Abstract
Rationale
Many decisions to admit patients to the ICU are not grounded in evidence regarding who benefits from such triage, straining ICU capacity and limiting its cost-effectiveness.
Objectives
To measure the benefits of ICU admission for patients with sepsis or acute respiratory failure.
Methods
At 27 United States hospitals across two health systems from 2013 to 2018, we performed a retrospective cohort study using two-stage instrumental variable quantile regression with a strong instrument (hospital capacity strain) governing ICU versus ward admission among high-acuity patients (i.e., laboratory-based acute physiology score v2 ⩾ 100) with sepsis and/or acute respiratory failure who did not require mechanical ventilation or vasopressors in the emergency department.
Measurements and Main Results
Among patients with sepsis (n = 90,150), admission to the ICU was associated with a 1.32-day longer hospital length of stay (95% confidence interval [CI], 1.01–1.63; P < 0.001) (when treating deaths as equivalent to long lengths of stay) and higher in-hospital mortality (odds ratio, 1.48; 95% CI, 1.13–1.88; P = 0.004). Among patients with respiratory failure (n = 45,339), admission to the ICU was associated with a 0.82-day shorter hospital length of stay (95% CI, –1.17 to –0.46; P < 0.001) and reduced in-hospital mortality (odds ratio, 0.75; 95% CI, 0.57–0.96; P = 0.04). In sensitivity analyses of length of stay, excluding, ignoring, or censoring death, results were similar in sepsis but not in respiratory failure. In subgroup analyses, harms of ICU admission for patients with sepsis were concentrated among older patients and those with fewer comorbidities, and the benefits of ICU admission for patients with respiratory failure were concentrated among older patients, highest-acuity patients, and those with more comorbidities.
Conclusions
Among high-acuity patients with sepsis who did not require life support in the emergency department, initial admission to the ward, compared with the ICU, was associated with shorter length of stay and improved survival, whereas among patients with acute respiratory failure, triage to the ICU compared with the ward was associated with improved survival.
Keywords: sepsis, acute respiratory failure, capacity strain, ICU admission, triage
At a Glance Commentary
Scientific Knowledge on the Subject
Many decisions to admit patients to the ICU are not grounded in evidence regarding who benefits from such triage, straining ICU capacity and limiting its cost-effectiveness.
What This Study Adds to the Field
Among high-acuity patients with sepsis who did not require life support in the emergency department, initial admission to the ward was associated with shorter hospital length of stay and improved hospital survival, whereas among acute respiratory failure patients, triage to the ICU was associated with improved survival.
A promising approach for optimizing and personalizing decisions regarding patient triage and improving the cost-effectiveness of acute care delivery is to only admit patients to the ICU who truly benefit from such resource-intensive care (1). However, despite the fact that sepsis and acute respiratory failure (ARF) account for more than 3 million U.S. hospitalizations annually (2–4), little evidence exists regarding which of these patients, especially those not requiring life support, benefit from ICU admission. Hospitals vary significantly in ICU admission rates for patients with many acute syndromes (5–7), and ICU admission rates also vary within hospitals over time because of changes in ICU occupancy (8–10) and hospital-wide strain (11).
Measuring the benefit of ICU care is methodologically challenging because traditional comparisons of patients admitted to ICUs and wards are susceptible to confounding by indication, and randomizing patients to ICU versus ward admission would face significant logistical and ethical hurdles. To address this challenge, we recently developed a composite, hospital-wide index of capacity strain that meets all criteria of a strong, within-hospital instrumental variable (IV)—a variable that changes over time within each hospital and strongly governs the triage of patients from the emergency department (ED) to ICUs or wards (11). Here we leverage that IV to estimate the benefit or harm of ICU admission for patients with sepsis and/or ARF presenting to the EDs of 27 hospitals in two health systems. Some of the results of these studies have been reported previously in the form of an abstract (12).
Methods
We extracted electronic health record data from all patients treated at 22 Kaiser Permanente Northern California hospitals and 5 Penn Medicine hospitals between 2013 and 2,018. Both systems’ institutional review boards approved this study.
Sepsis and ARF Clinical Cohorts
The cohorts included adult patients (age ⩾ 18 yr) with sepsis and/or ARF admitted from the ED to a medical or medical-surgical ward, step-down unit, or ICU. We used previously specified clinical and temporal definitions for sepsis or ARF during the ED stay (11, 13, 14). Sepsis was defined based on an adaptation of the Sepsis-3 criteria (suspected or confirmed infection and evidence of end-organ dysfunction), and ARF was defined based on etiology-agnostic measures of hypoxemia, hypercarbia, or respiratory support. Because our focus was on patients who might be admitted to either the ward or the ICU, we restricted to higher-acuity patients (admission laboratory-based acute physiology score v2 [LAPS2] ⩾ 100) (11, 15, 16) who did not receive invasive mechanical ventilation or vasopressors in the ED (11). This LAPS2 cutoff was selected based on a step-up in observed hospital mortality in the study sample before application of exclusion criteria (LAPS2 0–49, 1.8% mortality; LAPS 2 50–99, 4.7%; LAPS2 ⩾ 100, 17.3%) and was also used in the strain index derivation and validation study (11). We excluded patients with orders for comfort measures only or hospice in the ED. Appendix E1 in the online supplement provides additional detail.
Variable Definitions
The primary exposure was the first inpatient location (i.e., ICU or ward, with step-down units considered wards in primary analyses) after hospital admission from the ED (8). All analyses included the multivariable index of hospital capacity strain as an IV (Appendix E2) (11). This IV incorporates 22 measures of occupancy, acuity, turnover, discharges, and utilization of life support, each captured on up to an hourly basis across EDs, wards, step-down units, and ICUs (11). The IV value for a particular patient was calculated using the mean of hourly strain measures from 1 hour before to 3 hours after the first collection of routine laboratory studies in the ED. The strain index meets all assumptions of a strong within-hospital IV, as demonstrated previously (11) and summarized in Table E1.
