Abstract
Graphene‐based materials have shown immense pertinence for sensing/imaging, gene/drug delivery, cancer therapy/diagnosis, and tissue engineering/regenerative medicine. Indeed, the large surface area, ease of functionalization, high drug loading capacity, and reactive oxygen species induction potentials have rendered graphene‐ (G‐) and graphene oxide (GO)‐based (nano)structures promising candidates for cancer therapy applications. Various techniques namely liquid‐phase exfoliation, Hummer's method, chemical vapor deposition, chemically reduced GO, mechanical cleavage of graphite, arc discharge of graphite, and thermal fusion have been deployed for the production of G‐based materials. Additionally, important criteria such as biocompatibility, bio‐toxicity, dispersibility, immunological compatibility, and inflammatory reactions of G‐based structures need to be systematically assessed for additional clinical and biomedical appliances. Furthermore, surface properties (e.g., lateral dimension, charge, corona influence, surface structure, and oxygen content), concentration, detection strategies, and cell types are vital for anticancer activities of these structures. Notably, the efficient accumulation of anticancer drugs in tumor targets/tissues, controlled cellular uptake properties, tumor‐targeted drug release behavior, and selective toxicity toward the cells are crucial criteria that need to be met for developing future anticancer G‐based nanosystems. Herein, important challenges and future perspectives of cancer therapy using G‐ and GO‐based nanosystems have been highlighted, and the recent advancements are deliberated.
Keywords: cancer nanotherapy, graphene oxide, graphene‐based nanomaterials, graphene
Important challenges, recent advancements, and future perspectives for deploying graphene‐ and graphene oxide‐based nanosystems in cancer therapy are deliberated. Notably, large surface area, ease of functionalization, high drug loading capacity, and reactive oxygen species induction potentials have rendered these structures promising candidates for cancer therapy applications. The ease of functionalization bodes well for their utility in treating assorted types of cancers.
1. INTRODUCTION
Graphene (G), the two‐dimensional and hexagonally bonded sp2 hybridized carbon structure with extraordinary characteristics, has garnered huge interdisciplinary attention in different fields of science and engineering over the last half‐century. The single, multi‐layered (less than 10) and flatted honeycomb structure of G possesses unique attributes namely high hardness, resistance, thermal and electrical conductivity, optical transmittance, infinite surface area, among others. 1 , 2 , 3 , 4 , 5 Graphene oxide (GO) is the oxidized version of G, which is usually produced under harsh oxidation conditions. GO possesses numerous oxygen‐bearing functionalities, such as hydroxyl, carboxylic, and epoxide groups on the carbon surface, rendering it more hydrophilic than G. 6 , 7 , 8 , 9 The incorporation of G layers in nanocomposites is one of the methods for controlling and improving their area of surface, mechanical/electrical attributes, and thermal conductivity. 10 , 11 , 12 These G‐ and GO‐based nanocomposites with high surface area, ease of functionalization, high drug loading capacity, and reactive oxygen species (ROS) induction potential are promising candidates aimed for the targeted transport of anticancer drugs/genes and diagnostic agents. 13 , 14 , 15 , 16 Assorted G‐ and GO‐based derivatives in association with multifunctionalization processes can help assist in improving the optical and electrical properties as well as poor solubility in aqueous solutions. 17 , 18 , 19 , 20 These nanosystems with better biodistribution of drugs, low adverse effects on healthy cells, high selectivity/sensitivity, and higher local therapeutic absorption have garnered a lot of attention. 21 However, complex synthetic procedures, potential inflammatory effects, possible accumulation in the spleen, likely immunogenicity, cell disruption in higher concentration, and the need for comprehensive in vivo studies/protein folding studies, are their important limitations. 22 , 23
G and GO and their corresponding nanocomposites have shown promising applicability for biosensing/bioimaging, 24 nano‐detecting/labeling, 25 gene/drug delivery, 26 and tissue engineering/regenerative medicine, 27 among others. Nanocarriers comprising G or GO have been deployed for the delivery of anticancer agents with high selectivity/specificity and drug loading capacity. 28 , 29 The G‐based advanced functional structures with large surface areas, ease of functionalization/modification, and photothermal features are attractive for cancer nanotherapy. 30 , 31 For instance, reduced‐GO structures with good biocompatibility have been fabricated using Euphorbia heterophylla, and their cytotoxicity evaluated against A549 and HepG2 human cancerous cells; high cytotoxic effects were observed in vitro, but further studies are warranted to analyze their other biomedical potentials. 32 Additionally, reduced‐GO materials with dose‐dependent cytotoxicity effects against MCF‐7 cells have been generated by applying Bacillus marisflavi as the stabilizer and reductant agent. These bacterially reduced‐GO materials (∼60 μg ml–1) could increase the formation of ROS and initiate the release of lactate dehydrogenase. 33 Han et al. 34 have reviewed the functionalization and optimization strategies of GO‐centered nanomaterials for drug/gene transport. Various strategies including non‐covalent and covalent (e.g., addition, condensation, and nucleophilic/electrophilic substitution) have been widely explored for the functionalization of G and GO. Increased electrical conductivity, enhanced dispersibility, improved functionality, and good biocompatibility have been reported as outstanding benefits of these functionalized materials. However, some of these functionalization techniques such as addition may suffer from difficulty in controlling, thus controllable selective strategies should be further explored by researchers. 35 , 36
For constructing advanced G‐based nanosystems for diagnosis and treatment of cancers, several challenging issues should be considered such as flexibility, biocompatibility, biodegradability, toxicity, surface functionizability, and fluorescence quenching potentials. 37 , 38 , 39 , 40 , 41 , 42 The surface modification and functionalization of G‐based materials can be performed by different polymeric materials. 20 , 43 Bioactive materials (e.g., L‐ascorbic acid, chitosan, and gelatin) can be deployed for surface functionalization of these G‐based materials for improving biocompatibility and targeting features. The surface modification of these materials has been reported by introducing a variety of functional groups, helping to adjust and manipulate their surfaces and improve their properties and activities in the form of hybrid materials; galactose, hyaluronic acid, and folic acid are some important compounds reported for improving the targeting and selectivity of anticancer delivery systems. 44 Herein, important challenges and future perspectives of G‐ and GO‐based materials for cancer therapy are highlighted, with deliberations on recent advances.
2. PREPARATION TECHNIQUES
The prodigious intrinsic properties of G and GO make them a high demand material for deployment in diverse research areas such as water treatment, 45 , 46 , 47 air purification, 48 , 49 , 50 , 51 bactericidal, 52 cell imaging, 53 , 54 medical and life science, 50 , 55 , 56 , 57 , 58 , 59 drug delivery, 60 , 61 tissue engineering, 62 , 63 energy‐related researches, 64 , 65 , 66 , 67 , 68 , 69 among others. In spite of the fact that G's and GO's potential has been promisingly ascertained; their preparation is relatively difficult and expensive that may restrict their utilization on large industrial scales. 70 Four basic methods have been established for oxidizing the G to produce GO, comprising Staudenmaier, 71 Hoffmann, 72 Brodie, 73 and Hummers processes, 74 and modification thereof to render them more efficient, cheaper, and environmentally friendlier, but the main challenges still exist. 75 , 76 , 77 Although other techniques such as chemical exfoliation 78 , 79 and chemical vapor deposition 80 have been developed for the synthesis of G and GO, these methods are expensive and require specialized instrumentation. Additionally, the generation of NO2, N2O4, ClO2, and other toxic or explosive gases during these methods is another drawback that must be considered seriously from an environmental viewpoint. 81 As the up‐scalable preparation of G‐based structures necessitates expensive materials, complex instruments, and sometimes is ecologically unfriendly; thus, there is an urgent demand for synthesizing these materials via simple and eco‐friendly methods. The sustainable production of these G‐based materials by applying agricultural wastes (e.g., walnut shells and husk) is one of them. Besides, the requirement of high temperature and production of some toxic syngas may cause some environmental problems that need to be addressed in future studies to make the final product more sustainable.
3. G‐ AND GO‐BASED NANOSYSTEMS FOR CANCER THERAPY
3.1. Photothermal therapy
Cancer is often a fatal disease that results in deaths worldwide, thus detection in primary stages and effective treatment strategies are very essential for improving the rate of survival in patients with cancers. 82 G‐based materials with their unique physicochemical properties can be employed for the detection and treatment of cancers. For instance, when GO was combined with polyethylene glycol (PEG), it exhibited photothermal therapy effects against cancers and tumors via the induction of heating effect in macrophages, in vitro and in vivo. 83 The macrophage cell lines RAW264.7 were treated with near‐infrared (NIR) light irradiation, and their polarization status was evaluated by flow cytometric and mRNA expression study. GO‐PEG had high thermal stability, improved biocompatibility, and significant photothermal influence. Notably, these photothermal structures alleviated interleukin‐4‐induced M2 polarization of macrophages and regulated their antitumor potentials. Thus, human osteosarcoma lost their migration and invasion potentials, instigating suitable antitumor effects. 83 Additionally, chitosan‐functionalized GO nanoplatforms were conjugated with folic acid with the purpose of photothermal cancer therapy guided by NIR fluorescence and photoacoustic imaging; the cancerous cells were completely destroyed under laser irradiation, in vitro. 84 Also, in vivo studies indicated that the tumors were totally obstructed with no recurrence within 20 days, after the deployment of this targeted nanosystem under laser irradiation (Figure 1). 84
FIGURE 1.
