Introduction
Most patients develop a single cancer in their lifetime. However, in individuals with cancer-predisposition syndromes,1-4 both metachronous and synchronous tumors can be observed. This poses a therapeutic challenge because of the lack of agents simultaneously efficacious for both tumors, and the intolerable toxicity of using multiple regimens at once.5 Therefore, synchronous cancers have poor outcome and are frequently lethal.
Biallelic germline variants in the DNA mismatch-repair (MMR) genes (MLH1/MSH2/MSH6/PMS2) leading to inability to repair mutations during DNA replication are the highlight of the cancer-predisposing constitutional MMR-deficiency syndrome (CMMRD). Individuals with CMMRD develop early, aggressive, hypermutant cancers, with > 40 different tumor types described.6-9 As high tumor mutation burden (TMB) can predict response to immunotherapy,10 CMMRD cancers are attractive candidates for immune checkpoint inhibition (ICI),11 regardless of tissue of origin or cancer type. Here, we report that in CMMRD, the prevalence and impact of metachronous/synchronous cancers are extremely high. Since all synchronous cancers are hypermutant, immunotherapy can result in objective responses and improved survival.
Methods
Clinical data (cancer incidence and time) were collected from 106 patients with CMMRD registered in the International Replication Repair Deficiency Consortium6-9,12 between 2008 and 2019. Metachronous (arising sequentially and after completion of treatment for the first primary tumor) and synchronous tumors (distinct cancers that either coexist at the time of the original diagnosis or develop during the treatment of the original cancer and/or < 6 months from the primary diagnosis) were identified, and whole-exome sequencing (WES) was preformed from their paired tumor and germline samples to determine TMB, COSMIC mutational signatures,13 neoantigen burden, and microsatellite (MS)-indels14 using established tools (Data Supplement). Immunohistochemistry for programmed death ligand-1 and CD8 were performed for patients receiving ICI.
Ethics.
The study was reviewed and approved by the SickKids REB (Number: 1000048813). Written and informed consent and/or assent were obtained from each patient to report their clinical course and publish the images.
Results
