Abstract
Tonic activation of excitatory and inhibitory receptors, by the ambient concentration of neurotransmitters in the extracellular space of the brain, has been suggested to underlie phenomena as diverse as relapse to cocaine use by reward pathways in the striatum, sparse coding of motor information in the cerebellum, and control of the development of the cerebral and cerebellar cortices. Here we assess the mechanisms which may determine the ambient levels of excitatory and inhibitory neurotransmitters, and consider their likely effect on information processing.
Keywords: Glutamate, GABA, Uptake, Release, Tonic, Ambient, Extracellular
1. Introduction
The average extracellular concentration of CNS neurotransmitters is determined by the balance between their rate of release and the rate at which they are removed by uptake. If the release rate is low, then in principle transporters can lower the extracellular transmitter concentration to a value determined by the ionic stoichiometry of the transporters. For example, glutamate transporters, which power glutamate accumulation by the co-transport of 3 Na+ and 1 H+ and the counter-transport of 1 K+, could in principle lower the extracellular glutamate concentration to ~2 nM (Fig. 1; Zerangue and Kavanaugh, 1996; Levy et al., 1998), while GABA transporters, which power GABA accumulation by the co-transport of 2 Na+ and 1 Cl−, could theoretically lower [GABA]o to ~0.4 μM (Attwell et al., 1993), and the similarly powered GlyT1b transporters could lower the extracellular glycine concentration to about 0.15 μM (Attwell et al., 1993; Supplisson and Roux, 2002). Microdialysis experiments in the in vivo brain report extracellular concentrations higher than these theoretical minimum values, typically a few μM (0.2–7μM (typically 2 μM) for glutamate, 0.1–2μM for GABA, 2.5 μM for glycine: Benveniste et al., 1984; Hagberg et al., 1985; Takagi et al., 1993; Phillis et al., 1994; Wahl et al., 1994), which may reflect a high rate of transmitter release (Bernander et al., 1991; Rapp et al., 1992; Borg-Graham et al., 1998; Hirsch et al., 1998; Destexhe and Pare, 1999; Jabaudon et al., 1999, Baker et al., 2002), but could alternatively reflect some cell damage or leakage from the blood caused by the microdialysis probe (Westergren et al., 1995) as discussed below.
Fig. 1.
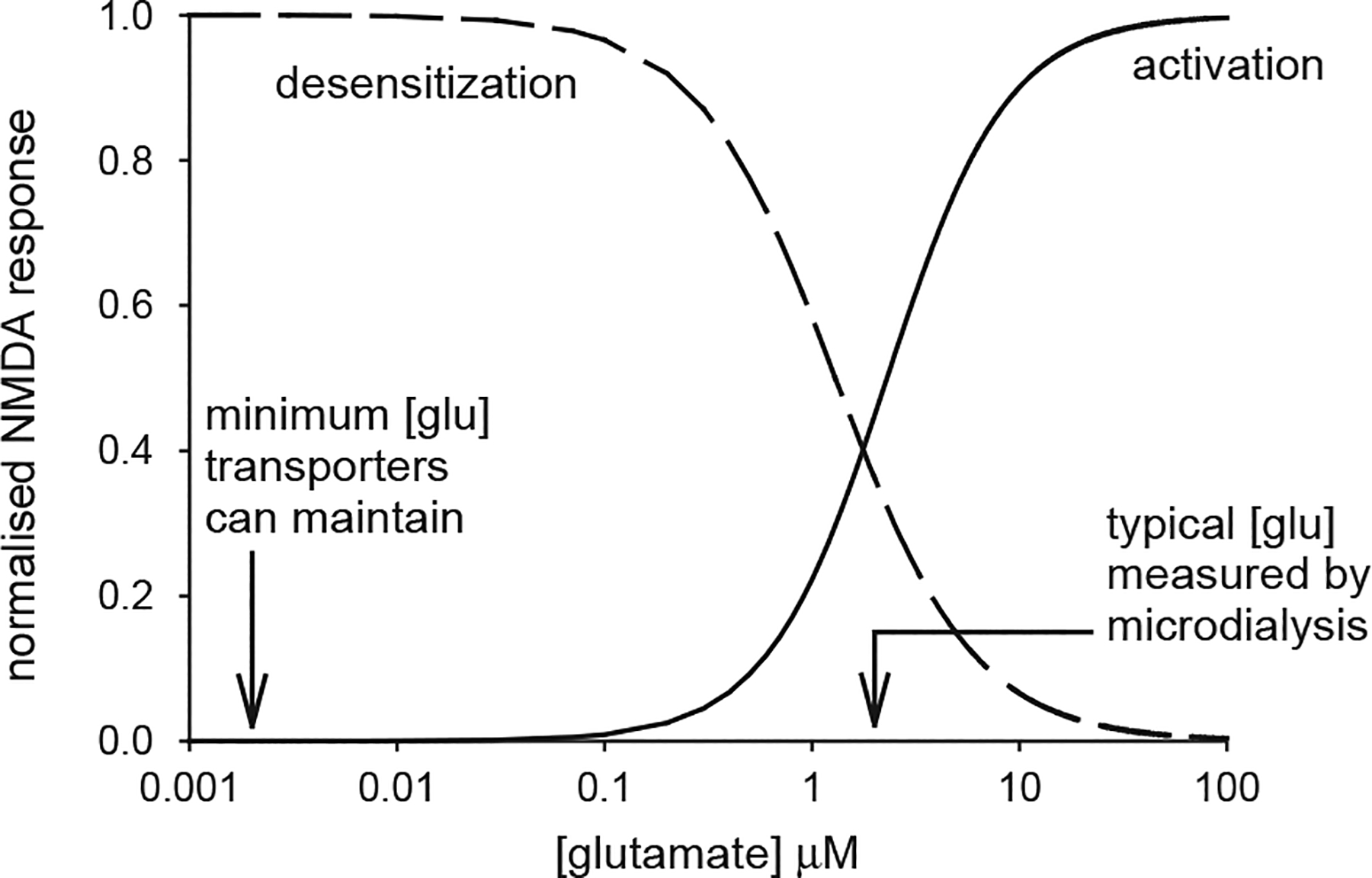
The effect of a non-zero baseline extracellular glutamate concentration on the activation of NMDA receptors. Continuous curve is the activation curve for NMDA receptors (, with EC50 = 2.3 μM, from Patneau and Mayer, 1990). Na+-dependent transporters with the stoichiometry measured for EAAC1 and GLT-1 can theoretically lower [glu]o to ~2 nM (left arrow). Microdialysis experiments typically measure a resting [glu]o of ~2 μM (right arrow), which is sufficient to activate NMDA receptors by 45% of their maximum activation, so that subsequent rises of [glu]o produce a smaller change of activation than would occur with no glutamate present initially. Prolonged presence of glutamate also desensitises NMDA receptors. The dashed line, given by , with IC50=1.3 μM, shows the inhibition of the NMDA component of EPSCs measured by Zorumski et al. (1996): this curve overestimates the amount of desensitization occurring, because pre-synaptic actions of glutamate contribute a small amount to the EPSC depression (e.g. at 1 μM glutamate, presynaptic inhibition alone inhibits the response by about 12% while the combination of pre-synaptic inhibition and desensitization inhibits by about 42%: see Zorumski et al. (1996) Fig. 6B).