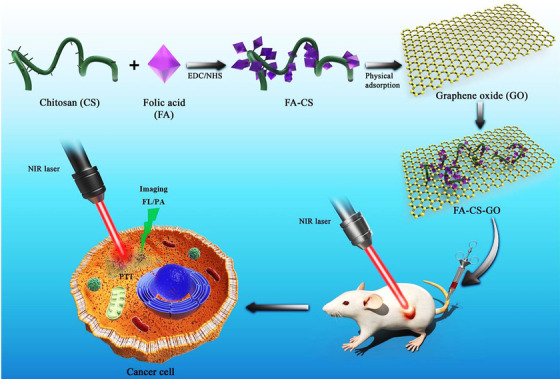
The chitosan (CS)‐functionalized graphene oxide (GO) nanosheets were conjugated with folic acid (FA) for targeted photothermal tumor therapy guided by photoacoustic imaging. Reproduced with permission from Elsevier, 2020 84
3.2. Delivery of anticancer agents
G‐based nanostructures incorporated with anticancer drugs were constructed from GO, G quantum dots (GQDs), and curcumin, with high stability and effective delivery of curcumin inside the cancerous cells. 85 The complexes, GO‐curcumin and GQDs‐curcumin were evaluated in various ratios against human breast cancer cell lines MDA‐MB‐468 and MCF‐7. As a result, cell viability of more than 75% could be detected from these samples after 48 h of incubation with the cell lines, whereas, by applying curcumin alone (about 100 μg ml–1), the cell viability was ∼40%. The corresponding cell death results were ∼60, 80, and 95% at 100 μg ml–1 after 48 h of the treatment process, respectively. 85 In addition, silver‐GO nanocomposites (∼20‐100 μg ml–1) have been studied against cancerous cells, 86 where they displayed suitable cytotoxic effects, but their efficiency was lower than free silver (Ag) nanoparticles with smaller size and better uptake. 87
Cu2O nanoparticles (∼4 nm) were decorated on GO for efficient and selective cancer nanotherapy. 87 The anticancer effects of these Cu2O‐GO nanocomposites were studied against HK‐2, 231, and A549 cells in vitro beneath the visible light irradiation. 87 Besides, the formulated GO‐PEGylated folate nanocarrier was examined in acidic (pH = 5.0) and physiological (pH = 7.4) environments. 88 Consequently, ∼21.5% and 71.0% of loaded camptothecin anticancer drug could be released under physiological and acidic conditions, after 48 h of treatment, respectively. The images of confocal microscopy obtained from the treated HeLa cells (after 8 h) by the GO‐PEGylated folate nanosystem illustrated the blue and green fluorescence emission of camptothecin from the nucleus and cytoplasm, respectively, demonstrating targeted drug delivery to the cells. 88 Zhou et al. 89 introduced a smart multifunctional MnO2‐doped GO nanosystem for the delivery of cisplatin and photosensitizer (Ce6). Consequently, the decomposition of hydrogen peroxide into oxygen was catalyzed to ease the hypoxia of the tumor, and the level of glutathione was reduced in targeted tumors; Mn2+ was continuously generated for progressing Fenton‐like reaction, thus providing improved antitumor effects. Notably, hyaluronic acid was applied for modifying the surface of the prepared nanosystem to improve its targeting properties, causing increased cellular toxicity and growth inhibition of tumors. 89
Folic acid has been decorated on the GO‐based nanocomposite functionalized with PEG for delivering paclitaxel. 90 The cellular toxicity analysis of this nanosystem demonstrated good biocompatibility and low cytotoxicity; the cell viability being ∼60% and 30% after treating by free paclitaxel drug and the designed nanosystem, respectively. The images of fluorescence microscopy evaluations from the nanosystem proved paclitaxel delivery into the targeted tumor cells with high efficiency. Consequently, by increasing the concentration of a drug, the blue fluorescence emission was also decreased, indorsing the reduction in cell number and the successful entrance of drug nanocarrier into the cells. 90 In another study, a nanosystem was developed for targeted delivery of doxorubicin, based on the strong conjunction between acidic functional groups of tumors and the hydroxyl groups of G, 91 when PEG was added for enhancing the biocompatibility of hydroxylated G fabricated via the solid‐state ball milling technique. The cell viability of tumor cells (OCM‐1) and normal cells (ARPE‐19) were less than 10% and 80%, respectively by treating with the nanosystem (10 μg ml–1) after 48 h. The confocal microscopy analysis revealed that the hydroxylated GO could be detected inside the cells after 12 h; however, the hydroxylated GO could be found around the cells after 48 h, and then it disappeared after 60 h. Results of this study illustrated that the nanocomposite had suitable antitumor effects against OCM‐1 tumors and exhibited low toxicity to the normal cells. 91
A GO‐based nanosystem was developed for targeted fluorouracil (FU) delivery. 92 The cell viability evaluations demonstrated no noticeable toxicity at various concentrations; therefore, further in vivo analysis should be conducted on the prepared nanosystem, especially for anticancer drug delivery. It was indicated that by loading FU on this nanosystem, the cellular viability was increased as nanocarrier could reduce the toxic influence of FU on normal tissues thus improving the biocompatibility. 92 Besides, an innovative nanocarrier with controllable release features was developed for delivering chlorambucil anticancer drug, 93 which was prepared using gelatin and reduced‐GO functionalized with folic acid. In vitro drug release was analyzed in 3 mediums including phosphate buffer solution as simulated blood (pH = 7.4), colonic fluid (pH = 5.4), and gastric fluid (pH = 1.2) by applying varying reduced‐GO concentrations; higher release rates could be detected under acidic conditions compared to the neutral conditions. The cell viability evaluations revealed that the prepared nanocomposite had low cytotoxicity; the results from cellular viability analysis (500 μg ml–1) were ∼11.7% and 28% for free chlorambucil and chlorambucil‐loaded nanocomposite, respectively. 93
The phospholipid‐based amphiphilic polymer was deployed for modifying the reduced‐GO to improve the transfection of small interfering RNA (siRNA). 94 The prepared nanocarrier could deliver siRNA without enzymatic degradation when compared to the free siRNA. 94 Additionally, the modified GO could be employed as a nanocarrier for transferring siRNA into cells. 95 After formulating the GO/poly‐L‐lysine hydrobromide/folic acid and GO/poly‐L‐lysine hydrobromide platforms, doxorubicin and siRNA were loaded on them and the corresponding delivery and cytotoxicity issues were analyzed on HeLa cells; no significant toxic effects could be detected on HeLa cells even at a high concentration of G‐based nanosystem (∼120 μg ml–1). The tumor growth was inhibited in the presence of the siRNA gene, and the nanocarrier for siRNA had positive effects while free siRNA demonstrated no noticeable gene silencing effect. Therefore, this nanosystem can be suggested for delivering siRNA genes and silencing the specified gene expression. 95
Doxorubicin has been loaded onto GO hybridized nanogels, which were employed for photothermal therapy by NIR laser irradiation (wavelength of 808 nm). 96 As a consequence, the transport of anticancer drugs into A549 cells was improved by applying nanogels. Remarkably, the toxicity effects of these prepared nanogels against the A549 cancer cells were improved by laser treatment, due to the thermal absorption of GO under laser irradiation. 96 Besides, the G hydrogels functionalized with branched polyethyleneimine were explored for delivering doxorubicin with good biocompatibility and photothermal therapy of breast cells. A combination of chemotherapy with photothermal therapy reduced the cancer cells to ∼33 % while the utilization of doxorubicin‐loaded G hydrogels without laser irradiation decreased the breast cancer cells to ∼66.7%. 97
Magnetic GO nanostructures were coated by poly lactic‐co‐glycolic acid for the delivery of 5‐iodo‐2‐deoxyuridine to stimulate radio‐sensitizing influences on patients with glioblastoma. 98 Analytical studies showed that suitable magnetic targeting and improved penetration through the blood‐brain barrier could be obtained. Also, the synergistic outcome was reported by applying this nanocarrier, providing the effective inhibition and apoptosis against C6 glioma tumor, extended circulation half‐life (more than 140 h), increased dose enhancement factor, and enhanced radio‐sensitizing effects. 98 Additionally, gold nanorods were loaded on GO nanocomposites using polydopamine for targeted doxorubicin delivery to the cancerous cells. These nanosheets had low cytotoxicity and significant biocompatibility even at a 250 μg ml–1 concentration after 48 h of the treatment. 99
Multifunctionality is one of the important criteria for controlling and treating cancers, as it was indicated that the multifunctionalized GO‐based platforms had efficient doxorubicin drug delivery as well as inhibitory effects against hepatocarcinoma cancerous cells. The surface functionalization was performed by deploying polyethyleneimine (PEI) modified with PEG‐linked lactobionic acid and fluorescein isothiocyanate, followed by acetylation of the residual amine groups from PEI. 100 Among the important properties of this nanocarrier has been suitable cell feasibility in the examined strength span, thus the nanosystem demonstrated improved target specificity and pH‐sensitive release behavior with high growth inhibition effects to the cancerous cells (Figure 2). 100 Reduced GO nanocarriers were fabricated for pH‐sensitive doxorubicin drug delivery. The prepared nanosystem demonstrated suitable safety/stability profile and high drug loading capacity with pH‐sensitive and sustainable/controllable release behavior. This nanohybrid system illustrated cytotoxicity activity to MCF‐7 and A549 cells via a nonspecific endocytosis mechanism (Figure 3). 101 It was revealed that the conjugation of GO‐based nanoplatforms with zoledronic acid could lead to producing nanosystems with optimum performance against breast cancer, providing synergistic effects for treating osteoporosis and metastasis. 102
FIGURE 2.
