Abstract
The role of electrolysis products, including protons, hydroxyl ions, reactive oxygen intermediates, oxygen, hydrogen, and heat, in mediating electrical enhancement of killing of Pseudomonas aeruginosa biofilms by tobramycin (the bioelectric effect) was investigated. The log reduction in biofilm viable cell numbers compared to the numbers for the untreated positive control effected by antibiotic increased from 2.88 in the absence of electric current to 5.58 in the presence of electric current. No enhancement of antibiotic efficacy was observed when the buffer composition was changed to simulate the reduced pH that prevails during electrolysis. Neither did stabilization of the pH during electrical treatment by increasing the buffer strength eliminate the bioelectric effect. The temperature increase measured in our experiments, less than 0.2°C, was far too small to account for the greatly enhanced antibiotic efficacy. The addition of sodium thiosulfate, an agent capable of rapidly neutralizing reactive oxygen intermediates, did not abolish electrical enhancement of killing. The bioelectric effect persisted when all of the ionic constituents of the medium except the two phosphate buffer components were omitted. This renders the possibility of electrochemical generation of an inhibitory ion, such as nitrite from nitrate, an unlikely explanation for electrical enhancement. The one plausible explanation for the bioelectric effect revealed by this study was the increased delivery of oxygen to the biofilm due to electrolysis. When gaseous oxygen was bubbled into the treatment chamber during exposure to tobramycin (without electric current), a 1.8-log enhancement of killing resulted. The enhancement of antibiotic killing by oxygen was not due simply to physical disturbances caused by sparging the gas because similar delivery of gaseous hydrogen caused no enhancement whatsoever.
The striking enhancement of antibiotic efficacy against microbial biofilm by application of a weak direct electric current was first reported by Costerton and coworkers (2, 11), who termed this phenomenon the “bioelectric effect.” Subsequent research has confirmed this effect over a range of conditions (2, 5, 9–11, 19, 20). The significance of the bioelectric effect is that it affords a means to overcome the nearly universally observed reduced susceptibility of microorganisms when they are growing in the biofilm state compared to their susceptibility in suspension cultures (3).
The mechanism of electrical enhancement of antibiotic action remains unclear but is interesting for at least two reasons. First, knowledge of the mechanism will facilitate design of technological applications of electrical enhancement of biofilm killing. Second, data on the mechanism of the bioelectric effect may shed light on the still obscure mechanisms by which biofilms resist antimicrobial challenge. Some of the mechanisms of electrical enhancement of biofilm killing that have been postulated include electrophoretic augmentation of antimicrobial transport (11), membrane permeabilization (11), reduction of biofilm capacity for binding to the antimicrobial agent (2), electrochemical generation of potentiating oxidants (1, 5), increased bacterial growth—and hence increased antibiotic susceptibility—due to electrolytic oxygen generation (9), increased convective transport due to contraction and expansion of the biofilm (15), and increased antimicrobial efficacy due to pH changes resulting from electrolysis reactions (15). Other potential mechanisms of the bioelectric effect include increased transport through electroosmosis (4), physical removal of the biofilm with electrolytically generated gas bubbles, and increased susceptibility due to a temperature increase arising from resistive heating.
The multiple hypotheses on this daunting list are not easily discriminated experimentally. Costerton et al. (5) argued against the electrochemical generation of antimicrobial molecules or ions on the basis of the absence of antimicrobial activity immediately downstream of an electrified chamber. This interpretation is consistent with reports that electric current alone does not result in discernible killing (2, 5, 9, 10). In the experimental system used in the work reported in this article, a slight deleterious effect of the current alone was detected (13). Jass et al. (9) measured a plateau in the electrical enhancement versus current dose-response and suggested that this implied a mechanism other than enhanced transport, which they postulated would behave linearly with current. Stoodley et al. (15) have shown by direct microscopic examination the remarkable expansion and contraction of a biofilm growing on a wire electrode when it is subjected to current polarity reversal. Antimicrobial susceptibility was not measured.