All patients had developed at least one malignancy by their third decade. Demographic details and cancer types are elaborated in Figures 1A-1D. Thirty-three patients (31%) had died rapidly of their initial cancer (median survival: 12 months), whereas 21 (20%) were still alive following treatment of their first cancer (median survival: 26 months; Figs 1A and 1B). As the median time interval for a metachronous second cancer development was 39 months, the latter patients remain at risk of developing a second cancer. Furthermore, over the time period of 11 years, two independent cancers had already been diagnosed in half of the patients (n = 52; 49%), with synchronous tumors being observed in one quarter (n = 26; 24.5%; Figs 1C and 1D).
FIG 1.
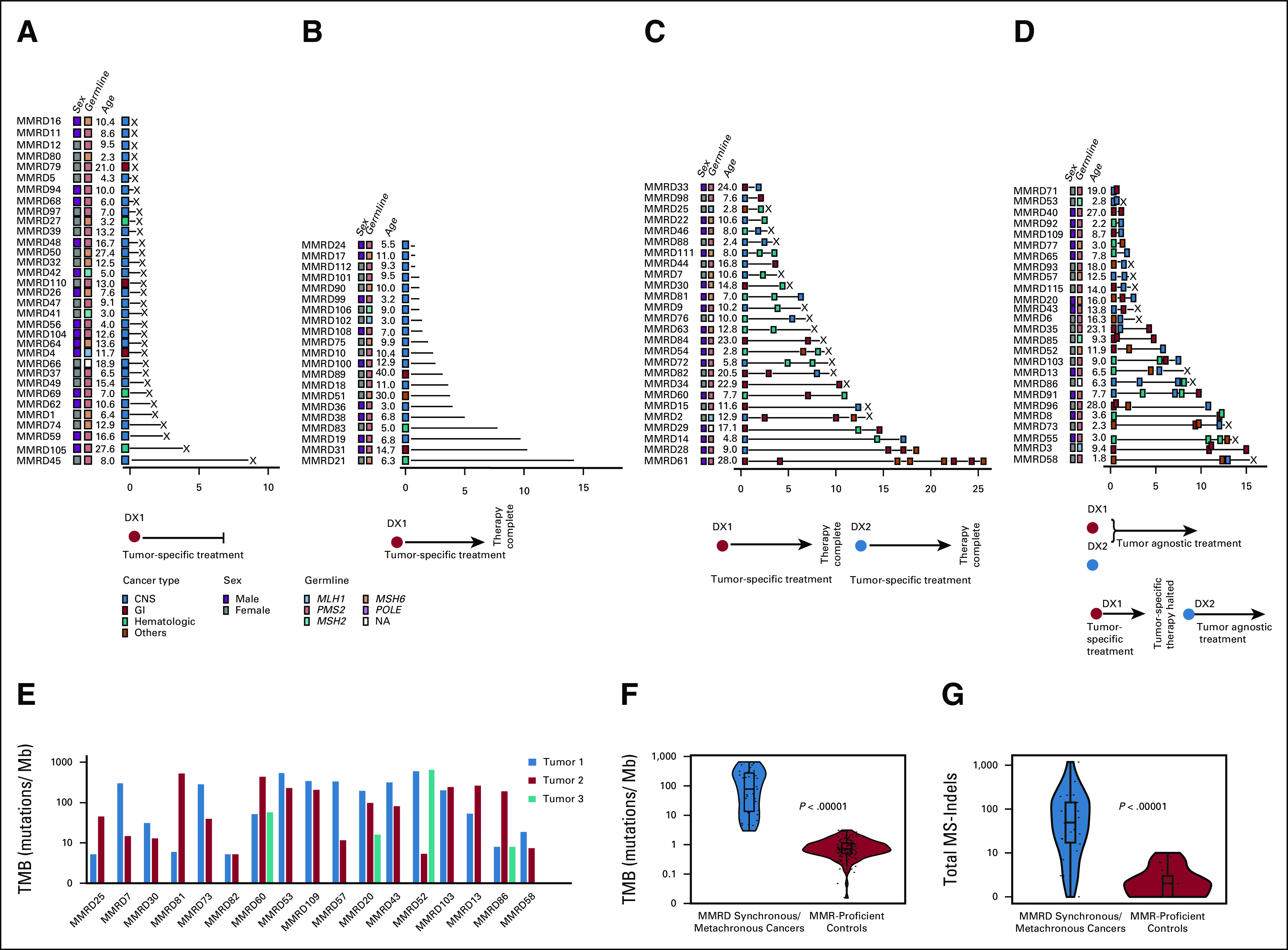
Prevalence and mutational burden of synchronous and metachronous cancers in patients with CMMRD. (A-D) Tumor burden in 106 patients with CMMRD. (A) Patients with a single lethal tumor (n = 33; 31%; X = deceased) and (B) patients with a single tumor to date (n = 21; 20%). (C) Patients with metachronous tumors (n = 26; 24.5%) and (D) synchronous tumors (n = 26; 24.5%). (E) TMB for individual CMMRD patients with synchronous and metachronous tumors (n = 38 tumors from 17 patients). (F) TMB of all synchronous and metachronous MMR-deficient cancers compared with MMR-proficient pediatric cancers. (G) MS-indel burden in exomes of all synchronous and metachronous MMR-deficient cancers compared with MMR-proficient pediatric cancers (all genomic data were derived from WES of tumors). CMMRD, constitutional mismatch repair deficiency syndrome; DX1, first cancer diagnosis; DX2, second cancer diagnosis; MMR, DNA mismatch repair; MMRD, mismatch repair deficiency; MS-indels, microsatellite indels; NA, not available; TMB, tumor mutation burden; WES, whole-exome sequencing.
Regardless of the tissue of origin, all cancers harbored high TMB (median: 155 mutations/Mb; range: 5-651.68), significantly higher than primary cancers arising in children and young adults outside the context of CMMRD (Fig 1E).9 Furthermore, their genomic MS-indel burden was significantly higher than that in MMR-proficient cancers (Fig 1G). Half exhibited ultrahypermutation (> 100 mutations/Mb; Fig 1F), which results from secondary somatic polymerase-proofreading deficiency because of mutations in POLE or POLD19, and is considered predictive for ICI response.11 Importantly, available WES data for 38 paired synchronous cancers demonstrated TMB uniformly > 5 mutations/Mb (range: 5.2-651.68; Fig 1F), which is the threshold for the ongoing immunotherapy trial for children with hypermutant cancers (NCT02992964). We therefore proceeded to treat two patients with synchronous, progressive, and metastatic malignancies using ICI.
Patient 1.
