Abstract
Background
A high incidence of functional decline (deterioration in physical or cognitive function) during hospitalisation of older adults is reported. The role of exercise in preventing these deconditioning effects is unclear.
Objectives
To determine the effect of exercise interventions for acutely hospitalised older medical patients on functional status, adverse events and hospital outcomes.
Search methods
We searched MEDLINE (1966‐Feb 2006), CINAHL (1982‐Feb 2006), EMBASE (1988 to Feb 2006), Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2006), PEDro (1929‐ Feb 2006), Current Contents (1993‐ Feb 2006) and Sports Discus (1830‐Feb 2006). The Journal of the American Geriatrics Society was hand searched. Additional studies were identified through reference and citation tracking, personal communications with a content expert and contacting authors of eligible trials. There was no language restriction.
Selection criteria
Eligible studies were prospective randomised controlled trials (RCT) or prospective controlled clinical trials (CCT) comparing exercise for acutely hospitalised older medical patients to usual care or no treatment controls.
Data collection and analysis
Two independent reviewers extracted data relating to patient and hospital outcomes and assessed the method quality of included studies. Data were pooled in meta‐analysis using the relative risk (RR) and absolute risk reduction (ARR) for dichotomous outcomes and the standardised mean difference (SMD) or the weighted mean difference (WMD) for continuous outcomes.
Main results
Of 3138 potentially relevant articles screened, 7 randomised controlled trials and 2 controlled clinical trials were included. The effect of exercise on functional outcome measures is unclear. No intervention effect was found on adverse events. Pooled analysis of multidisciplinary interventions that included exercise indicated a small significant increase in the proportion of patients discharged to home at hospital discharge (Relative Risk 1.08, 95% CI 1.03 to 1.14 and Numbers Needed to Treat 16, 95% CI 11 to 43) and a small but important reduction in acute hospital length of stay (weighted mean difference, ‐1.08 days, 95% CI ‐1.93 to ‐0.22) and total hospital costs (weighted mean difference, ‐US$278.65, 95% CI ‐491.85 to ‐65.44) compared to usual care. Pooled analysis of exercise intervention trials found no effect on the proportion of patients discharged to home or acute hospital length of stay.
Authors' conclusions
There is 'silver' level evidence (www.cochranemsk.org) that multidisciplinary intervention that includes exercise may increase the proportion of patients discharged to home and reduce length and cost of hospital stay for acutely hospitalised older medical patients.
Plain language summary
Exercise for older patients in hospital
This summary of a Cochrane review presents what we know from research about the effects of exercise for older patients who are admitted to hospital. The review shows that:
For older patients who are admitted to hospital, exercise sessions
‐ may not lead to any difference in function, harms, length of stay in hospital or whether they go home or to a nursing home or other care facility.
For older patients who are admitted to hospital, a special care programme that includes exercise
‐ may not lead to any difference in function or harms. ‐ may slightly reduce the length of stay in hospital, may slightly increase the number of patients who go home instead of to a nursing home or another hospital. ‐ may slightly reduce the cost of care to the health system.
There is not enough evidence to be certain of these results. Why exercise for older patients when they are in hospital? It has been argued that older people often leave hospital less able to function or move than before they were admitted. For example, one study shows that many older patients, who were able to walk on their own two weeks before going into hospital, needed help to walk when they left hospital. This may be because they are resting in bed during their hospital stay. Usual care in hospitals does not always include exercise. It is thought that if older patients exercise more during their hospital stay they may not lose as much function. Usual care in hospitals does not always include exercise.
What are the effects of exercise? The studies included patients who were 65 years or older and were admitted to hospital with a medical illness. While in hospital they received either usual hospital care, usual care plus exercise sessions or a special overall care programme that included exercise. The exercise sessions and special programmes started within a few days of patients being admitted to hospital. Many of the programmes included walking.
Overall, there is not enough evidence to be certain of the benefits and harms of exercise sessions or programmes for older patients in hospital.
Function and harms (falls, move to an intensive care unit (ICU), or death): There may be little or no difference with exercise sessions or with an overall programme of care that includes exercise.
Going home and length of time in hospital: There may be little difference with exercise sessions. With a special care programme that includes exercise, patients may go home 1 day earlier and 6 more patients out of 100 may go home instead of to a nursing home or other care facility ‐ 81 patients out of 100 may go home after receiving an overall programme of care that includes exercise ‐ 75 patients out of 100 may go home after receiving usual care
Health care costs: Costs were not reported for studies of exercise sessions. A special care programme that includes exercise may reduce health care costs by approximately $300 per patient hospital stay.
Background
People aged 65 and older comprise 13% of the US population (Feachem 2002). Eighty‐five percent of this subgroup have at least one chronic health condition and this proportion increases with age (Hoffman 1996). In 2002, people aged 65 years and older accounted for 45% of US hospital bed days (deFrances 2004). The proportion of hospital bed days used by older people is likely to increase as the average population age continues to rise (Scott 1999). Consequently, healthcare costs and pressure on the health care system are also expected to escalate in the coming years. Interventions that may reduce dependence on healthcare services and improve patient outcomes warrant research attention.
Detrimental effects of hospitalisation on older adults have been reported (Covinsky 2003). Older patients have decreased physiological and functional reserve that renders them particularly vulnerable to the effects of bed rest during hospitalisation. Loss of muscle strength during bed rest has been estimated at 5% per day and may affect lower limbs more than upper limbs (Harper 1988). Cardiovascular response to exercise is also altered. After prolonged bed rest, heart rate is higher during exercise, the normal increased stroke volume response to exercise is reduced, as is cardiac output at maximal exercise (Harper 1988). Despite this, bed based hospital care is common, particularly in the acute setting.
Functional decline of hospitalised older patients has been consistently reported (Gillick 1982; Hirsch 1990; Inouye 1993; McVey 1989; Palmer 1995; Sager 1996) and this is argued to be more a consequence of hospitalisation than of the presenting medical illness (Creditor 1993; Gillick 1982; Inouye 1993). Mahoney et al (Mahoney 1998), for example, reported 17% of older medical inpatients, who were independently ambulant two weeks prior to hospital admission, required assistance to walk at hospital discharge. They identified six patient risk factors for functional decline during hospitalisation and five of these factors were independent of presenting medical illness (age > 85 years, functional impairment prior to hospitalisation, Caucasian race, use of a walker or wheelchair before admission, more than four comorbid conditions, a cancer diagnosis). Despite different methods of determining functional decline and varying trial designs across studies, the reported prevalence of functional decline is consistently high. However, it remains unclear how much functional decline is attributable to hospitalisation and is able to be prevented. Despite evidence that healthy older adults respond positively to strength training (Latham 2004; Morris 2004) little appears known about the types of exercise that might limit functional decline in acutely hospitalised older patients or the magnitude of effects associated with these programs.
Objectives
Primary objective: to determine the effects of exercise interventions for acutely hospitalised older medical inpatients on functional status, adverse events and hospital outcome measures. Secondary objective: to describe the exercise programs that have been provided to older medical inpatients and to summarise the outcomes used to measure intervention effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
Prospective randomised controlled trials (RCT) or prospective controlled clinical trials (CCT) (e.g. alternate allocation, date of birth, medical record number) comparing exercise for medical inpatients to usual care or no treatment controls were eligible for inclusion.
Types of participants
Participants included were patients aged 65 years or older admitted to a hospital medical ward or unit with an acute exacerbation of a medical condition. Therefore, this review did not include patients who were admitted to inpatient rehabilitation hospitals. We included trials if 95% of the study participants were aged at least 65 years and were randomised within three days of hospital admission. Animal studies and studies of subjects suffering exclusively from cerebrovascular accidents or a non‐general medical condition (e.g. orthopaedic condition) were excluded.
Types of interventions
Any trial that investigated the effects of exercise or exercise prescribed as a component of a multidisciplinary intervention was considered for inclusion. Exercise was defined as any physical intervention program designed to maintain or improve patient strength or function. We excluded trials where a multidisciplinary intervention was tested but it was not clear that all patients in the intervention group were prescribed exercise.
Types of outcome measures
To be eligible for inclusion, at least one measure of functional (that included activities of daily living, mobility or cognition) or hospital outcome must have been reported.
Primary outcome measure Measures of patient function and adverse events were the primary outcome measures. Examples of functional outcome measures include the timed up and go test, functional reach test, 10 metre walk test, elderly mobility scale, functional ambulation classification, cognitive outcome measures and activity of daily living scales such as the Barthel Index and Functional Independence Measure. Adverse events included complications, patient mortality, falls, medical deterioration requiring admission to the intensive care unit or musculoskeletal injury.
Secondary outcome measures Secondary outcome measures were hospital outcomes. Acceptable hospital outcome measures were length of stay (LOS), discharge destination after hospitalisation, readmission rates, patient satisfaction or costs associated with patient care.
Search methods for identification of studies
A sensitive search was conducted to identify any study reporting a RCT or CCT that investigated the effect of exercise for general medical patients in the acute care setting (see below). MEDLINE (1966‐Feb 2006), CINAHL (1982‐Feb 2006), EMBASE (1988 to Feb 2006), Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2006), PEDro (1929‐ Feb 2006), Current Contents (1993‐ Feb 2006) and Sports Discus (1830‐Feb 2006) were searched. The Journal of the American Geriatrics Society was hand searched. Additional studies were identified through reference and citation tracking, personal communications with a content expert (Morris 2004) and contacting authors of eligible trials. There was no language restriction. Search Strategy for MEDLINE 1 exp Inpatients/ 2 (Medical adj2 Inpatient$).mp. 3 Hospitalized.mp. or exp AGED, HOSPITALIZED/ 4 Immobili#ed.mp. 5 1 or 2 or 3 or 4 6 exp "AGED, 80 AND OVER"/ or exp AGED/ 7 Old$.mp. 8 Elder$.mp. 9 exp FRAIL ELDERLY/ 10 exp Aging/ or exp GERIATRICS/ 11 6 or 7 or 8 or 9 or 10 12 exp EXERCISE/ or exp EXERCISE THERAPY/ or exp EXERCISE MOVEMENT TECHNIQUES/ 13 strength$.mp. 14 train$.mp. 15 (exercise$ adj10 train$).mp. 16 (strength$ adj10 train$).mp. 17 exp Physical Therapy/ or Physiother$.mp. 18 exp REHABILITATION/ 19 rehabilitate.mp. 20 exp Walking/ 21 Ambulat$.mp. 22 12 or 13 or 15 or 16 or 17 or 18 or 19 or 20 or 21 23 exp Treatment Outcome/ or Function$ Outcome$.mp. 24 Mobility.mp. 25 exp GAIT ANALYSIS/ or GAIT/ 26 stride length.mp. 27 step length.mp. 28 Barthel Index.mp. 29 (Timed Up and Go).mp. 30 TUG.mp. 31 Functional Ambulation Classification.mp. 32 FAC.mp. 33 Functional Independence Measure.mp. 34 exp Clinical Assessment Tools/ or FIM.mp. or exp "Activities of Daily Living"/ 35 pedometer.mp. 36 (Six Metre Walk Test or 6MWT).mp. 37 (Ten Metre Walk Test or 10MWT).mp. 38 exp Cognition Disorders/ or Cognitive outcome$.mp. 39 exp "ACUTE CONFUSION (NANDA)"/ or exp CONFUSION/ 40 exp Delirium/ 41 Independence.mp. 42 exp "Outcome and Process Assessment (Health Care)"/ or exp "Outcome Assessment (Health Care)"/ 43 (Length of Stay or LOS).mp. 44 exp PATIENT READMISSION/ 45 exp Patient Discharge/ or Discharge Destination.mp. 46 exp Health Care Costs/ 47 exp Health Services for the Aged/ 48 exp "Utilization Review"/ 49 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 50 5 and 11 and 22 and 49 51 (Child$ or paediatric$).mp. 52 50 not 51 53 (Cerebrovascular accident or CVA or stroke).mp. 54 52 not 53 55 limit 54 to human
Data collection and analysis
Selection of Studies Title and/or abstract were examined by two independent reviewers (NdeM and KJ). If a reason for exclusion was not evident, the full paper was obtained. Two independent reviewers examined hard copies of all remaining papers (NdeM and JK). Disagreement was resolved with discussion. Quality Assessment Included studies were independently rated for quality by two reviewers (NdeM and KJ) using the PEDro scale (PEDro 1999). This scale contains ten items that are scored to provide an estimate of methodological rigour of randomised controlled trials (Maher 2003). These items are random allocation, concealed allocation, similarity at baseline, subject blinding, therapist blinding, assessor blinding, greater than 85% follow up for at least one key outcome, intention to treat analysis, between group statistical analysis for at least one key outcome and point estimates of variability for at least one key outcome. Items are marked as either present (yes/1) or absent (no/0) and a score out of ten is obtained.
