PURPOSE
There is limited evidence on the clinical utility of monitoring measurable residual disease (MRD) in patients with acute myeloid leukemia treated with lower-intensity therapy. Herein, we explored the outcomes of patients treated with venetoclax and azacitidine who achieved composite complete remission (CRc; complete remission + complete remission with incomplete hematologic recovery) and MRD < 10–3 in the VIALE-A trial.
METHODS
The patients included in this report were treated with venetoclax and azacitidine. Bone marrow aspirate samples for multiparametric flow cytometry assessments were collected for central analysis at baseline, end of cycle 1, and every three cycles thereafter. MRD-negative response was defined as < 1 residual blast per 1,000 leukocytes (< 10–3 or 0.1%) with an estimated analytic sensitivity of 0.0037%-0.0027%. CRc, duration of remission (DoR), event-free survival (EFS), and overall survival (OS) were assessed. A multivariate Cox regression analysis identified prognostic factors associated with OS.
RESULTS
One hundred sixty-four of one hundred ninety (86%) patients with CRc were evaluable for MRD. MRD < 10–3 was achieved by 67 of 164 (41%), and 97 of 164 (59%) had MRD ≥ 10–3. The median DoR, EFS, and OS were not reached in patients with CRc and MRD < 10–3, and the 12-month estimates for DoR, EFS, and OS in this group were 81.2%, 83.2%, and 94.0%. Among patients with CRc and MRD ≥ 10–3, the median DoR, EFS, and OS were 9.7, 10.6, and 18.7 months. Multivariate analysis showed that CRc with MRD < 10–3 was a strong predictor of OS (adjusted hazard ratio = 0.285; 95% CI, 0.159 to 0.510; P < .001).
CONCLUSION
Patients who achieved CRc and MRD < 10–3 with venetoclax and azacitidine had longer DoR, EFS, and OS, than responding patients with MRD ≥ 10–3.
INTRODUCTION
The phase III VIALE-A trial established the treatment of venetoclax and azacitidine combination as a new standard of care for older patients with treatment-naïve acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy.1,2 Results from the trial demonstrated that the combination of venetoclax and azacitidine led to a significant improvement in overall survival (OS) and composite complete remission (CRc; complete remission [CR] + complete remission with incomplete hematologic recovery [CRi]). The rates of CRc with measurable residual disease (MRD) response (< 10–3) were also significantly higher in the venetoclax and azacitidine group as compared with azacitidine alone (23.4% v 7.6%, P < 0.001), providing evidence that deep responses are attainable in patients treated with lower-intensity therapy.2
CONTEXT
Key Objective
To evaluate the outcomes of patients who achieved composite complete remission (CRc, defined as complete remission and complete remission with incomplete hematologic recovery) and were evaluable for a measurable residual disease (MRD) response by multiparametric flow cytometry in patients with acute myeloid leukemia treated with venetoclax and azacitidine.
Knowledge Generated
The patients who achieved CRc + MRD-negative (< 10–3) response with the combination had a longer duration of remission, overall survival (OS), and event-free survival. Achievement of CRc + MRD < 10–3 response was a prognostic factor for OS, providing evidence that MRD monitoring could be informative for patients who are treated with lower-intensity therapies.
Relevance
The outcomes of this analysis provide evidence that MRD assessments among patients treated with lower-intensity therapy could be conducted to understand the prognostic impact of MRD on OS. MRD< 10–3 response in patients treated with venetoclax and azacitidine is valuable and warrants further investigation to establish its role in clinical management.
Despite the achievement of morphological remission in most older patients with AML receiving standard intensive therapy, approximately 80% experience relapse because of residual leukemic cells in the bone marrow (BM).3–6 In intensively treated patients, the achievement of a deeper MRD-negative response in CR is a prognostic marker of superior outcomes, including OS and relapse-free survival, compared with conventional remission criteria alone (< 5% blasts by morphology).7,8 A retrospective study reported that in patients age > 60 years and treated with hypomethylating agents, the cumulative incidence of relapse was lower among patients who had achieved a MRD-negative (< 10–3) response, but failed to establish a relationship between MRD and OS or relapse-free survival.9 However, there is limited literature on the clinical significance of MRD, survival outcomes, and the utility of monitoring in treatment-naive patients receiving lower-intensity therapy when ineligible for intensive induction chemotherapy.
Immunophenotyping with multiparametric flow cytometry (MFC) is a powerful technique used to quantify MRD in AML.10,11 Although limitations of MFC-based MRD analyses include the lack of standardization, reliance on a high-quality marrow aspirate, and variable sensitivity, the method is applicable to a majority of patients after a treatment-induced morphologic remission, even for those who do not harbor an appropriate molecular target for quantitative polymerase chain reaction12 and next-generation sequencing (NGS) MRD approaches.13
In this report, we evaluated the outcomes of patients who achieved CRc and were evaluable for an MRD response by MFC in the aforementioned cohort of patients with AML treated with venetoclax and azacitidine in the VIALE-A trial. Among patients treated with placebo and azacitidine, only 11 of 145 patients achieved CRc with an MRD-negative (< 10–3) response and thus were excluded from this exploratory analysis because of small numbers.
METHODS
Patients and Treatment
Patients enrolled in the ongoing randomized phase III VIALE-A study (NCT02993523) who received venetoclax and azacitidine (venetoclax arm) were included in this analysis. Enrolled patients were ≥ 18 years with a confirmed diagnosis of AML by the WHO criteria and were ineligible for standard induction chemotherapy either because of age ≥ 75 years or because of comorbidities. Additional eligibility criteria have been previously published.2 Patients in the venetoclax arm received venetoclax 400 mg orally once daily on days 1-28 and azacitidine at 75 mg/m2 intravenously once daily on days 1-7 once every 28-day cycle.
The study was approved by the local ethics committees and conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Outcomes
Disease responses were evaluated per modified International Working Group response criteria for AML.14 Efficacy was assessed as CRc, duration of CRc, event-free survival (EFS), and OS. CR was defined as absolute neutrophil count > 103/μL, platelets > 105/μL, red cell transfusion independence, and BM with < 5% blasts. CRi was defined as all criteria for CR, except for neutropenia ≤ 103/μL or thrombocytopenia ≤ 105/μL. Duration of remission (DoR) for CRc was defined as the number of days from the date of first response (CR or CRi) per modified International Working Group criteria for AML to the earliest evidence of confirmed morphologic relapse, confirmed progressive disease, or death because of disease progression. EFS was defined as the number of days from random assignment to the date of confirmed progressive disease, confirmed morphological relapse from CR or CRi, treatment failure (defined as a failure to achieve CR, CRi, or morphological leukemia-free state) after at least six cycles of study treatment, or death from any cause. OS was defined as the time from random assignment to the date of death from any cause. BM assessments were performed at screening, at end of cycle 1, and after every three cycles thereafter. Per protocol, for patients who had two successive disease assessments that indicated CR or CRi, BM aspirates were no longer required for response or biomarker assessments. The patients had to achieve an MRD-negative response before or after clinical remission, and the MRD response had to occur before study drug discontinuation. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4·0.15
Detection of MRD
MRD response was defined as one or fewer residual leukemic blasts per 1,000 leukocytes or 10–3. Patients who had one negative sample for MRD value below this cutoff at any time on the study were defined as patients with an MRD-negative response. Samples were collected at baseline from BM aspirates during the clinical assessment and after each course as described above. Patients were unevaluable for MRD if they had missing BM samples, were deemed a technical failure, or if the BM samples contained less than a hundred thousand CD45+ leukocytes (classified thereafter as MRD-indeterminate). The analysis was performed centrally by LabCorp Central Laboratory Services and was blinded to investigator-reported disease response assessments. The BM aspirates were analyzed by a five-tube, eight-color panel (CD45, CD34, HLA-DR, and CD13 as a backbone in all five tubes, with CD123, CD117, CD71, CD64, CD56, CD38, CD33, CD19, CD15, CD14, CD11b, CD7, CD4, and CD2 assessed across the remaining tubes). These markers align with the recent recommendations of the European LeukemiaNet consensus document for flow cytometry–based MRD assessments in AML.11 The integrated leukemia-associated immunophenotypes and different than normal procedures were used.10,11 The assay validation established the analytical sensitivity of the MRD panel at the upper limit of 0.0037% and the lower limit of 0.0027%. For specimens in which the identified aberrant phenotypes were quantified below 7%, the data were reviewed and approved by a board-certified hematopathologist; a total of four hematopathologists reviewed MRD assessments for this trial.