The purpose of the work reported in this article was to investigate the role of electrolysis products in mediating the bioelectric effect. Electrolysis of aqueous solutions leads to the generation of molecular oxygen, molecular hydrogen, hydrogen cations, hydroxyl anions, other reactive oxygen species, and heat. The first few of these effects can be seen by examining the principal cathodic and anodic reactions:
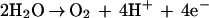 |
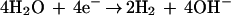 |
The net reaction in a closed system is
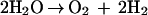 |
Since the test systems used to study the bioelectric effect are all continuous-flow devices, the pH in the system can fall out of balance if one of the electrodes is closer to the reactor effluent than the other. Additional reactions can lead to the formation of reactive oxygen intermediates such as superoxide anion, peroxide, and hydroxyl radicals:
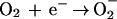 |
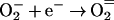 |
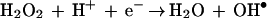 |
Another product of electrolysis is heat. Energy dissipated by resistive heating could raise the temperature of the fluid bathing the biofilm. Because disinfection and growth rates are highly dependent on temperature, it is possible that relatively small increases in temperature could account for part or all of the bioelectric effect. The experiments reported in this paper were designed to test the specific roles of oxygen, hydrogen, pH, active oxygen intermediates, and heat in contributing to the electrical enhancement of antibiotic efficacy.
MATERIALS AND METHODS
Biofilm development.
Pseudomonas aeruginosa ERC1, an environmental isolate maintained in the Center for Biofilm Engineering culture collection, was used in pure culture throughout. Biofilms were grown as described previously (13). The growth medium contained (per liter) 20 mg of glucose, 426 mg of Na2HPO4, 205 mg of KH2PO4, 13.6 mg of KNO3, 1.0 mg of MgSO4, 1.0 mg of CaCO3, 200 μg of nitrilotriacetic acid, 159 μg of FeSO4, 142 μg of ZnSO4, 11.4 μg of MnSO4, 2.8 μg of CuSO4, 2.3 μg of Co(NO3)2, 1.4 μg of Na2B4O7, and 1.4 μg of ammonium molybdate. Experiments were conducted at ambient temperature, which was 18 to 20°C. A continuous-flow stirred reactor containing eight polycarbonate coupons (1.7 by 7.2 cm each) was filled with 32-fold-concentrated medium and inoculated with 1 ml of frozen stock culture. The reactor was operated in batch mode for 24 h with magnetic stirring. After this period of batch growth, continuous flow of regular-strength medium was initiated at a dilution rate of 3.84 h−1. Biofilms were allowed to develop for 72 h in the continuous-flow mode.
Antimicrobial agent-electric current challenge.
The apparatus and protocol for biofilm treatment have been described in detail elsewhere (13). Biofilms developed on polycarbonate slides were transferred aseptically to rectangular treatment chambers with a working fluid volume of approximately 30 ml. The treatment chamber was filled with nutrient medium, amended where indicated with 5 μg of tobramycin per ml, and then a slow continuous flow, approximately 2.8 ml/h, of this same solution was initiated through the chamber. Where indicated, an electric current of 2 mA was delivered through the chamber by means of a circuit containing a current controller and two stainless steel wires at opposite ends of the long axis of the treatment chamber. Electric current flowed approximately parallel to the substratum to which the biofilm was attached at a current density of 4 × 10−4 A/cm2. The potential required to establish this current was approximately 9 to 11 V. The treatment (either untreated control, antibiotic alone, electric current alone, or antibiotic plus electric current) lasted 24 h.
Analytical methods.
At the end of the treatment period, biofilm sample slides were removed from their individual treatment chambers and immediately processed. Biofilm was scraped into a sterile beaker with a stainless steel scraper. The biofilm was resuspended in 10 ml of phosphate buffer, and serial dilutions were drop-plated (7, 14) onto R2A agar (Difco, Detroit, Mich.). The number of CFU were counted after incubation of the plates at 35°C for 18 h. Biofilm areal cell density (numbers of CFU centimeter−2) was calculated by dividing the total number of viable bacteria on the sample slide by the surface area of the slide.
