were approved in combination with, or for use as, intensive induction chemotherapy in patients with newly diagnosed AML. Midostaurin, a kinase inhibitor, was approved first for adults with newly diagnosed Fms-like tyrosine kinase 3–mutated AML in combination with intensive induction and consolidation on the basis of improvement in overall survival (OS) in the randomized, placebo-controlled RATIFY trial.2,3 CPX-351, the new liposomal formulation of daunorubicin and cytarabine, was approved for treatment of therapy-related AML or AML with myelodysplasia-related changes on the basis of better OS and a higher complete remission (CR) rate compared with standard therapy in these populations.4 Finally, GO, a CD33-directed antibody-drug conjugate, was approved on the basis of improved event-free survival (EFS) when given in combination with intensive induction and consolidation chemotherapy to patients with newly diagnosed CD33-positive AML.5,6 GO was the first FDA approval in AML on the basis of a benefit in EFS, and use of this end point was discussed at a 2017 Oncologic Drugs Advisory Committee (ODAC) meeting.7
Although durable CR rate and OS have long been the preferred end points for assessment of new therapies for AML,8-10 several novel therapeutics have demonstrated effects on lesser response rates (eg, CR with incomplete hematologic recovery [CRi] and CR with incomplete platelet recovery [CRp]) and the time-to-event end point EFS. EFS, in particular, can be difficult to interpret on the basis of varied definitions used across trials, due in part to published guidelines being relatively vague with respect to how treatment failure should be captured.11,12 Furthermore, the relationships between the different response rates, EFS, and OS in newly diagnosed AML have not been conclusively established.
Herein, we performed an analysis of clinical trial data submitted to the FDA between 2007 and 2017 to examine the association between response rates, a harmonized definition of EFS, and OS in patients with newly diagnosed AML who were treated with intensive induction chemotherapy.
METHODS
Trial Selection Criteria
We searched for clinical trial data submitted to the FDA as part of New Drug or Biologics License Applications for treatment of newly diagnosed AML between 2007 and 2017. Criteria for inclusion were randomized, active-controlled (head-to-head or add-on), multicenter trials of intensive AML induction and consolidation chemotherapy with a sample size of at least 100 participants.
Outcome Measures
Efficacy end points including response rates, EFS, and OS were identified and analyzed by FDA reviewers using raw clinical trial data. For the purposes of this analysis, the definition of EFS was harmonized across trials by FDA reviewers and defined as the time from random assignment to treatment failure, relapse from CR, or death from any cause, whichever occurred earlier, as per International Working Group criteria.11 The harmonization of EFS in the primary analysis included (1) use of CR responses (ie, CRi or CRp counted as treatment failure), (2) definition of treatment failure at the date of random assignment for patients in whom therapy did not yield a CR following induction, and (3) censoring at the date last known to be alive for patients without an event at the data cutoff. OS was defined as the time from random assignment to death from any cause. For patients alive at the data cutoff date, OS was censored at the last date known to be alive. Responses of CR, CRi, and CRp were defined per the individual study protocols, which were roughly in line with International Working Group criteria.11 Patients who achieved partial response, stable disease, were unevaluable, or had unknown response data were considered nonresponders. Unless otherwise specified, the analyses were performed on an intent-to-treat basis, with all randomly assigned patients being included.
Statistical Analysis
The association between treatment effects on CR rate, CR + CRi or CRp, and the time-to-event end point, EFS, and OS were examined on the trial level using weighted linear regression models, with weights equal to sample size of each randomized trial. The analysis was performed on a log scale, weighted by sample size of each comparison. Coefficient of determination (R2) and 95% CIs were calculated to measure the strength of the association. Treatment effects on EFS and OS were measured as hazard ratios (HRs), estimated using Cox proportional hazards regression models. Treatment effects on CR rate and CR + CRi or CRp were measured using odds ratios (ORs) estimated from logistic regression models. An HR of < 1 indicates a treatment benefit to the experimental arm for EFS and OS. An OR of > 1 indicates a favorable result for CR rate or CR + CRi or CRp on the experimental arm.
