To the Editor:
Cystic fibrosis (CF), a genetic disease related to mutations in the gene encoding for the CF transmembrane conductance regulator (CFTR) protein, often results in progressive development of respiratory failure. Lung transplantation appears as a therapeutic option that prolongs survival and improves quality of life in patients with advanced CF pulmonary disease in whom medical therapy is not sufficient to control disease progression (1), and CF remains one of the major indications for lung transplantation worldwide.
Over the past 10 years, small molecules directly targeting the CFTR defect, called CFTR modulators, have been developed and have provided clinical benefits to patients with CF (2). The first CFTR modulator, ivacaftor, is considered a highly effective CFTR modulator, including in patients with advanced CF pulmonary disease, with the potential of preventing evolution to end-stage disease and lung transplantation (3). However, only a limited number of patients with CF have CFTR mutations eligible for ivacaftor (∼5% in France). Double combination therapy (lumacaftor–ivacaftor and tezacaftor–ivacaftor) target approximately 40–50% of patients with CF but have only a moderate effect on lung function (4, 5), especially in patients with advanced pulmonary disease. Furthermore, lumacaftor–ivacaftor had to be discontinued in up to 28% of patients with advanced pulmonary disease, in most cases owing to the occurrence of respiratory adverse effects (4). In marked contrast with these double combinations, triple combination of elexacaftor–tezacaftor–ivacaftor has been developed for patients with at least one Phe508del CFTR allele (corresponding to 80–85% of patients with CF) and induces large improvement in lung function, respiratory symptoms, exacerbation frequency, and nutritional status.
In a recent study, our group described the effects of elexacaftor–tezacaftor–ivacaftor in 245 French patients with CF with advanced pulmonary disease (6). Our data showed rapid improvement in lung function and body mass index with an acceptable safety profile. Initiation of elexacaftor–tezacaftor–ivacaftor was further associated with improvement in gas exchange leading to discontinuation of long-term oxygen and noninvasive ventilation in 30–50% of patients (6). Importantly, most patients who were listed for lung transplantation were removed from the transplant list, and those who were under active evaluation for transplantation listing showed such an improvement that they were no longer considered for lung transplantation at the end of our study (6). Data obtained from the French Agence de la Biomédecine Registry, which collects all transplant-related data in France, further indicated that lung transplantation for CF was reduced by 55% in 2020 as compared with 2018–2019 (6). Altogether, these data suggested an effect of elexacaftor–tezacaftor–ivacaftor in reducing the need for lung transplantation in patients with advanced CF pulmonary disease. However, the study was performed at the time when the coronavirus disease (COVID-19) pandemic was surging in France and was having a profound effect on the ability to perform lung transplantation (7–9). Thus, it was suggested that at least some of the reduction in lung transplantation observed in our study could have been related to the COVID-19 pandemic (10). Indeed, data from the United Network for Organ Sharing registry also reported a decrease in lung transplant volume at the onset of the COVID-19 pandemic (11).
Here, we present more recent data on first lung transplantation (excluding lung retransplantation as patients with CF living with a lung transplant are not currently eligible to receive elexacaftor–tezacaftor–ivacaftor) in France (Figure 1). Figure 1A shows that a marked decrease in lung transplant volume occurred in the first 6 months of 2020, but that lung transplant activity largely resumed, although to a lower level, during the second half of 2020 and the first half of 2021. Figure 1B shows the major decrease in lung transplantation for CF from approximately 20 patients/quarter before 2020 to 2 patients/quarter in the last quarter of 2020 and in the first 6 months of 2021 (∼10-fold reduction). The initial decrease occurred in the first half of 2020, corresponding not only to the availability of elexacaftor–tezacaftor–ivacaftor (available from December 24, 2019) but also to the onset of the COVID-19 pandemic in France in January 2020, leading to the first COVID-19–related lockdown (from March 17 to May 10, 2020). The initial decrease in lung transplantation for CF was paralleled by a less marked decrease in lung transplantation observed for other diseases (including chronic obstructive pulmonary disease, pulmonary fibrosis, and pulmonary hypertension and other [rare] lung diseases). Importantly, as the ongoing COVID-19 pandemic became more controlled with less restriction on the healthcare system and shorter periods of less restrictive lockdowns in October 2020 and April 2021, lung transplantation became easier to organize and to perform during the last 6 months of 2020 and the beginning of 2,021. Analysis of the curves depicting lung transplant numbers showed that lung transplantation numbers resumed for patients with other (non-CF) diseases starting in the second half of 2020. In striking contrast with these findings, lung transplantation for patients with CF kept decreasing, further suggesting the profound impact of the highly effective CFTR modulator combination, elexacaftor–tezacaftor–ivacaftor, in patients with advanced pulmonary disease. Of note, the number of patients with CF who died without lung transplantation, which was on average 20 patients/year before the release of elexacaftor–tezacaftor–ivacaftor, was reduced to 16 deaths in 2020 and 3 deaths over the first 6 months of 2021.