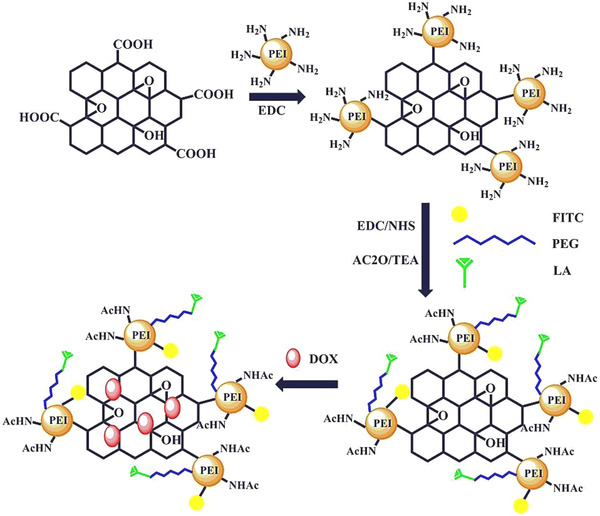
Preparative process of multifunctional graphene oxide (GO)‐based structures with pH‐sensitive and controllable drug delivery properties. Reproduced with permission from Elsevier, 2016 100
FIGURE 3.
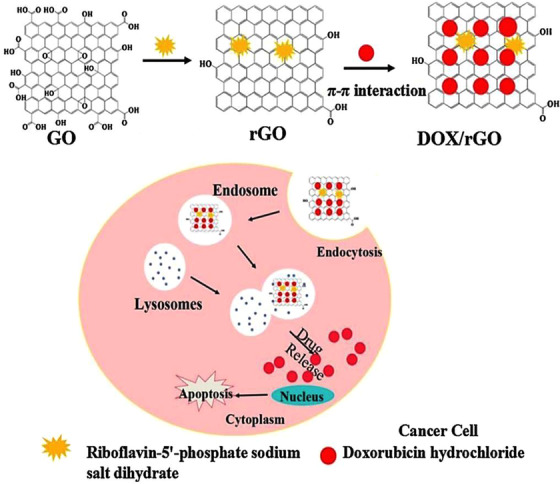
Reduced graphene oxide (GO) for doxorubicin anticancer delivery with pH‐dependent behavior. rGO: reduced GO, DOX: doxorubicin. Reproduced with permission from Elsevier, 2015 101
Functionalized GO‐based nanocomposites have been designed with the purpose of anticancer drug delivery. For instance, a nanocarrier with good biocompatibility and biodegradability features was constructed using gelatin and reduced GO nanosheets functionalized with folic acid for the delivery of chlorambucil anticancer drug. This nanosystem showed controlled release behavior with significant loading capacity. Consequently, the drug release rate was higher under acidic conditions in comparison with the neutral environments. 93 Additionally, the non‐covalent functionalized GO by Pluronic F127 molecules was introduced for tumor‐targeting therapy. Doxorubicin was loaded onto the prepared nanosystem with high loading capacity and efficiency (∼83%) could induce a higher apoptosis rate (∼12.27 %) of U251 cells compared with free doxorubicin (∼8.20 %). 103 Hamblin and co‐workers 104 have designed GO‐based polymeric nanocomposites (∼51 nm) for the delivery of doxorubicin against breast cancer; the drug release being 24.7% and 41.2% under neutral and acidic environments after 72 h. 104 To develop a dual‐drug loaded nanosystem for combinational cancer therapy, cisplatin and doxorubicin were loaded into a nanoplatform constructed from GO and PEG. 105 Consequently, the designed nanosystems could be efficiently delivered into tumor cells, introducing noticeable cell apoptosis and necrosis; these agents could inhibit the growth of tumor cells with enhanced efficacy, the rate of promoted apoptosis and necrosis effects on cancerous cells being ∼18.6%. 105
3.3. Combinational cancer therapy
3.3.1. Radiotherapy and photothermal therapy
Combination therapy with lower toxicity and improved targeting benefits through functionalized nanostructures has been one of the topics of interest for scientists in the field of cancer treatment. It was indicated that Fe3O4@Au/reduced GO nanomaterials could be designed via hydrothermal reaction for combinational therapy via both radiotherapy and photothermal therapy approaches. Accordingly, the efficiency of photothermal conversion was about 61%. These nanosystems showed good biocompatibility with suitable cytotoxicity against oral squamous carcinoma KB cell lines. 106
3.3.2. Chemo‐photothermal and chemo‐photodynamic therapy
A nanoplatform was developed via the attachment to Fe3O4‐GO polymers emanating from β‐cyclodextrin‐hyaluronic acid; 107 it could be simply separated magnetically and demonstrated significant biocompatibility, suitable dispersibility in water, and photothermal heating via high NIR. The application of hyaluronic acid‐β‐cyclodextrin combination could increase the drug (doxorubicin) packing capacity to more than 485.43 mg g–1. Notably, the prepared nanosystem provided a rapid photothermal reaction to perform the NIR‐stimulated release of anticancer drugs in solvents with low acidity. The doxorubicin‐loaded nanocomposites revealed CD44 receptor facilitated active‐directing identification together with chemo‐photothermal synergistic antitumor effects (Figure 4). 107
FIGURE 4.
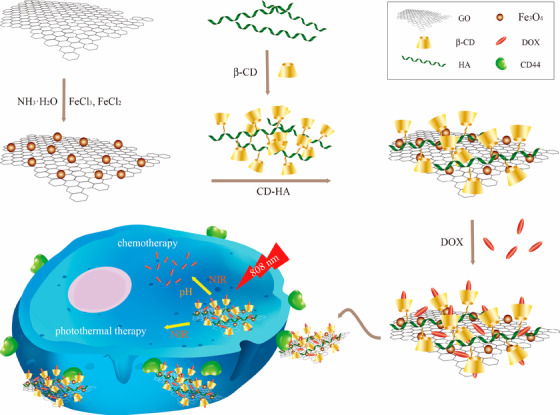
The polymers of β‐cyclodextrin (β‐CD)‐hyaluronic acid (HA) were attached to Fe3O4‐GO nanocomposites for targeted chemo‐photothermal therapy of tumors cells. DOX: doxorubicin. Reproduced with permission from MDPI (CC BY 4.0) 107
Liu et al. 108 reported a drug delivery nanosystem for the synergistic chemo‐photothermal therapy of cancers using GO nanosheets; the tumor intracellular environment and photothermal heating had stimulatory effects on the release of anticancer agents from nanocarriers. 108 On the other hand, during the non‐invasive treatment of cancers, photodynamic therapy can form significantly toxic ROS. 109 , 110 The suppression of MutT homolog 1 protein function (a DNA oxidative damage repair protease) can enhance the efficacy of photodynamic therapy via the improvement of cellular sensitivity to ROS. Thus, in one study, functionalized GO‐based nanosystems were prepared using PEG, folic acid, and photosensitizer indocyanine green for delivering the MTH1 inhibitor and doxorubicin. These nanosystems demonstrated chemo‐photodynamic therapy effects for inhibiting the osteosarcoma cells proliferation and migration. The improved chemo‐photodynamic therapy stimulated the apoptosis and autophagy pathways via the suppression of MutT homolog 1 protein and stimulation of ROS accumulation (Figure 5). 109 In another study, the decoration of reduced‐GO was performed with the purpose of combinational chemo‐photodynamic cancer treatment using magnetic nanoparticles and camptothecin drug by connecting 4‐hydroxy coumarin to reduced‐GO via an allylamine linker. 109 The nanocarriers demonstrated pH‐depended release behavior and suitable cytotoxic effects against the human breast cancer cell lines. It was revealed that free camptothecin had higher toxic effects on normal cells, and could damage the DNA. In contrast, the prepared nanosystem demonstrated good biocompatibility and no remarkable toxic effects on the normal cells (WS‐1 cells); the cell viability was ∼75% in 100 μg ml–1 following 24 h of the therapy. The photodynamic therapy deploying UV‐Visible irradiation (> 365 nm) could reduce the cancer cell viability to ∼38%. The laser irradiation could lead to generating higher amounts of ROS for significantly inhibition of cancerous cells; this combinational therapy strategy exhibited synergistic anti‐tumor efficiency and significant apoptosis of targeted cells. 111
FIGURE 5.
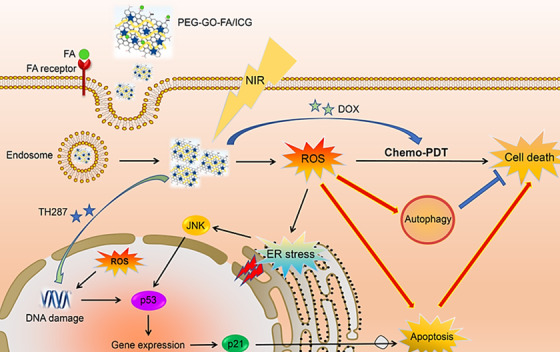
Chemo‐photodynamic cancer nanotherapy using a nanocomposite prepared from GO, polyethylene glycol (PEG), folic acid (FA), and photosensitizer indocyanine green (ICG). The nanosystem could stimulate the apoptosis and autophagy pathways through the suppression of MutT homolog 1 protein and stimulation of reactive oxygen species (ROS) accumulation; ROS helped to the endoplasmic reticulum (ER) stress‐promoted apoptosis via the JNK/p53/p21 trail. Reproduced with permission from Elsevier, 2020 109
4. IMPORTANT CHALLENGES AND FUTURE PERSPECTIVES
Overall, the use of G‐ and GO‐based materials with elevated electrical conductivity, mechanical strength, and stiffness in the design and fabrication of anticancer nanosystems has promising advantages and unique features. 110 , 112 Table 1 summarizes some important examples of G‐ and GO‐based materials with their advantages and properties. However, crucial challenges regarding their cellular long‐term cytotoxicity/histopathology, immunogenicity, bio‐persistency, multi‐drug resistance, clearance mechanism, intracellular uptake, and bioaccumulation are still need to be systematically evaluated; the effects of particle size on the viability of cells have not been much examined by researchers. 22 , 113 Bi et al. 114 have discussed the possibility of lung cancer metastasis/progression after long‐term pulmonary exposure of G and carbon black. Accordingly, the cell necrosis and discharge of damage‐associated molecular patterns (such as mitochondrial DNA) could have happened; the mitochondrial DNA can potently stimulate the secretion of Wnt ligands in alveolar macrophages. 114
TABLE 1.