Statistical Analysis
Demographics were summarized by descriptive statistics. DoR, EFS, and OS were estimated by the Kaplan-Meier methodology between the CRc + MRD< 10–3 and CRc + MRD≥ 10–3 groups. Median and 95% CIs were reported. For subgroup analyses, hazard ratios (HRs) and 95% CIs were generated using an unstratified Cox proportional hazards model. To improve the precision or HR estimates of OS, a multivariate Cox regression analysis was performed using adjustments of key prognostic factors as determined in the analysis of VIALE-A, including age, AML type, and cytogenetic risk.
RESULTS
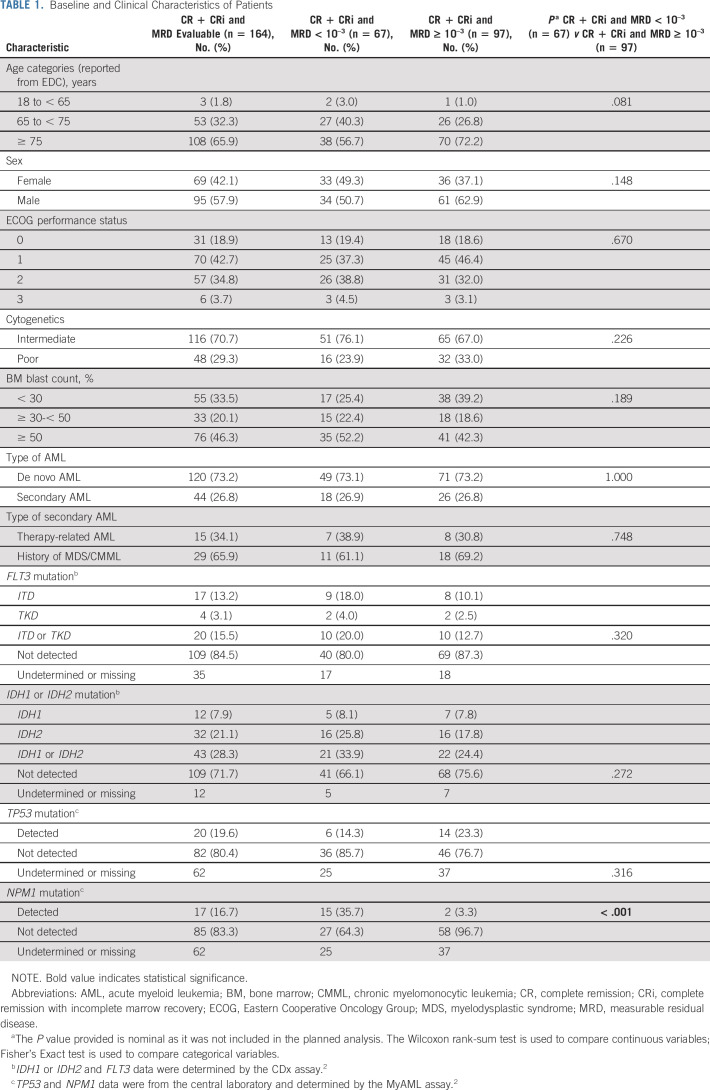
As of January 04, 2020, 286 patients were randomly assigned into the venetoclax arm, of which 190 achieved CRc (Fig 1). Among patients who achieved CRc, 164 were evaluable for MRD, 22 patients had a missing MRD assessment, and four patients had indeterminate MRD values (Data Supplement, online only). An MRD-negative response was achieved by 67, and 97 had persistent MRD ≥ 10–3. The clinical and molecular characteristics of patients are summarized in Table 1. Among patients with molecular mutations, the CRc and MRD-negative response rates were 50% (10 of 20) for patients with FLT3, 49% (21 of 43) for patients with IDH1/2, 30% (6 of 20) for patients with TP53, and 88% (15 of 17) for patients with an NPM1 mutation. Overall, the baseline characteristics were similar among groups. However, the patients in the MRD-negative response group had a significantly higher rate of NPM1 mutation than did patients in the MRD ≥ 10–3 group (36% v 3%).
FIG 1.