Micromolar concentrations of glutamate and GABA are expected to produce tonic activation of both ionotropic and G protein coupled receptors. NMDA receptors have an EC50 of ~2 μM (Fig. 1; Patneau and Mayer, 1990), while GABAA receptors containing α6 and δ subunits have an EC50 of 0.2 μM (Saxena and MacDonald, 1996), and these high affinity receptors are expected to be very significantly activated by micromolar levels of glutamate and GABA. The steady state dose-response curves for AMPA receptors, metabotropic glutamate receptors (mGluRs), GABAA and GABAB receptors have EC50 values which are somewhat higher (e.g. ~16 μM for AMPA receptors: Patneau and Mayer, 1990; 4–56 μM for mGluR1-6: Pin and Duvoisin, 1995), but might also be activated to some extent by the ambient extracellular transmitter level. (Note that synaptic and extrasynaptic receptors may have different subunit compositions, and hence different EC50 values for activation and desensitization, and may also be exposed to different ambient transmitter concentrations). In this review we assess the likely effects of this tonic activation of glutamate and GABA receptors on CNS information processing, and highlight some problems in our understanding of the transmitter release mechanisms underlying the observed average extracellular glutamate and GABA concentrations.
2. Actions of ambient glutamate on NMDA, AMPA and kainate receptors
Sah et al. (1989) reported that NMDA receptors in pyramidal cells in hippocampal slices can be tonically activated by the background level of glutamate present in the extracellular space. Similarly, Dalby and Mody (2003) found that dentate gyrus granule cell NMDA receptors are activated by spontaneous (action potential independent) glutamate release. This receptor activation by ambient glutamate generates an inward current, suppressible by NMDA receptor blockers, which increases the excitability of the neurons. Furthermore, because of the voltage-dependent Mg2+-block of NMDA receptor channels, the size of this inward current increases with depolarization from the resting potential, increasing the tendency of the cell to show a regenerative depolarization, which may boost synaptic potentials. In midbrain dopamine-secreting neurons, and in supraoptic vasopressin- and oxytocin-secreting neurons, this NMDA receptor mediated current is reported to control the bursting pattern of the cells’ spiking activity, and thus control secretory activity (Chergui et al., 1993; Moos et al., 1997).
The ~2 μM extracellular glutamate concentration measured in vivo with microdialysis is expected to produce about a 45% activation of NMDA receptors, from the glutamate dose–response curve for the receptors shown in Fig. 1 (the ambient level of endogenous modulators of NMDA receptors (e.g. Mg2+, glycine, Zn2+, polyamines, pH: reviewed by Dingledine et al., 1999) will also influence the level of activation of these receptors by the ambient glutamate concentration present). This would explain the tonic activation of NMDA receptors observed by Sah et al. (1989), but implies that the rise of glutamate concentration to ~1 mM during synaptically evoked NMDA receptor activation (including that produced by spontaneous transmitter release in the study of Dalby and Mody, 2003) would evoke a current that was only 55% of what could occur if the ambient glutamate concentration was at the bottom of the receptors’ dose–response curve (Fig. 1). Furthermore, the IC50 for pre-application of glutamate desensitizing hippocampal NMDA receptors is only ~1.3 μM (Zorumski et al., 1996; see Fig. 1 legend for details), so an ambient glutamate concentration of 2 μM would lead to an even greater reduction in the amplitude of the NMDA component of synaptic currents. Similarly, pre-application of glutamate desensitizes hippocampal AMPA and kainate receptors with an IC50 of 4 and 2.8–13 μM, respectively (Colquhoun et al., 1992; Zorumski et al., 1996; Wilding and Huettner, 1997; Paternain et al., 1998), so an ambient glutamate concentration of 2 μM would also reduce the amplitude of the AMPA and kainate components of synaptic currents.