The first patient was diagnosed with a frontotemporal glioblastoma (GBM) and treated with subtotal resection and radiotherapy (59.4 Gy in 33 fractions) with concomitant temozolomide. Evaluation of a pathologic fracture at the distal ulna diagnosed 6 months later (Fig 2B) revealed this to be related to a metastatic lesion from an adenocarcinoma in the distal rectum, the latter being diagnosed in the subsequent weeks. The mass in the rectum was not resected as the family opted for palliative care. WES of both tumors revealed ultrahypermutation (Fig 2B), prompting treatment using pembrolizumab (2 mg/kg, once every 3 weeks). To control the worsening pain, radiotherapy (20 Gy in five fractions) was administered to the distal ulna. Dramatic responses were noted, with complete disappearance of the metastatic ulnar lesion with extensive bony remodeling. Colonoscopy performed a year later confirmed resolution of the rectal mass (Fig 2B). The local site remained stable for > 20 months while in the interim, a new thoracic spinal (T8/9) lesion was treated with concomitant radiotherapy (25 Gy, 10 fractions; boost: 5 Gy, two fractions) alongside ICI, with 67% reduction in size on imaging. Unfortunately, pembrolizumab was recurrently interrupted over the next 6 months because of autoimmune pneumonitis and pancreatitis. Disseminated progression of GBM detected 24 months from diagnosis, and the family opted for palliative care. Overall, the patient had prolonged survival of over 30 months from his diagnosis of GBM, and 24 months from the diagnosis of a metastatic adenocarcinoma, both the primary and metastatic sites for which showed no evidence of disease for 21 months from treatment initiation.
FIG 2.
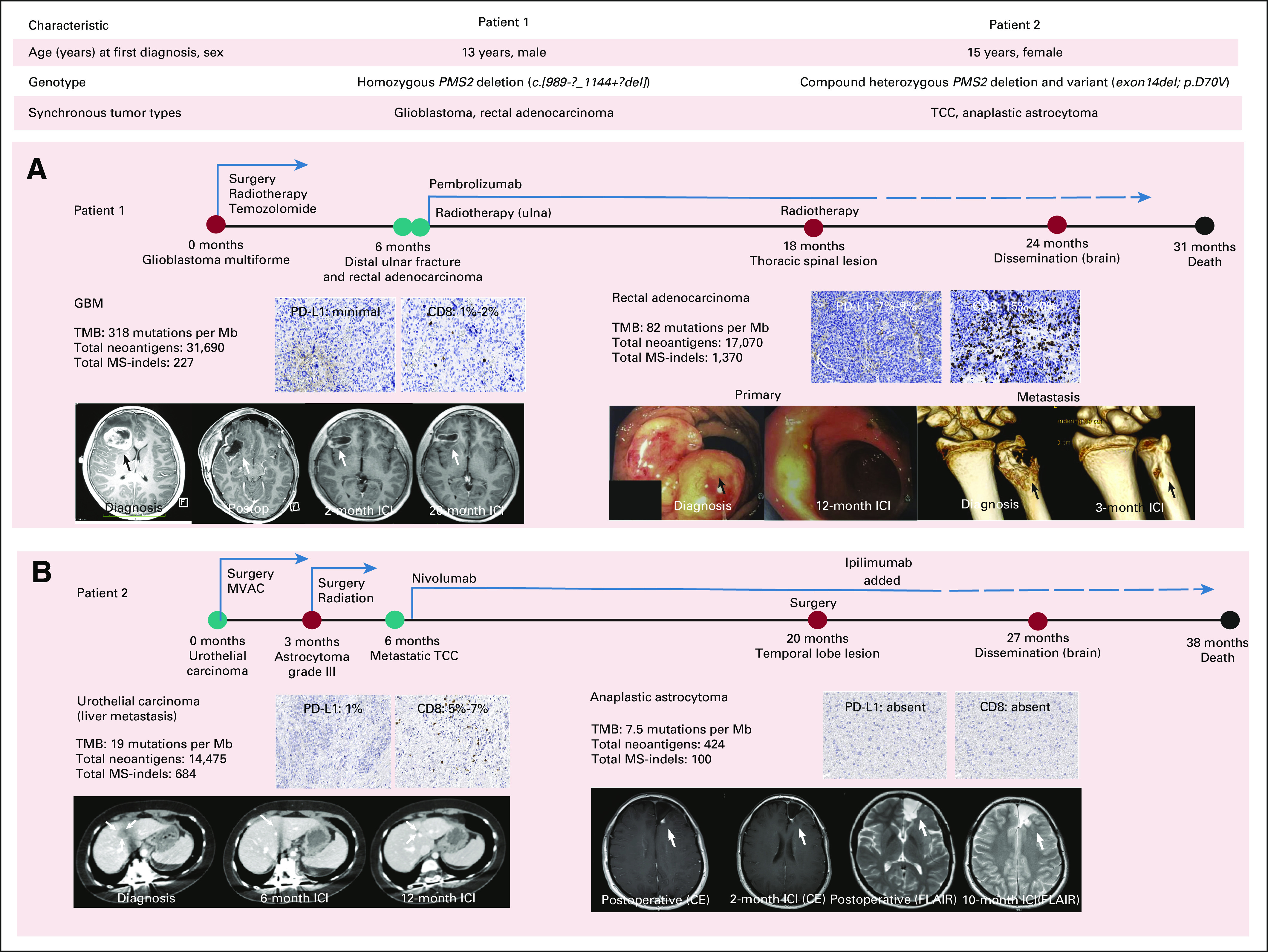
Response to immune checkpoint blockade and outcome of CMMRD patients with synchronous cancers. Clinical and germline alterations in the two patients. Timeline and tumor response for (A) patient 1 and (B) patient 2. Tumor genomic biomarkers and microenvironment findings are outlined. Arrows denote tumor at diagnosis and response to immune checkpoint blockade at the specified time points (all genomic data were derived from WES of tumors). Colored circles denote diagnoses for separate cancers and/or recurrences, with red suggesting central nervous system, and teal suggesting extracranial site of the cancer. The dashed line represents toxicity-related interruptions in treatment. CMMRD, constitutional mismatch repair deficiency syndrome; CE, contrast-enhanced; FLAIR, fluid-attenuated inversion recovery images; GBM, glioblastoma; ICI, immune checkpoint inhibition; MS-indels, microsatellite indels; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; PD-L1, programmed death ligand-1; TCC, transitional cell carcinoma; TMB, tumor mutation burden; WES, whole-exome sequencing.
Patient 2.