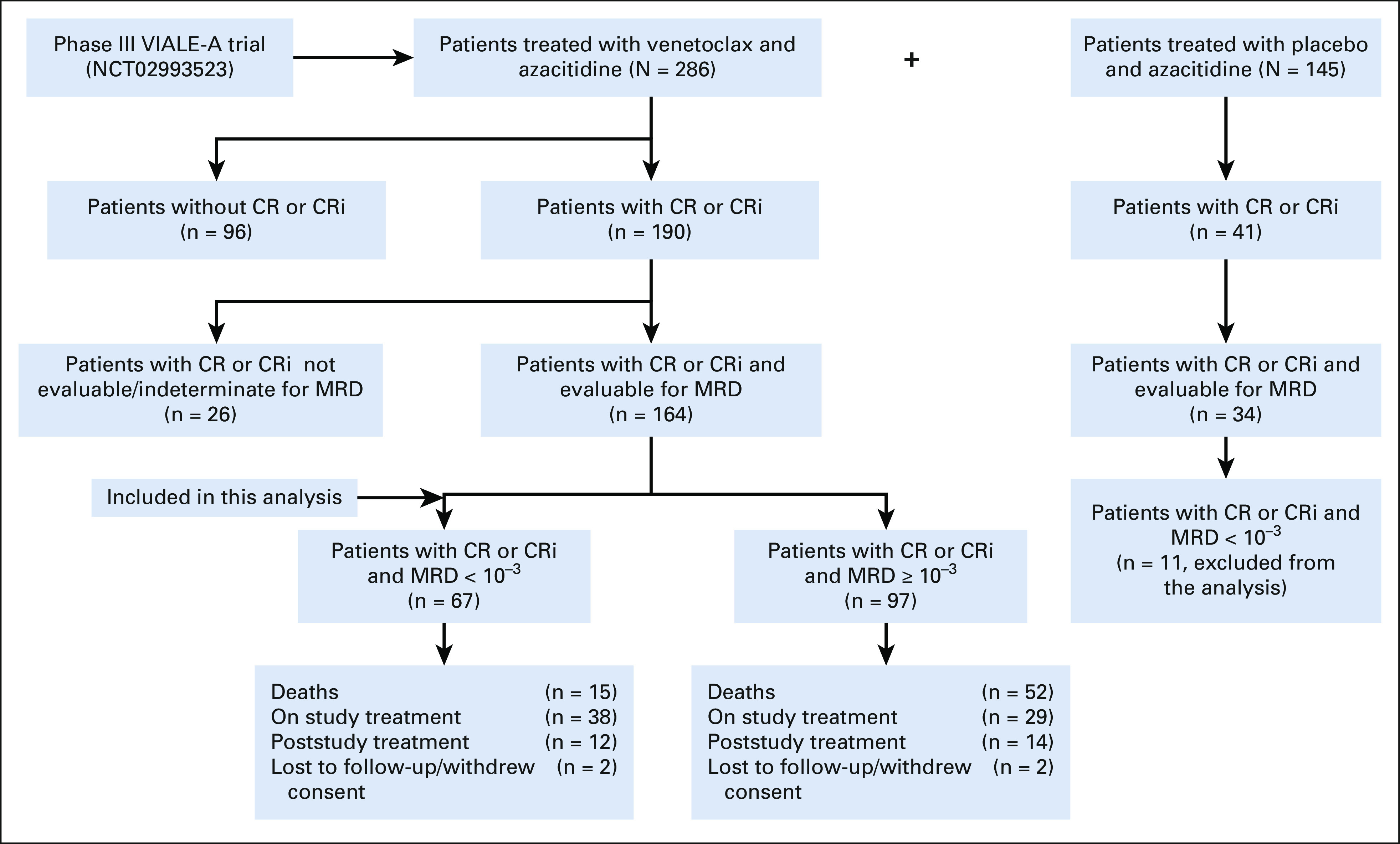
Profile of patients. CR, complete remission; CRi, complete remission with incomplete marrow recovery; MRD, measurable residual disease.
TABLE 1.
Baseline and Clinical Characteristics of Patients
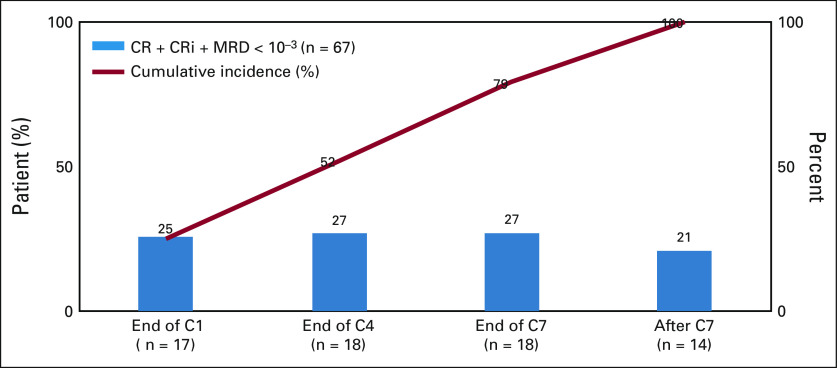
Patients with CRc and evaluable for MRD had a median of 3.0 (range: 1.0-8.0) MRD assessments postbaseline. CRc and MRD-negative responses were achieved by 17 (25%) patients by end of cycle 1, 18 (27%) patients by end of cycle 4, 18 (27%) patients by end of cycle 7, and 14 (21%) patients after cycle 7. The rate of CRc and MRD-negative response by treatment cycle and the cumulative incidence is depicted in Figure 2.
FIG 2.
MRD by treatment cycle and cumulative incidence. End of C1: MRD < 10–3 from C1 day 1 to the end day of C1 + 7 days. End of C4: MRD < 10–3 from the end day of C1 + 8 days to minimum value between end day of C4 and the last dose + 7 days. End of C7: MRD < 10–3 from the end day of C4 + 1 day to minimum value between end day of C7 and last dose + 7 days. After C7: end day of C7 + 1 day and onward up to cutoff date. CR was defined as the absolute neutrophil count > 103/μL, platelets > 105/μL, red cell transfusion independence, and bone marrow with < 5% blasts; CRi was defined as all criteria for CR, except for neutropenia ≤ 103/μL or thrombocytopenia ≤ 105/μL. C, cycle; CR, complete remission; CRi, complete remission with incomplete hematologic remission; MRD, measurable residual disease.
The median follow-up was 22.1 (range: 1.3-30.1) and 20.8 (range: 2.3-30.7) months in patients with MRD-negative response and MRD ≥ 10–3, respectively. Patients who attained an MRD-negative response at any time received a median treatment with 16.0 (range: 1.0-28.0) cycles of venetoclax and azacitidine, whereas patients with MRD ≥ 10–3 received a median treatment of 9.0 (range: 2.0-30.0) cycles.
The median DoR for CRc was not reached among patients with an MRD-negative response, and the 12-month estimated DoR for CRc and MRD-negative response was 81.2% (95% CI, 69.3 to 88.9). The median DoR for CRc was 9.7 (95% CI, 8.0 to 15.8) months in patients with MRD ≥ 10–3 (Fig 3A). Subgroup analysis by age (≥ 75 v < 75 years), AML subtype (de novo v secondary), and cytogenetic risk (poor v intermediate) showed that the median DoR for CRc was longer among patients who achieved an MRD-negative response than in those who had MRD ≥ 10–3 in all categories (Fig 3B and Data Supplement).
FIG 3.