Figure 1.
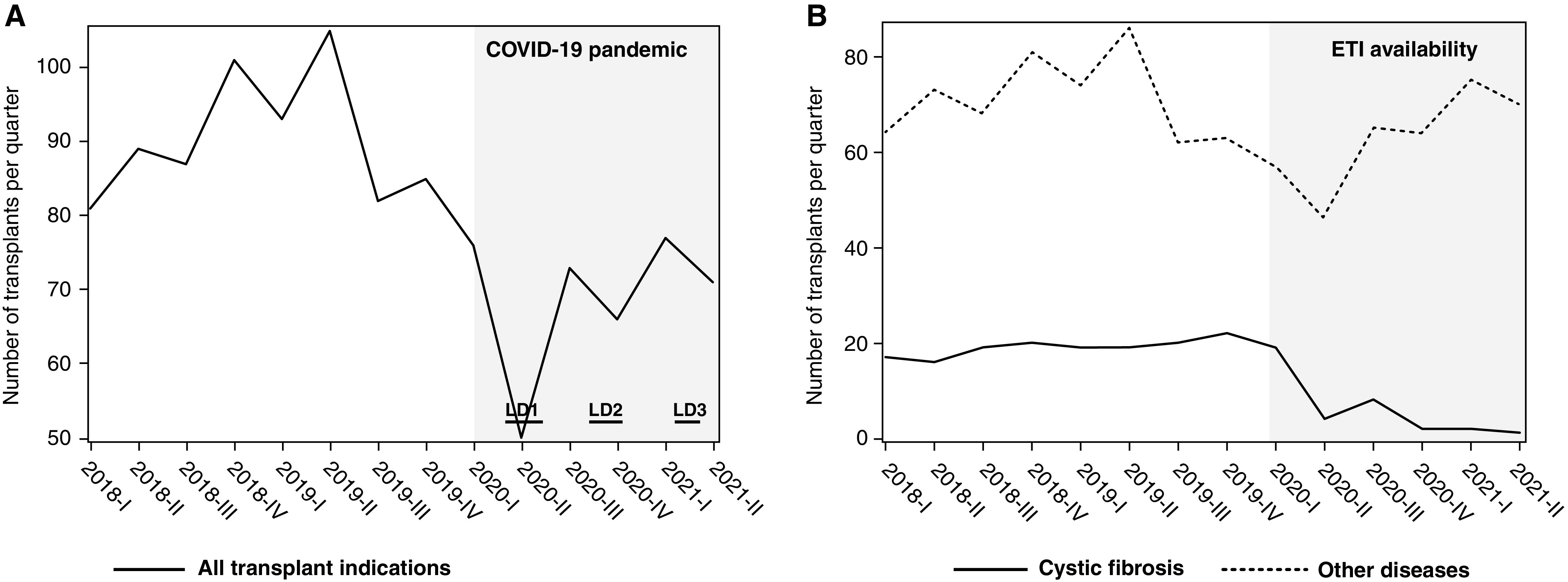
Lung transplantation in France from 2018 to 2021. Data are shown as number of first lung transplantation (excluding retransplantation) per quarter. (A) All indications. (B) Cystic fibrosis (CF) versus other diseases. The coronavirus disease (COVID-19) pandemic progressively developed in France during the first quarter of 2020, leading to the first lockdown (LD1) from March 17 to May 10, 2020; less restrictive lockdowns occurred from October 30 to December 15, 2020 (LD2), and from April 3 to May 3, 2021 (LD3). Elexacaftor–tezacaftor–ivacaftor (ETI) was made available on December 24, 2019, for patients with advanced CF pulmonary disease. Note that the decrease in lung transplantation that occurred during the first COVID-19–related lockdown for all indications persisted for CF over 2020 and 2021, whereas lung transplantation for other lung diseases resumed as the COVID-19 pandemic became better controlled in France.