The PEDro scale has been reported to be adequately reliable (Maher 2003). Differing opinions between independent reviewers for item scores were resolved with discussion including a third independent reviewer (JK) where necessary. Authors were contacted for additional information as required (NdeM).
Data Extraction Relevant data was extracted by three independent reviewers (NdeM, JK and KJ) from included trials. Extracted data included the study location, population description, intervention description and dosage (frequency, intensity, repetition, duration), hospital environment modifications, outcome measures used and patient and hospital outcome data. Disagreement was resolved with discussion. Authors were contacted for additional information as required (NdeM).
Data Analysis For dichotomised data, the relative risk (RR) and absolute risk reduction (ARR) and associated 95% confidence intervals were calculated.
For continuous data, the standardised mean difference (SMD) or the weighted mean difference (WMD) and associated 95% confidence intervals were calculated. The WMD was employed when outcome measures in pooled trials were measured using the same scale. The SMD was employed when different instruments were used to assess comparable factors. For SMD calculations, pooled post intervention standard deviations were employed. However, when significant differences between post intervention standard deviations were identified using a 2‐tailed F test, the control group post intervention standard deviation was employed (Hedges 1985).
Where only medians were reported, these were used as direct best estimates of the group mean. Associated interquartile ranges (25%‐75%) were converted to best estimates of the standard deviation by using half the 25th to 75th percentile score range. Standard deviations were derived from related statistics when necessary. For example, if the standard error in the estimate of the mean was reported, the standard deviation was calculated by multiplying the standard error by the square root of the number of patients in the group.
For hospital cost (Asplund 2000; Covinsky 1997) and LOS (Asplund 2000; Slaets 1997) data, standard deviations, where reported, were observed to be similar in magnitude to mean scores, indicating skewed data. Where the standard deviation for cost and LOS data were not reported, effect size calculations were performed using estimates of standard deviations that were equal to reported mean or median scores. Intention to treat data were preferentially used for analysis when per protocol data were also provided (Counsell 2000).
One trial, conducted at two hospitals, provided results for each hospital and these were treated as two independent trials (Collard (C) 1985; Collard (S) 1985). For the same trial, the reported standard error associated with mean LOS and costs appeared to be a typographical error, as it contradicted reported t test results. Hence the group mean was used as the best estimate of the standard deviation for effect size calculations. For this study, sample sizes reported in figures were used in calculations.
Another trial (Asplund 2000) reported mean LOS and cost data and an associated 95% confidence interval. It appeared that the confidence interval provided was the 95% CI for the error in the estimate of the mean, and the raw score standard deviation was estimated based on this assumption.
Clinical relevance tables were compiled. For statistically significant outcomes, the numbers needed to treat (NNT) were also reported. For dichotomous outcomes (e.g. complications), the number needed to treat were calculated from the control group event rate and the relative risk using the Visual Rx NNT calculator (Cates 2003). Continuous outcome tables were also presented. Absolute benefit was calculated as the improvement in the intervention group minus the improvement in the control group, in the original units. Relative difference in the change from baseline was calculated as the absolute benefit divided by the baseline mean of the control group. Data for comparable trials were pooled in meta‐analysis using a fixed effect model (FE) and associated 95% confidence interval. However, if significant between trial variation was detected, data were pooled in meta‐analysis using a random effects (RE) model and potential sources of heterogeneity investigated. Statistical heterogeneity was assessed and considered likely if p<0.1 for chi‐squared testing of the Q statistic. Heterogeneity was quantified using the I2 statistic (Higgins 2003) and was considered substantial if found to be greater than 50%.
A priori, subgrouping by intervention type was planned. Trials were separated into two groups for pooling, multidisciplinary interventions including exercise and exercise only interventions. To assess the possibility of publication bias, funnel plots were planned (Egger 1997). Meta‐analysis was performed using the Cochrane Collaboration Review Manager 4.3.8 software.
Grading of evidence We used the grading system recommended by the Cochrane Musculoskeletal Group (Tugwell 2004): Platinum: A published systematic review that has at least two individual controlled trials each satisfying the following : ·Sample sizes of at least 50 per group ‐ if these do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. ·Blinding of patients and assessors for outcomes. ·Handling of withdrawals >80% follow up (imputations based on methods such as Last Observation Carried Forward (LOCF) are acceptable). ·Concealment of treatment allocation.
Gold: At least one randomised clinical trial meeting all of the following criteria for the major outcome(s) as reported: ·Sample sizes of at least 50 per group ‐ if these do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. ·Blinding of patients and assessors for outcomes. ·Handling of withdrawals > 80% follow up (imputations based on methods such as LOCF are acceptable). ·Concealment of treatment allocation.
Silver: A randomised trial that does not meet the above criteria. Silver ranking would also include evidence from at least one study of non‐randomised cohorts that did and did not receive the therapy, or evidence from at least one high quality case‐control study. A randomised trial with a 'head‐to‐head' comparison of agents would be considered silver level ranking unless a reference was provided to a comparison of one of the agents to placebo showing at least a 20% relative difference. Bronze: The bronze ranking is given to evidence if at least one high quality case series without controls (including simple before/after studies in which patients act as their own control) or if the conclusion is derived from expert opinion based on clinical experience without reference to any of the foregoing (for example, argument from physiology, bench research or first principles).
Results
Description of studies
The search strategy retrieved 3138 potentially relevant papers. After screening of title and/or abstract, 143 papers remained and were obtained in full text. Predetermined inclusion and exclusion criteria were applied. Nine trials were included in this review (Asplund 2000; Collard (C) 1985; Collard (S) 1985; Counsell 2000; de Morton 2006; Jones 2006; Landefeld 1995; Siebens 2000; Slaets 1997). One included paper provided additional data (Covinsky 1997) for a trial already included in the review (Landefeld 1995). Another included paper reported two trials that were each suitable for inclusion (Collard (C) 1985; Collard (S) 1985). All included trials were published in English.
See the Characteristics of Included Studies Table for full details.
Participants This review includes data from seven published and two unpublished trials (one submitted for publication (de Morton 2006) and one now published (Jones 2006)). Trials were conducted at hospitals in the USA (5 trials), Australia (2 trials), Netherlands (1 trial) and Sweden (1 trial). All trials were similar with regard to patient characteristics including age, gender and population description. In total, the review considers trials of 4223 participants. Most studies had large samples with all but one (Jones 2006) recruiting more than 200 participants. Participant inclusion and exclusion criteria were similar across trials. Only one trial excluded all patients that lived in residential care prior to hospital admission (Counsell 2000). Three trials excluded patients who lived in a nursing home prior to hospital admission (de Morton 2006; Jones 2006; Siebens 2000).
Exercise Intervention All trials were compared to a "usual hospital care" control group. Most trials did not describe or described with minimal detail, usual hospital physiotherapy or exercise/mobility care procedures. One trial reported that usual hospital care included physiotherapy by referral from other hospital staff (de Morton 2006). Another trial reported usual care to consist of "medical, nursing and allied health intervention and discharge planning consistent with the patient's diagnosis and resources available on the acute general medical wards" (Jones 2006). For one trial, usual care included an occasional early start to rehabilitation and occasional physiotherapy and occupational therapy assessment (Asplund 2000).
Exercise intervention varied considerably across trials. Six trials were multidisciplinary interventions that included exercise and three trials were exercise only interventions. For the three exercise intervention trials, each provided a walking program and exercises that were individually tailored by a physiotherapist and then administered by a physiotherapy assistant. These programs were commenced within 2 to 3 days of hospital admission and encouraged strengthening and mobility. Each of the exercise only trials provided some information regarding exercise frequency and duration. Frequency of the exercise intervention was reported to be twice per day during hospitalisation and for a duration of up to 30 minutes across trials. Two trials reported exercise repetition. One trial reported a maximum of 10 repetitions (de Morton 2006) and the other reported 5 to 10 repetitions (Siebens 2000). Only one trial reported exercise intensity (Siebens 2000) and this was reported to be 60 to 80% of the patients age adjusted maximum heart rate and the ability of the patient to talk while walking. Two of the exercise intervention trials reported adherence to the exercise program (Jones 2006; Siebens 2000). Siebens et al. reported 82% of patients to receive a proportion of the in hospital exercise program and Jones et al. reported 6.3% of intervention group patients to be "non compliant, that is, they participated in the intervention less than half of the time (that was planned)".
The six multidisciplinary intervention trials all provided increased medical and/or nursing care and the staff to administer or supervise the additional exercise were reported in each trial. Exercise was reported to be prescribed and/or supervised by nursing staff (Collard (C) 1985; Collard (S) 1985), a physiotherapist (Slaets 1997), physiotherapist and occupational therapist (Asplund 2000) or families and/or ward staff (Collard (C) 1985; Collard (S) 1985; Counsell 2000; Landefeld 1995). All trials included in this review reported exercise intervention to either commence "early" (Asplund 2000), at hospital admission or within three days of admission. However, the content and dosage (frequency, repetition, duration or intensity) of these exercise programs were generally not well described. None of the trials described the exercise program repetition, duration, intensity or adherence to the exercise program. Two reports provided details of exercise type and frequency (Counsell 2000; Landefeld 1995). The exercise program in these trials consisted of a walk or stand 3 times per day and a daily walk to the activity room for exercises.
Outcomes Functional, hospital or mortality outcomes were the primary or secondary outcome measures for the included trials. Some trials reported multiple functional outcome measures (Counsell 2000; de Morton 2006; Jones 2006; Landefeld 1995; Siebens 2000). All authors reported functional status at hospital admission. In three trials, patient self reported functional status 2 weeks prior to hospital admission was measured (Counsell 2000; Landefeld 1995; Siebens 2000). All trials reported functional or hospital outcomes at hospital discharge. Three trials did not report any measures of patient functional status at hospital discharge (Asplund 2000; Collard (C) 1985; Collard (S) 1985). Two papers reported cognitive outcomes after the intervention (Asplund 2000; Landefeld 1995). Two trials reported readmission rates within 28 days of hospital discharge (Counsell 2000; de Morton 2006) and one trial reported readmission rate within 3 months of hospital discharge (Asplund 2000). Mortality was measured at varying time points across trials, from hospital discharge up to 12 months after hospital discharge.
Excluded studies Papers that required detailed reading to be excluded are listed in the Characteristics of Excluded Studies Table and their reason for exclusion provided.
Risk of bias in included studies
Study quality ranged from 4 to 8 with a mean score of 6/10. Seven of the included studies were considered to be adequately randomised. One trial reported using "an alternating random procedure" (Slaets 1997). Another trial reported consecutive eligible patients to be allocated to one of two similar wards based on bed availability and the intervention ward was determined by a coin toss prior to commencement of the trial (de Morton 2006). These studies were both therefore considered to be controlled clinical trials. Four trials provided adequate detail to consider that concealed allocation occurred (Asplund 2000; Counsell 2000; Jones 2006; Siebens 2000). All except for one trial (Jones 2006) were similar at baseline regarding important outcomes. A median 10 point difference in admission Barthel Index scores were reported between groups at hospital admission for this trial. Blinding of subjects is difficult to implement in exercise intervention trials. Reviewers agreed that one of the included trials reported patient blinding because patients were considered unable to identify if they were receiving the intervention of interest or usual care (de Morton 2006). It is neither possible, nor appropriate, to blind the therapist providing exercise. Therefore, none of the trials reported therapist blinding to occur. Assessor blinding was reported to have occurred for at least one key outcome for the three exercise only intervention trials (de Morton 2006; Jones 2006; Siebens 2000). All trials reported at least 85% of hospital discharge data for one key outcome and four studies reported or provided evidence that intention to treat analysis occurred (Counsell 2000; de Morton 2006; Jones 2006; Siebens 2000). For each trial, between group statistical comparison data, point estimates and measures of variability for outcomes were provided.