The primary outcome was hospital length of stay (LOS). To prevent biased estimates because of informatively missing data as a result of deaths (17), we used a “placement of death” approach, ranking in-hospital deaths or hospice discharges as equivalent to the 99th percentile of hospital LOS by clinical cohort (18, 19). Secondary outcomes were rates of in-hospital mortality and hospital discharges to home (compared with other dispositions, including in-hospital deaths and transfers to hospice, skilled nursing or rehabilitation facilities, long-term acute care hospitals, or another hospital).
Statistical Analysis
All analyses were performed separately for the sepsis and ARF cohorts. Patients meeting both sepsis and ARF inclusion criteria were included in both cohorts in primary analyses and were excluded and evaluated on their own in secondary analyses. Primary analyses of the LOS outcome used two-stage IV multivariable median quantile regression adjusted for hospital as a fixed effect (18–22) and included these a priori–designated, patient-level covariates: age, gender, race, ethnicity, insurance status, ED LAPS2 score (a 24-variable acuity score incorporating vital signs, neurologic assessment, laboratory values, and demographics; possible range, 0–414), and admission comorbidity point score v2 (COPS2; a comorbidity burden score based on 12 months of preceding International Classification of Diseases diagnosis coding; possible range, 0–1,014) (13, 15, 16, 23–27). LAPS2 was selected a priori for acuity adjustment because we required a score validated for all patients at hospital presentation rather than scores applicable only to ICU patients; it also has discrimination and calibration for hospital mortality in this cohort superior the sepsis-related organ failure assessment score (14). Hospital was treated as a fixed effect because IV quantile regression cannot include mixed effects. For median quantile regression, β-coefficient results represent the expected change in the median hospital LOS for admission to the ICU compared with admission to the ward, with deaths considered a specific undesirable LOS.
The secondary outcomes of hospital mortality and hospital discharge home were analyzed using two-stage IV multivariable residual inclusion logistic regression with the same adjustment strategy (28). Exposure, covariate, and outcome variables were missing at low rates (<1%), enabling complete-case analyses. Patients with missing mortality status (sepsis, n = 384 [0.43%]; ARF, n = 169 [0.37%]) because of rare transfers to another acute care hospital were excluded from the mortality analysis. Confidence intervals (95% CIs) were calculated using bootstrapping with a minimum of 500 runs. P values of less than 0.05 were considered statistically significant.
Robustness and Sensitivity Analyses
To support a causal argument and to evaluate for bias from the IV, we assessed the association between the IV and key outcomes. Known as “reduced form” analysis, this approach is analogous to an intention-to-treat analysis in a randomized trial with intervention nonadherence in that the analysis includes all ED patients regardless of whether their triage is influenced by the IV (29). Thus, if the IV is working properly and not introducing bias, then the point estimates of the effects would have the same direction but attenuated magnitude compared with those in the two-stage IV analysis because the inclusion of noncompliers (i.e., patients who would always be admitted to the ward or the ICU regardless of strain) moves the “reduced form” result toward the null.
Because our placement-of-death approach to LOS analyses produces an effect estimate without a familiar clinical interpretation, we repeated the LOS analyses using seven alternate approaches, as detailed in Appendix E3.
ED LOS was not included as part of the primary LOS outcome (i.e., the time between inpatient hospital admission and hospital discharge) because it occurs before the exposure (i.e., ICU versus ward admission), and so its inclusion could introduce immortal time bias. However, because increased ED boarding times are associated with worse clinical outcomes (30–37), we performed sensitivity analyses adjusting for ED LOS as a patient-level covariate and calculating the relationship between ED LOS and inpatient LOS. We also performed a number of prespecified sensitivity analyses (Appendix E3): restricting to patients who only met criteria for a single clinical cohort and to only those who met criteria for both cohorts, repeating analyses without patient-level adjustment, alternatively assigning deaths to the 95th percentile instead of the 99th percentile LOS, treating step-down units as ICUs instead of wards when assigning exposure status, stratifying the ARF cohort by whether patients utilized bilevel positive airway pressure in the ED, and adjusting for admission code status. Finally, as a preliminary step toward a mechanistic understanding of pooled results in heterogeneous populations, we performed post hoc analyses stratifying IV regression models by quintiles of age, LAPS2, and COPS2.
Results
During the study period, 90,150 patients met criteria for sepsis and 45,339 met criteria for ARF with LAPS2 ⩾ 100 (26,404 patients [24.2%] were included in both clinical cohorts). Among patients with sepsis and ARF, mean age was 73.6 and 72.9 years, 60.7% and 61.1% were of White race, 20.5% and 29.5% were admitted to the ICU, and observed in-hospital mortality was 17.2% and 20.2%, respectively (Table 1). Raw data in both cohorts reveal that patients admitted to the ICU had higher overall acuity and were more likely to die (Table E2), confirming that analyses without use of an IV would be subject to important selection effects. Among patients initially admitted to the ward, 8.5% of patients with sepsis and 9.7% of patients with ARF required subsequent ICU transfer, with a median time from hospital admission to first ICU transfer of 30.3 hours (interquartile range, 14.0–73.2) and 28.2 hours (interquartile range, 13.1–67.5), respectively.
Table 1.