The fact that micromolar levels of ambient glutamate will tend to cause saturation of NMDA receptor activation, and induce desensitization of NMDA, AMPA and kainate receptors, suggests that alteration of the ambient glutamate concentration may have profound consequences for the information processing carried out by neurons. Such a high ambient glutamate level (if correct, and not an artefact of cell damage, or leakage from the blood (Westergren et al., 1995) caused by the microdialysis cannula) also raises the question of why the resulting calcium influx through activated NMDA receptors does not lead to neuronal death, as occurs when exogenous NMDA or glutamate is applied (Choi, 1987).
3. Tonic activation of metabotropic glutamate receptors
Activation of presynaptic mGluRs in hippocampus has been found either to inhibit glutamate release (Forsythe and Clements, 1990) or to facilitate it (McBain et al., 1994). The inhibitory effect is seen at glutamate concentrations less than 1 μM, and so would occur at the ambient glutamate concentrations found in vivo. Indeed, Losonczy et al. (2003) report that blocking mGluRs leads to a potentiation of postsynaptic EPSCs, implying that there is a tonic inhibition of glutamate release by the ambient glutamate concentration. Activation of postsynaptic mGluRs leads to an inward current in hippocampal interneurons (McBain et al., 1994), possibly mediated by activation of a TRP channel (Kim et al., 2003).
Tonic activation of mGluRs with externally applied glutamate or ACPD, or stimulation trains, leads to 40 Hz oscillations generated by GABAA receptors in slices of hippocampus and neocortex (Whittington et al., 1995). Since coherent 40 Hz oscillations have been proposed to underly the “binding” together of different cognitive aspects of stimuli (Singer, 1993), this raises the possibility that tonic activation of mGluRs could modulate significant aspects of hippocampal and cortical processing.
4. Can ambient glutamate affect neuronal information processing if it produces no membrane current?
Sah et al. (1989) found that NMDA receptor block produced a large current change in hippocampal slice CA1 pyramidal cells (~200 pA at −35 mV in 400 μm thick slices (age not specified); <60 pA in 100 μm slices) which was attributed to activation of NMDA receptors by the ambient glutamate level present. However, not all workers have found such a large effect, indeed we find that, at post-natal day 12, the NMDA receptor antagonist D-AP5 only blocks an inward current of ~5 pA at −35 mV in whole-cell clamped pyramidal cells in 225 μm thick slices. The reason for this difference is unclear, but it could reflect the sharp electrode recordings made by Sah et al. (1989) being from cells very deep in the slice, possibly at a location with compromised oxygen supply, while those studied with patch-clamp techniques are from more superficial cells.
Nevertheless, even if receptor block produces a negligible current, this does not rule out a possible influence of ambient glutamate on ionotropic glutamate receptors. The non-linear dose–response curve of NMDA and AMPA receptors (Patneau and Mayer, 1990), which results from 2–4 glutamate molecules being required to bind for full activation to occur (Smith and Howe, 2000; Banke and Traynelis, 2003), will result in a rise of extracellular glutamate concentration produced by exocytosis having a different effect if it is superimposed on an ambient glutamate level that results in some of the receptors’ binding sites being tonically occupied. Whether binding of ambient glutamate produces an increased or a decreased response to exocytotic glutamate release will depend on whether partial activation or desensitization of the receptors dominates, and whether desensitization can occur from a closed state (Lin and Stevens, 1994; Colquhoun and Hawkes, 1995).
5. Possible sources of ambient glutamate
Conventionally, glutamate is assumed to be released into the extracellular space as a result of exocytosis from neurons, either evoked by action potentials or spontaneously released as miniature events. Recently, however, a diverse range of other possible glutamate release mechanisms have been discovered, including Ca2+-dependent (probably exocytotic) release from glia (Bezzi et al., 1998; Krzan et al., 2003), release via swelling-activated anion channels (Kimelberg et al., 1990; Rutledge and Kimelberg, 1996), gap junction hemi-channels (Ye et al., 2003; Bennett et al., 2003), P2X7 receptors (Sperlagh et al., 2002; Duan et al., 2003), and cystine–glutamate exchange (Cho and Bannai, 1990). Of these, a role for cystine–glutamate exchange has recently gained prominence in the context of setting the ambient extracellular glutamate concentration.