Effects of interventions
See Forest plots for results of meta‐analysis and Additional Table 1, Table 2 and Table 3 for clinical relevance tables.
1. Clinical relevance table for dichtomous outcomes.
| Outcome | Comparison | #patients (#trials) | Control event rate | ARD (95%CI), % | Wt Rel % change | NNT (95% CI) | Statistically sig | Quality of evidence |
| Maintain or improve ADL scores between admission and discharge | MDI v UC ‐ function | 2271 (3) | 901/1111 (82.0%) | 0.05 (‐0.03, 0.12) , 5% | 5% (I) | NA | Not statistically significant | Silver |
| Maintain or improve mobility scores between admission and discharge | MDI v UC ‐ function | 2119 (3) | 894/1032 (86.6%) | 0.03 (0.00, 0.06), 3% | 3% (I) | NA | Not statistically significant | Silver |
| Maintain or improve ADL scores between 2 weeks prior to hospital admission and discharge | MDI v UC ‐ function | 2001 (2) | 648/990 (65.5%) | 0.04 (0.00, 0.04), 4% | 7% (I) | NA | Not statistically significant | Silver |
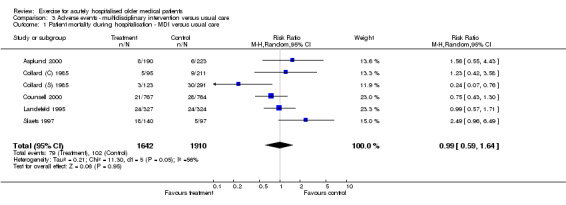
| Mortality during hospitalisation | MDI v UC ‐ adverse events | 3552 (6) | 102/1910 (5.3%) | 0.00 (‐0.03, 0.03), 0% | 1% (I) | NA | Not statistically significant | Silver |
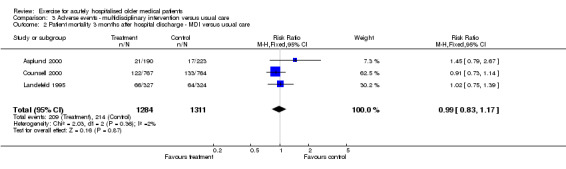
| Mortality 3 months after hospital discharge | MDI v UC ‐ adverse events | 2595 (3) | 214/1311 (16.3%) | 0.00 (‐0.03, 0.03), 0% | 1% (I) | NA | Not statistically significant | Silver |
| Complications during hospitalisation | MDI v UC ‐ adverse events | 550 (2) | 88/372 (23.7%) | ‐0.01 (‐0.09, 0.06), ‐1% | 6% (I) | NA | Not statistically significant | Silver |
| Mortality during hospitalisation | Exercise only versus UC ‐ adverse events | 696 (3) | 4/357 (1.1%) | 0.01 (‐0.01, 0.03), 1% | 98% (W) | NA | Not statistically significant | Silver |
| Admission to ICU during hospitalisation | Exercise only versus UC ‐ adverse events | 396 (2) | 4/206 (1.9%) | 0.01 (‐0.07, 0.09), 1% | 6% (W) | NA | Not statistically significant | Silver |
| Falls during hospitalisation | Exercise only versus UC ‐ adverse events | 384 (2) | 7/203 (3.5%) | 0.00 (‐0.04, 0.04), 0% | 12% (W) | NA | Not statistically significant | Silver |
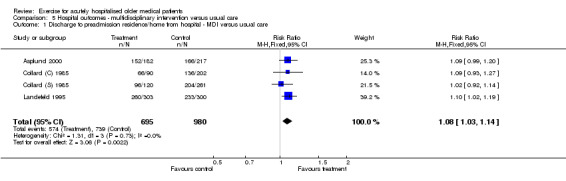
| Discharge to preadmission residence/home | MDI v UC ‐ hospital outcomes | 1675 (4) | 739/980 (75.4%) | 0.06 (0.02, 0.10), 6%, 6 more patients out of 100 | 8% (I) | 16 (11, 43) | Statistically significant | Silver |
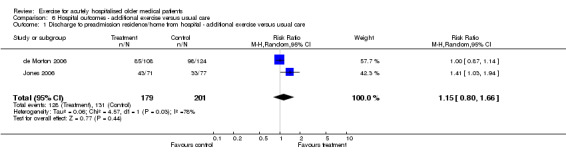
| Discharge to preadmisison residence/home | Exercise only versus UC ‐ hospital outcomes | 380 (2) | 131/201 (65.2%) | 0.08 (‐0.10, 0.26), 8% | 15% (I) | NA | Not statistically significant | Silver |
| Legend: ADL=activities of daily living; ICU=intensive care unit | MDI=multidisciplinary intervention; UC=usual care | ARD=absolute risk difference | Wt Rel=weighted relative change | NNT=number needed to treat | sig=significant |
2. Clinical Relevance Table for continuous outcomes (using the same scale).
| Outcome (scale) | #patients (#trials) | Control baseline m | Wt absolute change | Relative % change | NNT | Statistical sig | Quality of evidence |
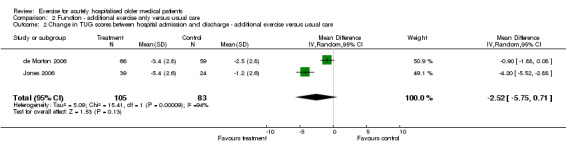
| Change in TUG score (seconds)‐ additional exercise versus usual care | 188 (2) | 20.59 (de Morton et al.) | ‐2.52 seconds | 2.52/20.59 = 0.12 = 12% | NA | Not statistically significant | Silver |
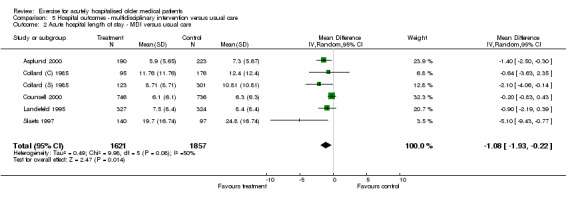
| Acute hospital LOS ‐ MDI versus usual care | 3478 (6) | 6.3 days (Counsell et al.) | ‐1.08 days | 1.08/6.3 = 0.17 = 17% | 16 | Significant | Silver |
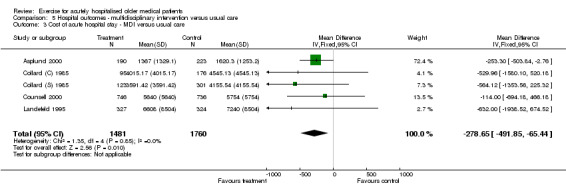
| Cost of acute hospital stay ‐ MDI versus usual care | 3241 (5) | 0 | ‐$278.65 | NA | 26 | Significant | Silver |
| Acute hospital LOS ‐ additional exercise versus usual care | 680 (3) | 6.0 (de Morton et al.) | 0.01 | 0.01/6.0 = 0.002 =0.2% | NA | Not statistically significant | Silver |
| Total LOS (acute plus subacute)‐ additional exercise versus usual care | 380 (2) | 6.0 (de Morton et al.) | ‐0.70 days | 0.70/6.0 = 0.12 = 12% | NA | Not statistically significant | Silver |
| Legend: TUG=timed up and go test; LOS=length of stay; MDI=multidisciplinary intervention | m=mean | Wt=weighted | NNT=number needed to treat | sig=signficant |
3. Clinical Relevance Table for continuous outcomes (using different scale).
| Outcome (scale) | # patients (#trials) | Control baseline m | Wt absolute change | Relative % change | NNT | Statistical sig | Quality of evidence |
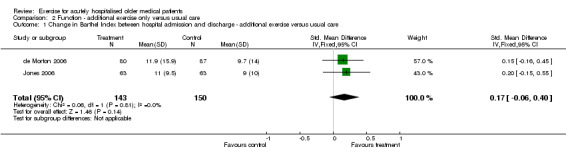
| Barthel Index (original and modified versions, scale range 0‐100) ‐ additional exercise versus usual care | 293 (2) | 68.09 (de Morton et al.) | 0.17 x 26.08 = 4.43. This represents 4.45 more points on a 100 point Barthel Index scale. | 4.43/68.09 = 0.06 = 6% | NA | Not statistically significant | Silver |
| Legend: | m=mean | wt=weighted | NNT=number needed to treat | sig=significant |
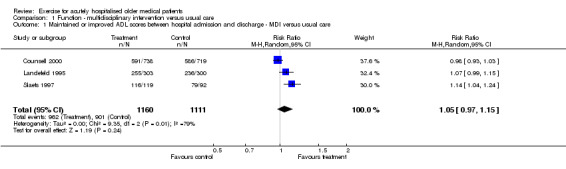
01. Functional status‐ Multidisciplinary intervention compared to usual care Three multidisciplinary intervention trials assessed changes in independence with Activities of Daily Living (ADL) between hospital admission and discharge compared to usual hospital care (n = 2271) (Counsell 2000; Landefeld 1995; Slaets 1997)(Comparison 01‐01). One of the trials reported a significant effect on ADL score (Slaets 1997) (RR 1.14, 95%CI 1.04 to 1.24) compared to evidence of no significant effect from the other two trials (Counsell 2000; Landefeld 1995). Slaets et al. examined the effect of a multidisciplinary team that included a geriatrician, also trained in geriatric psychiatry, a specialised liaison nurse and a physiotherapist, in addition to usual care. The other two trials investigated the effect of a nursing led Acute Care Elders (ACE) unit compared to usual care. Pooled analysis indicated an inconclusive treatment effect compared to usual care on change in ADL between hospital admission and discharge (RE model (RR) 1.05, 95% CI 0.97 to 1.15). Significant statistical heterogeneity was apparent in pooling these trial data sets (I2 = 78.6%) and warrants further enquiry regarding possible sources of observed differences.
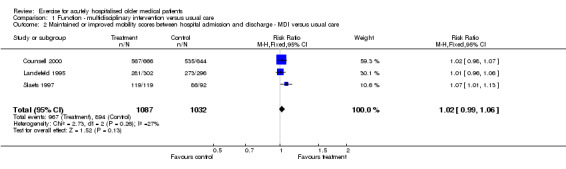
The same three trials were pooled for change in mobility scores between hospital admission and discharge for multidisciplinary intervention including exercise compared with usual care (n = 2119)(Counsell 2000; Landefeld 1995; Slaets 1997)(Comparison 01‐02). Only the trial reported by Slaets et al. showed a significant treatment effect. Low to moderate inconsistency across trials was detected for this comparison (I2 = 30.9%). Under fixed effects pooling, the effect was inconclusive (FE model (RR) 1.02, 95% CI 0.99 to 1.06).
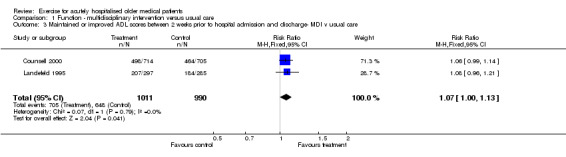
Two multidisciplinary trials reported change in ADL scores between two weeks prior to hospital admission (patient self report at hospital admission) and discharge (Counsell 2000; Landefeld 1995)(Comparison 01‐03). Both trials reported similar relative risks, described the same acute care unit interventions and had large samples (n = 2001). Pooled analysis showed favourable outcomes for the intervention group but this did not reach statistical significance (FE model (RR) 1.07, 95% CI 1.00 to 1.13).
