Abstract
Human papillomavirus (HPV) prevalence and genotype distribution data is important for HPV vaccine monitoring. This study investigated the prevalence and distribution of HPV genotypes in cervical lesions of unvaccinated women referred to Nelson Mandela Academic Hospital Gynaecology Department due to different abnormal cervical conditions. A total of 459 women referred to the Nelson Mandela Academic Hospital Gynaecology department were recruited. When the cervical biopsy was collected for histopathology, an adjacent biopsy was provided for HPV detection. Roche Linear Array HPV genotyping assay that detects 37 HPV genotypes was used to detect HPV infection in cervical biopsies. HPV infection was detected in 84.2% (383/455) of participants. The six most dominant HPV types were HPV-16 (34.7%), followed by HPV-35 (17.4%), HPV-58 (12.1%), HPV-45 (11.6%), HPV-18 (11.4%) and HPV-52 (9.7%). HPV-35 was the third most dominant type among women with cervical intraepithelial lesion (CIN)-2 (12.6%; single infection: 5.7% and multiple infection: 6.9%), the second most dominant type among women with CIN3 (22.2%; single infection: 8.0% and multiple infection: 14.2%); and the fourth most dominant type among women with cervical cancer (12.5%; single infection: 7.1% and multiple infection: 5.4%). A proportion of 41.1% (187/455) was positive for HPV types targeted by the Cervarix®, 42.4% (193/455) by Gardasil®4, and 66.6% (303/455) by Gardasil®9. There was a statistically significant increase when the prevalence of women infected with HPV-35 only or with other HPV types other than Gardasil®9 types was included to those infected with Gardasil®9 HPV types (66.6%, 303/455 increase to 76.0%, 346/455, p = 0.002). High HPV-35 prevalence in this population, especially among women with CIN3 warrants attention since it is not included in current commercially available HPV vaccines.
Introduction
Cervical cancer is the second most common cancer in South African women [1]. The National Cancer Registry (NCR) of South Africa reported an age-standardized rate (ASR) of 22.56 per 100,000 for all South African women in 2014 and an ASR of 27.01 per 100,000 for black African women [2]. According to Somdyala et al. (2020), in the rural Eastern Cape Province of South Africa, the annual cervical cancer ASR per 100,000 increased from 22.0 in 1998–2002 to 29.2 in 2008–2012 [3]. Of the African countries, South Africa has the largest population affected by human immunodeficiency virus (HIV) infection, with 7.8 million people living with HIV and 230 000 new infections reported in 2020 [4]. Both cervical cancer and HIV burden are high in African countries [5–7].
Human papillomavirus (HPV) is the most common sexually transmitted virus [8–11], with its peak prevalence observed in adolescents and young women soon after sexual debut and decreasing with increasing age in women [12,13]. Persistent infection with HR-HPV genotypes is associated with the development of cervical lesions and cervical cancer [14]. Compared to HIV-negative individuals, the HIV-infected individuals are more likely to be infected by HPV, co-infected with multiple HPV types, persistent infection, reactivation, and develop HPV-associated cancers on different anatomical sites [7,15–18]. Among HPV types known to be dominant in cervical cancer cases worldwide, HPV-16 is the most common type worldwide, followed by HPV-18 [19–21]. It is important to note that among women of African ancestry origin, HPV-35 is detected in approximately 10% of cervical cancer cases, while it is detected in approximately 2% of worldwide cases [1,21,22]. Studies in Sub-Saharan African populations have reported an HPV-35 prevalence of up to 40% among women with cervical intraepithelial neoplasia (CIN) or cervical cancer [21,31,35–38].
Currently, there are three HPV vaccines approved by the U.S Food and Drug Administration. They are Cervarix® (GlaxoSmithKline), Gardasil®4 (Merck Inc), and Gardasil®9 (Merck Inc). They target a different combination of HPV types; Cervarix® targets HPV-16/18, the most carcinogenic HPV types associated with approximately 70% cervical cancer cases; Gardasil®4 targets HPV-16/18 as well as two low-risk (LR) types, HPV-6/11, associated with genital warts and recurrent respiratory papillomatosis; and Gardasil®9 (Merck Inc) targets same types as Gardasil®4 and the other five high-risk (HR) HPV types HPV-31/33/45/52/58 [6,23–25]. Sub-Saharan African HIV-positive women were reported to have decreased HPV types targeted by Gardasil®9 HPV vaccine (HPV-6/11/16/18/31/33/45/52/58) when compared with Sweden-born HIV-negative and HIV-positive women. These observations were influenced by decreased HPV-16 and increased HPV-35 prevalence in sub-Saharan African women [26]. Unfortunately, the currently commercially available HPV vaccines are not targeting HPV-35 [6].
Many countries have implemented HPV vaccination programs since the first licensure of Gardasil®4 in 2006 [24,25]. In South Africa, the school-based national HPV vaccination program was introduced in 2014, targeting girls aged nine years or older (mostly in grade-4), and the Cervarix® HPV vaccine two-dose schedule is used in this program. The vaccine schedules are 6-months apart within the academic calendar year [23,27,28]. Different strategies are implemented to prevent pre-invasive lesions and cervical cancer, mainly through HPV vaccination and cervical cancer screening [29].