Analysis of lung transplantation by indication in France during the ongoing COVID-19 pandemic strengthens our previously reported findings that suggested a reduction in the need for lung transplantation over the first months following the availability of elexacaftor–tezacaftor–ivacaftor in patients with advanced CF pulmonary disease. Until more data are available regarding the long-term benefit of elexacaftor–tezacaftor–ivacaftor in patients with CF with advanced lung disease, referral for transplant should continue (12), and although listing for transplant may be safely delayed, CF and lung transplant programs should collaborate with patients for shared decision-making on the appropriate time to proceed with lung transplantation.
Footnotes
Author Contributions: C.M., C.L., and P.-R.B. designed the study and contributed to data management and analysis. C.M. and P.-R.B. wrote the first draft of the manuscript. All authors revised and approved the first draft of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202109-2121LE on December 15, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Thabut G, Christie JD, Mal H, Fournier M, Brugière O, Leseche G, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med . 2013;187:1335–1340. doi: 10.1164/rccm.201303-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med . 2020;8:65–124. doi: 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax . 2018;73:731–740. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burgel PR, Munck A, Durieu I, Chiron R, Mely L, Prevotat A, et al. French Cystic Fibrosis Reference Network Study Group Real-life safety and effectiveness of lumacaftor-ivacaftor in patients with cystic fibrosis. Am J Respir Crit Care Med . 2020;201:188–197. doi: 10.1164/rccm.201906-1227OC. [DOI] [PubMed] [Google Scholar]
- 5. Burgel PR, Durieu I, Chiron R, Mely L, Prevotat A, Murris-Espin M, et al. French Cystic Fibrosis Reference Network study group Clinical response to lumacaftor-ivacaftor in patients with cystic fibrosis according to baseline lung function. J Cyst Fibros . 2021;20:220–227. doi: 10.1016/j.jcf.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 6. Burgel PR, Durieu I, Chiron R, Ramel S, Danner-Boucher I, Prevotat A, et al. French Cystic Fibrosis Reference Network Study Group Rapid improvement after starting elexacaftor-tezacaftor-ivacaftor in patients with cystic fibrosis and advanced pulmonary disease. Am J Respir Crit Care Med . 2021;204:64–73. doi: 10.1164/rccm.202011-4153OC. [DOI] [PubMed] [Google Scholar]
- 7. Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet . 2020;395:e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picard C, Le Pavec J, Tissot A, Groupe Transplantation Pulmonaire de la Société de Pneumologie de Langue Française (SPLF) Impact of the Covid-19 pandemic and lung transplantation program in France. Respir Med Res . 2020;78:100758. doi: 10.1016/j.resmer.2020.100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aubert O, Yoo D, Zielinski D, Cozzi E, Cardillo M, Dürr M, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health . 2021;6:e709–e719. doi: 10.1016/S2468-2667(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Myerburg M, Pilewski JM. CFTR modulators to the rescue of individuals with cystic fibrosis and advanced lung disease. Am J Respir Crit Care Med . 2021;204:7–9. doi: 10.1164/rccm.202103-0674ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan EG, Chan PG, Harano T, Ryan JP, Morrell MR, Sanchez PG. Trends in lung transplantation practices across the United States during the COVID-19 pandemic. Transplantation . 2021;105:187–192. doi: 10.1097/TP.0000000000003522. [DOI] [PubMed] [Google Scholar]
- 12. Ramos KJ, Smith PJ, McKone EF, Pilewski JM, Lucy A, Hempstead SE, et al. CF Lung Transplant Referral Guidelines Committee Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. J Cyst Fibros . 2019;18:321–333. doi: 10.1016/j.jcf.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