There were two multidisciplinary intervention trials that reported cognitive outcomes. One of these trials reported mental status score at hospital discharge (Landefeld 1995) and the other reported Mini Mental State Examination score at three months after hospital discharge (Asplund 2000). Due to the differing assessment times, these trials were not pooled in meta‐analysis. However, individual trial effect sizes indicated a significant effect of the intervention on cognition at three months after hospital discharge in the trial reported by Asplund et al. (SMD 0.40, 95% CI 0.19 to 0.61) but not for cognition at hospital discharge in the trial reported by Landefeld et al. (SMD ‐0.10, 95% CI ‐0.32 to 0.12).
02 Functional status‐ Exercise intervention compared to usual care Functional outcomes at hospital discharge were reported in two of the exercise intervention trials (de Morton 2006; Jones 2006). Both trials used the Barthel Index to measure functional independence and the Timed Up and Go Test (TUG) to assess mobility (Comparison 02‐01 and 02‐02). The two pooled trials implemented similar exercise interventions and were well matched for patient characteristics. Both trials had greater than 15% loss to follow up for Barthel and TUG scores. For Barthel Index and TUG scores, individual trial effect sizes were non significant but favoured outcomes in the intervention group. Pooled analysis indicated an inconclusive intervention effect on change in Barthel Index (n = 293, FE model (SMD), 0.17, 95% CI ‐0.06 to 0.40) or change in TUG scores (n = 188, RE model (WMD), ‐2.52, 95%CI ‐5.75 to 0.71) between hospital admission and discharge. Significant statistical heterogeneity was observed between trials for comparing change in TUG scores (I2 = 93.5%).
03 Adverse events‐ Multidisciplinary intervention compared to usual care Each multidisciplinary intervention trial provided mortality data at hospital discharge. Pooling of six trials with a random effects model indicated no effect of the intervention on patient mortality at hospital discharge (n = 3552, RE model (RR) 0.99, 95% CI 0.59 to 1.64)(Comparison 03‐01). Large inconsistency across trials was identified for this comparison (I2 = 55.8%). One trial showed a significant effect of the intervention in reducing mortality at hospital discharge (Collard (S) 1985). However, data from another trial (Slaets 1997) indicated a lower incidence of mortality in the control group compared to the intervention group but was not statistically significant. Three multidisciplinary intervention trials provided mortality data for three months after discharge (Asplund 2000; Counsell 2000; Landefeld 1995)(Comparison 03‐02). Pooled analysis indicated no effect of the intervention on patient mortality three months after hospital discharge (n = 2595, FE model (RR) 0.99, 95% CI 0.83 to 1.17). Only one trial reported 6 and 12 month after hospital discharge mortality data and therefore could not be pooled in meta‐analysis. For the trial reported by Landefeld et al. and Counsell et al., patients lost to follow up between hospital discharge and three months after discharge were assumed to have survived for this comparison.
Only two multidisciplinary intervention trials provided complication during hospitalisation data (Collard (C) 1985; Collard (S) 1985)(Comparison 03‐03). Pooled analysis indicated no effect of multidisciplinary intervention on hospital complication rate (pneumonia, skin breakdown, confusion, falls, infection or other) under fixed effects modelling (n = 550, FE model (RR) 0.94, 95% CI 0.68 to 1.29).
04 Adverse events‐ Exercise intervention compared to usual care Each exercise intervention trial reported mortality data at hospital discharge (de Morton 2006; Jones 2006; Siebens 2000). Pooled analysis indicated no intervention effect on patient mortality at hospital discharge (3 trials, n = 696, FE model, RR 1.98, 95% CI 0.64 to 6.18)(Comparison 04‐01). Similarly, pooled analysis of two exercise intervention trials indicated no effect of the intervention on the incidence of admission to the intensive care unit (n = 396, RE model, 1.06 95% CI 0.04 to 30.44)(Comparison 04‐02) or falls (n = 384, FE model (RR) 1.12, 95% CI 0.40 to 3.15)(Comparison 04‐03) during hospitalisation. The same two trials reported no incidence of musculoskeletal injuries as a result of exercise intervention (2 trials, n = 396).
05 Hospital outcomes‐ Multidisciplinary intervention compared to usual care Four trials reported discharge destination data at hospital discharge (Asplund 2000; Collard (C) 1985; Collard (S) 1985; Landefeld 1995)(Comparison 05‐01). Asplund et al. reported patients who returned to their preadmission residence, Landefeld et al. reported patients discharged to a private home and Collard et al. reported routine discharge or home health care at hospital discharge. Pooled analysis indicated that multidisciplinary intervention significantly increased the proportion of patients discharged directly home compared to geriatric rehabilitation/transfer to another acute hospital/sheltered living/nursing home under FE modelling (n = 1675, FE model, 1.08 (RR), 95% CI 1.03 to 1.14). For this comparison, the NNT indicated that, on average, after treating 16 (95% CI 11 to 43) patients with multidisciplinary intervention that includes exercise, one more patient is discharged to home compared to the control group. Alternatively, the ARR indicates a significant 6% (95% CI 2% to 10%) increase in the number of patients discharged to home in the intervention group compared to the control group.
Each multidisciplinary intervention trial included in the review provided acute hospital LOS data (Asplund 2000; Collard (C) 1985; Collard (S) 1985; Counsell 2000; Landefeld 1995; Slaets 1997)(Comparison 05‐02). The RE model indicated a significant effect of multidisciplinary intervention on reducing acute hospital LOS (n = 3478, WMD ‐1.08, 95% CI ‐1.93 to ‐0.22). The observed effect is a reduction in LOS of approximately one day. Inconsistency across trials was moderately large (I2 =37.6%).
Pooled analysis of five trials (n = 3241) showed a significant effect of multidisciplinary intervention compared to usual care on reducing cost of hospital stay (Asplund 2000; Collard (C) 1985; Collard (S) 1985; Counsell 2000; Landefeld 1995)(Comparison 05‐03). An estimated mean saving of US$278 per patient per hospital stay was indicated using a fixed effects model (WMD,‐$278.65, 95% CI ‐491.85 to ‐65.44).
For acute hospital LOS and cost comparisons, multidisciplinary intervention trials reported data that included patients who had died during hospitalisation. Since mortality was not significantly influenced by the intervention, it is likely that the effect of mortality on LOS and cost data were similar across control and intervention groups. Thus, those who died were included in meta‐analysis.
Two multidisciplinary intervention trials provided hospital readmission data. One trial reported the incidence of readmission within one month of hospital discharge (Counsell 2000) and the other within three months of hospital discharge (Asplund 2000). Due to the differing follow up times, these trials were not pooled in meta‐analysis. No effect of the intervention was identified for either trial on the incidence of hospital readmission. 06 Hospital outcomes‐ Exercise intervention compared to usual care Pooled relative risks of discharge to home compared to hostel/nursing home/subacute care for two exercise trials (de Morton 2006; Jones 2006) indicated no significant exercise effect under fixed or random effects modelling (n = 380, RE model (RR) 1.15, 95% CI 0.80 to 1.66)(Comparison 06‐01). The trial by de Morton et al. reported the number of patients discharged to preadmission residence compared to Jones et al. who reported the number of patients discharged to home at hospital discharge. Large inconsistency was identified between trials (I2 = 78.1%).
Each exercise intervention trial provided acute LOS data (de Morton 2006; Jones 2006; Siebens 2000)(Comparison 06‐02). Pooled analysis with RE modelling indicated that exercise intervention alone does not influence acute hospital length of stay (n = 680, RE model (WMD) 0.01, 95%CI ‐1.23 to 1.26). Significant statistical heterogeneity was detected for this comparison (I2 = 74.4%). It was unclear in the trial reported by Siebens et al. whether the two patients who died in the intervention group were included in the data reported. The other two trials reported LOS data that did not include the patients who had died.
Two of the exercise intervention trials provided total LOS data (acute plus subacute LOS) (de Morton 2006; Jones 2006)(Comparison 06‐03). Random effects analysis indicated no effect of the intervention on total hospital LOS (n = 380, RE model (WMD) ‐0.70 days, 95% CI ‐2.59 to 1.17).
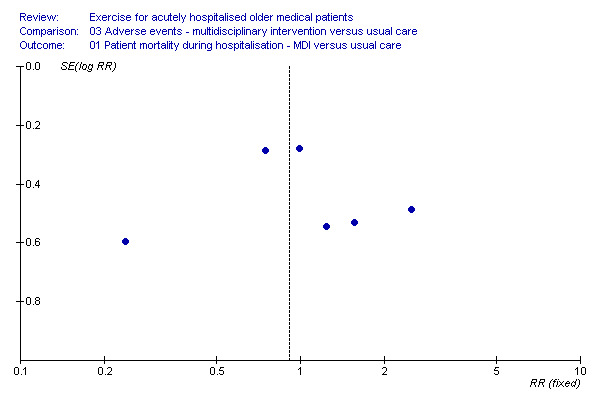
Publication Bias Publication bias was assessed where three or more outcomes from published trials could be pooled. There was no evidence to suggest that publication bias influenced the outcomes of this review. Representative data used to reach this conclusion is shown in Additional Figure 1.
1.
Discussion
Previous studies of exercise interventions in older adults have predominantly targeted participants in the community, institutions or those hospitalised for rehabilitation. Older patients with acute medical illness, who often have multiple co‐morbidities, are typically excluded from exercise intervention trials. Seven randomised controlled trials and two controlled clinical trials met inclusion for this review. Exercise programs intended to minimise functional decline during hospitalisation and the methods used to assess outcomes varied considerably.
Multidisciplinary intervention resulted in significant and important reductions in health care utilisation compared to usual care. However, the effects were small. Costs of hospitalisation were reduced by approximately US$278 per patient hospital stay. Since older people occupy approximately half of US hospital bed days (deFrances 2004), these figures potentially represent a large cost saving to the health care system. With random effects analysis, acute hospital LOS was reduced by approximately one day. This is a small but important reduction. Reduced LOS can increase hospital bed availability as well as reduce hospital costs (Feachem 2002). However, these results need to be interpreted with caution considering the skewed distribution of cost and LOS data. For outcomes that have a minimum possible value such as costs and LOS, the best method for pooling these skewed data in meta‐analysis is unclear. Parametric analysis, such as those applied in this meta‐analysis, may be robust to violations of the assumption of normality (Alderson 2002). Nevertheless, the validity of conclusions based on pooling skewed data are unclear.
Significant statistical heterogeneity was also found for LOS analysis. Differing discharge policies and procedures across hospitals and countries are likely to account for some of the observed heterogeneity. It is also possible that the decreased LOS results from better co‐ordination of care provision, rather than measurable improvements in patient health, as it is unclear whether patient function was influenced by the programs.
A significantly greater proportion of patients were discharged to home at hospital discharge following multidisciplinary intervention. The magnitude of the effect uniquely attributable to exercise cannot be partitioned. The weighted absolute risk difference indicates that 6 more patients out of 100 are discharged to home if they receive multidisciplinary intervention compared to usual care. Similarly, the weighted relative change indicates an 8% relative improvement in the proportion of patients discharged to home compared to the control group and the numbers needed to treat indicate that after treating 16 patients with multidisciplinary intervention compared to usual care, one more patient is discharged to home compared to the control group. It is possible that if patients most at risk of not being discharged back to their home could be identified at hospital admission, more cost effective targeting of the intervention could be employed. However, the magnitude of the effect uniquely attributable to exercise cannot be partitioned. Discharge to home data was only available for two exercise intervention trials and results differed. The two trials were of similar quality and had similar interventions. However, the proportion of patients discharged to home in the control group was 78% in the trial reporting a smaller effect size (de Morton 2006) and 43% in the trial reporting a larger effect size (Jones 2006). Population differences or differing hospital policies and procedures for discharge planning may explain some of the between study variation.
Despite these indicators of program benefit, the effect of the intervention on patient function was inconclusive. Methods used to assess function may be insensitive to change occurring in this population. The Barthel Index demonstrated a ceiling effect and the Timed Up and Go Test (TUG) showed a floor effect in two trials (de Morton 2006; Jones 2006). These trials reported 39.4% (Jones 2006) and 23.3% (de Morton 2006) of patients unable to complete a TUG at either hospital admission or discharge. A high loss to follow up of hospital discharge functional measures in trials was also reported (de Morton 2006; Jones 2006).