G‐ and GO‐based nanosystems for cancer therapy with promising advantages
| G‐ and GO‐based nanosystems | Applications | Important features | Refs. |
|---|---|---|---|
| Multifunctionalized GO | Targeted cancer therapy and drug delivery |
– No noticeable toxic effects – Higher drug stacking capability – pH‐responsive drug discharge features – Particular target transport and effectual cell inhibition |
100 |
| Carboxymethyl cellulose‐GO | Targeted and sustained drug delivery |
– No noticeable toxicity with sustained and prolonged release of doxorubicin – Incorporation of GO nanosheets highly improved the swelling capacity of hydrogels |
118 |
| GO | Cancer therapy and drug delivery system |
– Sustained‐release nanoformulation – Improved suppression of cancer cell growth |
119 |
| GO‐hyaluronic acid‐Arg‐Gly‐Asp peptide | Targeted cancer therapy and anticancer drug delivery |
– Low toxicity – High drug loading – Improved specificity and efficiency of anticancer drug delivery |
120 |
| Magnetic GO‐chitosan‐PEG‐N‐Hydroxysuccinimide | Anticancer drug delivery system |
– Good biocompatibility – Low cytotoxicity – pH‐responsive controllable drug release behavior – High drug loading potentials |
121 |
| polyvinylpyrrolidone‐ and β‐cyclodextrin‐modified GO | Targeted anticancer drug delivery |
– Low toxicity – pH‐dependent drug release |
122 |
| GO@soy phosphatidylcholine‐folic acid nanohybrid | Antitumor therapy and targeted drug delivery |
– No noticeable toxicity – pH‐dependable drug release – Improved steadiness and good biocompatibility – Higher drug packing ability – Effectual cellular uptake – Regulated drug discharge |
123 |
| Chitosan‐grafted‐poly(methacrylic acid)/GO | Anticancer drug delivery |
– No detectable toxicity – Significant biocompatibility – High drug packing capacity – pH‐dependent drug delivery performance |
124 |
| GO/chitosan oligosaccharide/γ‐polyglutamic acid | Anticancer drug delivery |
– No detectable toxicity – Simple delivery and controllable anticancer drug release behavior |
125 |
| Superparamagnetic iron oxide‐GO | Smart nanotheranostics platform |
– Good biocompatibility – pH‐dependable drug release |
126 |
| Chitosan‐carboxylated GO | Gene delivery | – High gene transferring properties | 127 |
| Modified GO | Gene delivery |
– Low toxicity – Improved release of DNA – Suitable interaction with DNA and hydrophobic immune adjuvant |
128 |
| GO/ethylene glycol‐polycaprolactone | Anticancer drug delivery; tumor therapy |
– Low cytotoxicity – Improved biocompatibility and biodegradability – High drug release and inhibition of tumor growth |
129 |
| GO‐nanoscale hydroxyapatite | Cancer therapy (chemotherapy and photothermal therapy) |
– High biocompatibility – High photothermal therapy activity – Improved drug release behavior – High drug loading capacity |
130 |
| Polymer G nano‐aerogels | Anticancer drug delivery | – High anticancer drug‐releasing with pH‐dependable behavior | 131 |
| Starch‐G nanosheets | Anticancer drug delivery |
– High anticancer drug loading capacity – Sustained‐release behavior – Good biocompatibility – Low toxicity with improved therapeutic efficacy |
132 |
| Reduced‐GO nanostructures | Cancer therapy and anti‐inflammatory effects | – Anti‐proliferative activity with high efficacy | 133 |
| Reduced‐GO nanostructures | Anticancer drug delivery |
– Sustained pH‐sensitive drug release – Improved therapeutic efficacy – High drug loading capacity – High hemolytic toxicity to rabbit red blood cells |
101 |
| Nanoscale GO loaded with HN‐1 (a tumor‐targeted peptide) | Anticancer drug delivery |
– High stability to the biological solution – High tumor‐targeting behavior – pH‐responsive drug release – High cellular uptakes and cytotoxicity toward tumor cells |
134 |
| D‐mannose‐mediated chitosan‐functionalized GO nanosystems | Anticancer drug delivery |
– Good biocompatibility – Targeted and controlled delivery – Intracellular discharge of marine algae‐mediated anticancer drugs versus glioblastoma cancers (e.g., ulvan) |
135 |
| 5‐Fluorouracil and curcumin loaded chitosan/reduced GO nanocomposites | Anticancer drug delivery |
– Synergistic inhibitory effects against the growth of HT‐29 colon cancerous cells – Dual‐drug loading properties – Improved targeting properties |
136 |
Another important issue is biological membranes that can function as barriers and restrict the diffusion of various molecules. Thus, innovative drug delivery nanosystems have to be developed with improved membrane permeability features, as it has been indicated that GO nanosheets loaded Tegafur drug had beneficial cell membrane permeability properties. 115 Besides, the lack of enough stability in bio‐medium can hinder the cancer photothermal therapy using G‐based materials. Thus, various polymers need to be explored for the functionalization of these materials, as has been exemplified in the case of functionalized GO being modified by an amphiphilic polymer which displayed improved colloidal stability with enough cytocompatibility, suitable size distribution, and neutral surface charge. 116 Furthermore, hybrid functional G‐based nanocomposites have been studied by researchers to improve the biocompatibility and cellular uptake features. 118 For instance, the complex of GO and GQDs exhibited excellent photothermal effects, improved biocompatibility, and high cytotoxic performances against cancers, indicating that hybrid G‐based nanocomposites may be promising candidates for cancer theranostics and cell imaging. However, these types of hybrid nanostructures with synergistic and optimized properties need to be still further examined, and more analytical explorations are nonetheless needed for the improvement of specificity and reduction of possible toxicity. 117
For a step toward the improvement of stability and bioactivity, natural polyphenols have been utilized in combination with the G‐based nanocomposites. Sivaperumal and co‐workers 137 have described the preparation of silver and gold nanoparticles hybridized with reduced GO nanocomposites and deploying the anticancer flavone, chrysin with improved stability, bioactivity, and biocompatibility. These nanohybrids had enhanced cytotoxic effects against breast carcinoma cell lines with low toxicity to the normal cells; the formation of ROS discharged by the co‐existing metal ions on the reduced GO promoted apoptosis. 137 Some natural polyphenolic flavonoids with anticancer effects (e.g., quercetin) have been utilized to generate G‐ and GO‐based nanosystems with controlled drug delivery; high cytotoxicity toward cancerous cells and targeted drug delivery properties could be attained by these nanosystems. 138
Because of the serious adverse effects and non‐targeting disadvantages of chemotherapy tactics, researchers have instigated extensive investigations into innovative nanostructures that have led to the design of a wide range of effective nanosystems for the treatment of cancers. However, important challenges regarding the effects of particle size/morphology, chemical structures, reaction/physiological conditions, and surface chemistry on efficacy and biosafety of designed anticancer systems are crucial. 139 Furthermore, the usage of hazardous or toxic compounds should be avoided in the process of designing these anticancer systems, preventing possible adverse health effects, skin irritations, immune reactions, and toxicity. 140 Green and sustainable synthesis methods, as well as green functionalization processes with reproducibility and up‐scalability advantages, can be deployed for the preparation of G‐based nanosystems. 141 Such protocols based on green chemistry for the reduction and prevention of potential environmental and health risks as well as the enhancement of biocompatibility and sustainability should be given more consideration by researchers. For instance, the fluorinated G constructed via a simple and green technique has been used as a nanocarrier for the targeted transport of curcumin to the cancerous cells with good biocompatibility in the concentration range of 100–500 μg ml–1; the toxic effects were dependent on the concentration of the sample. 142 The images of confocal spectroscopy have revealed blue and red emission from the nuclei of cancerous cells because of the attendance of G‐based nanocarrier and curcumin, respectively, after 12 h of the treatment. 142 Besides, green‐synthesized copper oxide (CuO) nanoparticles were decorated on GO nanoplatforms to perform against HCT‐116 human colon cancer cell lines (the cytotoxicity was ∼70%). 143
Important criteria such as pharmacokinetics, pharmacodynamic biomarkers, and tumor responses must be assessed, especially for targeted anticancer nano‐delivery. 144 , 145 , 146 For industrial manufacturing of G‐based nanomaterials with anticancer applicability, simplicity, cost‐effectiveness, and environmentally benign routes with excellent productivity are a prerequisite; both practical and theoretical studies must be specifically emphasized for the development of optimized synthesis techniques to have a seamless transition from lab‐scale to industrial production in the adaptation of conventional lab‐scale techniques. 147 Clinical and long‐term assessments are vital after the production, which has been infrequently attended; long‐term cytotoxicity of G‐based materials and their effects on cell signaling should be clarified. Importantly, the understanding of mechanisms responsible for toxic effects can help to identify the means to reduce them, providing functionalized G‐based materials with high biocompatibility. The selection of rational criteria is of immense importance for the development of clinically successful and translatable nanomedicines. A disease‐driven strategy to develop smart drug delivery nanosystems with emphasis on significant parameters related to the drug‐delivery system and target patient population can be deployed to balance different variables to enhance the therapeutic activity (Figure 6). 148 Furthermore, immunogenicity, inflammatory reactions, and hemocompatibility are vital criteria for anticancer employment of G‐based materials. 149 It has been indicated that G‐based nanocomposites could have DNA or mitochondrial damage, inflammatory reactions, autophagy, necrosis, and apoptosis effects; these materials have also validated dose‐dependent toxicity behavior. 150 In one study, hemolytic effects of GO structures were typically initiated via electrostatic interaction between these materials and red blood cell membrane, which can be circumvented by suitable surface functionalization or modification to improve the hemocompatibility. 151 It was revealed that GO caused significant immunogenicity as confirmed by a notable upsurge of tumor necrosis factor‐α, interleukin‐6, and interleukin‐1; however, the functionalized GO structures illustrated improved immunological compatibility. 152 For instance, interleukin‐6, interleukin‐12, tumor necrosis factor‐α, interferon γ, and monocyte chemotactic protein 1 were remarkably enhanced by applying GO structures, causing significant inflammatory effects. 153 GO‐induced inflammatory cytokines via interaction with toll‐like receptors activated the NF‐κB trail; however, functionalized G‐based nanomaterials could evade such inflammatory effects by macrophages through weakening the opsonin‐protein interaction. 151
FIGURE 6.