Clinical Cohort Patient Characteristics
| Characteristics | Sepsis Cohort (n = 90,150) | ARF Cohort (n = 45,339) |
|---|---|---|
| Age, mean years (SD) | 73.6 (15.1) | 72.9 (14.7) |
| Male, n (%) | 46,994 (52.1) | 22,232 (49.0) |
| Race, n (%) | ||
| White | 54,732 (60.7) | 27,715 (61.1) |
| Black | 11,171 (12.4) | 6,807 (15.0) |
| Asian | 9,073 (10.1) | 4,169 (9.2) |
| Other* | 15,174 (16.8) | 6,648 (14.7) |
| Hispanic ethnicity, n (%) | 8,869 (9.8) | 3,662 (8.1) |
| Insurance, n (%) | ||
| Private | 70,939 (78.7) | 33,348 (73.6) |
| Medicare/Medicaid | 12,252 (13.6) | 7,563 (16.7) |
| Other/unknown | 6,959 (7.7) | 4,428 (9.8) |
| LAPS2 score,† mean (SD) | 130.2 (24.3) | 132.3 (25.7) |
| COPS2 score,‡ mean (SD) | 109.9 (61.4) | 114.8 (60.2) |
| ED LOS, median hours (IQR) | 5.8 (4.3–8.3) | 5.5 (3.9–8.0) |
| ED disposition decision, n (%) | ||
| Ward | 65,218 (72.3) | 26,941 (59.4) |
| Step-down unit | 6,476 (7.2) | 5,028 (11.1) |
| ICU | 18,456 (20.5) | 13,370 (29.5) |
| Hospital LOS, median days (IQR) | 3.9 (2.4-6.7) | 3.9 (2.2–5.8) |
| Discharge home, n (%) | 51,880 (57.6) | 26,240 (57.9) |
| Hospital mortality,§ n (%) | 15,465 (17.2) | 9,146 (20.2) |
Definition of abbreviations: ARF = acute respiratory failure; COPS2 = comorbidity point score v2; ED = emergency department; IQR = interquartile range; LAPS2 = laboratory-based acute physiology score v2; LOS = length of stay.
Includes Hawaiian/Pacific Islander, American Indian/Native American, self-reported race as “multiple” or “other,” or unknown.
LAPS2 possible range 0–414 and univariate relationship with hospital mortality: 0–49, 1.8%; 50–99, 4.7%; ⩾100, 17.3%. Inclusion criteria restricted patients to those without mechanical ventilation or vasopressors and with LAPS2 ⩾ 100 in the emergency department.
COPS2 possible range 0–1,014 and univariate relationship with hospital mortality: 0–64, 5.0%; ⩾65, 13.4%.
Defined as death or transition to hospice.
Primary and Secondary Analyses
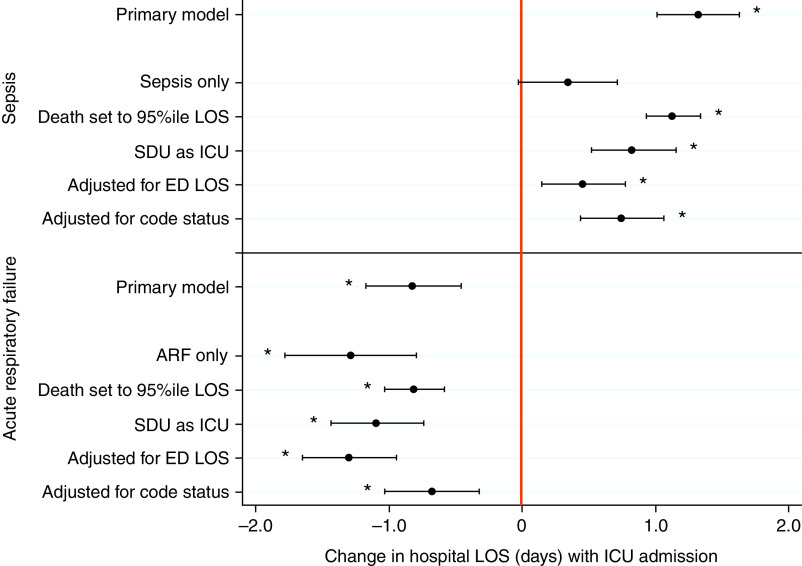
In fully adjusted IV regression analyses among patients with sepsis, admission to the ICU versus the ward was associated with a 1.32-day (31.7-h) longer median hospital LOS (95% CI, 1.01–1.63 days; P < 0.001), with deaths ranked at the 99th percentile of the LOS distribution (31.9 d) (Figure 1 and Table E3). Admission to the ICU was also associated with increased in-hospital mortality (odds ratio [OR], 1.48; 95% CI, 1.13–1.88; P = 0.004) (Figure 2 and Table E4), equaling a 5.1% absolute mortality increase (predicted mortality of 18.3% for ICU admissions versus 13.2% for ward admissions).
Figure 1.
Association of ICU versus ward admission with hospital length of stay (LOS) among patients with sepsis and acute respiratory failure: primary and sensitivity analyses. Among high-acuity patients with sepsis who did not require mechanical ventilation or vasopressors in the emergency department, admission to the ICU was associated with a 1.32-day longer hospital LOS (95% confidence interval, 1.01–1.63 days; P < 0.001), with deaths ranked at the 99th percentile of the LOS distribution. Among high-acuity patients with acute respiratory failure who did not require mechanical ventilation or vasopressors in the emergency department, admission to the ICU was associated with a 0.82-day shorter hospital LOS (95% confidence interval, –1.17 to –0.46 days; P < 0.001). Overall, the directionality of sensitivity analyses results are consistent with the primary results throughout. *P < 0.05. ARF = acute respiratory failure; ED = emergency department; SDU = step-down unit.
Figure 2.
Association of ICU versus ward admission with in-hospital mortality among patients with sepsis and acute respiratory failure (ARF): primary and sensitivity analyses. Among high-acuity patients with sepsis who did not require mechanical ventilation or vasopressors in the emergency department, admission to the ICU was associated with 48% increased odds of in-hospital mortality (odds ratio, 1.48; 95% confidence interval, 1.13–1.88; P = 0.004). Among high-acuity patients with ARF who did not require mechanical ventilation or vasopressors in the emergency department, admission to the ICU was associated with 25% decreased odds of in-hospital mortality (odds ratio, 0.75; 95% confidence interval, 0.57–0.96; P = 0.04). Overall, the directionality of sensitivity analyses results are consistent with the primary results throughout. Of note, restricting to patients who only met criteria for a single clinical cohort (sepsis only or ARF only) amplified the divergent results for each cohort further away from the null. *P < 0.05. ED = emergency department; LOS = length of stay; SDU = step-down unit.