One multidisciplinary intervention trial (Slaets 1997) reported a significant beneficial effect on ADL and mobility scores between hospital admission and discharge. However, this trial involved a multidisciplinary team that was led by a geriatrician who was trained in geriatric psychiatry, and included a full time physiotherapist. The other trials pooled in meta‐analysis (Counsell 2000; Landefeld 1995) were nursing led acute care units, with exercise intervention provided by ward staff and families. A psychogeriatric specialist and/or the provision of specialised exercise intervention may explain some of the between trial variation on change in reported functional outcomes.
The number of patients who declined in ADL scores between two weeks prior to hospital admission and discharge was reported in two multidisciplinary intervention trials. Favourable outcomes for the intervention group were shown. However, results of meta‐analysis did not reach statistical significance with 95% confidence. Preadmission functional ability was reported retrospectively by patients or families at hospital admission and the validity of these measurements is not known.
There was no effect of exercise on adverse events that included patient mortality, hospital readmission following acute hospital discharge, intensive care admissions, falls or musculoskeletal injuries. Since only a small number of trials provided details regarding adverse events other than mortality, results should be interpreted with caution.
The minimally clinically important difference (MCID) represents the smallest change score on an outcome measure that is required to indicate a clinically important change. For some continuous outcomes, Norman et al. (Norman 2003) recommend that half the baseline standard deviation of raw scores provides an appropriate estimate of the MCID, and this form of estimation is useful in the absence of direct measurements of important change. For the Barthel Index and Timed Up and Go, this calculation was performed for the two trials that were pooled in meta‐analysis (de Morton 2006; Jones 2006). Using this method, the MCID was calculated to be approximately 12 points for the Barthel Index and 9.5 seconds for the TUG. This analysis indicates that 49% of patients in the intervention group and 42% in the control group improved by 12 points or greater on the Barthel Index. For the TUG, approximately 3% of patients in the intervention group and 0.5% of patients in the control group improved by 9.5 seconds or more. However, given the floor and ceiling effects that were identified in the individual trials for each of these outcome measures, the validity of applying Norman's recommendations for calculating the MCID is unknown and differences between groups may be underestimated. An outcome measure that can measure across the broad spectrum of abilities for older medical patients is required to explore the effects of exercise on patient function in future trials.
For continuous hospital outcomes that were reported in this review, such as costs or LOS, there is no clear consensus regarding the MCID. However, any small significant differences between groups for these outcomes are likely to be important for patients or the healthcare system and therefore warrant further research attention. Similarly, for dichotomous outcomes, such as discharge destination or adverse events, any significant differences between groups are likely to represent clinical importance.
Exercise was provided as a component of a multidisciplinary intervention in six of the trials identified in this review and therefore the effect of exercise in isolation could not be estimated. In addition, few details were reported regarding exercise type and dosage for these trials and therefore the most effective components of these exercise programs (e.g.. frequency, intensity, duration, repetition) could not be identified. For the three exercise only trials, although more program details were provided, there were too few trials available to investigate the effects of the individual exercise program components.
This review has limitations. Some effect size estimations were based on assumptions due to limited published data for some outcomes. Exercise programs were not clearly described in many of the included trials and older general medical patients are a typically heterogenous patient population. Program and population differences may explain some of the heterogeneity in the data. However, there are many potential sources of heterogeneity in trials, and their influence on differences in outcomes is not clear.
Authors' conclusions
Implications for practice.
Results of meta‐analysis in this review indicate that multidisciplinary intervention that includes exercise may result in a small but significant reduction in acute hospital LOS and cost of hospital stay and a small but significant increase in the proportion of patients discharged directly to home. There was a reduction in length of hospital stay of approximately one day, reduction in direct hospital costs of approximately US$278 and for every 16 patients who are treated with multidisciplinary intervention that included exercise, one more person was discharged to home compared to the control group. These statistically significant findings are all small but might indicate that patients who receive multidisciplinary intervention can return home from hospital sooner, have a higher probability of being discharged directly home from hospital and direct costs associated with their hospitalisation reduced.
It is not known which components of multidisciplinary intervention might cause these small positive effects. Given that exercise only interventions did not significantly improve hospital LOS, costs or the proportion of patient discharges to home, it is possible that the multidisciplinary intervention components other than exercise may explain improved hospital outcomes. These results could be explained by increased medical, nursing or allied health intervention, a combination of improved team goal setting and discharge planning and/or increased patient contact time during acute hospitalisation. However, few exercise only trials were available for pooling. These preliminary findings indicate 'silver level evidence' that multidisciplinary interventions that include additional exercise may result in an increase in the proportion of patients discharged directly home from hospital and an accompanying reduction in acute hospital LOS and costs.
Implications for research.
Few randomised trials have investigated the effects of exercise for acutely hospitalised older medical patients. The trials that met inclusion in this review were of varying method quality and there were too few trials available to conduct sensitivity analysis or meta‐regression. Further high quality trials are required to investigate the effects of exercise in this patient population and it is recommended that a detailed description of the exercise program and dosage is given. Adverse outcomes other than mortality were generally poorly reported and need to be closely monitored in future trials. In addition, since older general medical patients are a typically heterogenous population, the response to exercise across participants is likely to be variable. Individual patient data meta‐analysis or sensitivity analysis may identify patients that derive most benefit from multidisciplinary or exercise intervention during acute hospitalisation. Such analyses would allow the effect of patient level characteristics such as admission diagnosis, age and gender to be investigated and may enable effective targeting of healthcare services. In addition, improved reporting of exercise programs in future trials would allow the effects of differing exercise program dosage components to be investigated and therefore facilitate the development of recommendations for the most effective exercise programs for older acute general medical patients.
What's new
| Date | Event | Description |
|---|---|---|
| 17 September 2008 | Amended | Converted to new review format. C034‐R |
Acknowledgements
The authors of this review would like to thank the Cochrane Musculoskeletal Editorial Team for their support during the development of this review and the journal of Clinical Rehabilitation for feedback on an earlier published version of this review.
Data and analyses
Comparison 1. Function ‐ multidisciplinary intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintained or improved ADL scores between hospital admission and discharge ‐ MDI versus usual care | 3 | 2271 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.15] |
| 2 Maintained or improved mobility scores between hospital admission and discharge ‐ MDI versus usual care | 3 | 2119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.99, 1.06] |
| 3 Maintained or improved ADL scores between 2 weeks prior to hospital admission and discharge‐ MDI v usual care | 2 | 2001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.00, 1.13] |
1.1. Analysis.
Comparison 1 Function ‐ multidisciplinary intervention versus usual care, Outcome 1 Maintained or improved ADL scores between hospital admission and discharge ‐ MDI versus usual care.
1.2. Analysis.
Comparison 1 Function ‐ multidisciplinary intervention versus usual care, Outcome 2 Maintained or improved mobility scores between hospital admission and discharge ‐ MDI versus usual care.
1.3. Analysis.
Comparison 1 Function ‐ multidisciplinary intervention versus usual care, Outcome 3 Maintained or improved ADL scores between 2 weeks prior to hospital admission and discharge‐ MDI v usual care.
Comparison 2. Function ‐ additional exercise only versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Barthel Index between hospital admission and discharge ‐ additional exercise versus usual care | 2 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.06, 0.40] |
| 2 Change in TUG scores between hospital admission and discharge ‐ additional exercise versus usual care | 2 | 188 | Mean Difference (IV, Random, 95% CI) | ‐2.52 [‐5.75, 0.71] |
2.1. Analysis.
Comparison 2 Function ‐ additional exercise only versus usual care, Outcome 1 Change in Barthel Index between hospital admission and discharge ‐ additional exercise versus usual care.
2.2. Analysis.
Comparison 2 Function ‐ additional exercise only versus usual care, Outcome 2 Change in TUG scores between hospital admission and discharge ‐ additional exercise versus usual care.
Comparison 3. Adverse events ‐ multidisciplinary intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient mortality during hospitalisation ‐ MDI versus usual care | 6 | 3552 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.59, 1.64] |
| 2 Patient mortality 3 months after hospital discharge ‐ MDI versus usual care | 3 | 2595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.17] |
| 3 Patient complications during hospitalisation ‐ MDI versus usual care | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.29] |
3.1. Analysis.
Comparison 3 Adverse events ‐ multidisciplinary intervention versus usual care, Outcome 1 Patient mortality during hospitalisation ‐ MDI versus usual care.
3.2. Analysis.
Comparison 3 Adverse events ‐ multidisciplinary intervention versus usual care, Outcome 2 Patient mortality 3 months after hospital discharge ‐ MDI versus usual care.
3.3. Analysis.
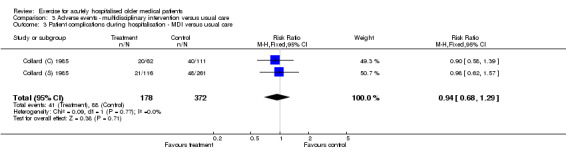
Comparison 3 Adverse events ‐ multidisciplinary intervention versus usual care, Outcome 3 Patient complications during hospitalisation ‐ MDI versus usual care.
Comparison 4. Adverse events ‐ additional exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient mortality during hospitalisation ‐ additional exercise versus usual care | 3 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.64, 6.18] |
| 2 Admission to the Intensive Care Unit (ICU) ‐ additional exercise versus usual care | 2 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.04, 30.44] |
| 3 Falls during hospitalisation ‐ additional exercise versus usual care | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.40, 3.15] |
4.1. Analysis.
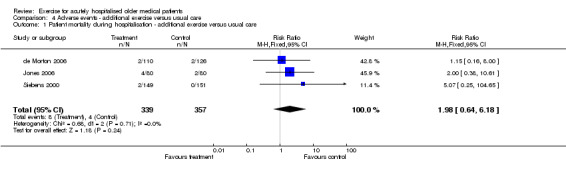
Comparison 4 Adverse events ‐ additional exercise versus usual care, Outcome 1 Patient mortality during hospitalisation ‐ additional exercise versus usual care.
4.2. Analysis.
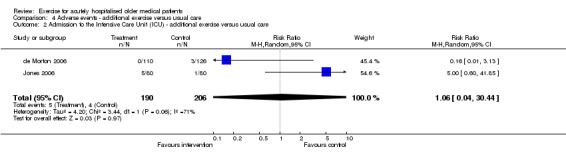
Comparison 4 Adverse events ‐ additional exercise versus usual care, Outcome 2 Admission to the Intensive Care Unit (ICU) ‐ additional exercise versus usual care.
4.3. Analysis.
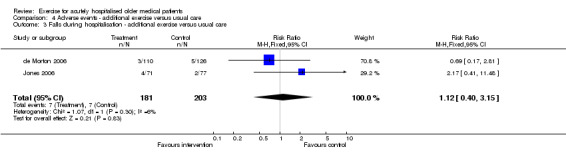
Comparison 4 Adverse events ‐ additional exercise versus usual care, Outcome 3 Falls during hospitalisation ‐ additional exercise versus usual care.
Comparison 5. Hospital outcomes ‐ multidisciplinary intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discharge to preadmission residence/home from hospital ‐ MDI versus usual care | 4 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.03, 1.14] |
| 2 Acute hospital length of stay ‐ MDI versus usual care | 6 | 3478 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.93, ‐0.22] |
| 3 Cost of acute hospital stay ‐ MDI versus usual care | 5 | 3241 | Mean Difference (IV, Fixed, 95% CI) | ‐278.65 [‐491.85, ‐65.44] |
5.1. Analysis.
Comparison 5 Hospital outcomes ‐ multidisciplinary intervention versus usual care, Outcome 1 Discharge to preadmission residence/home from hospital ‐ MDI versus usual care.
5.2. Analysis.
Comparison 5 Hospital outcomes ‐ multidisciplinary intervention versus usual care, Outcome 2 Acute hospital length of stay ‐ MDI versus usual care.
5.3. Analysis.
Comparison 5 Hospital outcomes ‐ multidisciplinary intervention versus usual care, Outcome 3 Cost of acute hospital stay ‐ MDI versus usual care.