The second patient, previously treated for neuroblastoma and colonic polyposis, presented with hematuria and severe unilateral hydronephrosis, leading to a diagnosis of an invasive ureteric papillary transitional cell carcinoma (TCC), stage pT3N1M0 (Fig 2A). While on chemotherapy, a frontal lobe anaplastic astrocytoma was diagnosed and treated with subtotal resection and local radiotherapy (59.4 Gy in 33 fractions) without any chemotherapy. Hepatic metastatic recurrence of TCC was noted (Fig 2C). Following confirmation of high TMB in both cancers, treatment with nivolumab (3 mg/kg, once every 2 weeks) was initiated. Reimaging after 12 weeks demonstrated a 40% reduction in the liver lesion (Fig 2C), and a decrease in the nodular enhancement in the intracranial surgical cavity (Fig 2C). While the frontal lesion remained stable, a new temporal lesion detected at 20 months was treated with surgical resection and addition of the CTLA-4 inhibitor, ipilimumab (Fig 2C). This combination had to be interrupted for autoimmune transaminitis followed by multifocal intracranial progression. Overall, the patient survived for 38 months after the diagnosis of the TCC and 35 months from malignant glioma diagnosis.
Discussion
This report demonstrates the efficacy of immunotherapy as a rational approach for patients with CMMRD and synchronous cancers. The excessive prevalence of both metachronous and synchronous cancers in CMMRD has not been previously reported in any human cancer syndrome. This is a major cause of the high mortality in these children, who rarely reach adulthood. As more than half of the survivors will experience other cancers, some even during therapy for their first cancer, the introduction of immunotherapy as the primary therapy may be extremely valuable for both limiting toxicity as well as cancer control.
Genomic analysis of pediatric cancers is still not routinely performed. In the context of synchronous tumors, therapies targeting pan-cancer genetic drivers can offer an attractive alternative to current tissue-specific chemoradiation approaches. However, specific mutations rarely fit synchronous cancers, and these patients are usually excluded from such clinical trials.15 The use of immune-based approach may be more broadly effective in the context of faulty DNA-damage repair leading to genomic instability and hypermutation, such as in CMMRD.11
Traditional chemoradiation results in a median survival of 8-9 months in metastatic colorectal cancer,16 9-13 months in metastatic TCC,17 and < 6 months in CMMRD gliomas.11 Treatment of cancers in CMMRD is inherently challenging,18 because of resistance to alkylating agents like temozolomide and mercaptopurines6 used in the treatment of GBM, colon, and urothelial cancers. However, as these cancers are inherently hypermutant7 and exhibit high MS-indels,14 ICI is more commonly used for these individuals at relapse. Indeed, in both of our patients, across diverse cancer types including malignant gliomas, metastatic adenocarcinoma, and TCCs, objective responses were noted in a tissue-agnostic manner, resulting in significant survival benefit > 30 months. Notably, even within these hypermutant synchronous cancers, those with relatively higher TMB demonstrated higher neoantigen load, immune infiltration, and robust objective responses to ICI (Fig 2).