Among patients with ARF, admission to the ICU was associated with a 0.82-day (19.7-h) shorter median hospital LOS (95% CI, –1.17 to –0.46 days; P < 0.001), with deaths ranked equivalent to a 32.9-day hospital LOS (Figure 1 and Table E3). ICU admission was also associated with reduced in-hospital mortality (OR, 0.75; 95% CI, 0.57–0.96; P = 0.04) (Figure 2 and Table E4), equaling a 3.8% absolute mortality reduction (predicted mortality of 14.0% for ICU admissions versus 17.8% for ward admissions).
ICU admission was not significantly associated with discharge home among patients with sepsis (OR, 0.81; 95% CI, 0.64–1.00; P = 0.06) or patients with ARF (OR, 1.09; 95% CI, 0.88–1.34; P = 0.46) (Table E5).
Sensitivity and Secondary Analyses
Reduced-form analyses comparing the strain index with the outcomes of hospital LOS and in-hospital mortality showed the same effect directions and remained statistically significant in both cohorts with the expected attenuation of the effect size. These results are as expected when using a valid IV (Tables E6 and E7).
ICU admission was associated with increased hospital LOS in all sensitivity analyses among patients with sepsis except that using two-stage least squares linear IV regression that ignored death (Table 2). For patients with ARF, ICU admission was associated with shorter hospital LOS in all models in which death was ranked as a long hospital LOS. In models that excluded, ignored, or censored deaths, effect estimates were in the opposite direction (i.e., longer hospital LOS with ICU admission) and of varied statistical significance (Table 2).
Table 2.
Association of ICU Admission with Hospital Length of Stay among Patients with Sepsis and Acute Respiratory Failure, Using Variable Modeling Approaches to Assess Length of Stay in the Setting of In-Hospital Mortality
| Model | Test Statistic | Sepsis | ARF | Interpretation |
|---|---|---|---|---|
| Analyses Coding Deaths as Long LOS | ||||
| IVQR with deaths set as 99th percentile LOS (primary analysis) | β-Coefficient (95% CI, P value) | 1.32 (1.01 to 1.63, <0.001)* | –0.82 (–1.17 to –0.46, <0.001)* | Sepsis: primary result is due to ICU admission associated with increased mortality and increased LOS. ARF: primary result is due to ICU admission associated with decreased mortality and decreased LOS. |
| 2SLS with deaths set as 99th percentile LOS†‡ | β-Coefficient (95% CI, P value) | 1.59 (0.13 to 3.06, 0.03)* | –2.04 (–3.53 to –0.57, 0.006)* | |
| 2SLS with deaths set as 99th percentile LOS and outcome log(LOS)†‡ | Log β-coefficient (95% CI, P value, % change in hospital LOS) | 0.25 (0.12 to 0.38, <0.001, +28.8%)*ǁ | –0.13 (–0.26 to –0.01, 0.04, −12.3%)* | |
| Anazlyses That Exclude, Ignore, or Censor Deaths | ||||
| Survival average causal effect IV 2SLS† | β-Coefficient (95% CI, P value) | 0.51 (0.46 to 0.56)* + α | 0.45 (0.39 to 0.50) + α | Sepsis: primary result is due to ICU admission associated with increased mortality and increased LOS. ARF: primary result due to ICU admission associated with decreased mortality but no change in LOS or accompanied by increased LOS. |
| Cox proportional hazard censoring on death§ | Hazard ratio of discharge (95% CI, P value) | 0.71 (0.70 to 0.73, <0.001)* | 0.72 (0.70 to 0.74, <0.001)* | |
| 2SLS with deaths ignored‡ | β-Coefficient (95% CI, P value) | 0.75 (–0.23 to 1.74, 0.13) | 0.16 (–0.90 to 1.22, 0.76) | |
| 2SLS with deaths excluded‡ | β-Coefficient (95% CI, P value) | 1.34 (0.28 to 2.40, 0.01)* | 0.26 (–0.79 to 1.30, 0.63) | |
| IVQR with deaths excluded | β-Coefficient (95% CI, P value) | 0.89 (0.68 to 1.11, <0.001)* | 0.06 (–0.18 to 0.31, 0.61) | |
| 2SLS with deaths ignored and outcome log(LOS)‡ | Log β-coefficient (95% CI, P value, % change in hospital LOS) | 0.14 (0.04 to 0.25, 0.009, +14.5%)*ǁ | 0.09 (–0.02 to 0.20, 0.10, +9.5%) | |
| 2SLS with deaths excluded and outcome log(LOS)‡ | Log β-coefficient (95% CI, P value, % change in hospital LOS) | 0.26 (0.15 to 0.37, <0.001, +29.1%)*ǁ | 0.04 (–0.06 to 0.15, 0.41, +4.5%) | |
| Linear regression with deaths ignored and outcome log(LOS)† | Log β-coefficient (95% CI, P value, % change in hospital LOS) | 0.31 (0.29 to 0.32, <0.001, 36.3%)*ǁ | 0.35 (0.33 to 0.37, <0.001, 41.9%)* | |
Definition of abbreviations: 2SLS = two-stage least squares linear instrumental variable regression; ARF = acute respiratory failure; CI = confidence interval; IV = instrumental variable; IVQR = instrumental variable quantile regression; LAPS2 = laboratory-based acute physiology score v2; LOS = length of stay.
P < 0.05. Inclusion criteria restricted patients to those without mechanical ventilation or vasopressors and with LAPS2 ⩾ 100 in the emergency department. All models were adjusted for patient-level covariates: age, gender, ethnicity, race, insurance, LAPS2, COPS2, and hospital. Log(LOS) was calculated as log(LOS + 0.01) to account for any LOS = 0.
Normality assumption is violated.
Anderson-Rubin test for P value and 95% CI.
Proportionality assumption is violated.
Percent changes are included for the log(LOS) outcomes to allow for comparison with non-log β-coefficients.