Comparison 6. Hospital outcomes ‐ additional exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discharge to preadmission residence/home from hospital ‐ additional exercise versus usual care | 2 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.80, 1.66] |
| 2 Acute hospital length of stay ‐ additional exercise versus usual care | 3 | 680 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐1.23, 1.26] |
| 3 Total (acute plus subacute) hospital length of stay‐ additional exercise versus usual care | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.58, 1.17] |
6.1. Analysis.
Comparison 6 Hospital outcomes ‐ additional exercise versus usual care, Outcome 1 Discharge to preadmission residence/home from hospital ‐ additional exercise versus usual care.
6.2. Analysis.
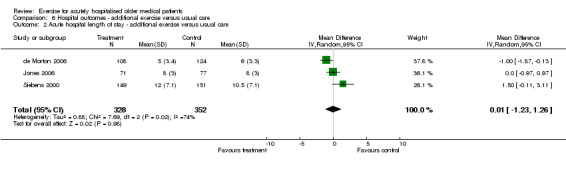
Comparison 6 Hospital outcomes ‐ additional exercise versus usual care, Outcome 2 Acute hospital length of stay ‐ additional exercise versus usual care.
6.3. Analysis.
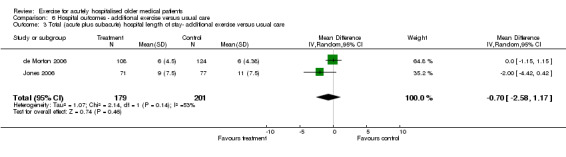
Comparison 6 Hospital outcomes ‐ additional exercise versus usual care, Outcome 3 Total (acute plus subacute) hospital length of stay‐ additional exercise versus usual care.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asplund 2000.
| Methods | ‐ RCT comparing an acute geriatric ward (AGW) with 2 usual care general medical wards (MW). ‐ randomisation in blocks of 12 patients ‐ Setting: Sweden, acute care and tertiary referral hospital. ‐ Funding: Vasterbotten County Council and Vardalstiftelsen and King Gustaf V's and Queen Victoria's Foundation. | |
| Participants | ‐ 444 older medical patients (190 AGW and 223 MW). 25 were excluded due to protocol violations. ‐ Mean age 81 years, 60% female and 16% resided in an institution prior to admission. ‐ Inclusion criteria: patients older than 70 years, acutely admitted to hospital for a medical ailment. ‐ Exclusion criteria: Admission to a specialised unit (intensive care, coronary care, acute stroke unit) or admission to a designated subspecialty unit. ‐ Main presenting symptom: chest pain (23%), dyspnoea (18%), other pain (11%), nausea/vomiting (11%), vertigo (11%), other (26%). | |
| Interventions | ‐ Acute geriatric based ward differed from the standard ward in that it provided a geriatrician, physiotherapist, occupational therapist. Interdisciplinary team work focussed on early and intensive rehabilitation and intense discharge planning. ‐ Exercise: Early start to rehabilitation. Physiotherapy and occupational therapy assessment and staffing of the ward to optimise early rehabilitation. | |
| Outcomes | ‐ Functional status (Barthel Index), cognitive status (MMSE), psychological well being, mortality, place of residence, length and cost of hospital stay, hospital readmission, healthcare costs, events after discharge, outpatient visits and personal assistance requirements. ‐ Time of outcome measure assessment: hospital discharge and 3 months after hospital discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 6 ‐ Cost data converted from SEK to US$ using the conversion rates reported by the author. ‐ Author conclusions: " A geriatric approach with greater emphasis on early rehabilitation and discharge planning in the AGW shortened the length of hospital stay and may have reduced the need for long‐term institutional living." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Collard (C) 1985.
| Methods | ‐ RCT of a geriatric special care unit compared to a traditional medical or surgical ward. ‐ randomisation: "the hospital admissions office randomly assigned eligible patients." ‐ Setting: USA, acute hospital. ‐ Funding: not stated. | |
| Participants | ‐ 271 medical/surgical patients (95 treatment, 176 control). ‐ Mean age 77 years, 60% female, 10% resided in a nursing home prior to admission. ‐ Inclusion criteria: aged at least 65 years, predicted length of stay of greater than 48 hours, under the care of a participating physician. ‐ Exclusion criteria: nil reported ‐ Major diagnostic categories (ordered from most common): Respiratory, cerebrovascular, cardiac, neurological, bowel/intestinal, fractures and metastatic malignancies. | |
| Interventions | ‐ Geriatric special care unit. Registered nurses/assistants trained for project. Emphasis on maximising patient independence. Multidisciplinary team meeting twice weekly. Early discharge planning and home visit 3 weeks after discharge. ‐ Exercise intervention: patients wear their own clothes, dine in a communal area and participate in an exercise program. Supervised by nursing staff and family. Role of the physiotherapist and occupational therapist not clearly defined. | |
| Outcomes | ‐ Discharge destination, mortality, complications during hospitalisation, length and cost of hospital stay, use of physical or chemical restraints. ‐ Time of outcome measure assessment: hospital discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 5 ‐ Additional information from authors: no. Attempted to contact authors regarding inconsistent sample sizes reported in tables but unable to locate, possibly due to paper having been published 20+ years ago. The authors reported the trial to be continuing but no further publications were identified. ‐ Authors conclusions: " the preliminary outcomes themselves are encouraging; they suggest that high‐quality hospital care can be delivered to the elderly for less money." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Collard (S) 1985.
| Methods | ‐ RCT of a geriatric special care unit compared to a traditional medical or surgical ward. ‐ randomisation: "the hospital admissions office randomly assigned eligible patients." ‐ Setting: USA, acute hospital. ‐ Funding: not stated. | |
| Participants | ‐ 424 medical/surgical patients (123 treatment, 301 control). ‐ Mean age 79 years, 65% female, 9% resided in a nursing home prior to admission. ‐ Inclusion criteria: aged at least 65 years, predicted length of stay of greater than 48 hours, under the care of a participating physician. ‐ Exclusion criteria: nil reported ‐ Major diagnostic categories (ordered from most common): Respiratory, cardiac, fractures, cerebrovascular, bowel/intestinal, metastatic malignancies and neurological. | |
| Interventions | ‐ Geriatric special care unit. Registered nurses/assistants trained for project. Emphasis on maximising patient independence. Multidisciplinary team meeting twice weekly. Early discharge planning and home visit 3 weeks after discharge. ‐ Exercise: patients wear their own clothes, dine in a communal area and participate in an exercise program. Supervised by nursing staff and family. Role of the physiotherapist and occupational therapist not clearly defined. | |
| Outcomes | ‐ Discharge destination, mortality, complications during hospitalisation, length and cost of hospital stay, use of physical or chemical restraints. ‐ Time of outcome measure assessment: hospital discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 5 ‐ Additional information from authors: no. Attempted to contact authors regarding inconsistent sample sizes reported in tables but unable to locate, possibly due to paper having been published 20+ years ago. The authors reported the trial to be continuing but no further publications were identified. ‐ Authors conclusions: " the preliminary outcomes themselves are encouraging; they suggest that high‐quality hospital care can be delivered to the elderly for less money." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Counsell 2000.
| Methods | ‐ RCT of an acute care elders unit (ACE) compared to usual care units. ‐ randomised using computer generated random numbers ‐ Setting: USA, community teaching hospital. ‐ Funding: Summa Health System Foundation. | |
| Participants | ‐ 1531 of 6609 eligible patients. ‐ Mean age 80 years, 60% female, none from institutions. ‐ Inclusion criteria: community dwelling persons aged 70 or older admitted to a medicine or family practice service. ‐ Exclusion criteria: transferred from a nursing facility or another hospital, required specialty unit admission, were admitted electively, had a length of stay of less than 2 days or had been previously enrolled in the study. Reason for admission: acute dyspnoea or pulmonary problem (24.1%), change in mental status or neurological abnormality (20.1%), gastrointestinal (18.75%), fever, pneumonia or infection (13.98%), diabetes mellitus, failure to thrive or other problem (11.56%), congestive heart failure, chest pain or cardiac problem (11.50%). | |
| Interventions | ‐ Multidisciplinary ACE unit. Specially designed environment, patient centred care, nursing care plans for prevention of functional decline, rehabilitation, patient discharge to home and review of medical care to prevent iatrogenic illness. Daily team rounds. Exercise: 3 times per day walk or stand. Daily ambulation to activity room for exercises and meals. Encouraged by staff. Patient or caregivers taught exercises. | |
| Outcomes | ‐ Function (ADL and IADL), mobility, mortality, discharge destination, hospital costs and LOS, satisfaction and use of at‐risk medications. ‐ Time of outcome measure assessment: hospital discharge and 1, 3, 6 and 12 months after discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 7 ‐ Additional information from authors: no ‐ Author conclusions: "ACE in a community hospital improved the process of care and patient and provider satisfaction without increasing hospital length of stay or costs." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Covinsky 1997.
| Methods | ‐ see Landefeld 1995 | |
| Participants | ‐ see Landefeld 1995 | |
| Interventions | ‐ see Landefeld 1995 | |
| Outcomes | ‐hospital length of stay and costs | |
| Notes | ‐ see Landefeld 1995 ‐ Covinsky et al. provided mean cost and standard deviation data for the trial reported by Landefeld et al. ‐ Covinsky et al. also provided LOS data that included patients that died and was therefore consistent with other trials for pooling. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
de Morton 2006.
| Methods | ‐ CCT of additional exercise intervention compared to usual care. ‐Consecutive eligible patients admitted to one of two similar wards based on bed availability. The intervention ward was determined by a coin toss prior to commencement of the trial. ‐ Setting: Australia, acute public hospital. ‐Funding: Department of Medicine, Northern Health. | |
| Participants | ‐ 236 of 251 eligible patients (110 intervention, 126 usual care). ‐ Mean age 79 years, 55% female, 10% from hostel. ‐ Inclusion: general medical patients aged 65 or older, were admitted to either of the two general medical wards and were assessed within 48 hours of admission. ‐ Exclusion: admitted to hospital from a nursing home, were assessed to be nursing home level of care or palliative care, had suffered a stroke or a condition for which mobilisation was contraindicated (e.g. deep vein thrombosis or fracture), were too medically unwell to ambulate or exercise or were readmitted during the data collection period and had previously participated in the study. Primary Diagnosis: Respiratory (30.9%), circulatory (20.8%), digestive (8.5%), genitourinary (6.8%) and other (33%). | |
| Interventions | ‐ Exercise only intervention ‐Exercise intervention was in addition to usual care physiotherapy. ‐ Individually tailored exercise intervention program prescribed by a physiotherapist and supervised by an allied health assistant. One of 4 levels of exercise program. Individually tailored. Twice per day walking and exercise program. Maximum of 10 repetitions of each exercise, 20‐30 minutes duration. | |
| Outcomes | ‐ Functional status (Barthel Index, Timed Up and Go and Functional Ambulation Classification), adverse events in hospital (mortality, falls, admission to the intensive care unit), discharge destination from hospital, hospital length of stay and readmission within 28 days of discharge. ‐ Time of outcome assessment: hospital discharge and 28 days after discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 7 ‐ unpublished dataset. Manuscript is now "in press" ‐ Language: English ‐ Author conclusions: "This trial did not identify significantly improved outcomes as a result of additional exercise for acutely hospitalised older medical patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Jones 2006.