Sensitivity analyses of the trial-level association between treatment effects on EFS and OS were examined by alternative EFS definitions (Table 1). These alternative definitions were revised from the primary definition using alternative events and alternative induction failure date. Sensitivity analysis 1 was the same as the primary definition, except that the event date for treatment failure was the date of permanent discontinuation of study treatment or the end of the induction period (if there was no information on the end of induction in submitted data, day 60 was used), whichever was earlier; sensitivity 2 was the same as the primary definition, except that CRi or CRp responses were not considered treatment failure and relapse from both CR and CRi or CRp were considered; sensitivity 3 was similar to sensitivity 1 except that CRi or CRp responses were not considered treatment failure and relapse from both CR and CRi or CRp were considered; sensitivity 4 considered relapse from CR or death as events only without considering treatment failure; and sensitivity 5 was similar to sensitivity 4 except that relapse from both CR and CRi or CRp were considered.
TABLE 1.
Sensitivity Analysis of Trial-Level Association Between EFS and Overall Survival
A responder analysis was performed to evaluate the patient-level association of OS between responders and nonresponders with pooled data from all trials. For the purposes of this analysis, two types of response were considered: patients with CR and those with CRi or CRp. To account for differences in time to response, we compared OS by response status using a landmark approach for patients alive at 2 months, to approximate the induction period. Cox proportional analysis hazards models stratified by study were used to obtain Kaplan-Meier estimates of OS by response.
RESULTS
We identified a total of eight multicenter trials (N = 4,482) submitted between 2007 and 2017 in support of New Drug or Biologics License Applications for use in combination with, or for use as, intensive induction and consolidation chemotherapy for the treatment of patients with newly diagnosed AML (Table 2). All studies used induction chemotherapy as the comparator, with all but two specifying that the induction therapy used should be 7 + 3, that is, 3 days of anthracycline with 7 days of cytarabine.
TABLE 2.
Trials Included in Meta-Analysis (N = 4,482)
Six out of the eight studies had an add-on design, with induction therapy being compared with induction therapy plus the investigational product. Of these, five investigated the use of GO with induction therapy in the first-line setting. The population was limited to de novo AML in three trials and further restricted to CD33+ de novo AML in one. The other add-on study investigated use of midostaurin with induction chemotherapy in Fms-like tyrosine kinase 3–mutated AML. The remaining two studies were head-to-head comparisons of CPX-351 with 7 + 3. Inclusion in one of these trials was limited to patients with therapy-related AML or AML with myelodysplasia related changes. Of the eight randomized comparisons, the primary end point(s) included OS in four, CR rate in three, EFS in two, and DFS in one.
Demographic and disease characteristics are shown in Table 3. Most trials included more men than women, with the exception of the trial examining midostaurin, for which women made up 56% of the population. Overall, 73% of patients were under age 65 years at the time of enrollment. However, age varied by trial, with 100% of those in the midostaurin trial being under age 65 years, compared with 26% of those in CPX-351 trials and 73% in trials involving GO. Cytogenetics were unknown for 30% of patients overall. Few patients in any trial had favorable cytogenetics reported, with 7% of patients overall. The majority of patients had intermediate cytogenetics. Unfavorable cytogenetics were most frequent in the CPX-351 trials at 44%. The majority (63%) of patients on the CPX trials had secondary AML, whereas the majority (88%) on the GO trials had de novo AML. A small number of patients (3%) had high-risk myelodysplastic syndrome, all of whom were treated on one GO trial, for which response definitions were per AML response criteria.15
TABLE 3.
Key Baseline Demographics and Disease Characteristicsa

Figures 1A and 1B are scatterplots of the treatment effects on a log-scale, showing trial-level associations among the end points. The association between the HR for OS and OR for CR at the trial level was moderate, with an R2 of 0.49 (95% CI, 0.05 to 0.86). The association between the HR for OS and OR for CR + CRi or CRp was similar, with an R2 of 0.48 (95% CI, 0.05 to 0.99; data not shown). On the basis of the individual protocol definitions of EFS in the clinical study reports, the association between the HR for OS and HR for EFS was moderate, at 0.60 (95% CI, 0.03 to 0.97). On the basis of the harmonized primary definition of EFS across trials, the association became stronger at 0.87 (95% CI, 0.47 to 0.98; Fig 1B).