RESULTS
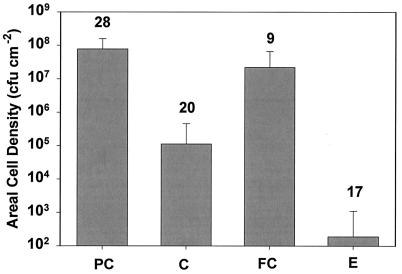
Biofilm viable cell densities after no treatment (which we denote by PC for positive control), treatment with antibiotic alone (denoted C, for control), electric current alone (denoted FC, for field control), and the combination of antibiotic and electric current (denoted E) are summarized in Fig. 1. The untreated positive control exhibited a mean cell density of 7.80 × 107 CFU/cm2. Treatment with antibiotic alone resulted in a mean log reduction of 2.88 ± 0.66 compared to the density of the untreated positive control, and this reduction was statistically significant (P < 10−4). Treatment of planktonic bacteria at an initial cell density of approximately 109 cfu/ml with the same antibiotic concentration for 24 h resulted in a log reduction of 4.9 ± 1.4. A significant reduction in viable cell numbers (log reduction of 0.65 ± 0.42) compared to the numbers for the untreated positive control was measured when the biofilm was exposed to an electric current alone (P = 0.0016). The electrical enhancement of antibiotic efficacy was calculated by comparing the combined treatment against the treatment with antibiotic alone (log [E/C]). The mean log reduction after combined treatment compared to that after antibiotic treatment alone was 2.75 ± 0.95, and this reduction was statistically significant (P < 10−4).
FIG. 1.
Effect of electric current and antibiotic on biofilm. Error bars indicate standard deviations. The number of replicates is indicated above each bar.
We performed four experiments in which the electrodes were placed outside the treatment chamber and a potential was applied. This established an electric field similar to that developed in the normal experiment, but there was no current flow. No enhancement of bacterial killing was measured in these experiments (Table 1).
TABLE 1.
Comparison of bioelectric enhancement of antibiotic efficacy with efficacies of other treatments
| Treatment | Mean log(E/C) | No. of replicates | P (mean = 0)a | P (mean = 2 mA)b |
|---|---|---|---|---|
| 2 mA | −2.75 | 15 | <10−4 | |
| Electric field, no current | 0.33 | 4 | 0.082 | <10−4 |
| 2 mA, 3× buffer | −2.30 | 3 | 0.077 | 0.27 |
| No current, pH 5.0 | 2.41 | 3 | 0.008 | <10−4 |
| 2 mA, 1 mg of thiosulfate per ml | −2.67 | 3 | 0.074 | 0.93 |
| 2 mA, 10 mg of thiosulfate per ml | −5.56 | 3 | <10−4 | <10−4 |
| 2 mA, salts omitted | −2.87 | 3 | 0.044 | 0.87 |
| No current, oxygen | −1.83 | 3 | 0.027 | 0.07 |
| No I, hydrogen | 0.51 | 3 | 0.11 | <10−4 |
The P value reported is that calculated by a two-sample, two-sided t test that compares the mean to zero.
The P value reported is that calculated by a two-sample, two-sided t test that compares the mean to the mean of the experiment with 2 mA alone.
When oxygen was sparged into a treatment chamber receiving antibiotic (but no electrical current), there was a significant (P = 0.027) enhancement of the antibiotic efficacy (Table 1). The enhancement was about 1.8 logs in these experiments, which was approximately two-thirds of the enhancement realized by 2 mA of direct current. No enhancement was detected when hydrogen was sparged during antibiotic challenge (Table 1).
Striking changes in pH occurred when an electric current was applied in this experimental system. The average pHs in the PC, C, FC, and E conditions were 7.16, 7.18, 4.52, and 4.74, respectively. The pH drop observed in experiments with current was statistically significant (P = 0.028).