The results of multiple sensitivity and secondary analyses were consistent with the primary results (Figures 1 and 2, Appendix E4, Tables E8–E21, and Figures E1 and E2). Adjustment for ED LOS attenuated the finding of longer hospital LOS among patients with sepsis admitted to the ICU, but this result remained statistically significant (β = 0.46 days; 95% CI, 0.15–0.77; P = 0.004). Similar adjustment magnified the finding of shorter hospital LOS among patients with ARF admitted to the ICU (β = –1.30 days; 95% CI, –1.65 to –0.94; P < 0.001) (Table E8). In these adjusted analyses, a 1-hour increase in ED LOS was associated with 1.02-hour (P < 0.001) and 1.55-hour (P < 0.001) decreases in median hospital LOS among patients with sepsis and ARF, respectively. Adjustment for ED LOS did not alter the mortality results for either cohort (Table E9).
Restricting to patients who only met criteria for a single clinical cohort amplified the estimates of increased in-hospital mortality among patients with sepsis admitted to the ICU (using 41.8% of the primary sepsis cohort without ARF) and of decreased mortality among patients with ARF admitted to the ICU (using 70.7% of the primary ARF cohort without sepsis) (Table E10). Repeating analyses without patient-level adjustment amplified the results in both cohorts (Table E11). No effect of ICU admission on LOS (β = 0.12 days; 95% CI, –0.95 to −1.20 using the sepsis strain index and β = 0.06 days; 95% CI, –0.47 to −0.59 using the ARF strain index) or mortality (OR = 0.95; 95% CI, 0.65–1.39 using the sepsis strain index and OR = 0.86; 95% CI, 0.62–1.27 using the ARF strain index) was identified in analyses restricted to patients with both sepsis and ARF (Table E12).
Post hoc analyses stratifying by quintiles of age (Table E19), LAPS2 (Table E20), and COPS2 (Table E21) revealed several examples of heterogeneous effects of ICU versus ward admission. Specifically, only older patients with sepsis (age ⩾ 73 yr) and those with lower comorbidity burdens (COPS2 ⩽ 93) had significantly longer LOS with ICU admission. Among patients with ARF, reductions in LOS with ICU admission were generally greatest among older quintiles and those with higher acuity. Reductions in both LOS and mortality also appeared to be greater among patients with ARF with higher comorbidity burdens.
Discussion
This large cohort study leveraging granular electronic health record data from 27 hospitals over 6 years addresses a common clinical dilemma—whether patients with sepsis or ARF who present to EDs with high severity of illness but without requiring mechanical ventilation or vasopressors should be admitted to ICUs or wards. In analyses leveraging a strong IV, we found that initial ICU admission of patients with sepsis, compared with ward admission, was associated with longer LOS and higher mortality, whereas initial ICU admission of patients with ARF was associated with reduced mortality and equivocal effects on LOS, with reductions found only in analyses treating deaths as equivalent to long LOS.
Prospectively randomizing ED patients with sepsis or ARF to be admitted to ICUs or wards is logistically and ethically challenging. Thus, use of a strong, valid, within-hospital IV provides the most compelling way to quantify benefits or harms of ICU admission for groups of patients who could plausibly be managed in either setting. The IV we deployed comprises 22 measures of strain, is tailored to each of the 27 hospitals for optimal performance, and meets all of the criteria of a valid instrument (Table E1) (11).
One prior study analyzed ICU benefit using a putative within-hospital instrument—ICU occupancy (38). However, that study, which showed improved 90-day mortality with ICU admission, did not demonstrate the validity of ICU occupancy as an IV and studied deteriorating ward patients with heterogeneous diseases rather than ED patients with well-characterized diseases and physiology. Further, we showed previously that our composite strain index provides discrimination superior to ICU occupancy alone (11).
Another pair of studies by Valley and colleagues (7, 39) attempted to address the benefits of ICU admission in analyses using an IV of the differential distance between the closest hospital to the patient and a hospital that commonly admits such patients to ICUs. Benefits of ICU admission were found for patients with pneumonia but not for patients with exacerbation of chronic obstructive pulmonary disease or heart failure. Differences between the outcomes obtained by those authors versus in our analyses may stem from differences in the cohort definitions or the IV. Valley and colleagues (7, 39) used an among-hospital IV to pseudorandomize patients between one hospital’s ward and another hospital’s ICU, addressing a different question from that facing ED clinicians, and with susceptibility to confounding by unmeasured differences among hospitals. By contrast, the hourly index we developed met all criteria as a strong within-hospital IV (Table E1) (11), enabling us to address the more clinically relevant question of whether a patient in a given hospital’s ED should go to that hospital’s ICU or ward, and avoiding among-hospital confounding (40).
Our conclusions are further strengthened by their general consistency across the many sensitivity and secondary analyses we conducted, including approaches to LOS assessment that are least susceptible to bias (17). Furthermore, the “reduced form” analyses provide strong supporting evidence that the IV performed appropriately. Perhaps most importantly, the observations of disparate findings by cohort—benefits of ICU admission for patients with ARF and harm for patients with sepsis—argue against systematic bias from unmeasured patient-level confounders, which could only occur when the confounders differed between the cohorts. Further, the validity of our results is supported by the observed magnification of ICU admission effects among patients with only sepsis or ARF and the absence of effects among patients with both sepsis and ARF. These results do not prove causality but are precisely what would be expected if ICU admission caused harm in sepsis and benefit in ARF.
Of note, the results were also robust to adjustment for ED LOS. When hospitals operate under capacity strain, patients who require inpatient admission may instead board in the ED, during which time the care they receive may differ than if they had been transferred immediately to a ward or ICU (30–37). Although the results remained directionally consistent and statistically significant when adjusting for ED LOS in both cohorts, the fact that LOS estimates were attenuated for sepsis but amplified for ARF may suggest a differential impact of ED boarding time on care delivery by disease process. However, our observation that increases in ED LOS because of strain were accompanied by reductions in inpatient LOS of corresponding magnitudes for patients with sepsis and ARF suggests that ED boarding did not adversely affect either patient group in this study.