FIG 1.

Trial-level association between treatment effects on (A) OS and CR rate and (B) OS and EFS using the harmonized primary definition across trials. Circles represent trial size. CR, complete remission; EFS, event-free survival; HR, hazard ratio; OR, odds ratio; OS, overall survival.
To evaluate the impact of varying definitions of EFS on the association with OS, we performed a series of sensitivity analyses on the primary definition of EFS (Table 1 and Data Supplement [online only]). The strongest association of EFS and OS existed per the primary, harmonized definition of EFS. Defining treatment failure at the time of discontinuation or end of induction (sensitivity 1 and 3), inclusion of CRi or CRp in the definition of EFS (sensitivity 2, 3, and 5), and exclusion of treatment failure as an event (sensitivity 4 and 5) weakened the association with OS.
A scatterplot of EFS and OS for individual patients on one trial (ALFA-0701) was produced (Data Supplement) to better understand the association between EFS and OS. The data showed that a significant number of patients who relapsed did not die during the time on study, resulting in considerably longer OS compared with EFS. For example, three individuals experienced relapse at about 1 year but were still alive at 5 years. Furthermore, several patients whose leukemia did not respond to induction lived several years following the induction treatment failure.
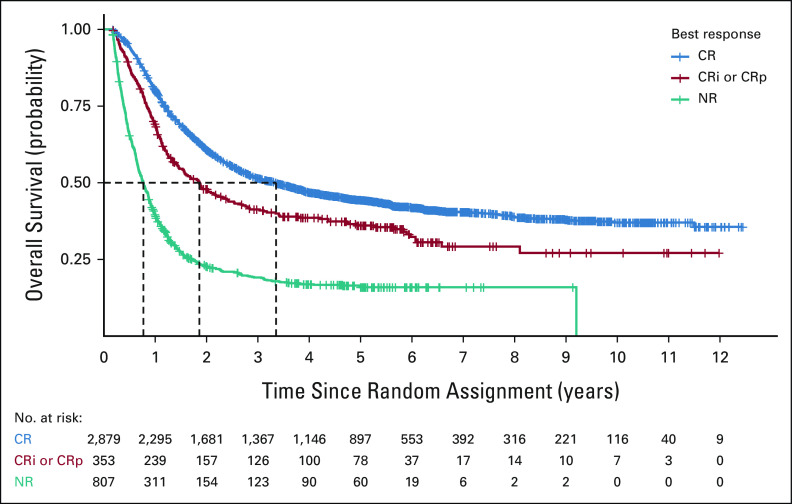
To further explore the relationship between response and survival, a patient-level analysis of response (CR v CRi or CRp v no response) was performed using a landmark approach from 2 months, to approximate the induction period (Fig 2). Patients who achieved a CR had a better OS compared with patients whose best response was CRi or CRp (HR 0.73; 95% CI, 0.64 to 0.84) or no response (HR, 0.33; 95% CI, 0.31 to 0.37), irrespective of treatment received. Patients who achieved a CRi or CRp as their best response had a better OS compared with no response (HR 0.46; 95% CI, 0.39 to 0.53) irrespective of treatment received. An analysis using all randomly assigned patients demonstrated similar results (Data Supplement). A total of 33% of patients with CR and 42% of patients with CRi or CRp underwent hematopoietic stem-cell transplantation (HSCT), suggesting that HSCT did not affect our finding of improved OS in patients with CR versus CRi or CRp responses. Of note, a small number of patients with no response to therapy experienced prolonged survival, with a few surviving up to 9 years, presumably because of successful second-line therapy.
FIG 2.
Kaplan-Meier estimates of overall survival between CR responders, CRi or CRp responders, and nonresponders who were alive at 2 months after initial induction chemotherapy. Dashes represent censored events. CR, complete remission; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; NR, no response.