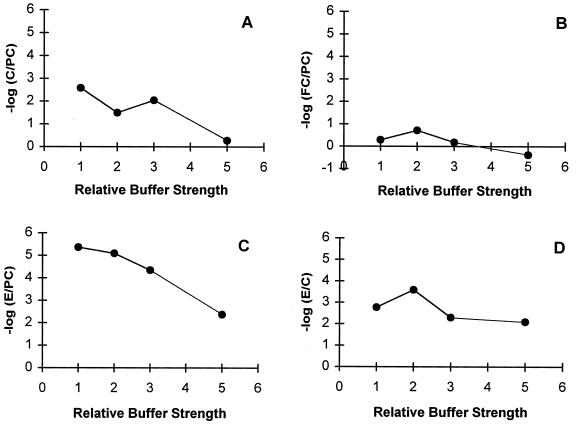
To test whether the pH decrease was responsible for the enhancement of antibiotic efficacy, we performed a series of experiments in which the buffer strength was increased. Increasing the buffer strength reduced the pH change when current was applied, but it also reduced the antibiotic efficacy (Fig. 2). At three times the normal buffer strength, the mean pH in experiments with 2 mA of current was 6.7, whereas in regular buffer the mean pH in experiments with current flow was 4.7. Increasing the buffer strength did not diminish the electrical enhancement of antibiotic action (Fig. 2D). With three times the normal buffer strength the mean log reduction observed in a comparison of the effect of current and antibiotic with the effect of antibiotic alone was slightly less than that for the standard experiment, but it was not significantly different (Table 1).
FIG. 2.
Effect of relative buffer strength on the efficacy of the antibiotic alone (C/PC denotes the ratio of the number of viable cells after treatment with antibiotic alone to the number of viable cells for the untreated positive control), (A), the action of the electric current alone (FC/PC denotes the ratio of the number of viable cells after treatment with the electric current alone to the number of viable cells for the untreated positive control) (B), the combined action of electric current and antibiotic (E/PC denotes the ratio of the number of viable cells after treatment with antibiotic and electric current combined to the number of viable cells for the untreated positive control) (C), and the enhancement of killing by adding an electric current (E/C denotes the ratio of the number of viable cells after treatment with antibiotic and electric current combined to the number of viable cells after treatment with antibiotic alone) (D). Relative buffer strength refers to the multiple by which the concentration of the two phosphate salt constituents of the buffer were changed.
A further test of the role of pH was undertaken by artificially forcing a pH change by altering the relative proportions of the two buffer constituents. The phosphate buffer was formulated to have a pH of 5.0 with the same total phosphate concentration. This forced reduction in pH actually reduced antibiotic efficacy rather than enhanced it (Table 1).
To test the possibility that active oxygen intermediates, such as peroxide, were responsible for potentiating antibiotic efficacy, sodium thiosulfate was added to the medium. Thiosulfate at 1 mg/ml did not abolish the bioelectric effect (Table 1), nor did it affect the efficacy of the antibiotic alone. Thiosulfate at 10 mg/ml reduced the efficacy of the antibiotic alone, but the electrical enhancement of killing was even more dramatic than that in the standard experiment (Table 1). Thiosulfate did not potentiate the killing effect of the electric current alone (Table 2). When a skeletal medium consisting only of glucose and the two phosphate buffer components was used, the electrical enhancement also remained the same (Table 1).
TABLE 2.
Comparison of effects of various treatments in the absence of antibiotic
The measured temperature increase brought about by the delivery of 2 mA for 24 h compared to the temperature in an identical treatment chamber not receiving current was 0.18 ± 0.05°C.
A definitive experiment to preclude the intrusion of electrolysis products into the experimental treatment chamber without the elimination of current flow was attempted. This was done by replacing each wire electrode with a salt bridge, in this case, a flexible tube filled with agar containing sufficient potassium sulfate to conduct 2 mA. These experiments were unsuccessful because the salt leached from the agar bridge and interfered strongly with the action of the antibiotic.
DISCUSSION
We have reproduced the bioelectric effect, the electrical enhancement of antibiotic efficacy against a biofilm, using a model system of P. aeruginosa and tobramycin. In this system, under standard operating conditions the log reduction (compared to the numbers for the untreated positive control) effected by the antibiotic increased from 2.88 logs in the absence of electric current to 5.58 logs in the presence of electric current.