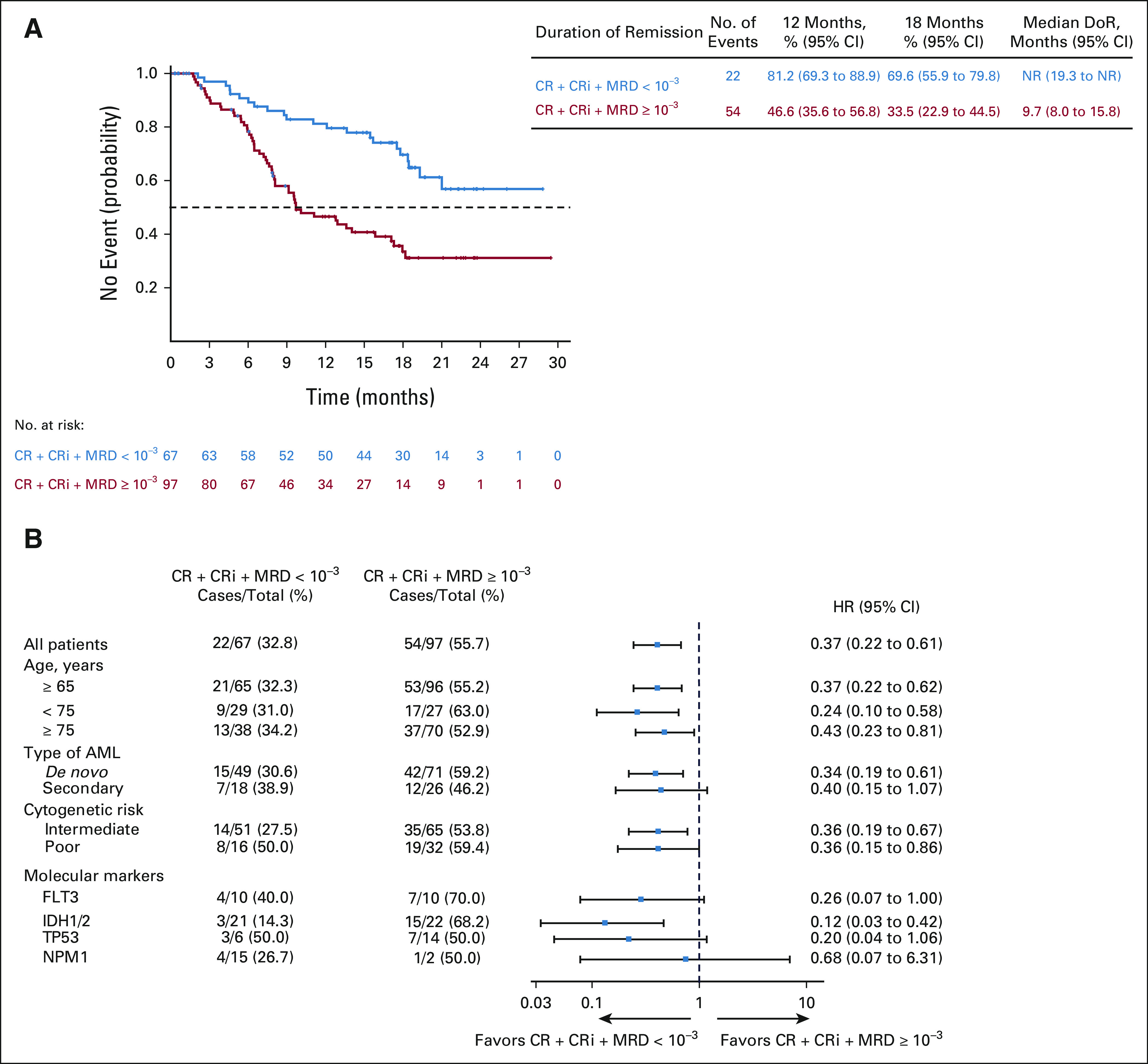
(A) DoR among patients with composite complete remission. (B) Forest plot for DoR in subgroups. CR, complete remissions; CRi, complete remission with incomplete hematologic recovery; DoR, duration of remission; MRD, measurable residual disease; NR, not reached.
The median EFS for CRc was not reached in patients with an MRD-negative response, and the estimated 12-month EFS was 83.2% (95% CI, 71.6 to 90.3). The median EFS was 10.6 months (95% CI, 9.0 to 13.9) in patients with CRc and MRD ≥ 10–3 (Figs 4A and 4B and Data Supplement). EFS was similar among patients who achieved CRc with an MRD-negative response after cycle 1 and thereafter (Fig 4C).
FIG 4.
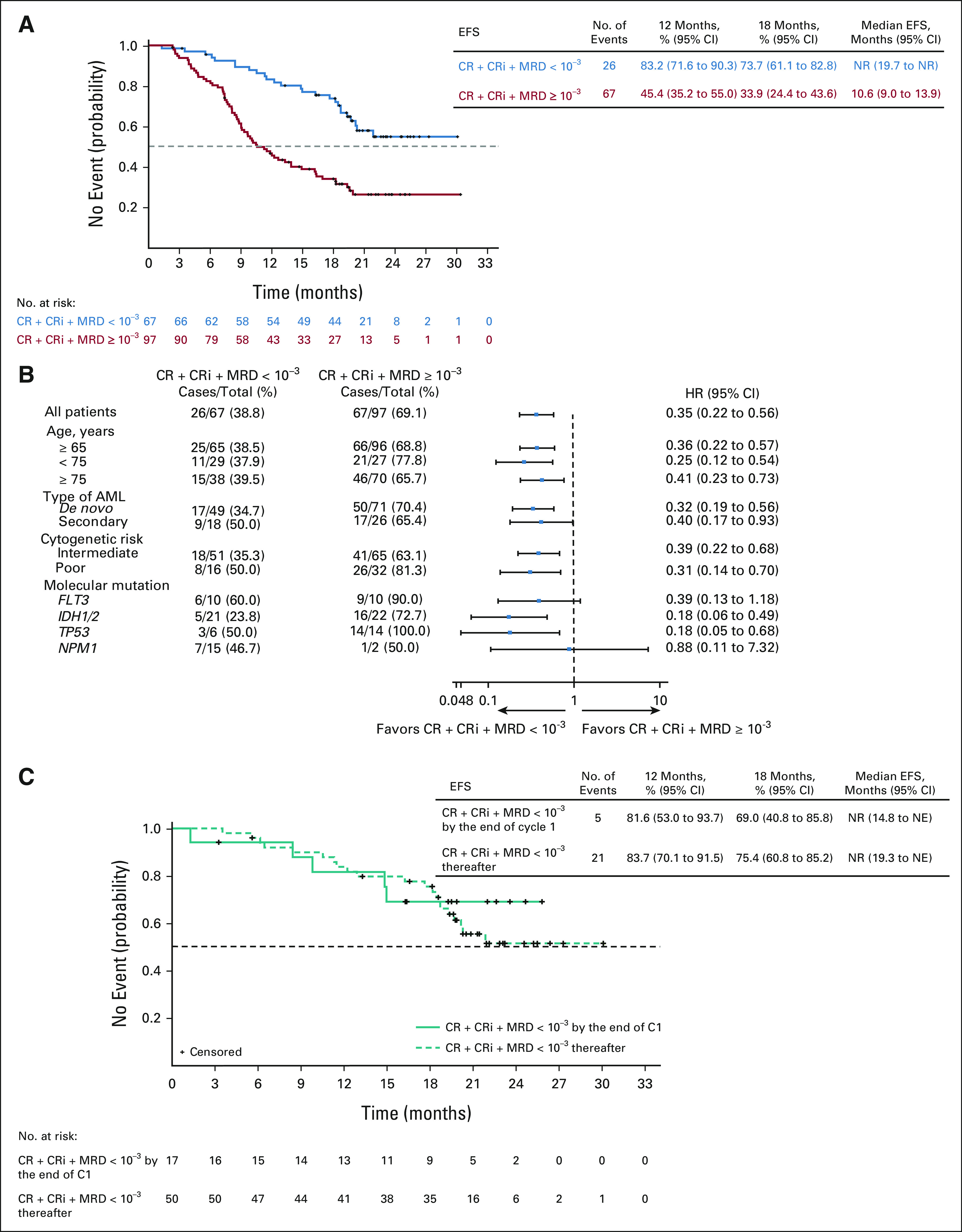
(A) EFS among patients. (B) Forest plot for EFS in subgroups. (C) Kaplan-Meier curves of EFS for patients who achieved composite complete remission and MRD-negative response by treatment cycles. CR, complete remission; CRi, complete remission with incomplete hematologic recovery; EFS, event-free survival; MRD, measurable residual disease; NE, not evaluable; NR, not reached.