As both patients eventually succumbed to their CNS tumors while the extracranial cancers were well controlled, organ-specific immune surveillance still needs to be addressed. That the CNS is an ‘immune-privileged’ site was supported by both gliomas exhibiting lower CD8+T-lymphocyte infiltration and programmed death ligand-1 expression than the synchronous ICI-responsive extracranial cancers (Figs 2B and 2C). Combinatorial immune-based therapies have shown higher efficacy in several tumor types including GBM19,20 and in patients with CMMRD.21 However, the tolerability of ICI can be limited by immune toxicities and treatment interruptions, allowing immune-escape, as was observed in both of our patients, although overall, this is relatively uncommon in children and in CMMRD. Finally, loss of immune surveillance can develop over time because of the ongoing changes in the mutational landscape of these genomically unstable cancers.9 The waxing and waning of responses that we observed in both patients likely reflect the loss of certain neoantigens, and the subsequent sensitization/education toward novel antigens.9 Importantly, when compared with responses to traditional chemotherapy or targeted inhibitors, where clonal evolution leads to permanent resistance to treatment, this dynamic and adaptive response to ICI is unique and needs to be better harnessed by physicians. Despite the limitations of an observational registry study, these intriguing results from our global consortium of a rare yet increasingly recognized disease should pave the path for future collaborative prospective trials for these patients.
In conclusion, this is the first report of an immune-based, tissue-agnostic approach for the treatment of synchronous hypermutant cancers, which can improve survival while reducing morbidity and toxicity from less-effective conventional therapies, particularly in the setting of cancer predisposition syndromes such as CMMRD.
ACKNOWLEDGMENT
AD would like to acknowledge the kind support of the St Baldrick's Foundation International Scholar Program (with generous support from the Team Campbell Foundation; Grant number: 697257) and the SickKids Research Training Center Clinician-Scientist Training Program (Fall 2019 award).
Daniel A. Morgenstern
Honoraria: Ology Medical Education, WebMD, PlatformQ Health
Consulting or Advisory Role: Clarity Pharmaceuticals, EUSA Pharma, Ymabs Therapeutics Inc
Speakers' Bureau: Ymabs Therapeutics Inc
Research Funding: Bristol Myers Squibb (Inst), AbbVie (Inst), Lilly (Inst), Bayer (Inst), Cellectar (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Lilly
Sumedha Sudhaman
Employment: Strand Life Sciences
David E. Kram
Consulting or Advisory Role: Foundation Medicine
Cynthia Hawkins
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: IP for low-grade glioma and sarcoma fusion panels as well as medulloblastoma subgrouping panel
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst)
Yoon-Jae Cho
Expert Testimony: Barr and Mudford
No other potential conflicts of interest were reported.
Footnotes
J.J.H. and A.D. contributed equally to this work and are joint first authors.
AUTHOR CONTRIBUTIONS
Conception and design: Jacob J. Henderson, Anirban Das, David E. Kram, Eric Bouffet, Uri Tabori
Financial support: Anirban Das, Yoon-Jae Cho, Uri Tabori
Administrative support: Yoon-Jae Cho, Uri Tabori
Provision of study material or patients: Michael Osborn, Yoon-Jae Cho, Uri Tabori
Collection and assembly of data: Jacob J. Henderson, Anirban Das, Daniel A. Morgenstern, Sumedha Sudhaman, Vanessa Bianchi, Melissa Edwards, Michael Osborn, Cynthia Hawkins, Eric Bouffet, Yoon-Jae Cho, Uri Tabori
Data analysis and interpretation: Jacob J. Henderson, Anirban Das, Daniel A. Morgenstern, Sumedha Sudhaman, Jill Chung, Logine Negm, David E. Kram, Cynthia Hawkins, Yoon-Jae Cho, Uri Tabori
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel A. Morgenstern
Honoraria: Ology Medical Education, WebMD, PlatformQ Health
Consulting or Advisory Role: Clarity Pharmaceuticals, EUSA Pharma, Ymabs Therapeutics Inc
Speakers' Bureau: Ymabs Therapeutics Inc
Research Funding: Bristol Myers Squibb (Inst), AbbVie (Inst), Lilly (Inst), Bayer (Inst), Cellectar (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Lilly
Sumedha Sudhaman
Employment: Strand Life Sciences
David E. Kram
Consulting or Advisory Role: Foundation Medicine
Cynthia Hawkins
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: IP for low-grade glioma and sarcoma fusion panels as well as medulloblastoma subgrouping panel
Eric Bouffet
Consulting or Advisory Role: Novartis
Research Funding: Roche (Inst), Bristol Myers Squibb (Inst)
Yoon-Jae Cho
Expert Testimony: Barr and Mudford
No other potential conflicts of interest were reported.