DISCUSSION
Despite significant advancements for the treatment of AML in recent years, many challenges remain for the development of novel therapeutics. The presence of several molecular subsets of this already rare disease poses challenges for the conduct of large, randomized trials seeking the gold standard primary end point of OS. With the approval of GO on the basis of a primary end point of EFS,5 the FDA established a precedent for the use of EFS as a clinical benefit end point for patients treated with curative intent.10 CR rate has traditionally been considered a surrogate end point reasonably likely to represent clinical benefit for accelerated approval of new leukemia drugs.9 CRi and CRp rates are often evaluated on clinical trials and are included as response end points in consensus recommendations,11,12 but have not been accepted for contemporary regulatory decision making in AML on the basis of lack of adequate count recovery, association with measurable residual disease, and poorer outcomes.10,19,20
To our knowledge, our results represent the first direct examination of the relationship of response rate and EFS to OS using trial-level and patient-level data in patients with newly diagnosed AML treated with intensive induction chemotherapy. We demonstrated evidence for a moderate association between OS with CR, CR + CRi or CRp, and protocol-defined EFS. A strong association between OS and EFS (R2 = 0.87; 95% CI, 0.47 to 0.98) was evident when using a harmonized definition and censoring of EFS. Of note, there is no consensus of which R-square values are sufficient to assume strong association or surrogacy, but values of between 0.85 and 0.95 are often discussed.21-24 However, more stringent guidelines reference a high correlation having the lower limit of the 95% CI of R2 being ≥ 0.85, medium being > 0.7 and < 0.85, and low being ≤ 0.7.25 These stringent guidelines have not been met in our analysis. A meta-analysis of the five trials in the GO application determined the lack of a strong correlation between EFS and OS (ie, R2 0.6-0.7).5,7 However, despite the conclusion that EFS was not an established surrogate for OS, the FDA determined that EFS was itself a clinical benefit for patients with newly diagnosed AML on the basis of its reflection of durable CR and survival.5 In our analysis, we were surprised to see a strong association when we harmonized the primary definition of EFS across the eight clinical trials investigating GO, midostaurin, and CPX-351. However, given the wide CIs and considerable variation across sensitivity analyses for the definition of EFS, we cannot conclude with confidence strong surrogacy of EFS for OS on the basis of this analysis. However, the data suggest at least a moderate association on the basis of evaluation of these eight randomized, controlled trials. Furthermore, the results support the FDA's acceptance of EFS as a clinical benefit end point supportive of traditional approval for treatments with curative intent.10
The moderate association between both CR and CR + CRi or CRp with OS was expected, given that achievement of a CR response is the first step to potential cure for patients with AML. Perhaps unsurprisingly, the addition of CRi or CRp responses (n = 359; 8% pooled population) to CR responses (n = 2,892; 65% pooled population) only minimally affected the trial-level association with OS. However, the patient-level data indicated that patients with a CR response had a better OS expectation compared with those with CRi or CRp, and with no response. This is consistent with prior literature indicating poorer outcomes in patients with incomplete hematologic recovery.19,20 Although CR + CRi or CRp responses appear to have a moderate association with OS and patients with CRi or CRp responses live longer than patients with no response, it is clear that CR responses are superior. Thus, CR remains the preferred response end point for regulatory decision making in AML.10
Notably, CR with partial hematologic recovery with durability has recently emerged as an end point that may represent direct clinical benefit as temporary control of AML and relief from burdens of the disease during treatment with relatively nontoxic and nonmyelosuppressive drugs.10,26-28 We are not aware of any data evaluating CR with partial hematologic recovery, however, in trials of intensive induction chemotherapy. Thus, it remains to be seen whether this end point will have any relevance in the setting of curative induction chemotherapy.
To better understand the trial-level association between OS and EFS, we performed a series of sensitivity analyses on the basis of five alternative definitions of EFS. The alternative definitions varied the date at which induction treatment failure was called, included or excluded CRi and CRp responses, and eliminated treatment failure as part of the definition completely. The results demonstrated that the primary analysis had the strongest association. Sensitivity analyses 1 and 3 changed the time of treatment failure from the date of random assignment to the end of induction, which appeared to improve EFS for most smaller studies by extending the time to treatment failure. Sensitivity analyses 2 and 3, which involved use of CRi and CRp responses, weakened the association with OS, as may be expected. Sensitivity analyses 4 and 5 did not consider treatment failure at all and appeared to weaken the association with OS, which was a different finding from what was observed with the five GO studies examined during the ODAC.7 Of note, none of our analyses involved censoring at the time of HSCT, as per the FDA's guidance.10 Censoring at HSCT was examined across the GO studies during the ODAC, but did not appear to have a large impact on results.7 Overall, the sensitivity analyses performed indicate variability in the association between EFS and OS across definitions, but appear to support the FDA's primary preferred definition for EFS.