Cystine–glutamate exchange is mediated by a heterodimeric protein composed of the 12 transmembrane region protein xCT and the heavy chain of 4F2 cell surface antigen (Sato et al., 1999; Fukasawa et al., 2000). It is responsible for providing neurons with cystine, a precursor of the anti-oxidant glutathione (Bannai, 1984), and normally functions with the uptake of cystine being powered by the efflux of glutamate, down the concentration gradient set up by Na+-dependent glutamate transporters. Radiotracing studies on cerebellar slices have shown a maximum uptake rate for cystine of roughly 450 μmoles/l/h (Wyatt et al., 1996). Assuming that glutamate leaves cells at the same rate (i.e. a 1:1 exchange), this will raise the glutamate concentration in the extracellular space (with volume fraction 0.2) at a maximum rate of 450/(3600 × 0.2) μM/s=0.6 μM/s. Depending on the extent to which the released glutamate is taken up by Na+-dependent transporters, this efflux rate may be large enough to activate or desensitise NMDA and non-NMDA receptors. However, whether this occurs in practice will depend critically on the extracellular cystine concentration. The Km for extracellular cystine activating cystine–glutamate exchange is 77–140 μM (Wyatt et al., 1996; Warr et al., 1999) and, while the normal cystine concentration in the extracellular space of the brain is uncertain, it presumably lies closer to the value in the CSF than to the blood plasma value (0.2 and 80 μM, respectively: Murphy et al., 1989). The former value would provide minimal activation of cystine–glutamate exchange.
Despite the fact that there may not be sufficient cystine present in the extracellular space to produce a large fractional activation of cystine–glutamate exchange, Baker et al. (2002) have recently suggested that this release mechanism is responsible for at least 60% of the ambient extracellular glutamate measured by microdialysis in vivo in the striatum. Furthermore, they have suggested that a reduction of cystine–glutamate exchange activity after withdrawal from cocaine use leads to relapse into drug-seeking behaviour (Baker et al., 2003). This work depends critically on the use of the glutamate analogue CPG ((S)-4-carboxyphenylglycine) to block cystine–glutamate exchange. Unfortunately CPG also inhibits group I metabotropic glutamate receptors and, although Baker et al. (2002) checked that blocking these mGluRs did not alter the effect of CPG on cystine uptake (demonstrating that CPG was not blocking cystine–glutamate exchange via an action on the mGluRs), they did not do the crucial control experiment of testing whether CPG still reduced glutamate efflux when mGluRs were blocked. Furthermore, our experiments (Cavelier and Attwell, 2004) on hippocampal slices have found that, although CPG reduces tonic glutamate release when 300 μM cystine is applied (demonstrating that cystine–glutamate exchange is present), it does not detectably block it in the absence of exogenous cystine, presumably because cystine–glutamate exchange is not significantly activated by the ambient cystine concentration present in the extracellular space of the slice. It is possible that the ambient cystine is higher in the experiments of Baker et al. (2002, 2003), either because they are working in vivo or because introduction of the microdialysis tube cannula allows some cystine to pass from the blood to the extracellular space (Westergren et al., 1995). At present, therefore, the contribution of cystine–glutamate exchange to the ambient glutamate concentration remains uncertain.
The relative contribution of these different mechanisms to setting the ambient glutamate concentration may alter according to the intracellular signalling and metabolic status of neurons. For example, in ischaemia, the run-down of transmembrane ion gradients occurring leads to Na+-dependent transporters raising the ambient glutamate concentration to ~100 μM (Rossi et al., 2000).
6. Tonic activation of GABAA receptors
Cerebellar granule cells show a tonic activation of GABAA receptors, in addition to a phasic activation of receptors produced by synaptic GABA release from Golgi cells (Kaneda et al., 1995; Tia et al., 1996; Brickley et al., 1996; Wall and Usowicz, 1997). A similar tonic inhibition is seen in dentate gyrus granule cells and hippocampal interneurons (Stell and Mody, 2002; Semyanov et al., 2003), and appears in hippocampal pyramidal cells when GABA uptake is blocked (Semyanov et al., 2003).
In cerebellar granule cells the tonic inhibition is generated by high affinity GABAA receptors containing α6 and probably δ subunits (Laurie et al., 1992; Nusser et al., 1998; Brickley et al., 2001; Hamann et al., 2002; Stell et al., 2003) which have a low micromolar EC50 for GABA (0.2 μM for receptors containing the subunit combination α6β2δ and 2 μM for α6β2γ2: Saxena and MacDonald, 1996). The receptors mediating tonic inhibition in dentate gyrus granule cells may also contain δ subunits (Stell et al., 2003).
7. Sources of ambient transmitter causing tonic inhibition
In young animals the tonic inhibition of cerebellar granule cells is generated by the accumulation of GABA released by action potentials, but in adult animals it is not blocked by TTX and so is not caused by action potential-dependent release of transmitter (Kaneda et al., 1995; Tia et al., 1996; Brickley et al., 1996; Wall and Usowicz, 1997; Rossi et al., 2003). Rossi et al. (2003) showed that block of exocytotic GABA release (by inhibiting the H+-ATPase that powers vesicular GABA accumulation) also has no effect on the tonic inhibition, implying that spontaneous release of GABA-containing vesicles is not the cause of the tonic activation of GABAA receptors. Rossi et al. (2003) also ruled out release of GABA through a swelling activated anion channel, or through reversal of the GABA transporters GAT-1 and GAT-3 (despite the fact that these transporters are easily reversible: Richerson and Wu, 2003), as sources of GABA to activate the tonic inhibitory conductance. By exclusion, it was suggested that GABA may be released from Golgi cell synaptic terminals, or possibly from astrocytes, by a non-vesicular calcium- and action-potential independent mechanism. Interestingly, astrocytes have been suggested to release GABA: Liu et al. (2000) found that astrocyte conditioned medium activated GABA receptors in cultured hippocampal neurons, while Wang et al. (2002) reported GABA release via P2X7 receptors which was dependent on Cl− and .