| Methods | ‐ RCT of additional exercise intervention compared to usual care. ‐ computer generated random numbers (block randomisation). ‐ Setting: Australia, acute tertiary public hospital. ‐ Funding: Department of General Medicine, Royal Melbourne Hospital. | |
| Participants | ‐ 160 of 186 eligible patients (80 in each group). ‐ Mean age 82 years, 58% female and 18% from residence other than home prior to admission. ‐ Inclusion criteria: aged 65 years or older, admitted with a medical condition to a general medical ward. ‐Exclusion criteria: admitted from nursing home, received nursing home level of care at home, medically unstable or mobilisation was contra‐indicated by the treating medical team, admitted to the delirium management unit, non weight bearing, not assessed within 48 hours of admission, assessed to require palliative care, admitted to hospital with a diagnosis known to cause functional impairment or documented LOS of less than 48 hours. Primary Diagnosis: not reported. | |
| Interventions | ‐ Exercise only intervention ‐Exercise intervention was in addition to usual care physiotherapy. ‐ Individually tailored exercise program during hospitalisation. One of 4 levels of exercise program. Prescribed by a physiotherapist and supervised by a physiotherapy assistant. Twice per day for 30 mins. | |
| Outcomes | ‐ Functional status (Barthel Index and TUG), adverse events (mortality, admission to ICU and falls), discharge destination from hospital, hospital length of stay and readmission within 28 days of discharge. ‐ Time of outcome measurement: hospital discharge and 28 days after discharge. | |
| Notes | ‐ Language: English ‐ PEDro score: 7 ‐ Unpublished data obtained from authors from manuscript prepared for publication. Manuscript has now been published. ‐ Authors conclusions: "The intervention was effective at improving the function of hospitalised elderly general medical patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Landefeld 1995.
| Methods | ‐ RCT of an acute care elders (ACE) unit compared to usual care ‐ randomised by computer generated numbers ‐ Setting: USA, private, non profit teaching hospital. ‐ Funding: John A Hartford Foundation, National Institute on Ageing. | |
| Participants | ‐ 651 of 1794 eligible patients (327 intervention, 324 usual care). ‐ Mean age 80 years, 66% female and 8% from long term institutional care. ‐ Inclusion criteria: 70 years or older admitted for general medical care. ‐ Exclusion criteria: patients admitted to a specialty unit (eg. intensive care, cardiology‐telemetry or oncology). Reason for admission: Gastrointestinal (19.1%, fever, pneumonia or other infection (18.7%), congestive heart failure (16.4%), acute dyspnoea or other pulmonary problem (16.3%), diabetes mellitus, failure to thrive or other problem (15.8%), change in mental status or other neurologic abnormality (11.7%). | |
| Interventions | ‐ Multidisciplinary ACE unit. Specially designed environment, patient centred care, nursing care plans for prevention of functional decline, rehabilitation, patient discharge to home and review of medical care to prevent iatrogenic illness. Daily team rounds. Exercise: 3 times per day walk or stand. Daily ambulation to activity room for exercises and meals. Encouraged by staff. Patient or caregivers taught exercises. | |
| Outcomes | ‐ Function (ADL, IADL and ability to walk), mental status (subscore of MMSE), depression (Geriatric Depression Scale), overall health status, discharge destination from hospital, place or residence 3 months after discharge, acute hospital length and cost of hospital stay. ‐ Time of outcome measurement: hospital discharge and 3 months after discharge | |
| Notes | ‐ Language: English ‐ PEDro score: 5 ‐ Additional information from authors: No ‐ 3 patients assumed to be lost to follow up in the intervention group for change in ADL and mobility scores. ‐ Author conclusions: "Specific changes in the provision of acute hospital care can improve the ability of a heterogenous group of acutely ill older patients to perform basic activities of daily living." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Siebens 2000.
| Methods | ‐ RCT of an exercise program that included a hospital component and a self‐administered 1 month home component. ‐ stratified randomisation. ‐ Setting: USA, community‐based hospital. ‐Funding: John A Hartford Foundation. | |
| Participants | ‐ 300 subjects of 2198 eligible patients (151 control, 149 intervention) ‐ Mean age 78 years, 60% female and 7% admitted from institution. ‐ Inclusion criteria: 70 years or older admitted with a medical or surgical diagnoses. ‐ Exclusion criteria: nonambulatory or living in a nursing home prior to admission, had hospital admission diagnoses known to cause functional impairment, were likely to die within 12 months according to their primary physician, admitted with primary cardiac diagnoses, could not communicate clearly, had an admission Diagnostic Related Group average length of stay of less than 5 days. Medical Diagnostic Categories: digestive system (20%), circulatory system (7.3%), hepatobiliary system and pancreas (6.3%), male reproductive system (5.3%), skin, subcutaneous tissue or breast (4.7%), male reproductive system kidney and urinary tract (4.7%), female reproductive system (4.7%) and other (17.6%). | |
| Interventions | ‐ Exercise only intervention ‐ Exercise program during hospitalisation and 1 month after hospital discharge. 12 exercises for flexibility and strengthening and a walking program. Prescribed by a physiotherapist and supervised once per day by a physiotherapy aid and once per day unsupervised in hospital. Unsupervised for 1 month after discharge. ‐ Twice per day in hospital. Exercises 3 times per week at home. 5‐10 repetitions. Walking 5‐30mins at an intensity of 60‐80% of age adjusted maximum heart rate level and be able to talk while walking. Methods employed to encourage adherence to exercise after discharge eg. adherence cards and phone calls. | |
| Outcomes | ‐ Function (Functional Independence Measure, Locomotion Scale, frequency of leaving the neighbourhood, IADLs, National Health Interview Survey Physical Activity Scale), hospital length of stay, RAND General Health Scale, mortality. ‐ Time of outcome measurement: discharge and 1 month after discharge. | |
| Notes | ‐ Language: English ‐ PEDro scale: 8 ‐ only 20% of the intervention group had a high adherence level to the hospital and home exercises. ‐ Additional information from authors: No ‐ Author conclusions: "An exercise program started during hospitalisation and continued for 1 month did not shorten length of stay but did improve functional outcome at 1 month." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Slaets 1997.
| Methods | ‐ A CCT examining the effect of a psychogeriatric intervention group in addition to usual care compared to usual care. ‐ alternating randomisation procedure ‐ Setting: The Netherlands, teaching hospital. ‐ Funding: not stated. | |
| Participants | ‐ 237 patients enrolled (140 treatment group, 97 usual care). ‐ Mean age 83 years, 71% female and 28% from an institution. ‐ Inclusion criteria: 75 years or older, referred to the department of general medicine. ‐ Exclusion criteria: patients admitted for day treatment. Main diagnostic groups: Congestive heart failure (41.35%), diabetes or other endocrinological problems (27.8%), gastrointestinal (18.56%), cancer (12.2%), pneumonia (11.4%) and chronic lung disease (5.97%). | |
| Interventions | ‐ Multidisciplinary joint treatment by a psychogeriatric team leader in addition to usual care. Full time physiotherapist and additional 3 nurses on intervention ward. Aim to optimise patient function. Weekly team meetings. Exercise: Treatment for preventing functional decline and rehabilitation therapy. Supervised by a physiotherapist. Assessed daily by the physiotherapist. | |
| Outcomes | ‐ Length of stay, SIVIS dependency scales (Help index, Mobility, ADL+ continence), discharge destination, residence in a long‐term care facility ‐ Time of outcome measurement: hospital discharge. | |
| Notes | ‐ Language: English. ‐ PEDro scale: 4 ‐ Additional information: No ‐ Author conclusions: "By combining elements from a psychiatric and geriatric consultation service with elements of a unit‐driven service, we were able to improve health care for the elderly in our hospital in a feasible and cost effective way." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aschwanden 2001 | Participants too young |
| Bariola 1999 | No control group |
| Bogardus 2003 | Patients not randomly allocated to group. Prospectively matched intervention and control pairs. |
| Boyer 1986 | Physical exercise intervention not prescribed for all patients in the intervention group |
| Cohen 2002 | Patients not randomised during acute medical exacerbation |
| Cole 2002 | Not a physical exercise intervention program. Encouragement with self care and other personal activities provided |
| Curley 1998 | Participants too young |
| Gayton 1987 | Exercise intervention prescribed only if felt appropriate. Exercise not prescribed for all patients in the intervention group. |
| Germain 1995 | Participants too young. Patients were required to be 60 years of age or older. Mean age of approximately 80 years but standard deviation not reported. |
| Harris 1991 | Exercise not prescribed for all patients in the intervention group. |
| Hogan 1987 | Physical exercise intervention not prescribed for all patients in the intervention group |
| Hogan 1990 | Physical exercise intervention not prescribed for all patients in the intervention group |
| Inouye 1993a | No control group |
| Inouye 1993b | Patients not randomly allocated to group |
| Inouye 1999 | Patients not randomly allocated to group. Prospective individual matching of patients. |
| Landefeld 1988 | Published abstract only. Unclear if patients randomised within 48 hours of hospital admission. Correspondence with authors has occurred and further information to be obtained. |
| Landi 1997 | Historical control group |
| Meissner 1989 | Physical exercise intervention not prescribed for all patients in the intervention group |
| Mundy 2003 | Participants too young |
| Nagley 1986 | Patients not randomly allocated to group |
| Nikolaus 1999 | Patients not randomised within 3 days of hospital admission |
| Reuben 1995 | Physical exercise intervention not prescribed for all patients in the intervention group |
| Rizzo 2001 | Patients not randomly allocated to group. Prospective patient matching. |
| Rubenstein 1984a | Patients not randomised within 3 days of hospital admission |
| Rubenstein 1984b | Patients not randomised within 3 days of hospital admission |
| Rubenstein 1995 | Patients not randomised within 3 days of hospital admission |
| Saltvedt 2002 | Patients not randomised within 3 days of hospital admission |
| Wanich 1992 | Patients not randomly allocated to group |
| Yohannes 2003 | Participants too young |
Contributions of authors
Natalie de Morton conceived and designed the review, performed the literature search, contacted the authors of clinical trials, extracted data, appraised studies, performed the analyses and wrote the manuscript. Jenny Keating conceived and designed the review, extracted data, appraised studies and assisted with writing the manuscript. Kim Jeffs extracted data, appraised studies and made revisions to the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Australian National Health and Medical Research Council Dora Lush and Medical Post Graduate Scholarships, Australia.
Declarations of interest
The first and second authors of this review conducted one of the studies that met inclusion in this review. Other assessors evaluated the quality of this study's methods.
Edited (no change to conclusions)
References
References to studies included in this review
Asplund 2000 {published data only}
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric‐based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. Journal of the American Geriatrics Society 2000;48:1381‐8. [DOI] [PubMed] [Google Scholar]
Collard (C) 1985 {published data only}
- Collard AF, Bachman SS, Beatrice DF. Acute care delivery for the geriatric patient: an innovative approach. Quality Review Bulletin 1985;11:180‐5. [PubMed] [Google Scholar]
Collard (S) 1985 {published data only}
- Collard AF, Bachman SS, Beatrice DF. Acute care delivery for the geriatric patient: an innovative approach. Quality Review Bulletin 1985;11:180‐5. [PubMed] [Google Scholar]
Counsell 2000 {published data only}
- Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. Journal of the American Geriatrics Society 2000;48:1572‐81. [DOI] [PubMed] [Google Scholar]
Covinsky 1997 {published data only}
- Covinsky KE, King J T, Quinn LM, Siddique R, Palmer R, Kresevic DM, et al. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. Journal of the American Geriatrics Society 1997;45:729‐34. [DOI] [PubMed] [Google Scholar]
de Morton 2006 {unpublished data only}
- Morton NA, Keating JL, Berlowitz DJ, Lim WK, Jackson B. A controlled clinical trial of the effects of additional exercise on patient health and health care service utilisation: a pilot study. Australian Journal of Physiotherapy "in press".