Potential explanations for the observed increases in mortality and LOS for patients with sepsis admitted to the ICU include greater use of harmful interventions (e.g., excess intravenous fluid resuscitation [41]), increased incidence of complications (e.g., hospital-acquired infections, delirium, immobility, malnutrition, or acute kidney injury [42–47]), or different end-of-life care processes (e.g., palliative care consultations and goals-of-care discussions [48–50]). Potential mechanisms for the observed mortality reduction for patients with ARF admitted to the ICU include more frequent or superior respiratory care and monitoring, aggressive early pharmacologic treatment (e.g., antibiotics, inhaled bronchodilators, corticosteroids, or diuretics [51–55]), and greater use of noninvasive ventilatory support (e.g., bilevel positive airway pressure or high-flow nasal cannula [56]), leading to avoidance of invasive mechanical ventilation and early clinical turnaround. Alternatively, initial ICU admission may facilitate earlier mechanical ventilation when it is more effective among patients who ultimately need it (27, 57).
Future studies are needed to conclusively identify the mechanisms accounting for these findings. However, our observations that older, less comorbid patients with sepsis may be preferentially harmed by ICU care and that older, more acute, and more comorbid patients with ARF may preferentially benefit from ICU admission offer important initial guidance and suggest that triage guidelines should not treat all patients with specific clinical syndromes identically.
Going forward, causal mediation analyses that similarly leverage strong IVs or other quasiexperimental designs will be needed to identify specific mechanisms of harm and benefit. When conducting such analyses, we suggest that investigators focus on mediators that may act or be used differently between cohorts, given the different effects of ICU admission on patients with sepsis and those with ARF. For example, volume of intravenous fluid resuscitation is an intriguing area for future exploration, given that fluid resuscitation is explicitly part of sepsis resuscitation guidelines (58), whereas a restrictive fluid strategy and diuresis are often part of initial ARF management, such as when decompensated heart failure is suspected (59). Future studies of these and other plausible mechanisms would promote the goal of optimizing management of sepsis and ARF regardless of location and could guide trials of specific interventions that, unlike triage, are amenable to randomization.
Despite this study’s strengths in using rigorous analytic methods, having a robust sample size, and including patients from 27 different hospitals, the study results must be interpreted in the proper context. First, analyzing LOS in the setting of relatively high mortality, a common scenario in critical care studies, remains challenging (17, 60). To surmount this, we deployed a “placement of death” approach in which death was ranked as equivalent to an undesirable LOS (18). Although this approach is unbiased, it does not elucidate whether observed differences are due to changes in mortality, LOS independent of mortality, or both. Our interrogation of these data (Appendix E3 and Table 2) suggests that the primary sepsis result of longer hospital LOS with ICU admission is due to longer LOS and increased hospital mortality, whereas the ARF result of shorter hospital LOS with ICU admission is almost exclusively due to a reduction in mortality (and, therefore, fewer deaths that are assigned as long LOS in the ranked LOS outcome) with an uncertain impact on LOS itself.
Second, although we provide a robust approach to causal inference using observational data, even the best IV analyses are not equivalent to prospective randomized trials. Although our IV satisfied the assumption that it is not meaningfully associated with measured confounders, it cannot be proven that it is also not associated with unmeasured confounders. The possibility of residual confounding may be higher in the sepsis cohort because the strain index, although valid in both cohorts, was a comparatively weaker IV for the sepsis cohort (i.e., had lower ICU versus ward discrimination compared with the ARF cohort [11]). On the other hand, the findings of magnified effects when examining sepsis and patients with ARF in isolation and null effects when examining patients with both syndromes reduce the likelihood of important residual confounding.
Third, the results of these IV analyses only apply to patients whose ED disposition decision is modified by hospital capacity strain. Our prior work in the same cohort shows that 19.7% of patients with sepsis and 35.1% of patients with ARF meeting our eligibility criteria may have their admission dispositions changed by strain, which would equate to 17,760 patients with sepsis and 15,914 patients with ARF in the present study population (11). Our study does not address optimal admission disposition for patients who, in practice, are always admitted to the ICU or to the ward. For this reason, we excluded patients who required vasopressors or mechanical ventilation in the ED—patients who are unambiguously critically ill and to whom the results of this study do not apply. However, patients requiring noninvasive ventilatory support at a hospital that admits such patients to the ICU by policy may also not be importantly represented in the population of patients driving our results. Hospitals in this study had varying policies addressing noninvasive ventilatory support on the ward, although none adhered perfectly to an ICU-only policy.
Finally, sepsis and ARF are heterogeneous clinical syndromes with multiple etiologies and newly recognized phenotypes (61–63). Our limited post hoc exploratory analyses identified potentially important heterogeneity of treatment effects across age, acuity, and comorbidity quintiles. We have proposed potential mechanisms, but future studies are needed to analyze other important subgroups and to rigorously explore other sources of heterogeneous effects of ICU admission. Until a mechanistic understanding is established for specific clinical subgroups, clinicians may decide that these findings are not ready to be applied at the bedside.
Conclusions
In 27 hospitals across two health systems, among high-acuity patients with sepsis who did not require mechanical ventilation or vasopressors, initial admission to the ward compared with the ICU was associated with improved LOS and survival; among similar patients with ARF, initial admission to the ICU compared with the ward was associated with improved survival. These results suggest that triaging certain patients with sepsis to wards and more patients with ARF to ICUs may optimize outcomes for both populations. Such practices may improve overall patient outcomes and, as part of a comprehensive strategy to optimize critical care resource utilization, help preserve ICU beds for those who truly need them.
Acknowledgments
Acknowledgment
The authors thank Michael O. Harhay, Ph.D., (University of Pennsylvania Perelman School of Medicine) for additional guidance regarding statistics and Fernando Barreda (Kaiser Permanente Division of Research) for project management.