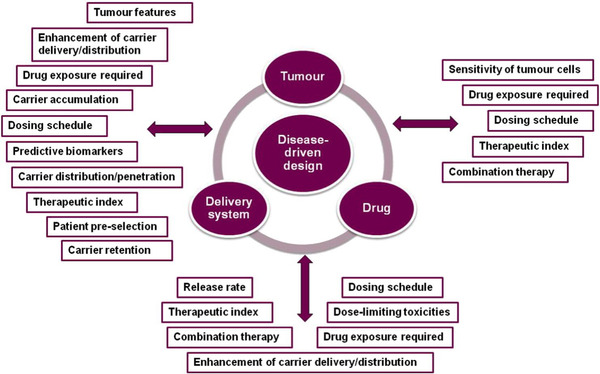
Some essential considerations for disease‐driven design and development of the nanosystem‐based delivery of antitumor or anticancer agents (therapeutics), the aim has to be on the criteria for selecting the delivery system, drug, and target patient population. Reproduced with permission from Elsevier, 2017 148
Given the widespread use of G‐based materials especially in biomedicine, precise deliberation of their toxicity is critical. 154 There are numerous studies showing the dose‐dependent toxicity of G‐based materials to animals and human cells, including lung granuloma generation, injury of liver/kidney, reduced viability of cells, and apoptosis. Some important parameters such as the functionalization, surface structure, aggregations, lateral size, corona effect, charge, and impurities have effects on the toxicological profile of these materials. On the other hand, distinct consideration should be exercised to study the possible events and mechanisms related to the toxicity of G‐ and GO‐centered entities such as apoptosis, DNA damage, oxidative stress, necrosis, physical destruction, inflammatory reactions, and autophagy. 154
5. CONCLUSION AND FUTURE OUTLOOKS
In conclusion, G‐ and GO‐based materials are of special interest in cancer therapy and have been widely studied in the past few decades. These structures have been studied for various pharmaceutical and biomedical appliances owing to their unique physicochemical properties such as two‐dimensional planar structures, large surface areas, high chemical/mechanical stability, and significant conductivity. However, pristine G and GO may suffer from unfavorable surface chemistry and low biocompatibility, thus various covalent or non‐covalent functionalization tactics have been deployed to improve their properties. Important themes associated with the sustained release of anticancer drugs as well as mechanistic insights of anticancer agents’ discharge from G‐based nanoplatforms still await comprehensive study. Likewise, the efficient accumulation of anticancer drugs in tumor targets/tissues, controlled cellular uptake properties, tumor‐targeted drug release behavior, and selective toxicity toward the cells are crucial criteria that need to be met for developing future anticancer G‐based nanosystems. Overall, functionalized G‐based nanosystems with better biodistribution of drugs, low adverse effects on healthy cells, high selectivity/sensitivity, and higher local therapeutic absorption have garnered a lot of attention. For biomedical and clinical applications of G‐based materials, precise deliberation of their biosafety and toxicity issues is critical.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST
Dr. Rajender S. Varma is on the editorial board of the journal, MedComm. The other authors declare no conflict of interest and no financial support toward this research, and/or for the authorship and publication of this article.
ETHICS APPROVAL
Not applicable.
AUTHOR CONTRIBUTIONS
All authors including A.S., S.I., and R.S.V., have contributed equally to conceptualize, review the papers, designing the discussion, writing the initial draft, and preparing the final document.
ACKNOWLEDGMENTS
Not applicable.
Shafiee A, Iravani S, Varma RS. Graphene and graphene oxide with anticancer applications: Challenges and future perspectives. MedComm. 2022;3:e118. 10.1002/mco2.118
Contributor Information
Siavash Iravani, Email: siavashira@gmail.com.
Rajender S. Varma, Email: varma.rajender@epa.gov.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Leong WS, Nai CT, Thong JT. What does annealing do to metal‐graphene contacts. Nano Lett. 2014;14(7):3840‐3847. [DOI] [PubMed] [Google Scholar]
- 2. Lee C, Xiaoding W, Jeffrey WK, James H. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321(5887):385‐388. [DOI] [PubMed] [Google Scholar]
- 3. Loh KP, Bao Q, Eda G, Chhowalla M. Graphene oxide as a chemically tunable platform for optical applications. Nat Chem. 2010;2(12):1015‐1024. [DOI] [PubMed] [Google Scholar]
- 4. Bolotin K, Sikes K, Jiang Z, et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146:351. [Google Scholar]
- 5. Sajibul M, Bhuyan A, Uddin MN, Islam MM, Bipasha FA, Hossain SS. Synthesis of graphene. Int Nano Lett. 2016;6(2):65. [Google Scholar]
- 6. Ali R, Hammad Aziz M, Gao S, et al. Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging. Ceram Int. 2022. 10.1016/j.ceramint.2021.12.290 [DOI] [Google Scholar]
- 7. Zhu Y, Murali S, Cai W, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010;22(25):3906‐3924. [DOI] [PubMed] [Google Scholar]
- 8. Ramanathan T, Abdala A, Stankovich S, et al. Functionalized graphene sheets for polymer nanocomposites. Nat Nanotechnol. 2008;3(6):327‐331. [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Ruan J, Song H, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dresselhaus MS, Dresselhaus G. Intercalation compounds of graphite. Adv Phys. 2002;51(1):1‐186. [Google Scholar]
- 11. Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water‐insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876‐10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666‐669. [DOI] [PubMed] [Google Scholar]
- 13. Alemi F, Zarezadeh R, Sadigh AR, et al. Graphene oxide and reduced graphene oxide: efficient cargo platforms for cancer theranostics. J Drug Deliv Sci Technol. 2020;60:101974. [Google Scholar]
- 14. Karki N, Tiwari H, Tewari C, et al. Functionalized graphene oxide as a vehicle for targeted drug delivery and bioimaging applications. J Mater Chem B. 2020;8(36):8116‐8148. [DOI] [PubMed] [Google Scholar]
- 15. Buskaran K, Hussein MZ, Mohd Moklas MA, Fakurazi S. Morphological changes and cellular uptake of functionalized graphene oxide loaded with protocatechuic acid and folic acid in hepatocellular carcinoma cancer cell. Int J Mol Sci. 2020;21(16):5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shafiee A, Aibaghi B, Carrier AJ, et al. Rapid photodegradation mechanism enabled by broad‐spectrum absorbing black anatase and reduced graphene oxide nanocomposites. Appl Surf Sci. 2022;575:151718. [Google Scholar]
- 17. Kuo W‐S, Chen H‐H, Chen S‐Y, et al. Graphene quantum dots with nitrogen‐doped content dependence for highly efficient dual‐modality photodynamic antimicrobial therapy and bioimaging. Biomaterials. 2017;120:185‐194. [DOI] [PubMed] [Google Scholar]
- 18. Alegret N, Criado A, Prato M. Recent advances of graphene‐based hybrids with magnetic nanoparticles for biomedical applications. Curr Med Chem. 2017;24(5):529‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masoudipour E, Kashanian S, Maleki N. A targeted drug delivery system based on dopamine functionalized nano graphene oxide. Chem Phys Lett. 2017;668:56‐63. [Google Scholar]
- 20. Pattnaik S, Swain K, Lin Z. Graphene and graphene‐based nanocomposites: biomedical applications and biosafety. J Mater Chem B. 2016;4(48):7813‐7831. [DOI] [PubMed] [Google Scholar]
- 21. Rahdar A, Hajinezhad MR, Hamishekar H, Ghamkhari A, Kyzas GZ. Copolymer/graphene oxide nanocomposites as potential anticancer agents. Polym Bull. 2021;78:4877‐4898. [Google Scholar]
- 22. Hoseini‐Ghahfarokhi M, Mirkiani S, Mozaffari N, et al. Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat. Int J Nanomed. 2020;15:9469‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varma RS, Len C. Glycerol valorization under continuous flow conditions‐recent advances. Curr Opin Green Sustain Chem. 2019;15:83‐90. [Google Scholar]
- 24. Wang Z, Huang P, Bhirde A, et al. A nanoscale graphene oxide–peptide biosensor for real‐time specific biomarker detection on the cell surface. ChemComm. 2012;48(78):9768‐9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo C, Book‐Newell B, Irudayaraj J. Protein‐directed reduction of graphene oxide and intracellular imaging. ChemComm. 2011;47(47):12658‐12660. [DOI] [PubMed] [Google Scholar]
- 26. Yang X, Wang Y, Huang X, et al. Multi‐functionalized graphene oxide based anticancer drug‐carrier with dual‐targeting function and pH‐sensitivity. J Mater Chem. 2011;21(10):3448‐3454. [Google Scholar]
- 27. Peng C, Hu W, Zhou Y, Fan C, Huang Q. Intracellular imaging with a graphene‐based fluorescent probe. Small. 2010;6(15):1686‐1692. [DOI] [PubMed] [Google Scholar]
- 28. Sheng Z, Song L, Zheng J, et al. Protein‐Assisted fabrication of nano‐reduced graphene oxide for combined vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34(21):5236‐5243. [DOI] [PubMed] [Google Scholar]