The median OS was not reached in patients with CRc and MRD-negative response, and the estimated 12-month OS was 94.0% (95% CI, 84.7 to 97.7). The median OS was 18.7 (95% CI, 12.9 to not reached) months in patients with CRc and MRD ≥ 10–3 (Figs 5A and 5B and Data Supplement). The results of the multivariate Cox regression analysis demonstrated a significant reduction in death rate with the achievement of CRc with MRD-negative response (HR = 0.285; 95% CI, 0.159 to 0.510; P < .001). Both CR and CRi with an MRD-negative response were significantly prognostic of OS (Fig 5C). Additional analysis showed that patients who achieved CRc with an MRD-negative response after cycle 1, and thereafter had similar baseline characteristics (Data Supplement), had significantly better OS outcomes; the median OS was not reached in either group, and the estimated 12-month OS was 87.8% (95% CI, 59.5 to 96.8) among patients who achieved an MRD-negative response after cycle 1 and 96.0% (95% CI, 84.9 to 99.0) among patients who achieved an MRD-negative response thereafter (Fig 5D).
FIG 5.
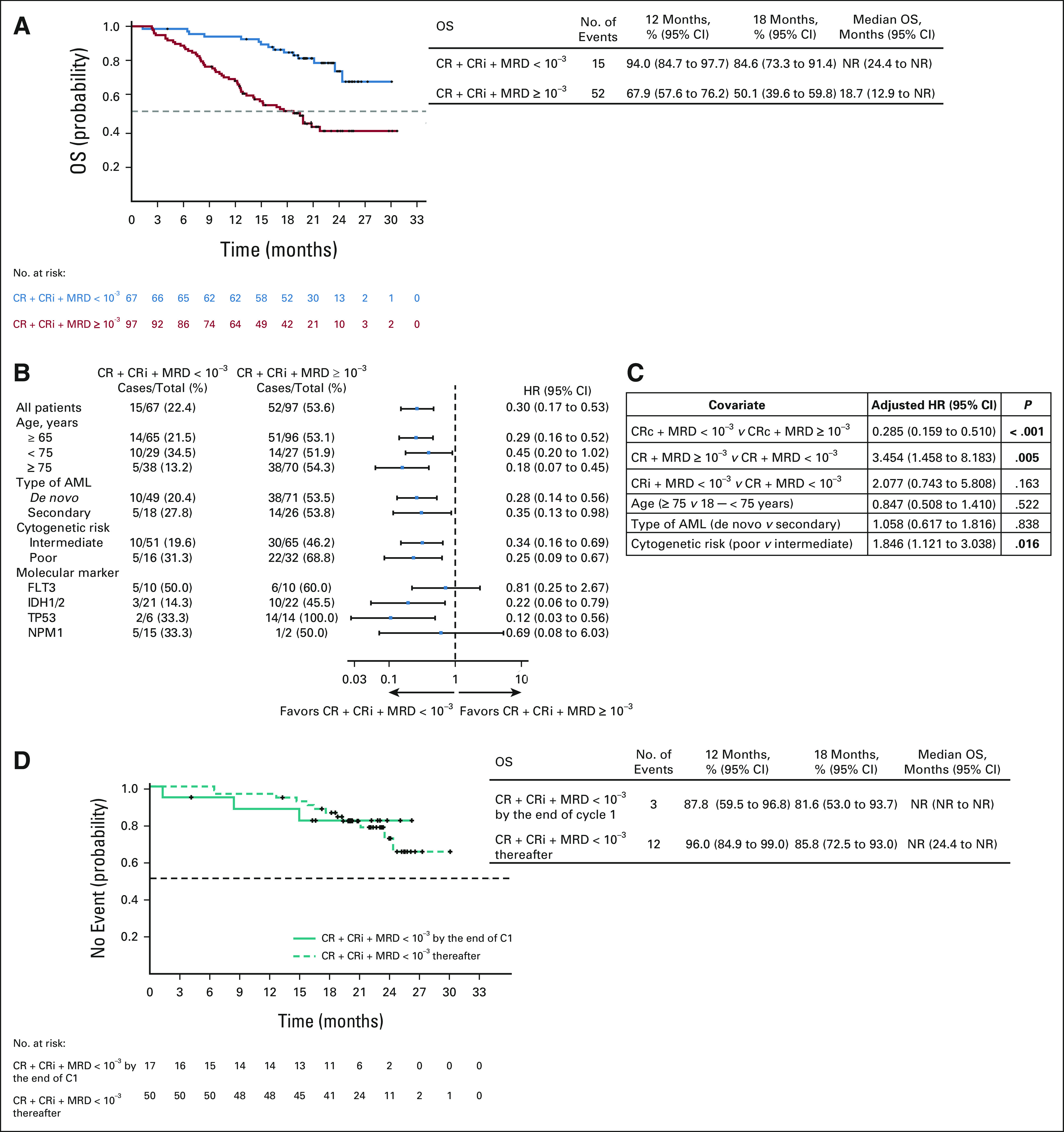
(A) Kaplan-Meier curves of OS among patients. (B) Forest plot for OS in subgroups. (C) Multivariate Cox regression analysis for OS. (D) Kaplan-Meier curves of OS for patients who achieved CRc and MRD-negative response by treatment cycles. AML, acute myeloid leukemia; C, cycle; CR, complete remission; CRc, composite complete remission; CRi, complete remission with incomplete hematologic recovery; HR, hazard ratio; MRD, measurable residual disease; NR, not reached; OS, overall survival.
The common treatment-emergent ≥ grade 3 adverse events occurring in ≥ 15% of patients in either group were febrile neutropenia, neutropenia, thrombocytopenia, anemia, pneumonia, and leukopenia (Data Supplement). Numerically higher rates of grade ≥ 3 neutropenia (54% v 37%) and febrile neutropenia (54% v 41%) were noted in patients with CRc with an MRD-negative response versus patients with CRc and MRD ≥ 10–3.
DISCUSSION
In the phase III VIALE-A trial, the combination treatment regimen of venetoclax and azacitidine resulted in superior remission rates and OS among treatment-naïve patients ineligible for intensive therapy.2 Among 190 patients who achieved CRc, 67 also achieved an MRD < 10–3 response. The results of this exploratory analysis further demonstrated that patients who had achieved CRc with MRD-negative response had longer DoR for CRc, EFS, and OS, than did patients who had MRD ≥ 10–3 in remission. This provides evidence that the lower-intensity combination of venetoclax and azacitidine can produce deep responses in patients ineligible for intensive therapy.