It is possible that part of the tonic activation of GABAA receptors is not produced by GABA, but by a related transmitter analogue such as taurine or β-alanine, as has been suggested for the tonic activation of glycine receptors observed in developing neocortex (Flint et al., 1998). However, Wall (2002) has shown, by enzymatically degrading extracellular GABA in cerebellar slices, that at least 30% of the tonic conductance in cerebellar granule cells is produced by GABA itself.
8. Computational consequences of tonic inhibition
Tonic inhibition, with a reversal potential near the resting potential (shunting inhibition) is expected to have several effects on the computational processing carried out by neurons. It will increase the membrane conductance, and thus reduce the voltage produced by an injected or excitatory input current; it will decrease the electrical space constant, so that there will be less spatial summation of signals; and it will reduce the membrane time constant, so that there is less temporal integration of different inputs (Bernander et al., 1991; Rapp et al., 1992; Hausser and Clark, 1997; Destexhe and Pare, 1999). As a result of these effects, tonic inhibition will make it harder to generate action potentials.
In adult rats the tonic inhibition of cerebellar granule cells generates a mean inhibitory conductance which is 3-fold larger than that generated by high frequency action potential evoked vesicular release of GABA (Hamann et al., 2002), suggesting that it may have a profound effect on information transfer through the granule cells. To investigate this, Hamann et al. (2002) took advantage of the fact that the high affinity receptors mediating tonic inhibition are selectively blocked by 100 μM furosemide. They found that blocking the tonic GABAA receptor mediated conductance altered the frequency of granule cell action potentials produced by injected current (Fig. 2A) or mossy fibre stimulation (Fig. 2B), and thus altered the dependence of Purkinje cell firing frequency on mossy fibre stimulation frequency (Fig. 2C). For action potentials produced by injected steady currents, tonic inhibition produces a shift of the frequency-current curve along the current axis (Fig. 2A) rather than a change of the gain of the curve, as has been predicted by theoretical models (Gabbiani et al., 1994; Holt and Koch, 1997). However, for excitatory synaptic input (actually a mixture of both excitatory and inhibitory synaptic input, since stimulating the mossy fibres with trains of action potentials will activate di-synaptic inhibitory synaptic input via Golgi cells) the granule cell output frequency—mossy fibre input frequency curve also shows a gain change (Fig. 2B). This is also seen in the transfer function for the whole cerebellar cortex (i.e. the Purkinje cell frequency—mossy fibre frequency relation: Fig. 2C). It results from there being frequency-dependent variance present in the excitatory current generated by synaptic input, that is absent in an injected constant current (Chance et al., 2002; Prescott and De Koninck, 2003; Mitchell and Silver, 2003).
Fig. 2.
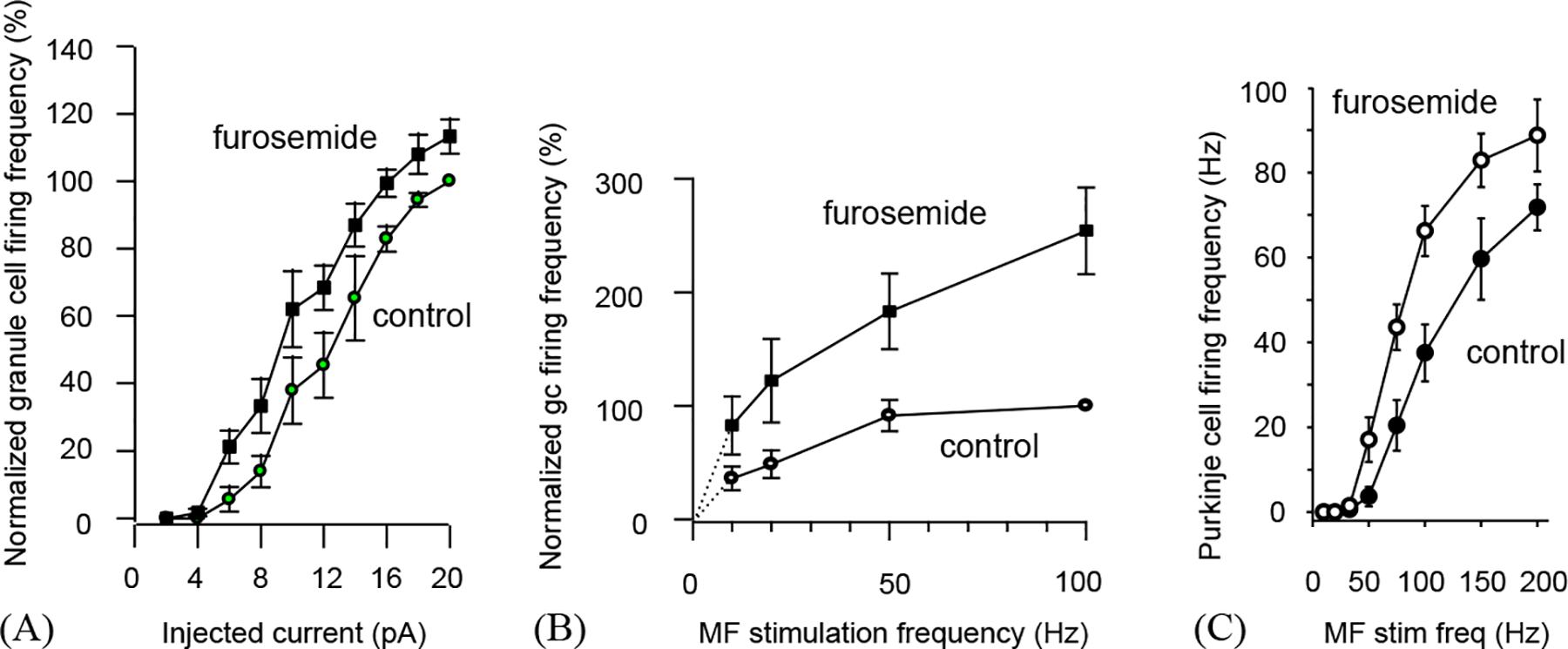
The effect of tonic inhibition of cerebellar granule cells on information transfer through the cerebellar cortex of rats (from the study of Hamann et al., 2002). (A) Granule cell firing rate (normalised to maximum rate in nine cells) as a function of injected current, in control conditions and with tonic inhibition by high affinity GABAA receptors blocked with furosemide. The input–output curve is shifted to the left by blocking the tonic inhibition. (B) Granule cell firing rate (normalized to maximum rate in four cells) as a function of mossy fibre stimulation rate, in the presence and absence (furosemide) of tonic inhibition. (C) Purkinje cell firing rate as a function of mossy fibre stimulation rate, in the presence and absence (furosemide) of tonic inhibition.