- 29. Yin PT, Shah S, Chhowalla M, Lee K‐B. Design, synthesis, and characterization of graphene‐nanoparticle hybrid materials for bioapplications. Chem Rev. 2015;115(7):2483‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. GhosalKishor K, Sarkar K. Biomedical applications of graphene nanomaterials and beyond. ACS Biomater Sci Eng. 2018;4:2653‐2703. [DOI] [PubMed] [Google Scholar]
- 31. Sajjadi M, Nasrollahzadeh M, Jaleh B, Jamalipour Soufi G, Iravani S. Carbon‐based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects. J Drug Target. 2021;29(7):716‐741. [DOI] [PubMed] [Google Scholar]
- 32. Lingaraju K, Naika HR, Nagaraju G, Nagabhushana H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in‐vitro cytotoxicity against human cancer cell lines. Biotechnol Rep. 2019;24:e00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gurunathan S, Han JW, Eppakayala V, Kim J‐H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int J Nanomedicine. 2013;8:1015‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han X‐M, Zheng K‐W, Wang R‐L, et al. Functionalization and optimization‐strategy of graphene oxide‐based nanomaterials for gene and drug delivery. Am J Transl Res. 2020;12(5):1515‐1534. [PMC free article] [PubMed] [Google Scholar]
- 35. Nobile MR, Raimondo M, Naddeo C, Guadagno L. Rheological and morphological properties of non‐covalently functionalized graphene‐based structural epoxy resins with intrinsic electrical conductivity and thermal stability. Nanomaterials. 2020;10(7):1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neri G, Fazio E, Nostro A, et al. Shedding light on the chemistry and the properties of münchnone functionalized graphene. Nanomaterials. 2021;11(7):1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9(12):9243‐9257. [DOI] [PubMed] [Google Scholar]
- 38. Chung C, Kim Y‐K, Shin D, Ryoo S‐R, Hong BH, Min D‐H. Biomedical applications of graphene and graphene oxide. Acc Chem Res. 2013;46:2211‐2224. [DOI] [PubMed] [Google Scholar]
- 39. Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS. Trimetallic nanoparticles: greener synthesis and their applications. Nanomaterials. 2020;10(9):1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS. Green‐synthesized nanocatalysts and nanomaterials for water treatment: current challenges and future perspectives. J Hazard Mater. 2021;401:123401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS. Carbon‐based sustainable nanomaterials for water treatment: state‐of‐art and future perspectives. Chemosphere. 2021;263:128005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasrollahzadeh M, Sajjadi M, Iravani S, Varma RS. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: a review. Carbohydr Polym. 2021;251:116986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang K, Hong K, Suh JM, et al. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res Chem Intermediat. 2019;45:599‐611. [Google Scholar]
- 44. Gadeval A, Maheshwar R, Raval N, Kalyane D, Kalia K, Tekade RK. Green graphene nanoplates for combined photo‐chemo‐thermal therapy of triple‐negative breast cancer. Nanomedicine. 2020;15(6):581‐601. [DOI] [PubMed] [Google Scholar]
- 45. Wang H, Mi X, Li Y, Zhan S. 3D graphene‐based macrostructures for water treatment. Adv Mater. 2020;32(3):1806843. [DOI] [PubMed] [Google Scholar]
- 46. Ali I, Alharbi OM, Tkachev A, Galunin E, Burakov A, Grachev VA. Water treatment by new‐generation graphene materials: hope for bright future. Environ Sci Pollut Res. 2018;25(8):7315‐7329. [DOI] [PubMed] [Google Scholar]
- 47. Ahn E‐Y, Hwang SJ, Choi M‐J, Cho S, Lee H‐J, Park Y. Upcycling of jellyfish (Nemopilema nomurai) sea wastes as highly valuable reducing agents for green synthesis of gold nanoparticles and their antitumor and anti‐inflammatory activity. Artif Cells Nanomed Biotechnol. 2018;46(sup 2):1127‐1136. [DOI] [PubMed] [Google Scholar]
- 48. Zou W, Gu B, Sun S, et al. Preparation of a graphene oxide membrane for air purification. Mater Res Express. 2019;6(10):105624. [Google Scholar]
- 49. Liang J, Cai Z, Li L, Guo L, Geng J. Scalable and facile preparation of graphene aerogel for air purification. RSC Adv. 2014;4(10):4843‐4847. [Google Scholar]
- 50. Raghav PK, Mohanty S. Are graphene and graphene‐derived products capable of preventing COVID‐19 infection. Med Hypotheses. 2020;144:110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ebrahimi A, Fatemi S. Titania‐reduced graphene oxide nanocomposite as a promising visible light‐active photocatalyst for continuous degradation of VVOC in air purification process. Clean Technol Environ Policy. 2017;19(8):2089‐2098. [Google Scholar]
- 52. Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4(10):5731‐5736. [DOI] [PubMed] [Google Scholar]
- 53. Akhavan O, Ghaderi E, Emamy H. Nontoxic concentrations of PEGylated graphene nanoribbons for selective cancer cell imaging and photothermal therapy. J Mater Chem. 2012;22(38):20626‐20633. [Google Scholar]
- 54. Li J‐L, Tang B, Yuan B, Sun L, Wang X‐G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials. 2013;34(37):9519‐9534. [DOI] [PubMed] [Google Scholar]
- 55. Srivastava A, Dwivedi N, Dhand C, et al. Potential of graphene‐based materials to combat COVID‐19: properties, perspectives and prospects. Mater Today Chem. 2020:100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kostarelos K, Novoselov KS. Graphene devices for life. Nat Nanotechnol. 2014;9(10):744‐745. [DOI] [PubMed] [Google Scholar]
- 57. Ye S, Shao K, Li Z, et al. Antiviral activity of graphene oxide: how sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7(38):21571‐21579. [DOI] [PubMed] [Google Scholar]
- 58. Priyadarsini S, Mohanty S, Mukherjee S, Basu S, Mishra M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J Nanostructure Chem. 2018;8(2):123‐137. [Google Scholar]
- 59. Shafiee A, Aibaghi B, Zhang X. Determination of ethambutol in biological samples using graphene oxide based dispersive solid‐phase microextraction followed by ion mobility spectrometry. Int J Ion Mobil Spectrom. 2020;23(1):19‐27. [Google Scholar]
- 60. Muthoosamy K, Bai RG, Manickam S. Graphene and graphene oxide as a docking station for modern drug delivery system. Curr Drug Deliv. 2014;11(6):701‐718. [DOI] [PubMed] [Google Scholar]
- 61. Daniyal M, Liu B, Wang W. Comprehensive review on graphene oxide for use in drug delivery system. Curr Med Chem. 2020;27(22):3665‐3685. [DOI] [PubMed] [Google Scholar]
- 62. Shadjou N, Hasanzadeh M, Khalilzadeh B. Graphene based scaffolds on bone tissue engineering. Bioengineered. 2018;9(1):38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Shang L, Qi Y, Lu H, et al. Graphene and graphene oxide for tissue engineering and regeneration. Theranostic Bionanomater. 2019:165‐185. [Google Scholar]
- 64. Renteria JD, Nika DL, Balandin AA. Graphene thermal properties: applications in thermal management and energy storage. Appl Sci. 2014;4(4):525‐547. [Google Scholar]
- 65. Kumar R, Sahoo S, Joanni E, et al. Heteroatom doped graphene engineering for energy storage and conversion. Mater Today. 2020;39:47‐65. [Google Scholar]
- 66. Geng P, Zheng S, Tang H, et al. Transition metal sulfides based on graphene for electrochemical energy storage. Adv Energy Mater. 2018;8(15):1703259. [Google Scholar]
- 67. Wang H, Feng H, Li J. Graphene and graphene‐like layered transition metal dichalcogenides in energy conversion and storage. Small. 2014;10(11):2165‐2181. [DOI] [PubMed] [Google Scholar]
- 68. Liang M, Luo B, Zhi L. Application of graphene and graphene‐based materials in clean energy‐related devices. Int J Energy Res. 2009;33(13):1161‐1170. [Google Scholar]
- 69. Zhu J, Yang D, Yin Z, Yan Q, Zhang H. Graphene and graphene‐based materials for energy storage applications. Small. 2014;10(17):3480‐3498. [DOI] [PubMed] [Google Scholar]
- 70. Caldas H, Ramos RO. Magnetization of planar four‐fermion systems. Phys Rev B. 2009;80(11):115428. [Google Scholar]
- 71. Staudenmaier L. Verfahren zur darstellung der graphitsäure. Berichte der deutschen chemischen Gesellschaft. 1898;31(2):1481‐1487. [Google Scholar]
- 72. Hoffmann U, Koenig E. Untersuchungen über Graphitoxid. Z Anorg Allg Chem. 1937;234:311‐336. [Google Scholar]
- 73. Brodie BC. On the atomic weight of graphite. Philos Trans R Soc Lond. 1859(149):249‐259. [Google Scholar]
- 74. William S, Hummers J, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80(6):1339‐1339. [Google Scholar]