The bioelectric effect requires current flow, not just an electric field. When electrodes were placed outside the treatment chamber to create essentially the same electric field but with zero current, the electrical enhancement of killing was completely abolished (Table 1). Previous experimenters with the bioelectric effect have implemented periodic reversal of the current flow direction, following the lead of the original discoverers. Current reversal is not necessary to obtain electrical enhancement of antibiotic action. In the experiments reported in this article, the current direction was unidirectional over the entire treatment period. This result eliminates enhanced convective transport via electrically driven contraction and expansion of the biofilm (15) as an explanation for the bioelectric effect in this case.
Other mechanisms ruled out in the present experimental system include potentiating effects due to electrolytically generated changes in pH, temperature, and reactive oxygen intermediates. No enhancement of antibiotic efficacy was observed when the buffer composition was changed to stimulate the pH that prevails during delivery of electric current. Neither did a reduction of the pH drop during electrical treatment by increasing the buffer strength eliminate the bioelectric effect. The temperature increase measured in our experiments, less than 0.2°C, is far too small to account for the greatly enhanced antibiotic efficacy. On the basis of the reported temperature dependence of the specific growth rate of P. aeruginosa (12), this temperature increase would translate into an enhancement of only approximately 0.15 log, whereas the measured electrical enhancement was 2.8 logs. The addition of sodium thiosulfate, an agent capable of rapidly neutralizing reactive oxygen intermediates, did not abolish the bioelectric effect. The bioelectric effect persisted when all of the ionic constituents of the medium except the two phosphate buffer components were omitted. This renders the possibility of electrochemical generation of an inhibitory ion, such as nitrite from nitrate, an unlikely explanation for electrical enhancement.
The one plausible explanation for the bioelectric effect revealed by this study was the increased delivery of oxygen to the biofilm due to its generation in situ by electrolysis, a mechanism previously suggested by Jass and colleagues (9). The flow of current established in bioelectric experiments exceeded that required theoretically to saturate the aqueous medium with oxygen. The appearance of gas bubbles in the treatment chamber was noted during these experiments. Measurements with a dissolved oxygen probe revealed clearly elevated levels of dissolved oxygen in electrified treatment chambers, although we have not reported specific values because the calibration of the oxygen meter was later shown to be faulty. When gaseous oxygen was bubbled into the treatment chamber during exposure to tobramycin (but with no electric current), a 1.8-log enhancement of killing resulted. Oxygen applied without antibiotic decreased the level of biofilm accumulation compared to that for the positive control by 0.47 log (Table 2), mimicking the effect of the direct current alone (0.65 log reduction). The enhancement of antibiotic killing by oxygen was not due simply to the physical disturbances caused by sparging the gas because similar delivery of gaseous hydrogen caused no enhancement whatsoever.
The mechanism by which oxygen enhances biofilm susceptibility remains to be established. One possibility is that oxygen reaches toxic levels, weakening the cells and making them more susceptible to the antibiotic. The observation that sparging with oxygen alone causes a small but statistically significant reduction in biofilm accumulation (Table 2) seems to support this hypothesis. On the other hand, increased delivery of oxygen could enhance growth in the biofilm, thereby overcoming the reduced susceptibility associated with slow growth (6). P. aeruginosa is an obligate aerobe, and biofilms of this microorganism have recently been shown to be readily oxygen limited, leading to zones of slow or no growth within the depths of the biofilm (8, 21). Furthermore, it is well known that aminoglycoside antibiotics are less effective under anaerobic conditions than under aerobic conditions (16, 18). If biofilm resistance to antibiotics is due to oxygen deprivation in the biofilm, then augmentation of the concentration of this or an alternative electron acceptor could make the biofilm more susceptible (17). Such hypotheses merit further investigation from the standpoint both of developing practical applications of the bioelectric effect and for understanding the fundamental mechanisms by which microorganisms in biofilms resist the actions of antimicrobial agents.