Footnotes
Supported by NHLBI grant R01HL136719 (S.D.H.), National Institute of General Medical Sciences grant R35GM128672 (V.X.L.). The Permanente Medical Group, Inc. (G.J.E.), and Agency for Healthcare Research and Quality grant K12HS026372 (G.L.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funders, including the Agency for Healthcare Research and Quality.
Author Contributions: Conception and design of study: G.L.A., V.X.L., D.S.S., M.K.D., B.B., E.D., G.J.E., and S.D.H. Data acquisition: G.L.A., V.X.L., M.C., B.B., and E.D. Analysis and data interpretation: G.L.A., V.X.L., M.C., D.S.S., W.W., M.K.D., B.B., G.J.E., and S.D.H. Drafting and revision of the manuscript: G.L.A., V.X.L., M.C., D.S.S., W.W., M.K.D., B.B., E.D., G.J.E., and S.D.H. All authors approve of the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202106-1350OC on November 24, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Anesi GL, Admon AJ, Halpern SD, Kerlin MP. Understanding irresponsible use of intensive care unit resources in the USA. Lancet Respir Med . 2019;7:605–612. doi: 10.1016/S2213-2600(19)30088-8. [DOI] [PubMed] [Google Scholar]
- 2. Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States: an analysis based on timing of diagnosis and severity level. Crit Care Med . 2018;46:1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med . 2013;8:76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med . 2017;45:1443–1449. doi: 10.1097/CCM.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest . 2014;146:1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Safavi KC, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, et al. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States. Circulation . 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA . 2015;314:1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anesi GL, Liu VX, Gabler NB, Delgado MK, Kohn R, Weissman GE, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc . 2018;15:1328–1335. doi: 10.1513/AnnalsATS.201804-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathews KS, Durst MS, Vargas-Torres C, Olson AD, Mazumdar M, Richardson LD. Effect of emergency department and ICU occupancy on admission decisions and outcomes for critically ill patients. Crit Care Med . 2018;46:720–727. doi: 10.1097/CCM.0000000000002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner J, Gabler NB, Ratcliffe SJ, Brown SE, Strom BL, Halpern SD. Outcomes among patients discharged from busy intensive care units. Ann Intern Med . 2013;159:447–455. doi: 10.7326/0003-4819-159-7-201310010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anesi GL, Chowdhury M, Small DS, Delgado MK, Kohn R, Bayes B, et al. Association of a novel index of hospital capacity strain with admission to intensive care units. Ann Am Thorac Soc . 2020;17:1440–1447. doi: 10.1513/AnnalsATS.202003-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anesi GL, Liu VX, Chowdhury M, Small DS, Wang W, Delgado MK, et al. Impact of intensive care unit admission on outcomes in sepsis and acute respiratory failure [abstract] Am J Respir Crit Care Med . 2021;203:A1677. doi: 10.1164/rccm.202106-1350OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anesi GL, Chelluri J, Qasim ZA, Chowdhury M, Kohn R, Weissman GE, et al. Association of an emergency department-embedded critical care unit with hospital outcomes and intensive care unit use. Ann Am Thorac Soc . 2020;17:1599–1609. doi: 10.1513/AnnalsATS.201912-912OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashana DC, Anesi GL, Liu VX, Escobar GJ, Chesley C, Eneanya ND, et al. Equitably allocating resources during crises: racial differences in mortality prediction models. Am J Respir Crit Care Med . 2021;204:178–186. doi: 10.1164/rccm.202012-4383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care . 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 16. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care . 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 17. Harhay MO, Ratcliffe SJ, Small DS, Suttner LH, Crowther MJ, Halpern SD. Measuring and analyzing length of stay in critical care trials. Med Care . 2019;57:e53–e59. doi: 10.1097/MLR.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin W, Halpern SD, Prasad Kerlin M, Small DSA. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res . 2017;26:292–311. doi: 10.1177/0962280214545121. [DOI] [PubMed] [Google Scholar]
- 19. Ranganathan P, Pramesh CS. Censoring in survival analysis: potential for bias. Perspect Clin Res . 2012;3:40. doi: 10.4103/2229-3485.92307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chernozhukov V, Hansen C. Instrumental variable quantile regression: a robust inference approach. J Econom . 2008;142:379–398. [Google Scholar]
- 21. He X. Quantile curves without crossing. Am Stat . 1997;51:186–192. [Google Scholar]
- 22.Kwak DW.https://sites.google.com/site/dwkwak/dataset-and-code
- 23. Liu V, Kipnis P, Gould MK, Escobar GJ. Length of stay predictions: improvements through the use of automated laboratory and comorbidity variables. Med Care . 2010;48:739–744. doi: 10.1097/MLR.0b013e3181e359f3. [DOI] [PubMed] [Google Scholar]
- 24. Escobar GJ, Plimier C, Greene JD, Liu V, Kipnis P. Multiyear rehospitalization rates and hospital outcomes in an integrated health care system. JAMA Netw Open . 2019;2:e1916769. doi: 10.1001/jamanetworkopen.2019.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lagu T, Pekow PS, Shieh MS, Stefan M, Pack QR, Kashef MA, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail . 2016;9:e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rondinelli J, Zuniga S, Kipnis P, Kawar LN, Liu V, Escobar GJ. Hospital-acquired pressure injury: risk-adjusted comparisons in an integrated healthcare delivery system. Nurs Res . 2018;67:16–25. doi: 10.1097/NNR.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delgado MK, Liu V, Pines JM, Kipnis P, Gardner MN, Escobar GJ. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med . 2013;8:13–19. doi: 10.1002/jhm.1979. [DOI] [PubMed] [Google Scholar]