- 75. Chen J, Yao B, Li C, Shi G. An improved Hummers method for eco‐friendly synthesis of graphene oxide. Carbon. 2013;64:225‐229. [Google Scholar]
- 76. Shamaila S, Sajjad AKL, Iqbal A. Modifications in development of graphene oxide synthetic routes. Chem Eng J. 2016;294:458‐477. [Google Scholar]
- 77. Yu W, Sisi L, Haiyan Y, Jie L. Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv. 2020;10(26):15328‐15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Akhavan O, Ghaderi E, Akhavan A. Size‐dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials. 2012;33(32):8017‐8025. [DOI] [PubMed] [Google Scholar]
- 79. Paredes J, Villar‐Rodil S, Fernández‐Merino M, Guardia L, Martínez‐Alonso A, Tascón J. Environmentally friendly approaches toward the mass production of processable graphene from graphite oxide. J Mater Chem. 2011;21(2):298‐306. [Google Scholar]
- 80. Kim K, Zhao Y, Jang H, et al. Large‐scale pattern growth of graphene films for stretchable transparent electrodes. Nature. 2009;457(7230):706‐710. [DOI] [PubMed] [Google Scholar]
- 81. Somanathan T, Prasad K, Ostrikov KK, Saravanan A, Krishna VM. Graphene oxide synthesis from agro waste. Nanomaterials. 2015;5(2):826‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sekhon SS, Kaur P, Kim Y‐H, Sekhon SS. 2D graphene oxide–aptamer conjugate materials for cancer diagnosis. NPJ 2D Mater Appl . 2021;5:21. 1–19. [Google Scholar]
- 83. Deng X, Liang H, Yang W, Shao Z. Polarization and function of tumor‐associated macrophages mediate graphene oxide‐induced photothermal cancer therapy. J Photochem Photobiol B. 2020;208:111913. [DOI] [PubMed] [Google Scholar]
- 84. Jun SW, Manivasagan P, Kwon J, et al. Folic acid–conjugated chitosan‐functionalized graphene oxide for highly efficient photoacoustic imaging‐guided tumor‐targeted photothermal therapy. Int J Biol. 2020;155:961‐971. [DOI] [PubMed] [Google Scholar]
- 85. De D, Kanta Das C, Mandal D, et al. Curcumin complexed with graphene derivative for breast cancer therapy. ACS Appl Bio Mater. 2020;3(9):6284‐6296. [DOI] [PubMed] [Google Scholar]
- 86. Khorrami S, Abdollahi Z, Eshaghi G, Khosravi A, Bidram E, Zarrabi A. An improved method for fabrication of Ag‐GO nanocomposite with controlled anti‐cancer and anti‐bacterial behavior. A comparative study. Sci Rep. 2019;9(1):9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hou C, Quan H, Duan Y, Zhang Q, Wang H, Li Y. Facile synthesis of water‐dispersible Cu2O nanocrystal–reduced graphene oxide hybrid as a promising cancer therapeutic agent. Nanoscale. 2013;5(3):1227‐1232. [DOI] [PubMed] [Google Scholar]
- 88. Tian J, Luo Y, Huang L, Feng Y, Ju H, Yu B‐Y. Pegylated folate and peptide‐decorated graphene oxide nanovehicle for in vivo targeted delivery of anticancer drugs and therapeutic self‐monitoring. Biosens Bioelectron. 2016;80:519‐524. [DOI] [PubMed] [Google Scholar]
- 89. Liu P, Xie X, Liu M, Hu S, Ding J, Zhou W. A smart MnO2‐doped graphene oxide nanosheet for enhanced chemo‐photodynamic combinatorial therapy via simultaneous oxygenation and glutathione depletion. Acta Pharm Sin. 2021;11(3):823‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhuang W, He L, Wang K, et al. Combined adsorption and covalent linking of paclitaxel on functionalized nano‐graphene oxide for inhibiting cancer cells. ACS Omega. 2018;3(2):2396‐2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin M, Shan S, Liu P, et al. Hydroxyl‐functional groups on graphene trigger the targeted delivery of antitumor drugs. J Biomed Nanotech. 2018;14(8):1420‐1429. [DOI] [PubMed] [Google Scholar]
- 92. Ashjaran M, Babazadeh M, Akbarzadeh A, Davaran S, Salehi R. Stimuli‐responsive polyvinylpyrrolidone‐NIPPAm‐lysine graphene oxide nano‐hybrid as an anticancer drug delivery on MCF7 cell line. Artif Cells Nanomed Biotechnol. 2019;47(1):443‐454. [DOI] [PubMed] [Google Scholar]
- 93. Singh G, Nenavathu BP, Imtiyaz K, Rizvi MMA. Fabrication of chlorambucil loaded graphene‐oxide nanocarrier and its application for improved antitumor activity. Biomed Pharmacother. 2020;129:110443. [DOI] [PubMed] [Google Scholar]
- 94. Imani R, Shao W, Taherkhani S, Emami SH, Prakash S, Faghihi S. Dual‐functionalized graphene oxide for enhanced siRNA delivery to breast cancer cells. Colloids Surf B. 2016;147:315‐325. [DOI] [PubMed] [Google Scholar]
- 95. Sun Q, Wang X, Cui C, Li J, Wang Y. Doxorubicin and anti‐VEGF siRNA co‐delivery via nano‐graphene oxide for enhanced cancer therapy in vitro and in vivo. Int J Nanomed. 2018;13:3713–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu X, Wang J, Wang Y, Zhao L, Li Y, Liu C. Formation of graphene oxide‐hybridized nanogels for combinative anticancer therapy. Nanomed Nanotechnol Biol Med. 2018;14(7):2387‐2395. [DOI] [PubMed] [Google Scholar]
- 97. Li Q, Wen J, Liu C, et al. Graphene‐nanoparticle‐based self‐healing hydrogel in preventing postoperative recurrence of breast cancer. ACS Biomater Sci Eng. 2019;5(2):768‐779. [DOI] [PubMed] [Google Scholar]
- 98. Shirvalilou S, Khoei S, Khoee S, et al. Enhancement radiation‐induced apoptosis in C6 glioma tumor‐bearing rats via pH‐responsive magnetic graphene oxide nanocarrier. J Photochem Photobiol B. 2020;205:111827. [DOI] [PubMed] [Google Scholar]
- 99. Qi Z, Shi J, Zhu B, Li J, Cao S. Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery. J Mater Sci. 2020:1‐14. [Google Scholar]
- 100. Lv Y, Tao L, Bligh SA, Yang H, Pan Q, Zhu L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater Sci Eng C. 2016;59:652‐660. [DOI] [PubMed] [Google Scholar]
- 101. Ma N, Zhang B, Liu J, Zhang P, Li Z, Luan Y. Green fabricated reduced graphene oxide: evaluation of its application as nano‐carrier for pH‐sensitive drug delivery. Int J Pharm. 2015;496(2):984‐992. [DOI] [PubMed] [Google Scholar]
- 102. Boran G, Tavakoli S, Dierking I, Kamali AR, Ege D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci Rep. 2020;10(1):7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang P, Wang X, Tang Q, et al. Functionalized graphene oxide against U251 glioma cells and its molecular mechanism. Mater Sci Eng C. 2020;116:111187. [DOI] [PubMed] [Google Scholar]
- 104. Ghamkhari A, Abbaspour‐Ravasjani S, Talebi M, Hamishehkar H, Hamblin MR. Development of a graphene oxide‐poly lactide nanocomposite as a smart drug delivery system. Int J Biol Macromol. 2021;169:521‐531. [DOI] [PubMed] [Google Scholar]
- 105. Pei X, Zhu Z, Gan Z, et al. PEGylated nano‐graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci Rep. 2020;10(1):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ardakani TS, Meidanchi A, Shokri A, Shakeri‐Zadeh A. Fe3O4@Au/reduced graphene oxide nanostructures: combinatorial effects of radiotherapy and photothermal therapy on oral squamous carcinoma KB cell line. Ceram Int. 2020;46:28676‐28685. [Google Scholar]
- 107. Liang W, Huang Y, Lu D, et al. β‐Cyclodextrin‐hyaluronic acid polymer functionalized magnetic graphene oxide nanocomposites for targeted photo‐chemotherapy of tumor cells. Polymers. 2019;11(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Du P, Yan J, Long S, et al. Tumor microenvironment and NIR laser dual‐responsive release of berberine 9‐O‐pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo‐photothermal synergetic cancer therapy. J Mater Chem B. 2020;8(18):4046‐4055. [DOI] [PubMed] [Google Scholar]
- 109. Huang X, Chen J, Wu W, et al. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo‐photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020;109:229‐243. [DOI] [PubMed] [Google Scholar]
- 110. Sharma H, Mondal S. Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: a promising material in nanomedicine. Int J Mol Sci. 2020;21(17):6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vinothini K, Rajendran NK, Rajan M, Ramu A, Marraiki N, Elgorban AM. A magnetic nanoparticle functionalized reduced graphene oxide‐based drug carrier system for a chemo‐photodynamic cancer therapy. New J Chem. 2020;44(14):5265‐5277. [Google Scholar]
- 112. Lalwani G, D'Agati M, Khan AM, Sitharaman B. Toxicology of graphene‐based nanomaterials. Adv Drug Deliv Rev. 2016;105:109‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Qu Y, He F, Yu C, et al. Advances on graphene‐based nanomaterials for biomedical applications. Mater Sci Eng C. 2018;90:764‐780. [DOI] [PubMed] [Google Scholar]
- 114. Bi Z, Li L, Yang J, et al. Graphene promotes lung cancer metastasis through Wnt signaling activation induced by DAMPs. Nanotoday. 2021;39:101175. [Google Scholar]