The achievement of an MRD-negative response predicted OS, even among patients with secondary AML. The median OS was not reached in either intermediate or poor cytogenetic risk groups attaining MRD-negative response, and the 12-month estimated OS was 94% in both subgroups, indicating that obtaining an MRD-negative response, even in patients with adverse risk cytogenetics, confers durable patient outcomes. Consistent with this, in a recent study, Maiti et al16 evaluated the prognostic impact of baseline patient factors for response to another lower-intensity treatment regimen, venetoclax plus decitabine, and reported that both patients with intermediate- and poor-risk cytogenetics who achieved MRD-negative status enjoyed a superior OS. Taken together, these data suggest that initial post-treatment MRD-negative response may be a valuable prognostic marker of OS in patients who are treated with lower-intensity regimens.
Given that a quarter of patients on treatment achieved MRD responses by the end of cycle 1, frequent late MRD responses were also observed; totally, 27% achieved an MRD-negative response by the end of cycle 7, with a further 21% achieving a response thereafter. Thus, the timing of MRD response may be independent of the time of achievement of clinical remission and may occur well after the patient has achieved clinical remission.
Another study reported a strong prognostic correlation used in combination between MRD response and OS with venetoclax and decitabine and noted that the benefit of MRD-negative status was similar across 1-, 2-, and 4-month time points.16 Furthermore, the median OS among patients who achieved an early versus late MRD-negative response was similar. Collectively, these results indicate that the attainment of a late MRD-negative response is not only possible but also associated with a better outcome. Consistent with this, unpublished data from the phase II studies before VIALE-A, in which MRD assessments were collected more frequently, indicate that MRD rates improved with longer time on therapy. Therefore, the first MRD values may not be indicative of the full effect of the venetoclax and azacitidine therapy. In addition, the current data show that patients who achieve later MRD negativity also have a survival advantage. For future studies, we would recommend at least two MRD assessments after achievement of remission.
We noted that the rates of neutropenia and febrile neutropenia were numerically higher in patients who achieved an MRD-negative response that warrants further investigation. However, these rates were similar to the overall study population of VIALE-A.2 Given the prognostic impact of an MRD-negative response on survival, with the proper use of prophylaxis and protocol-indicated cycle delays and dose reductions, the toxicities can be managed and ensure the safety of the regimen.
Molecular and MFC analyses are widely used techniques for MRD assessment in AML.11,13,17 The VIALE-A study is the first prospective study to show the utility of MRD assessment by MFC among patients treated with lower-intensity chemotherapy. Most patients were evaluable for MRD using MFC. In the venetoclax arm, of the 196 patients who achieved CRc, only 26 (13%) had either missing or indeterminate MRD assessments, whereas in the placebo and azacitidine arm, 17% (7 of 41) had missing MRD values.
In patients who present at diagnosis with genetic abnormalities associated with AML, the use of a combination of NGS and MFC has found that each technique had independent and additive prognostic value for predicting the rate of relapse and survival in younger (18-65 years) treatment-naïve patients treated with intensive regimens.17 Future studies can explore the use of both NGS and MFC in treatment-naïve patients with AML treated with lower-intensity therapies.
The interpretation of the data presented here is subject to a few limitations. The small number of patients who achieved CRc with MRD-negative response in the placebo and azacitidine arms made it difficult to compare between intervention and placebo arms and warrants further research. In this study, MRD was collected at specified time points and not continuously monitored; hence, we could not establish the most informative time point for MRD assessment in this population. In addition, patients who had two successive disease assessments that indicated CR or CRi, BM aspirates were no longer required for response or biomarker assessments; hence, it is possible that among these patients, MRD-negative responses may occur over time that warrants further evaluation. Furthermore, outcomes of the small numbers of patients in the molecular subsets should be interpreted with caution and warrant further prospective investigation.
For patients with AML ineligible for intensive therapy, the combination of venetoclax and azacitidine is now an established standard of care. Our observations reported herein establish the role of MRD assessment as an important predictor of outcomes in lower-intensity AML therapy and present an avenue for future investigation into patient-specific therapy on the basis of MRD response. Future studies of therapy deintensification/discontinuation in the MRD-negative subset may solidify the clinical utility of MRD monitoring in the unfit-for-intensive-chemotherapy subset, with hopes of identifying a subset with long-term EFS. Similarly, in the MRD-positive subset, future studies of the addition of novel agents to this regimen, with the end point of achieving MRD conversion, would be of high interest, as the bulk of patients treated with azacitidine and venetoclax remain MRD-positive despite the achievement of CRc. This study demonstrated the importance and utility of MRD monitoring as a prognostic factor for OS in the setting of lower-intensity therapy and warrants further investigation to establish its role in the management of patients with AML.
ACKNOWLEDGMENT
The authors would like to thank the patients and their families, the study coordinators, and the support staff. The authors would also like to acknowledge all investigators of Study M15-656. Medical writing support was provided by Dalia Majumdar, PhD, an employee of AbbVie.
Keith W. Pratz
Consulting or Advisory Role: Astellas Pharma, Boston Biomedical, AbbVie
Research Funding: Agios (Inst), Millennium (Inst), AbbVie (Inst), Daiichi Sankyo (Inst)
Brian A. Jonas
Consulting or Advisory Role: AbbVie, Jazz Pharmaceuticals, GlycoMimetics, Treadwell Therapeutics, Takeda, Genentech, Bristol Myers Squibb/Pfizer, Pfizer
Research Funding: Daiichi Sankyo (Inst), AbbVie (Inst), Pharmacyclics (Inst), Genentech/Roche (Inst), Glycomimetics (Inst), Celgene (Inst), FORMA Therapeutics (Inst), Incyte (Inst), Arog (Inst), Roche (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst), Sigma-Tau (Inst), Forty Seven¸ Amgen (Inst), Immune-Onc Therapeutics (Inst), Loxo (Inst), Gilead/Forty Seven (Inst)