REFERENCES
- 1.Compostella A, Toffolutti T, Soloni P, et al. Multiple synchronous tumors in a child with Fanconi anemia J Pediatr Surg 45e5–82010 [DOI] [PubMed] [Google Scholar]
- 2.Amayiri N, Al-Hussaini M, Swaidan M, et al. Synchronous glioblastoma and medulloblastoma in a child with mismatch repair mutation Childs Nerv Syst 32553–5572016 [DOI] [PubMed] [Google Scholar]
- 3.Moke DJ, Thomas SM, Hiemenz MC, et al. :Three synchronous malignancies in a patient with DICER1 syndrome Eur J Cancer 93140–1432018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schniederjan MJ, Shehata B, Brat DJ, et al. De novo germline TP53 mutation presenting with synchronous malignancies of the central nervous system Pediatr Blood Cancer 531352–13542009 [DOI] [PubMed] [Google Scholar]
- 5. Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: Challenges and approaches, a review. ESMO Open. 2017;2:e000172. doi: 10.1136/esmoopen-2017-000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabori U, Hansford JR, Achatz MI, et al. Clinical management and tumor surveillance recommendations of inherited mismatch repair deficiency in childhood Clin Cancer Res 23e32–e372017 [DOI] [PubMed] [Google Scholar]
- 7.Campbell BB, Light N, Fabrizio D, et al. Comprehensive analysis of hypermutation in human cancer Cell 1711042–1056.e102017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durno C, Ercan AB, Bianchi V, et al. Survival benefit for individuals with constitutional mismatch repair deficiency undergoing surveillance J Clin Oncol 392779–27902021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlien A, Campbell BB, de Borja R, et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers Nat Genet 47257–2622015 [DOI] [PubMed] [Google Scholar]
- 10.Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study Lancet Oncol 211353–13652020 [DOI] [PubMed] [Google Scholar]
- 11.Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency J Clin Oncol 342206–22112016 [DOI] [PubMed] [Google Scholar]
- 12.Bakry D, Aronson M, Durno C, et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: Report from the Constitutional Mismatch Repair Deficiency Consortium Eur J Cancer 50987–9962014 [DOI] [PubMed] [Google Scholar]
- 13.Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer Nature 57894–1012020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung J, Maruvka YE, Sudhaman S, et al. DNA polymerase and mismatch repair exert distinct microsatellite instability signatures in normal and malignant human cells Cancer Discov 111176–11912020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavacchi D, Roviello G, D'Angelo A.Tumor-agnostic treatment for cancer: When how is better than where Clin Drug Investig 40519–5272020 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Li S, Liu Y, et al. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis Cancer Med 9361–3732020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy J Clin Oncol 173173–31811999 [DOI] [PubMed] [Google Scholar]
- 18. Kebudi R, Amayiri N, Abedalthagafi M, et al. Position paper: Challenges and specific strategies for constitutional mismatch repair deficiency syndrome in low-resource settings. Pediatr Blood Cancer. 2020;67:e28309. doi: 10.1002/pbc.28309. [DOI] [PubMed] [Google Scholar]
- 19. Larouche V, Atkinson J, Albrecht S, et al. Sustained complete response of recurrent glioblastoma to combined checkpoint inhibition in a young patient with constitutional mismatch repair deficiency. Pediatr Blood Cancer. 2018;65:e27389. doi: 10.1002/pbc.27389. [DOI] [PubMed] [Google Scholar]
- 20.Wu A, Maxwell R, Xia Y, et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment J Neurooncol 143241–2492019 [DOI] [PubMed] [Google Scholar]
- 21.Campbell BB, Galati MA, Stone SC, et al. Mutations in the RAS/MAPK pathway drive replication repair deficient hypermutated tumors and confer sensitivity to MEK inhibition Cancer Discovery 111454–14672021 [DOI] [PMC free article] [PubMed] [Google Scholar]