- 115. Shahabi M, Raissi H. Payload delivery of anticancer drug Tegafur with the assistance of graphene oxide nanosheet during biomembrane penetration: molecular dynamics simulation survey. Appl Surf Sci. 2020;517:146186. [Google Scholar]
- 116. Leitão MM, Alves CG, Melo‐Diogo DD, Lima‐Sousa R, Moreira AF, Correia IJ. Sulfobetaine methacrylate‐functionalized graphene oxide‐IR780 nanohybrids aimed at improving breast cancer phototherapy. RSC Adv. 2020;10:38621‐38630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kumawat MK, Thakur M, Bahadur R, et al. Preparation of graphene oxide‐graphene quantum dots hybrid and its application in cancer theranostics. Mater Sci Eng C. 2019;103:109774. [DOI] [PubMed] [Google Scholar]
- 118. Rasoulzadeh M, Namazi H. Carboxymethyl cellulose/graphene oxide bio‐nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym. 2017;168:320‐326. [DOI] [PubMed] [Google Scholar]
- 119. Barahuie F, Saifullah B, Dorniani D, et al. Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater Sci Eng C. 2017;74:177‐185. [DOI] [PubMed] [Google Scholar]
- 120. Guo Y, Xu H, Li Y, et al. Hyaluronic acid and Arg‐Gly‐Asp peptide modified graphene oxide with dual receptor‐targeting function for cancer therapy. J Biomater Appl. 2017;32(1):54‐65. [DOI] [PubMed] [Google Scholar]
- 121. Huang Y‐S, Lu Y‐J, Chen J‐P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J Magn Magn Mater. 2017;427:34‐40. [Google Scholar]
- 122. Karki N, Tiwari H, Pal M, et al. Functionalized graphene oxides for drug loading, release and delivery of poorly water soluble anticancer drug: a comparative study. Colloids Surf B. 2018;169:265‐272. [DOI] [PubMed] [Google Scholar]
- 123. Ma K, Fu D, Liu Y, et al. Cancer cell targeting, controlled drug release and intracellular fate of biomimetic membrane‐encapsulated drug‐loaded nano‐graphene oxide nanohybrids. J Mater Chem B. 2018;6(31):5080‐5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abbasian M, Roudi M‐M, Mahmoodzadeh F, Eskandani M, Jaymand M. Chitosan‐grafted‐poly (methacrylic acid)/graphene oxide nanocomposite as a pH‐responsive de novo cancer chemotherapy nanosystem. Int J Biol Macromol. 2018;118:1871‐1879. [DOI] [PubMed] [Google Scholar]
- 125. Liu B, Che C, Liu J, et al. Fabrication and antitumor mechanism of a nanoparticle drug delivery system: graphene oxide/chitosan oligosaccharide/γ‐polyglutamic acid composites for anticancer drug delivery. ChemistrySelect. 2019;4(43):12491‐12502. [Google Scholar]
- 126. Luo Y, Tang Y, Liu T, et al. Engineering graphene oxide with ultrasmall SPIONs and smart drug release for cancer theranostics. ChemComm. 2019;55(13):1963‐1966. [DOI] [PubMed] [Google Scholar]
- 127. Jaleel JA, Ashraf SM, Rathinasamy K, Pramod K. Carbon dot festooned and surface passivated graphene‐reinforced chitosan construct for tumor‐targeted delivery of TNF‐α gene. Int J Biol Macromol. 2019;127:628‐636. [DOI] [PubMed] [Google Scholar]
- 128. Yin Y, Nguyen TL, Wang B, et al. Simultaneous delivery of DNA vaccine and hydrophobic adjuvant using reducible polyethylenimine‐functionalized graphene oxide for activation of dendritic cells. J Ind Eng Chem. 2019;80:870‐876. [Google Scholar]
- 129. Ma N, Song A, Li Z, Luan Y. Redox‐sensitive prodrug molecules meet graphene oxide: an efficient graphene oxide‐based nanovehicle toward cancer therapy. ACS Biomater Sci Eng. 2019;5(3):1384‐1391. [DOI] [PubMed] [Google Scholar]
- 130. Sang R, Chen M, Yang Y, et al. HAp@ GO drug delivery vehicle with dual‐stimuli‐triggered drug release property and efficient synergistic therapy function against cancer. J Biomed Mater Res A. 2019;107(10):2296‐2309. [DOI] [PubMed] [Google Scholar]
- 131. Ayazi H, Akhavan O, Raoufi M, Varshochian R, Motlagh NSH, Atyabi F. Graphene aerogel nanoparticles for in‐situ loading/pH sensitive releasing anticancer drugs. Colloids Surf B. 2020;186:110712. [DOI] [PubMed] [Google Scholar]
- 132. Liu K, Wang Y, Li H, Duan Y. A facile one‐pot synthesis of starch functionalized graphene as nano‐carrier for pH sensitive and starch‐mediated drug delivery. Colloids Surf B. 2015;128:86‐93. [DOI] [PubMed] [Google Scholar]
- 133. Yaragalla S, Rajendran R, Jose J, AlMaadeed MA, Kalarikkal N, Thomas S. Preparation and characterization of green graphene using grape seed extract for bioapplications. Mater Sci Eng C. 2016;65:345‐353. [DOI] [PubMed] [Google Scholar]
- 134. Li R, Wang Y, Du J, et al. Graphene oxide loaded with tumor‐targeted peptide and anti‐cancer drugs for cancer target therapy. Sci Rep. 2021;11:1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kesavan S, Meena KS, Sharmili SA, et al. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d‐mannose for targeted anticancer drug delivery. J Drug Deliv Sci Technol. 2021;65:102760. [Google Scholar]
- 136. Dhanavel S, Revathy TA, Sivaranjani T, et al. 5‐Fluorouracil and curcumin co‐encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym Bull. 2020;77:213‐233. [Google Scholar]
- 137. Gnanasekar S, Balakrishnan D, Seetharaman P, Arivalagan P, Chandrasekaran R, Sivaperumal S. Chrysin‐Anchored silver and gold nanoparticle‐reduced graphene oxide composites for breast cancer therapy. ACS Appl Nano Mater. 2020;3:4574‐4585. [Google Scholar]
- 138. Lee XJ, Lim HN, Gowthaman NSK, Abdul Rahman MB, Che Abdullah CA, Muthoosamy K. In‐situ surface functionalization of superparamagnetic reduced graphene oxide‐ Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl Surf Sci. 2020;512:145738. [Google Scholar]
- 139. Piperigkou Z, Karamanou K, Engin AB, et al. Emerging aspects of nanotoxicology in health and disease: from agriculture and food sector to cancer therapeutics. Food Chem Toxicol. 2016;91:42‐57. [DOI] [PubMed] [Google Scholar]
- 140. Lam P‐L, Wong W‐Y, Bian Z, Chui C‐H, Gambari R. Recent advances in green nanoparticulate systems for drug delivery: efficient delivery and safety concern. Nanomedicine. 2017;12(4):357‐385. [DOI] [PubMed] [Google Scholar]
- 141. Shi J, Kantoff P, Wooster R, Farokhzad O. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2016;17(1):20‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jahanshahi M, Kowsari E, Haddadi‐Asl V, et al. An innovative and eco‐friendly modality for synthesis of highly fluorinated graphene by an acidic ionic liquid: making of an efficacious vehicle for anti‐cancer drug delivery. Appl Surf Sci. 2020:146071. [Google Scholar]
- 143. Ganesan K, Jothi VK, Natarajan A, Rajaram A, Ravichandran S, Ramalingam S. Green synthesis of copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab J Chem. 2020;13:6802‐6814. [Google Scholar]
- 144. Banerji U, Workman P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin Oncol. 2016;43(4):436‐445. [DOI] [PubMed] [Google Scholar]
- 145. Tan DSW, Thomas GV, Garrett MD, et al. Biomarker‐driven early clinical trials in oncology: a paradigm shift in drug development. Cancer J. 2009;15(5):406‐420. [DOI] [PubMed] [Google Scholar]
- 146. Yap TA, Sandhu SK, Workman P, Bono JSd. Envisioning the future of early anticancer drug development. Nat Rev Cancer. 2010;10(7):514‐523. [DOI] [PubMed] [Google Scholar]
- 147. Chen F, Gao W, Qiu X, et al. Graphene quantum dots in biomedical applications: recent advances and future challenges. Front Lab Med. 2017;1:192‐199. [Google Scholar]
- 148. Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti‐cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25‐38. [DOI] [PubMed] [Google Scholar]
- 149. Liu L, Ma Q, Cao J, et al. Recent progress of graphene oxide‐based multifunctional nanomaterials for cancer treatment. Cancer Nano. 2021;12:1‐31. [Google Scholar]
- 150. Durán M, Durán N, Fávaro WJ. In vivo nanotoxicological profile of graphene oxide. J Phys Conf Ser. 2017;838:012026. [Google Scholar]
- 151. Kiew SF, Kiew LV, Lee HB, Imae T, Chung LY. Assessing biocompatibility of graphene oxide‐based nanocarriers: a review. J Control Release. 2016;226:217‐228. [DOI] [PubMed] [Google Scholar]
- 152. Zhi X, Fang H, Bao C, et al. The immunotoxicity of graphene oxides and the effect of PVP‐coating. Biomaterials. 2013;34(21):5254‐5261. [DOI] [PubMed] [Google Scholar]
- 153. Yue H, Wei W, Yue Z, et al. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012;33(16):4013‐4021. [DOI] [PubMed] [Google Scholar]
- 154. Ou L, Song B, Liang H, et al. Toxicity of graphene‐family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. 2016;13(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