- 28. Koladjo BF, Escolano S, Tubert-Bitter P. Instrumental variable analysis in the context of dichotomous outcome and exposure with a numerical experiment in pharmacoepidemiology. BMC Med Res Methodol . 2018;18:61. doi: 10.1186/s12874-018-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol . 2013;178:1177–1184. doi: 10.1093/aje/kwt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Qahtani S, Alsultan A, Haddad S, Alsaawi A, Alshehri M, Alsolamy S, et al. The association of duration of boarding in the emergency room and the outcome of patients admitted to the intensive care unit. BMC Emerg Med . 2017;17:34. doi: 10.1186/s12873-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andrew Taylor R, Venkatesh A, Parwani V, Chekijian S, Shapiro M, Oh A, et al. Applying advanced analytics to guide emergency department operational decisions: a proof-of-concept study examining the effects of boarding. Am J Emerg Med . 2018;36:1534–1539. doi: 10.1016/j.ajem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 32. DeLaney M. Emergency department boarding: the canary in the coal mine. J Am Coll Emerg Physicians Open . 2020;2:e12290. doi: 10.1002/emp2.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Falvo T, Grove L, Stachura R, Vega D, Stike R, Schlenker M, et al. The opportunity loss of boarding admitted patients in the emergency department. Acad Emerg Med . 2007;14:332–337. doi: 10.1197/j.aem.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34. Reznek MA, Upatising B, Kennedy SJ, Durham NT, Forster RM, Michael SS. Mortality associated with emergency department boarding exposure: are there differences between patients admitted to ICU and non-ICU settings? Med Care . 2018;56:436–440. doi: 10.1097/MLR.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 35. Schreyer KE, Martin R. The economics of an admissions holding unit. West J Emerg Med . 2017;18:553–558. doi: 10.5811/westjem.2017.4.32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singer AJ, Thode HC, Jr, Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med . 2011;18:1324–1329. doi: 10.1111/j.1553-2712.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 37. Viccellio P, Zito JA, Sayage V, Chohan J, Garra G, Santora C, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding. J Emerg Med . 2013;45:942–946. doi: 10.1016/j.jemermed.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 38. Harris S, Singer M, Sanderson C, Grieve R, Harrison D, Rowan K. Impact on mortality of prompt admission to critical care for deteriorating ward patients: an instrumental variable analysis using critical care bed strain. Intensive Care Med . 2018;44:606–615. doi: 10.1007/s00134-018-5148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Intensive care unit admission and survival among older patients with chronic obstructive pulmonary disease, heart failure, or myocardial infarction. Ann Am Thorac Soc . 2017;14:943–951. doi: 10.1513/AnnalsATS.201611-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anesi GL, Wagner J, Halpern SD. Intensive care medicine in 2050: toward an intensive care unit without waste. Intensive Care Med . 2017;43:554–556. doi: 10.1007/s00134-016-4641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, et al. CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med . 2018;72:457–466. doi: 10.1016/j.annemergmed.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA . 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 43. Hayashi Y, Morisawa K, Klompas M, Jones M, Bandeshe H, Boots R, et al. Toward improved surveillance: the impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis . 2013;56:471–477. doi: 10.1093/cid/cis926. [DOI] [PubMed] [Google Scholar]
- 44. Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med . 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 45. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med . 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubins HB, Moskowitz MA. Complications of care in a medical intensive care unit. J Gen Intern Med . 1990;5:104–109. doi: 10.1007/BF02600508. [DOI] [PubMed] [Google Scholar]
- 47. Steenbergen S, Rijkenberg S, Adonis T, Kroeze G, van Stijn I, Endeman H. Long-term treated intensive care patients outcomes: the one-year mortality rate, quality of life, health care use and long-term complications as reported by general practitioners. BMC Anesthesiol . 2015;15:142. doi: 10.1186/s12871-015-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med . 2015;175:1019–1026. doi: 10.1001/jamainternmed.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest . 2014;146:573–582. doi: 10.1378/chest.13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rolnick JA, Ersek M, Wachterman MW, Halpern SD. The quality of end-of-life care among ICU versus ward decedents. Am J Respir Crit Care Med . 2020;201:832–839. doi: 10.1164/rccm.201907-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Medicare & Medicaid Services. https://cmit.cms.gov/CMIT_public/ViewMeasure?MeasureId=1017
- 52. Daniel P, Rodrigo C, Mckeever TM, Woodhead M, Welham S, Lim WS, British Thoracic Society Time to first antibiotic and mortality in adults hospitalised with community-acquired pneumonia: a matched-propensity analysis. Thorax . 2016;71:568–570. doi: 10.1136/thoraxjnl-2015-207513. [DOI] [PubMed] [Google Scholar]
- 53. Dobler CC, Morrow AS, Beuschel B, Farah MH, Majzoub AM, Wilson ME, et al. Pharmacologic therapies in patients with wxacerbation of chronic obstructive pulmonary disease: a systematic review with meta-analysis. Ann Intern Med . 2020;172:413–422. doi: 10.7326/M19-3007. [DOI] [PubMed] [Google Scholar]
- 54. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet . 2015;385:1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 55. Woods JA, Wheeler JS, Finch CK, Pinner NA. Corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2014;9:421–430. doi: 10.2147/COPD.S51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med . 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 57. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med . 2012;7:224–230. doi: 10.1002/jhm.964. [DOI] [PubMed] [Google Scholar]
- 58. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med . 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 59. Murphy SP, Ibrahim NE, Januzzi JL., Jr Heart failure with reduced ejection fraction: a review. JAMA . 2020;324:488–504. doi: 10.1001/jama.2020.10262. [DOI] [PubMed] [Google Scholar]
- 60. Harhay MO, Ratcliffe SJ, Halpern SD. Measurement error due to patient flow in estimates of intensive care unit length of stay. Am J Epidemiol . 2017;186:1389–1395. doi: 10.1093/aje/kwx222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA . 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fohner AE, Greene JD, Lawson BL, Chen JH, Kipnis P, Escobar GJ, et al. Assessing clinical heterogeneity in sepsis through treatment patterns and machine learning. J Am Med Inform Assoc . 2019;26:1466–1477. doi: 10.1093/jamia/ocz106. [DOI] [PMC free article] [PubMed] [Google Scholar]