Travel, Accommodations, Expenses: AbbVie
Vinod Pullarkat
Consulting or Advisory Role: Novartis, Amgen, Pfizer, Jazz Pharmaceuticals, AbbVie/Genentech
Speakers' Bureau: Novartis, Amgen, Jazz Pharmaceuticals, AbbVie
Christian Recher
Consulting or Advisory Role: Celgene, Amgen, Novartis, Jazz Pharmaceuticals, AbbVie, Janssen, Astellas Pharma, MacroGenics¸ Daiichi Sankyo, Otsuka, Novartis¸ Incyte, Pfizer¸ Roche, Takeda
Research Funding: Celgene (Inst), Amgen (Inst), Jazz Pharmaceuticals (Inst), Astellas Pharma (Inst), Agios (Inst), Daiichi Sankyo (Inst), MaaT Pharma (Inst), Roche (Inst), AbbVie (Inst), Novartis (Inst), Chugai Pharma (Inst), IQVIA (Inst)
Travel, Accommodations, Expenses: Incyte, Celgene, Sanofi, Amgen, Novartis, Daiichi Sankyo, Gilead Sciences
Andre C. Schuh
Honoraria: Celgene, Amgen, Pfizer, Novartis, Jazz Pharmaceuticals
Research Funding: Celgene, Amgen, Agios, Pfizer
Michael J. Thirman
Consulting or Advisory Role: AstraZeneca, Genentech, AbbVie, Adaptive Biotechnologies, Celgene¸ Pharmacyclics
Research Funding: AbbVie (Inst), Syndax (Inst), Merck (Inst), TG Therapeutics (Inst)
Jacqueline S. Garcia
Consulting or Advisory Role: AbbVie, Takeda, Astellas Pharma
Research Funding: AbbVie (Inst), Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Prelude Therapeutics (Inst)
Courtney D. DiNardo
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Notable Labs
Stock and Other Ownership Interests: Notable Labs
Honoraria: Agios, Celgene, AbbVie, Jazz Pharmaceuticals, Daiichi Sankyo, Novartis, Takeda
Consulting or Advisory Role: Celgene, Agios, AbbVie
Research Funding: AbbVie, Agios, Celgene, Daiichi Sankyo
Vladimir Vorobyev
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Sanofi, Novartis, BeiGene, Roche, AbbVie, Takeda, Gilead Sciences
Speakers' Bureau: AbbVie, AstraZeneca, Amgen, BioCad, Janssen, Sanofi, Takeda¸ Novartis, BeiGene
Travel, Accommodations, Expenses: AstraZeneca
Nicola S. Fracchiolla
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals, Incyte, AbbVie
Travel, Accommodations, Expenses: Pfizer
Su-Peng Yeh
Honoraria: Novartis, Bristol Myers Squibb, Janssen¸ Takeda, AbbVie, Amgen, Sanofi, Bayer, Astellas Pharma
Consulting or Advisory Role: AbbVie, Sanofi, Novartis, Pfizer, Chugai Pharma
Jun Ho Jang
Speakers' Bureau: Novartis
Muhit Ozcan
Research Funding: Janssen, Celgene, Takeda, Bayer, Merck, Archigen Biotech, Roche, AbbVie, Acerta Pharma/AstraZeneca
Kazuhito Yamamoto
Honoraria: Kyowa Hakko Kirin, Takeda¸ Janssen, Bristol Myers Squibb, Celgene, Sumitomo Dainippon, Ono Pharmaceutical, Chugai Pharma, Novartis, Otsuka, Mundipharma, Eisai, MSD, Meiji Seika Kaisha, Sanofi, Nippon Shinyaku, AbbVie, GlaxoSmithKline, SymBio Pharmaceuticals, Micron, Daiichi Sankyo
Consulting or Advisory Role: Ono Pharmaceutical, Meiji Seika Kaisha, Chugai Pharma, Bristol Myers Squibb, Kyowa Hakko Kirin, Takeda, Celgene, HUYA Bioscience International, Stemline Therapeutics, Eisai, Janssen, AstraZeneca¸ Daiichi Sankyo, AbbVie,
Research Funding: Chugai Pharma (Inst), Novartis (Inst), ARIAD (Inst), Takeda (Inst), Gilead Sciences (Inst), AbbVie (Inst), Ono Pharmaceutical (Inst), Celgene (Inst), Solasia Pharma (Inst), MSD (Inst), Eisai (Inst), Zenyaku Kogyo (Inst), Bayer (Inst), SymBio Pharmaceuticals (Inst), AstraZeneca (Inst), Incyte (Inst), Mundipharma (Inst), Yakult Pharmaceutical (Inst)
Arpad Illes
Consulting or Advisory Role: Janssen, Takeda, Novartis, Pfizer, Roche, Celgene
Research Funding: Takeda, Seattle Genetics
Travel, Accommodations, Expenses: Novartis, Janssen, Pfizer, Roche
Ying Zhou
Stock and Other Ownership Interests: AbbVie
Monique Dail
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Brenda Chyla
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jalaja Potluri
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Hartmut Döhner
Consulting or Advisory Role: AbbVie, Agios¸ Amgen, Astellas Pharma, Celgene, Jazz Pharmaceuticals, Janssen Oncology, Novartis, AstraZeneca, Berlin-Chemie, GEMoaB, Bristol Myers Squibb/Celgene, Gilead Sciences, Syndax
Research Funding: Arog, Amgen, Bristol Myers Squibb, Novartis, Pfizer, Jazz Pharmaceuticals, AbbVie, Kronos Bio
No other potential conflicts of interest were reported.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
AUTHOR CONTRIBUTIONS
Conception and design: Keith W. Pratz, Brian A. Jonas, Michael J. Thirman, Jun Ho Jang, Monique Dail, Brenda Chyla, Jalaja Potluri, Hartmut Döhner
Provision of study materials or patients: Vinod Pullarkat, Christian Recher, Jacqueline S. Garcia, Courtney D. DiNardo, Vladimir Vorobyev, Su-Peng Yeh, Kazuhito Yamamoto, Arpad Illes, Hartmut Döhner
Collection and assembly of data: Keith W. Pratz, Brian A. Jonas, Christian Recher, Andre C. Schuh, Michael J. Thirman, Jacqueline S. Garcia, Courtney D. DiNardo, Vladimir Vorobyev, Nicola S. Fracchiolla, Su-Peng Yeh, Jun Ho Jang, Muhit Ozcan, Kazuhito Yamamoto, Arpad Illes, Brenda Chyla, Jalaja Potluri
Data analysis and interpretation: Keith W. Pratz, Brian A. Jonas, Vinod Pullarkat, Christian Recher, Andre C. Schuh, Michael J. Thirman, Jacqueline S. Garcia, Jun Ho Jang, Muhit Ozcan, Kazuhito Yamamoto, Arpad Illes, Ying Zhou, Monique Dail, Brenda Chyla, Jalaja Potluri, Hartmut Döhner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Measurable Residual Disease Response and Prognosis in Treatment-Naïve Acute Myeloid Leukemia With Venetoclax and Azacitidine
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Keith W. Pratz
Consulting or Advisory Role: Astellas Pharma, Boston Biomedical, AbbVie
Research Funding: Agios (Inst), Millennium (Inst), AbbVie (Inst), Daiichi Sankyo (Inst)
Brian A. Jonas
Consulting or Advisory Role: AbbVie, Jazz Pharmaceuticals, GlycoMimetics, Treadwell Therapeutics, Takeda, Genentech, Bristol Myers Squibb/Pfizer, Pfizer
Research Funding: Daiichi Sankyo (Inst), AbbVie (Inst), Pharmacyclics (Inst), Genentech/Roche (Inst), Glycomimetics (Inst), Celgene (Inst), FORMA Therapeutics (Inst), Incyte (Inst), Arog (Inst), Roche (Inst), Jazz Pharmaceuticals (Inst), Pfizer (Inst), Sigma-Tau (Inst), Forty Seven¸ Amgen (Inst), Immune-Onc Therapeutics (Inst), Loxo (Inst), Gilead/Forty Seven (Inst)
Travel, Accommodations, Expenses: AbbVie
Vinod Pullarkat
Consulting or Advisory Role: Novartis, Amgen, Pfizer, Jazz Pharmaceuticals, AbbVie/Genentech
Speakers' Bureau: Novartis, Amgen, Jazz Pharmaceuticals, AbbVie
Christian Recher
Consulting or Advisory Role: Celgene, Amgen, Novartis, Jazz Pharmaceuticals, AbbVie, Janssen, Astellas Pharma, MacroGenics¸ Daiichi Sankyo, Otsuka, Novartis¸ Incyte, Pfizer¸ Roche, Takeda
Research Funding: Celgene (Inst), Amgen (Inst), Jazz Pharmaceuticals (Inst), Astellas Pharma (Inst), Agios (Inst), Daiichi Sankyo (Inst), MaaT Pharma (Inst), Roche (Inst), AbbVie (Inst), Novartis (Inst), Chugai Pharma (Inst), IQVIA (Inst)