On the basis of the variable duration of the induction period and uncertainties on how to define treatment failure, the FDA has provided guidance recommending that treatment failure be defined on day one of treatment for patients in whom the therapy does not yield a CR following induction.10 Thus, we defined treatment failure for EFS on the date of random assignment. A variation of our methods compared with the published guidance was that patients alive and not known to have an event at the data cutoff were censored on the date they were last known to be alive, whereas the recommended guidance is to censor at the last assessment date.10 Our analysis was performed in this way since the date of last assessment was not known for all patients in the meta-analysis data set.
Limitations of our meta-analysis include the number of trials and the small sample size in some of the studies. There were only eight studies included in the meta-analysis, and four of eight studies had a sample size smaller than 500. Therefore, the studies with larger sample size could have more influence on the meta-analysis results than those with smaller sample size. Furthermore, five of the eight trials investigated GO. The limited number of trials affects the robustness of the trial-level association estimated, and the results could be influenced by including or excluding some of the trials. Only trials available to the FDA submitted with licensing applications were included. However, not all trials had statistically significant results leading to a labeled indication for the particular regimen(s) and/or patient population studied. Thus, despite the limited number of trials, inclusion of both positive and negative trials improved insight into the relationship between end points at the trial level. Another limitation would be varying patient characteristics across trials; for example, the different molecular and disease subgroups assessed across studies in our analysis. The relatively small sample size and heterogeneity limited our ability to examine subgroups and perform multivariate analyses. Thus, although OS was greater in patients with CR responses, we cannot conclude that response is completely determinative of survival, and as such, the effect of baseline covariates should be explored in future analyses. Furthermore, information on second-line therapy was generally not available. Finally, our analysis only included treatments with intensive chemotherapy, such that results may not apply to newer less-intensive regimens. Further research is needed to determine association of outcome measures with OS in the setting of lower-intensity treatment regimens (eg, venetoclax-based combinations).
Finally, Kaplan-Meier curves of EFS with the primary definition show the presence of nonproportional hazards, and it is challenging to interpret the HR estimate from the Cox proportional hazard model. Currently, there is not a standard way to summarize the treatment effect in the presence of nonproportional hazard. Future research should address this issue with alternate methods.
In conclusion, we demonstrated a moderate trial-level association between response rate (CR and CR + CRi or CRp) and OS in patients receiving intensive chemotherapy for treatment of newly diagnosed AML. The association between EFS and OS was strong when using a harmonized definition of EFS across trials, but notable variability was observed. At the patient level, patients with CR responses had better OS compared with those with CRi or CRp responses. With the emergence of novel induction therapies for the treatment of patients with AML, a re-evaluation of the end points examined here, as well as novel end points such as measurable residual disease–negative CR, and their association with OS should be examined to better understand their long-term efficacy in patients with AML.
Yutao Gong
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Jonathon Vallejo
Employment: AstraZeneca (I)
Gideon M. Blumenthal
Employment: Merck
Stock and Other Ownership Interests: Merck
No other potential conflicts of interest were reported.
See accompanying editorial on page 811
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
This article reflects the views of the authors and should not be construed to represent the FDA's view or policies.
The study was completed while Y.G. and G.M.B. were FDA employees.