REFERENCES
- 1.Armstrong S. Electric fields (biofilm killing) ASM News. 1993;59:270–271. [Google Scholar]
- 2.Blenkinsopp S A, Khoury A E, Costerton J W. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 1992;58:3770–3773. doi: 10.1128/aem.58.11.3770-3773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown M R W, Gilbert P. Sensitivity of biofilms to antimicrobial agents. J Appl Bacteriol Symp Suppl. 1993;74:87S–97S. doi: 10.1111/j.1365-2672.1993.tb04345.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y-H, D, Grodzinsky A J, Wang D I C. Augmentation of mass transfer through electrical means for hydrogel-entrapped Escherichia coli cultivation. Biotechnol Bioeng. 1995;48:149–157. doi: 10.1002/bit.260480209. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J W, Ellis B, Lam K, Johnson F, Khoury A E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–2809. doi: 10.1128/aac.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert P, Brown M R W. Mechanisms of the protection of bacterial biofilms from antimicrobial agents. In: Lappin-Scott H M, Costerton J W, editors. Microbial biofilms. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 118–130. [Google Scholar]
- 7.Hoben H J, Somasegaran P. Comparison of the pour, spread, and drop plate methods for enumeration of Rhizobium spp. in inoculants made from presterilized peat. Appl Environ Microbiol. 1948;44:1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C-T, Xu K D, McFeters G A, Stewart P S. Spatial patterns of alkaline phosphatase expression within bacterial colonies and biofilms in response to phosphate starvation. Appl Environ Microbiol. 1998;64:1526–1531. doi: 10.1128/aem.64.4.1526-1531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jass J, Costerton J W, Lappin-Scott H M. The effect of electrical currents and tobramycin on Pseudomonas aeruginosa biofilms. J Indust Microbiol. 1995;15:234–242. doi: 10.1007/BF01569830. [DOI] [PubMed] [Google Scholar]
- 10.Jass J, Lappin-Scott H M. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. J Antimicrob Chemother. 1996;38:987–1000. doi: 10.1093/jac/38.6.987. [DOI] [PubMed] [Google Scholar]
- 11.Khoury A E, Lam K, Ellis B, Costerton J W. Prevention and control of bacterial infections associated with medical devices. Am Soc Artif Organs J. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Leitão J H, Fialho A M, Correia I S. Effects of growth temperature on alginate synthesis and enzymes in Pseudomonas aeruginosa variants. J Gen Microbiol. 1992;138:605–610. doi: 10.1099/00221287-138-3-605. [DOI] [PubMed] [Google Scholar]
- 13.McLeod, B. R., S. M. Fortun, and P. S. Stewart. A standard test system and a dose response for the electrical enhancement of antibiotic efficacy against biofilms. Submitted for publication.
- 14.Reed R W, Reed G B. “Drop plate” method of counting viable bacteria. Can J Res. 1948;26:317–326. [Google Scholar]
- 15.Stoodley P, de Beer D, Lappin-Scott H M. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob Agents Chemother. 1997;41:1876–1879. doi: 10.1128/aac.41.9.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tack K J, Sabath L D. Increased minimum inhibitory concentration with anaerobiasis for tobramycin, gentamicin, and amikacin, compared to latamoxef, piperacillin, chloramphenicol, and clindamycin. Chemotherapy (Basel) 1985;31:204–210. doi: 10.1159/000238337. [DOI] [PubMed] [Google Scholar]
- 17.Tresse O, Jouenne T, Junter G-A. The role of oxygen limitation in the resistance of agar-entrapped sessile-like Escherichia coli to aminoglycoside and betalactam antibiotics. J Antimicrob Chemother. 1995;36:321–326. doi: 10.1093/jac/36.3.521. [DOI] [PubMed] [Google Scholar]
- 18.Verklin R M, Mandell G L. Alteration of effectiveness of antibiotics by anaerobiosis. J Lab Clin Med. 1977;89:65–71. [PubMed] [Google Scholar]
- 19.Wellman N, Fortun S M, McLeod B R. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother. 1996;40:2012–2014. doi: 10.1128/aac.40.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitham T S. Assessment of the potential of the bioelectric effect to control microbial biofilm in mild steel pipelines. In: Wimpenny J, Handley P, Gilbert P, Lappin-Scott H, editors. The life and death of biofilm. Cardiff, United Kingdom: BioLine; 1995. pp. 133–136. [Google Scholar]
- 21.Xu K D, Stewart P S, Xia F, Huang C-T, McFeters G A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998;64:4035–4039. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]