Travel, Accommodations, Expenses: Incyte, Celgene, Sanofi, Amgen, Novartis, Daiichi Sankyo, Gilead Sciences
Andre C. Schuh
Honoraria: Celgene, Amgen, Pfizer, Novartis, Jazz Pharmaceuticals
Research Funding: Celgene, Amgen, Agios, Pfizer
Michael J. Thirman
Consulting or Advisory Role: AstraZeneca, Genentech, AbbVie, Adaptive Biotechnologies, Celgene¸ Pharmacyclics
Research Funding: AbbVie (Inst), Syndax (Inst), Merck (Inst), TG Therapeutics (Inst)
Jacqueline S. Garcia
Consulting or Advisory Role: AbbVie, Takeda, Astellas Pharma
Research Funding: AbbVie (Inst), Pfizer (Inst), Genentech/Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Prelude Therapeutics (Inst)
Courtney D. DiNardo
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Notable Labs
Stock and Other Ownership Interests: Notable Labs
Honoraria: Agios, Celgene, AbbVie, Jazz Pharmaceuticals, Daiichi Sankyo, Novartis, Takeda
Consulting or Advisory Role: Celgene, Agios, AbbVie
Research Funding: AbbVie, Agios, Celgene, Daiichi Sankyo
Vladimir Vorobyev
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Sanofi, Novartis, BeiGene, Roche, AbbVie, Takeda, Gilead Sciences
Speakers' Bureau: AbbVie, AstraZeneca, Amgen, BioCad, Janssen, Sanofi, Takeda¸ Novartis, BeiGene
Travel, Accommodations, Expenses: AstraZeneca
Nicola S. Fracchiolla
Consulting or Advisory Role: Amgen, Jazz Pharmaceuticals, Incyte, AbbVie
Travel, Accommodations, Expenses: Pfizer
Su-Peng Yeh
Honoraria: Novartis, Bristol Myers Squibb, Janssen¸ Takeda, AbbVie, Amgen, Sanofi, Bayer, Astellas Pharma
Consulting or Advisory Role: AbbVie, Sanofi, Novartis, Pfizer, Chugai Pharma
Jun Ho Jang
Speakers' Bureau: Novartis
Muhit Ozcan
Research Funding: Janssen, Celgene, Takeda, Bayer, Merck, Archigen Biotech, Roche, AbbVie, Acerta Pharma/AstraZeneca
Kazuhito Yamamoto
Honoraria: Kyowa Hakko Kirin, Takeda¸ Janssen, Bristol Myers Squibb, Celgene, Sumitomo Dainippon, Ono Pharmaceutical, Chugai Pharma, Novartis, Otsuka, Mundipharma, Eisai, MSD, Meiji Seika Kaisha, Sanofi, Nippon Shinyaku, AbbVie, GlaxoSmithKline, SymBio Pharmaceuticals, Micron, Daiichi Sankyo
Consulting or Advisory Role: Ono Pharmaceutical, Meiji Seika Kaisha, Chugai Pharma, Bristol Myers Squibb, Kyowa Hakko Kirin, Takeda, Celgene, HUYA Bioscience International, Stemline Therapeutics, Eisai, Janssen, AstraZeneca¸ Daiichi Sankyo, AbbVie,
Research Funding: Chugai Pharma (Inst), Novartis (Inst), ARIAD (Inst), Takeda (Inst), Gilead Sciences (Inst), AbbVie (Inst), Ono Pharmaceutical (Inst), Celgene (Inst), Solasia Pharma (Inst), MSD (Inst), Eisai (Inst), Zenyaku Kogyo (Inst), Bayer (Inst), SymBio Pharmaceuticals (Inst), AstraZeneca (Inst), Incyte (Inst), Mundipharma (Inst), Yakult Pharmaceutical (Inst)
Arpad Illes
Consulting or Advisory Role: Janssen, Takeda, Novartis, Pfizer, Roche, Celgene
Research Funding: Takeda, Seattle Genetics
Travel, Accommodations, Expenses: Novartis, Janssen, Pfizer, Roche
Ying Zhou
Stock and Other Ownership Interests: AbbVie
Monique Dail
Employment: Genentech/Roche
Stock and Other Ownership Interests: Roche/Genentech
Brenda Chyla
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Jalaja Potluri
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
Hartmut Döhner
Consulting or Advisory Role: AbbVie, Agios¸ Amgen, Astellas Pharma, Celgene, Jazz Pharmaceuticals, Janssen Oncology, Novartis, AstraZeneca, Berlin-Chemie, GEMoaB, Bristol Myers Squibb/Celgene, Gilead Sciences, Syndax
Research Funding: Arog, Amgen, Bristol Myers Squibb, Novartis, Pfizer, Jazz Pharmaceuticals, AbbVie, Kronos Bio
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network : NCCN Guidelines for Patients with Acute Myeloid Leukemia, Version 3.2021, 2021. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf [Google Scholar]
- 2.DiNardo CD, Jonas BA, Pullarkat V, et al. : Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383:617-629, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, et al. : Age and acute myeloid leukemia. Blood 107:3481-3485, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, O'Brien S, Cortes J, et al. : Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090-1098, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Freeman SD, Jovanovic JV, Grimwade D: Development of minimal residual disease-directed therapy in acute myeloid leukemia. Semin Oncol 35:388-400, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Buccisano F, Maurillo L, Piciocchi A, et al. : Minimal residual disease negativity in elderly patients with acute myeloid leukemia may indicate different postremission strategies than in younger patients. Ann Hematol 94:1319-1326, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Terwijn M, van Putten WL, Kelder A, et al. : High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: Data from the HOVON/SAKK AML 42A study. J Clin Oncol 31:3889-3897, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Freeman SD, Virgo P, Couzens S, et al. : Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 31:4123-4131, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Boddu P, Jorgensen J, Kantarjian H, et al. : Achievement of a negative minimal residual disease state after hypomethylating agent therapy in older patients with AML reduces the risk of relapse. Leukemia 32:241-244, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Döhner H, Estey E, Grimwade D, et al. : Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424-447, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuurhuis GJ, Heuser M, Freeman S, et al. : Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 131:1275-1291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thermo Fisher Scientific : Real-Time PCR. https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr.html?cid=corp_ask-a-scientist_blog [Google Scholar]
- 13.Dix C, Lo TH, Clark G, et al. : Measurable residual disease in acute myeloid leukemia using flow cytometry: A review of where we are and where we are going. J Clin Med 9:1714, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, et al. : Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642-4649, 2003 [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services: National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE) v4.03, 2018. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm [Google Scholar]
- 16.Maiti A, DiNardo CD, Wang SA, et al. : Prognostic value of measurable residual disease after venetoclax and decitabine in acute myeloid leukemia. Blood Adv 5:1876-1883, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jongen-Lavrencic M, Grob T, Hanekamp D, et al. : Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 378:1189-1199, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.