The reduction of synaptic transmission from mossy fibres to granule cells which tonic inhibition produces, and the consequent reduction in information transfer through the cerebellar cortex, from the mossy fibres to the output Purkinje neurons, may have several functional consequences. Phasic and tonic activation of granule cell GABAA receptors are predicted by modelling work to have different effects on the pattern of firing of granule and Golgi cells, with phasic activation (due to synaptic GABA release from Golgi cells) producing more synchronous rhythmic firing, and tonic inhibition reducing synchronicity and rhythmicity (Maex and De Schutter, 1998). Furthermore, computational models of cerebellar cortex have emphasized that decreasing the number of granule cells which are simultaneously active increases the number of motor programmes that can be stored in the cerebellum (Marr, 1969; Tyrrell and Willshaw, 1992), so tonic inhibition of granule cells may be an important determinant of the animals’ motor repertoire.
9. Modulation of tonic inhibition
Since tonic inhibition has a profound effect on cerebellar information processing, and is likely to have similar effects in the hippocampus, it seems likely that its magnitude will be capable of being modulated. In the cerebellum, modulation of GABA transporters has been shown to alter the amount of inhibition occurring, presumably by altering the ambient level of extracellular GABA (Rossi et al., 2003). Modulation of GABA release by ACh, and of the GABAA receptors generating the tonic inhibition by neurosteroids, may have a similar effect (Hamann et al., 2002; Rossi et al., 2003; Stell et al., 2003).
10. Tonic activation of neurotransmitter receptors during development
Neurotransmitters play a key role in CNS development, controlling cell growth and division, gene expression, synapse formation and cell migration. Many of the developmental actions of transmitters precede the formation of anatomically defined synapses (Komuro and Rakic, 1993; Catsicas et al., 1998; Demarque et al., 2002), and are mediated by elevations of [Ca2+]i. In the cerebellum, Ca2+ entry through NMDA receptor channels modulates neurite growth, migration of granule cells, and the number of climbing fibre to Purkinje cell synapses (Pearce et al., 1987; Komuro and Rakic, 1993; Rossi and Slater, 1993; Rabacchi et al., 1992). In neocortex, neuronal proliferation is inhibited (LoTurco et al., 1995) by the depolarization and [Ca2+]i rise produced by activation of glutamate receptors or GABA receptors (which are depolarizing early in development). The mode of release of neurotransmitters early in development is uncertain, since even stimulation-evoked release is independent of calcium and the normal vesicular release machinery (Demarque et al., 2002). Reversed uptake is a possibility (Taylor and Gordon-Weeks, 1991), although Demarque et al. (2002) ruled out reversal of the GABA transporter GAT-1 (though not of GAT-3) as a mode of GABA release in embryonic and neonatal hippocampus. Further investigations of the possible roles of cystine/glutamate exchange, Ca2+-dependent release from glia, P2X7 receptors, gap junctional hemichannels and swelling-activated channels are needed.
11. Conclusions
Tonic activation of glutamate and GABA receptors appears to play an important role in the development of the CNS, and there is a clear role for the tonic activation of GABA receptors in the adult nervous system, where they contribute to information processing in the cerebellum and probably the hippocampus. The importance of tonic activation of glutamate receptors in the adult nervous system is less certain, and will depend critically on the true value of extracellular glutamate concentration [glu]o. The value of [glu]o measured in vivo by microdialysis seems high enough to severely reduce NMDA receptor function by saturation and desensitization, and to induce neuronal death, and so might reflect an artefactual elevation of [glu]o caused by damage induced by the microdialysis procedure. Furthermore, the mode of tonic glutamate release proposed for the striatum, cystine/glutamate exchange, would probably require an unphysiologically high value of cystine concentration in the extracellular space. Nevertheless, the increasing evidence that the spontaneous activity of the brain provides a context within which incoming sensory signals are analysed (Kenet et al., 2003) emphasizes the importance of establishing how excitatory and inhibitory receptors are activated by tonic transmitter release mechanisms.