AUTHOR CONTRIBUTIONS
Conception and design: Kelly J. Norsworthy, E. Dianne Pulte, R. Angelo de Claro, Richard Pazdur
Administrative support: R. Angelo de Claro, Richard Pazdur
Collection and assembly of data: Kelly J. Norsworthy, Jiaxi Zhou, Yutao Gong
Data analysis and interpretation: Kelly J. Norsworthy, Xin Gao, Chia-Wen Ko, E. Dianne Pulte, Jiaxi Zhou, Yuan Li Shen, Jonathon Vallejo, Thomas E. Gwise, Rajeshwari Sridhara, Albert B. Deisseroth, Ann T. Farrell, Gideon M. Blumenthal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Response Rate, Event-Free Survival, and Overall Survival in Newly Diagnosed Acute Myeloid Leukemia: US Food and Drug Administration Trial-Level and Patient-Level Analyses
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yutao Gong
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Jonathon Vallejo
Employment: AstraZeneca (I)
Gideon M. Blumenthal
Employment: Merck
Stock and Other Ownership Interests: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML) 2021. https://seer.cancer.gov/statfacts/html/amyl.html [Google Scholar]
- 2.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation N Engl J Med 377454–4642017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rydapt Prescribing Information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207997s000lbl.pdf [Google Scholar]
- 4.Krauss AC, Gao X, Li L, et al. FDA approval summary: (daunorubicin and cytarabine) Liposome for injection for the treatment of adults with high-risk acute myeloid leukemia Clin Cancer Res 252685–26902019 [DOI] [PubMed] [Google Scholar]
- 5.Jen EY, Ko CW, Lee JE, et al. FDA approval: Gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia Clin Cancer Res 243242–32462018 [DOI] [PubMed] [Google Scholar]
- 6.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study Lancet 3791508–15162012 [DOI] [PubMed] [Google Scholar]
- 7.FDA Briefing Document: Oncologic Drugs Advisory Committee Meeting, BLA 761060, Mylotarg (Gemtuzumab Ozogamicin) 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM566013.pdf [Google Scholar]
- 8.Guidance for Industry . Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. 2018. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071590.pdf [Google Scholar]
- 9.Appelbaum FR, Rosenblum D, Arceci RJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia Blood 1091810–18162007 [DOI] [PubMed] [Google Scholar]
- 10.Guidance for Industry: Acute Myeloid Leukemia: Developing Drugs and Biological Products for Treatment. 2020. https://www.fda.gov/media/140821/download [Google Scholar]
- 11.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia J Clin Oncol 214642–46492003 [DOI] [PubMed] [Google Scholar]
- 12.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel Blood 129424–4472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delaunay J, Recher C, Pigneux A, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study. Blood. 2011;118:79. [Google Scholar]
- 14.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial J Clin Oncol 29369–3772011 [DOI] [PubMed] [Google Scholar]
- 15.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia J Clin Oncol 303924–39312012 [DOI] [PubMed] [Google Scholar]
- 16.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia Blood 1214854–48602013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML Blood 1233239–32462014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia J Clin Oncol 362684–26922018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Xie H, Wood BL, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia J Clin Oncol 331258–12642015 [DOI] [PubMed] [Google Scholar]
- 20.Walter RB, Kantarjian HM, Huang X, et al. Effect of complete remission and responses less than complete remission on survival in acute myeloid leukemia: A combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study J Clin Oncol 281766–17712010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients' data Lancet Oncol 14619–6262013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials J Clin Oncol 238664–86702005 [DOI] [PubMed] [Google Scholar]
- 23.Michiels S, Le Maitre A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data Lancet Oncol 10341–3502009 [DOI] [PubMed] [Google Scholar]
- 24.Oba K, Paoletti X, Alberts S, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: A meta-analysis J Natl Cancer Inst 1051600–16072013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute for Quality and Efficiency in Health Care . Validity of Surrogate Endpoints in Oncology: Executive Summary of Rapid Report A10-05, Version 1.1. 2005. https://www.ncbi.nlm.nih.gov/books/NBK198799/pdf/Bookshelf_NBK198799.pdf [PubMed] [Google Scholar]
- 26.Norsworthy KJ, Luo L, Hsu V, et al. FDA approval summary: Ivosidenib for relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-1 mutation Clin Cancer Res 253205–32092019 [DOI] [PubMed] [Google Scholar]
- 27.Enasidenib Multi-Discipline Review/Summary, Clinical, Non-clinical. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209606Orig1s000MultidisciplineR.pdf [Google Scholar]
- 28.Pulte ED, Norsworthy KJ, Wang Y, et al. FDA approval summary: Gilteritinib for relapsed or refractory acute myeloid leukemia with a FLT3 mutation Clin Cancer Res 273515–35212021 [DOI] [PMC free article] [PubMed] [Google Scholar]