Jones 2006 {unpublished data only}
- Jones C, Lowe A, McGregor L, Brandt C, Tweddle N, Russell D. A randomised controlled trial of an exercise intervention to reduce functional decline and health service utilization in the hospitalized elderly. Australasian Journal of Ageing 2006;25(3):126‐33. [Google Scholar]
Landefeld 1995 {published data only}
- Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. New England Journal of Medicine 1995;332:1338‐44. [DOI] [PubMed] [Google Scholar]
Siebens 2000 {published data only}
- Siebens H, Aronow H, Edwards D, Ghasemi Z. A randomized controlled trial of exercise to improve outcomes of acute hospitalization in older adults. Journal of the American Geriatrics Society 2000;48:1545‐52. [DOI] [PubMed] [Google Scholar]
Slaets 1997 {published data only}
- Slaets JP, Kauffmann RH, Duivenvoorden HJ, Pelemans W, Schudel, WJ. A randomized trial of geriatric liaison intervention in elderly medical inpatients. Psychosomatic Medicine 1997;59:585‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aschwanden 2001 {published data only}
- Aschwanden M, Labs KH, Engel H, Schwob A, Jeanneret C, Mueller‐Brand J, et al. Acute deep vein thrombosis: Early mobilization does not increase the frequency of pulmonary embolism. Thrombosis & Haemostasis 2001;85:42‐6. [PubMed] [Google Scholar]
Bariola 1999 {published data only}
- Bariola JR, Sullivan DH, Wall PT, Hite R, Frost M, McClellan JL. Effects of muscle strength training in recuperating elderly. Journal of the American Geriatrics Society 1999;47:S30‐S30. [Google Scholar]
Bogardus 2003 {published data only}
- Bogardus ST, Desai MA, Williams CS, Leo‐Summers L, Acampora D, Inouye SK. The effects of a targeted multicomponent delirium intervention on post discharge outcomes for hospitalized older adults. American Journal of Medicine 2003;114:383‐90. [DOI] [PubMed] [Google Scholar]
Boyer 1986 {published data only}
- Boyer N, Chuang JL, Gipner D. An acute care geriatric unit. Nursing Management 1986;17:22‐5. [PubMed] [Google Scholar]
Cohen 2002 {published data only}
- Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. New England Journal of Medicine 2002;346:905‐12. [DOI] [PubMed] [Google Scholar]
Cole 2002 {published data only}
- Cole MG, McCusker J, Bellavance F, Primeau FJ, Bailey RF, Bonnycastle MJ, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. Canadian Medical Association Journal 2002;167:753‐9. [PMC free article] [PubMed] [Google Scholar]
Curley 1998 {published data only}
- Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards ‐ An intervention designed using continuous quality improvement. Medical Care 1998;36:AS4‐AS12. [DOI] [PubMed] [Google Scholar]
Gayton 1987 {published data only}
- Gayton D, Wood‐Dauphinee S, Lorimer M, Tousignant P, Hanley J. Trial of a geriatric consultation team in an acute care hospital. Journal of the American Geriatrics Society 1987;35:726‐36. [DOI] [PubMed] [Google Scholar]
Germain 1995 {published data only}
- Germain M, Knoeffel F, Wieland D, Rubenstein LZ. A Geriatric Assessment and Intervention Team for Hospital Inpatients Awaiting Transfer to a Geriatric Unit ‐ a Randomized Trial. Aging‐Clinical and Experimental Research 1995;7:55‐60. [DOI] [PubMed] [Google Scholar]
Harris 1991 {published data only}
- Harris RD, Henschke PJ, Popplewell PY, Radford AJ, Bond, MJ, Turnbull RJ, et al. A randomised study of outcomes in a defined group of acutely ill elderly patients managed in a geriatric assessment unit or a general medical unit. Australian & New Zealand Journal of Medicine 1991;21:230‐4. [DOI] [PubMed] [Google Scholar]
Hogan 1987 {published data only}
- Hogan DB, Fox RA, Badley BW, Mann OE. Effect of a geriatric consultation service on management of patients in an acute care hospital. Canadian Medical Association Journal 1987;136:713‐7. [PMC free article] [PubMed] [Google Scholar]
Hogan 1990 {published data only}
- Hogan DB, Fox RA. A prospective controlled trial of a geriatric consultation team in an acute‐care hospital. Age & Ageing 1990;19:107‐13. [DOI] [PubMed] [Google Scholar]
Inouye 1993a {published data only}
- Inouye SK, Acampora D, Miller RL, Fulmer T, Hurst LD, Cooney LM. The Yale Geriatric Care Program: a model of care to prevent functional decline in hospitalized elderly patients. Journal of the American Geriatrics Society 1993;41:1345‐52. [DOI] [PubMed] [Google Scholar]
Inouye 1993b {published data only}
- Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Tinetii ME. A controlled trial of a nursing‐centered intervention in hospitalized elderly medical patients ‐ The Yale Geriatric Care Program. Journal of the American Geriatrics Society 1993;41:1353‐60. [DOI] [PubMed] [Google Scholar]
Inouye 1999 {published data only}
- Inouye SK, Bogardus ST, Charpentier PA, Leo‐Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine 1999;340:669‐76. [DOI] [PubMed] [Google Scholar]
Landefeld 1988 {published data only}
- Landefeld CS, Palmer RM, Fortinsky RH, Kresevic DM, Kowal J. A randomised trial of acute care for elders (ACE) in the current era: lower hospital costs without advers effects on functional outcomes at discharge. Journal of General Internal Medicine 1998; Vol. 13:45.
Landi 1997 {published data only}
- Landi F, Zuccala G, Bernabei R, Cocchi A, Manigrasso L, Tafani A, et al. Physiotherapy and occupational therapy: a geriatric experience in the acute care hospital. American Journal of Physical Medicine & Rehabilitation 1997;76:38‐42. [DOI] [PubMed] [Google Scholar]
Meissner 1989 {published data only}
- Meissner P, Andolsek K, Mears, PA, Fletcher B. Maximizing the functional status of geriatric patients in an acute community hospital setting. Gerontologist 1989;29:524‐8. [DOI] [PubMed] [Google Scholar]
Mundy 2003 {published data only}
- Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest 2003;124:883‐9. [DOI] [PubMed] [Google Scholar]
Nagley 1986 {published data only}
- Nagley SJ. Predicting and preventing confusion in your patients. Journal of Gerontological Nursing 1986;12:27‐31. [DOI] [PubMed] [Google Scholar]
Nikolaus 1999 {published data only}
- Nikolaus T, Specht‐Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age & Ageing 1999;28:543‐50. [DOI] [PubMed] [Google Scholar]
Reuben 1995 {published data only}
- Reuben DB, Borok GM, Wolde‐Tsadik G, Ershoff DH, Fishman LK, Ambrosini VL, et al. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. New England Journal of Medicine 1995;332:1345‐50. [DOI] [PubMed] [Google Scholar]
Rizzo 2001 {published data only}
- Rizzo JA, Bogardus ST, Leo‐Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients ‐ What is the economic value?. Medical Care 2001;39:740‐52. [DOI] [PubMed] [Google Scholar]
Rubenstein 1984a {published data only}
- Rubenstein LZ, Josephson KR, Wieland GD, English PA, Sayre JA, Kane RL. Effectiveness of a geriatric evaluation unit. A randomized clinical trial. New England Journal of Medicine 1984;311:1664‐70. [DOI] [PubMed] [Google Scholar]
Rubenstein 1984b {published data only}
- Rubenstein LZ, Wieland D, English P, Josephson K, Sayre JA, Abrass IB. The Sepulveda VA Geriatric Evaluation Unit: data on four‐year outcomes and predictors of improved patient outcomes. Journal of the American Geriatrics Society 1984;32:503‐12. [DOI] [PubMed] [Google Scholar]
Rubenstein 1995 {published data only}
- Rubenstein LZ, Josephson KR, Harker JO, Miller DK, Wieland D. The Sepulveda GEU Study revisited: long‐term outcomes, use of services, and costs. Aging‐Clinical & Experimental Research 1995;7:212‐7. [DOI] [PubMed] [Google Scholar]
Saltvedt 2002 {published data only}
- Saltvedt I, Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. Journal of the American Geriatrics Society 2002;50:792‐8. [DOI] [PubMed] [Google Scholar]
Wanich 1992 {published data only}
- Wanich CK, Sullivan‐Marx EM, Gottlieb GL, Johnson JC. Functional status outcomes of a nursing intervention in hospitalized elderly. Image ‐ the Journal of Nursing Scholarship 1992;24:201‐7. [DOI] [PubMed] [Google Scholar]
Yohannes 2003 {published data only}
- Yohannes AM, Connolly MJ. Early mobilization with walking aids following hospital admission with acute exacerbation of chronic obstructive pulmonary disease. Clinical Rehabilitation 2003;17:465‐71. [DOI] [PubMed] [Google Scholar]
Additional references
Alderson 2002
- Alderson P, Green S. Cochrane Collaboration open learning material for reviewers Version 1.1. www.cochrane‐net.org/openlearning/HTML/modA1‐5.htm.
Cates 2003
- Dr. Chris Cates. EBM Website. www.nntonline.net 2003.
Covinsky 2003
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. Journal of the American Geriatrics Society 2003;51:451‐8. [DOI] [PubMed] [Google Scholar]
Creditor 1993
- Creditor, M. Hazards of hospitalization of the elderly. Annals of Internal Medicine 1993;118:219‐23. [DOI] [PubMed] [Google Scholar]
deFrances 2004
- deFrances C, Hall M. 2002 National Hospital Discharge Survey. Advance Data From Vital and Health Statistics 2004;342. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis is detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feachem 2002
- Feachem RK, Neelam KS, White KL. Getting more for their dollar: a comparison of the NHS with California's Kaiser Permanente. British Medical Journal 2002;324:135‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillick 1982
- Gillick M, Serrell N, Gillick LS. Adverse consequences of hospitalization in the elderly. Social Science & Medicine 1982;16:1033‐8. [DOI] [PubMed] [Google Scholar]
Harper 1988
- Harper CM, Lyles YM. Physiology and complications of bed rest. Journal of the American Geriatrics Society 1988;36:1047‐54. [DOI] [PubMed] [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical methods for meta‐analysis. United States of America: Academic Press, 1985. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks J, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 1990
- Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. Journal of the American Geriatrics Society 1990;38:1296‐303. [DOI] [PubMed] [Google Scholar]
Hoffman 1996
- Hoffman C, Rice D. Persons with chronic conditions:their prevalence and costs. Journal of the American Medical Association 1996;276:1478‐9. [PubMed] [Google Scholar]
Inouye 1993
- Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Tinetii ME. A controlled trial of a nursing‐centered intervention in hospitalized elderly medical patients ‐ The Yale Geriatric Care Program. Journal of the American Geriatrics Society 1993;41:1353‐60. [DOI] [PubMed] [Google Scholar]
Latham 2004
- Latham NK, Anderson C, Bennett D, Stretton C. Progressive resistance strength training for physical disability in older people. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Maher 2003
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Physical Therapy 2003;83(8):713‐21. [PubMed] [Google Scholar]
Mahoney 1998
- Mahoney JE, Sager MA, Jalaluddin M. New walking dependence associated with hospitalisation for acute medical illness: incidence and significance. Journal of Gerontology 1998;53A(4):M307‐M312. [DOI] [PubMed] [Google Scholar]
McVey 1989
- McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen H. Effects of a geriatric consult team on functional status in elderly hospitalized patients. Annals of Internal Medicine 1989;110:78‐84. [DOI] [PubMed] [Google Scholar]
Morris 2004
- Morris M, Schoo A. Optimizing exercise and physical activity in older people. Butterworth Heinemann, 2004. [Google Scholar]
Norman 2003
- Norman G, Sloan J, Wyrwich K. Interpretation of changes on health related quality of life. The remarkable universality of half a standard deviation. Medical Care 2003;41(5):582‐92. [DOI] [PubMed] [Google Scholar]
Palmer 1995
- Palmer, R. Acute hospital care of the elderly: minimizing the risk of functional decline. Cleveland Clinical Journal of Medicine 1995;62(2):117‐28. [DOI] [PubMed] [Google Scholar]
PEDro 1999
- Physiotherapy Evidence Database. http://www.pedro.fhs.usyd.edu.au/scale_item.html 1999.
Sager 1996
- Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, et al. Functional outcomes of acute medical illness and hospitalisation in older persons. Archives of Internal Medicine 1996;156:645‐52. [PubMed] [Google Scholar]
Scott 1999
- Scott I. Optimising care of the hospitalised elderly ‐ A literature review and suggestions for future research. Australian and New Zealand Journal of Medicine 1999;29(2):254‐64. [DOI] [PubMed] [Google Scholar]
Tugwell 2004
- Tugwell P, Shea B, Boers M, Brooks P, Simon L, Strand V, et al. Evidence‐based Rheumatology. London: BMJ books, 2004. [Google Scholar]