Acknowledgements
We thank Angus Silver for comments on the manuscript. Supported by the Wellcome Trust, a Burroughs-Wellcome Fellowship to DJR, a Wolfson–Royal Society Award to DA, and the European Union.
References
- Attwell D, Barbour B, Szatkowski M, 1993. Nonvesicular release of neurotransmitter. Neuron 11, 401–407. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z, Shen H, Swanson CJ, Kalivas PW, 2002. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci 22, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW, 2003. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci 6, 743–749. [DOI] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF, 2003. Activation of NR1/NR2B NMDA receptors. Nat. Neurosci 6, 144–152. [DOI] [PubMed] [Google Scholar]
- Bannai S, 1984. Transport of cystine and cysteine in mammalian cells. Biochem. Biophys. Acta 779, 289–306. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Contreras JE, Bukauskas FF, Saez JC, 2003. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 26, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH, 1984. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem 43, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Bernander O, Douglas RJ, Martin KAC, Koch C, 1991. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc. Natl. Acad. Sci. USA 88, 11569–11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A, 1998. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391, 281–285. [DOI] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y, 1998. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393, 369–372. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M, 1996. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol 497, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M, 2001. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92. [DOI] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P, 1998. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr. Biol 8, 283–286. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D, 2004. Tonic release of glutamate in rat hippocampal slices by a DIDS-sensitive mechanism. J. Physiol. Proc, 555p, C46 (http://www.physoc.org/publications/proceedings/archive/article.asp?ID=555PC46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD, 2002. Gain modulation from background synaptic input. Neuron 35, 773–782. [DOI] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G, 1993. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur. J. Neurosci 5, 137–144. [DOI] [PubMed] [Google Scholar]
- Cho Y, Bannai S, 1990. Uptake of glutamate and cystine in C-6 glioma cells and in cultured astrocytes. J. Neurochem 55, 2091–2097. [DOI] [PubMed] [Google Scholar]
- Choi DW, 1987. Ionic dependence of glutamate neurotoxicity. J. Neurosci 7, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG, 1995. Desensitization of NMDA receptors: a problem of interpretation. Proc. Natl. Acad. Sci. USA 92, 10327–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Jonas P, Sakmann B, 1992. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J. Physiol 458, 261–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO, Mody I, 2003. Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. J. Neurophysiol 90, 786–797. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L, 2002. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36, 1051–1061. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Pare D, 1999. Impact of network activity on the integrative properties of neocortical pyramidal cells in vivo. Journal of Neurophysiology 81, 1531–1547. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF, 1999. The glutamate receptor ion channels. Pharmacol. Rev 51, 7–61. [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA, 2003. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint AC, Liu X, Kriegstein AR, 1998. Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20, 43–53. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Clements JD, 1990. Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neurones. J. Physiol 429, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H, Kanai Y, 2000. Identification and characterization of a Na+-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral d- and l-amino acids. J. Biol. Chem 275, 9690–9698. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Midtgaard J, Knopfel T, 1994. Synaptic integration in a model of cerebellar granule cells. J. Neurophysiol 72, 999–1009. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A, 1985. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J. Cerebral Blood Flow Metab 5, 413–419. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi D, Attwell D, 2002. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33, 625–633. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA, 1997. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665–678. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Alonso J-M, Reid RC, Martinez LM, 1998. Synaptic integration in striate cortical simple cells. J. Neurosci 18, 9517–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR, Koch C, 1997. Shunting inhibition does not have a divisive effect on firing rates. Neural Comput. 9, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U, 1999. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc. Natl. Acad. Sci. USA 96, 8733–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG, 1995. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J. Physiol 485, 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A, 2003. Spontaneously emerging cortical representations of visual attributes. Nature 425, 954–956. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ, 2003. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426, 285–291. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA, 1990. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J. Neurosci 10, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P, 1993. Modulation of neuronal migration by NMDA receptors. Science 260, 95–97. [DOI] [PubMed] [Google Scholar]
- Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R, 2003. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J. Neurosci 23, 1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W, 1992. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain II: olfactory bulb and cerebellum. J. Neurosci 12, 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D, 1998. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci 18, 9620–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Stevens CF, 1994. Both open and closed NMDA receptors desensitize. J. Neurosci 14, 2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Schaffner AE, Chang YH, Maric D, Barker JL, 2000. Persistent activation of GABAA receptor/Cl− channels by astrocyte-derived GABA in cultured embryonic rat hippocampal neurons. J. Neurophysiol 84, 1392–1403. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Somogyi P, Nusser Z, 2003. Reduction of excitatory postsynaptic responses by persistently active metabotropic glutamate receptors in the hippocampus. J. Neurophysiol 89, 1910–1919. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR, 1995. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298. [DOI] [PubMed] [Google Scholar]
- Maex R, De Schutter E, 1998. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J. Neurophysiol 80, 2521–2537. [DOI] [PubMed] [Google Scholar]
- Marr D, 1969. A theory of cerebellar cortex. J. Physiol 202, 437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, DiChiara TJ, Kauer JA, 1994. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J. Neurosci 14, 4433–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA, 2003. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445. [DOI] [PubMed] [Google Scholar]
- Moos FC, Rossi K, Richard P, 1997. Activation of N-methyl-d-aspartate receptors regulates basal electrical activity of oxytocin and vasopressin neurons in lactating rats. Neuroscience 77, 993–1002. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT, 1989. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2, 1547–1558. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P, 1998. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci 18, 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J, 1998. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology 37, 1249–1259. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML, 1990. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-d-aspartate and quisqualate receptors. J. Neurosci 10, 2385–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce IA, Cambray-Deakin MA, Burgoyne RD, 1987. Glutamate acting on NMDA receptors stimulates neurite outgrowth from cerebellar granule cells. FEBS Lett. 223, 143–147. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Smith-Barbour M, Perkins LM, O’Regan MH, 1994. Characterization of glutamate, aspartate, and GABA release from ischemic rat cerebral cortex. Brain Res. Bull 34, 457–466. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R, 1995. The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34, 1–26. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y, 2003. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl. Acad. Sci. USA 100, 2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J, 1992. Involvement of the NMDA receptor in synapse elimination during cerebellar development. Science 256, 1823–1825. [DOI] [PubMed] [Google Scholar]
- Rapp M, Yarom Y, Segev I, 1992. The impact of parallel fibre background activity on the cable properties of cerebellar Purkinje cells. Neural Comput. 4, 518–532. [Google Scholar]
- Richerson GB, Wu Y, 2003. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol 90, 1363–1374. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Slater NT, 1993. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology 32, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Rossi D, Oshima T, Attwell D, 2000. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D, 2003. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol 548, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EM, Kimelberg HK, 1996. Release of [3H]-d-aspartate from primary astrocyte cultures in response to raised external potassium. J. Neurosci 16, 7803–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA, 1989. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science 246, 815–818. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S, 1999. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem 274, 11455–11458. [DOI] [PubMed] [Google Scholar]
- Saxena NC, MacDonald RL, 1996. Properties of putative cerebellar GABAA receptor isoforms. Mol. Pharmacol 49, 567–579. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, 2003. GABA uptake regulates cortical excitability via cell type–specific tonic inhibition. Nat. Neurosci 6, 484–490. [DOI] [PubMed] [Google Scholar]
- Singer W, 1993. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol 55, 349–374. [DOI] [PubMed] [Google Scholar]
- Smith TC, Howe JR, 2000. Concentration-dependent substate behavior of native AMPA receptors. Nat. Neurosci 3, 992–997. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Kofalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES, 2002. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem 81, 1196–1211. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I, 2002. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J. Neurosci 22, RC223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I, 2003. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 100, 14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supplisson S, Roux MJ, 2002. Why glycine transporters have different stoichiometries. FEBS Lett. 529, 93–101. [DOI] [PubMed] [Google Scholar]
- Takagi K, Ginsberg MD, Globus MYT, Dietrich D, Martinez E, Kraydieh S, Busto R, 1993. Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J. Cerebral Blood Flow Metab 13, 575–585. [DOI] [PubMed] [Google Scholar]
- Taylor J, Gordon-Weeks PR, 1991. Calcium-independent gamma-aminobutyric acid release from growth cones: role of gamma-aminobutyric acid transport. J. Neurochem 56, 273–280. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S, 1996. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J. Neurosci 16, 3630–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell T, Willshaw D, 1992. Cerebellar cortex: its simulation and the relevance of Marr’s theory. Philos. Trans. Roy. Soc. London B 336, 239–257. [DOI] [PubMed] [Google Scholar]
- Wahl F, Obrenovitch TP, Hardy AM, Plotkine M, Boulu R, Symon L, 1994. Extracellular glutamate during focal cerebral ischaemia in rats: time course and calcium-dependency. J. Neurochem 63, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Wall MJ, 2002. Furosemide reveals heterogeneous GABAA receptor expression at adult rat Golgi cell to granule cell synapses. Neuropharmacology 43, 737–749. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM, 1997. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur. J. Neurosci 9, 533–548. [DOI] [PubMed] [Google Scholar]
- Wang CM, Chang YY, Kuo JS, Sun SH, 2002. Activation of P2X7 receptors induced [3H]GABA release from the RBA-2 type-2 astrocyte cell line through a -dependent mechanism. Glia 37, 8–18. [DOI] [PubMed] [Google Scholar]
- Warr O, Takahashi M, Attwell D, 1999. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. J. Physiol 514, 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren L, Nystrom B, Hamberger A, Johansson BB, 1995. Intracerebral dialysis and the blood brain barrier. J. Neurochem 64, 229–234. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG, 1995. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE, 1997. Activation and desensitization of hippocampal kainate receptors. J. Neurosci 17, 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt I, Gyte A, Simpson MG, Widdowson PS, Lock EA, 1996. The role of glutathione in L-2-chloropropionic acid induced cerebellar granule cell necrosis in the rat. Arch. Toxicol 70, 724–735. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR, 2003. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci 23, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP, 1996. Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Que J, 1996. Modulation of excitatory synaptic transmission by low concentrations of glutamate in cultured rat hippocampal neurons. J. Physiol 494, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]