As part of the HPV vaccination strategy in South Africa, it is essential to have information on HPV prevalence and HPV types distribution among the unvaccinated population to inform vaccination campaigns and monitor the impact on HPV types after vaccination [30]. The data needs to come from population-based surveillance and women with cervical disease in unvaccinated women. Information on the prevalence of HPV and the distribution of HPV types in women residing in the Eastern Cape Province of South Africa is limited [31] and based on cervical specimens collected by cervical brush. The current report presents HPV genotyping data in cervical biopsies and this is significant because HPV types detected in cervical biopsies are more likely to be integrated into tissue and associated with the observed lesion. In contrast, in cytobrush specimens, all the HPV genotypes that are on the cervix will be detected including transient types [32,33]. Therefore, this study aimed to investigate the prevalence and distribution of HPV genotypes in cervical biopsy of HPV unvaccinated women referred to Gynaecology Department Nelson Mandela Academy Hospital in Mthatha, Eastern Cape.
Materials and methods
Ethical statement
Participation in the study was voluntary, with written informed consent. This study was approved by the Human Research Ethics Committees of the University of Cape Town (HREC: 079/2014) and Walter Sisulu University (reference: 090/2016). Permission to conduct research in the Eastern Cape was granted by the Eastern Cape Provincial Health Research Committee (EC_2016RP29_562). Participation in the study was voluntary, with written informed consent.
Study setting, population, and recruitment
This project is a hospital-based project among women with high-grade cervical lesions and cervical cancer referred to Nelson Mandela Academy Hospital in Mthatha, Eastern Cape. Nelson Mandela Academic Hospital serves most of the population residing in the former Transkei region of the Eastern Cape Province (OR Tambo, Chis Hani, Alfred Nzo, Amathole, and Joe Gqabi municipality). Between February 2018 and March 2020, women aged ≥18 years with atypical squamous cells cannot exclude high-grade lesions (ASC-H), low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL), and cervical cancer referred to Nelson Mandela Academic Hospital Gynaecology department were recruited. When the cervical biopsy was collected for histopathology, an adjacent piece was provided for HPV detection. Histopathology was conducted by National Health Laboratory Service, Histopathology Laboratory at the Nelson Mandela Academic Hospital. Cervical biopsy for HPV detection was stored in Digene transport medium at -20°C and transported to the University of Cape Town, HPV Laboratory.
Nucleic acid extraction and HPV detection
A cervical biopsy specimen was lysed using MagNA Pure 96 tissue lysis buffer. Nucleic acid was extracted using an automated procedure of MagNA Pure Compact (Roche Molecular Systems, Inc., Branchburg, NJ, USA) and MagNA Pure Compact Nucleic Acid Isolation Kit (Roche Molecular Systems, Inc., Branchburg, NJ, USA). Roche Linear Array HPV Genotyping Test (Roche Molecular Systems, Inc., Branchburg, NJ, USA) was used to detect HPV genotypes in extracted nucleic acid from cervical biopsy specimens and manufacturer instructions were followed. The Linear Array HPV Genotyping Test amplifies the target HPV DNA for 37 anogenital HPV genotypes, namely, HPV-6, -11, -16, -18, -26, -31, -33, -35, -39, -40, -42, -45, -51, -52, -53, -54, -55, -56, -58, -59, -61, -62, -64, -66, -67, -68, -69, -70, -71, -72, -73, -81, -82, -83, -84, -IS39 and -CP6108 (HPV-89). The Linear Array HPV Genotyping Test also amplifies the β-globin gene to monitor sample adequacy, extraction, amplification, and hybridization.
Data analysis
All variables were captured and coded in Microsoft Excel 2013. Participants were counted more than once when determining the prevalence of LR-HPV, HR-HPV, and probable HR-HPV if they have types that belong to more than one category. Single infection was defined as infection with one HPV type. Multiple HPV infections were defined as the detection of two or more HPV types in the same sample. Statistical analysis was performed using chi-squared for trends and Fisher’s exact for comparison of the HPV prevalence (GraphPad Prism Software v6.01). The level of significance was set at 5% (p-value ≤ 0.05) for statistical significance.
Results
Demographic characteristics of study participants
A total of 459 women were recruited. Four samples were negative for the β-globin gene and were therefore excluded from the analysis. Study participants were between the ages of 18 and 90 years, with a median of 42 years. The majority of study participants were HIV-positive (65.7%, 299/455), and 88.3% were on ARVs. Only 19.8 (90/455) had training after high school education. A higher proportion of study participants had three to four lifetime sexual partners (Table 1).
Table 1. Demographic and behavioural characteristics of study participants.
| Characteristics | % | n/N |
|---|---|---|
| Age in years, median (range) | 42 (18–90) | |
| Age group | ||
| 18–30 years | 5.9 | 27 |
| 31–50 years | 32.3 | 147 |
| 51–90 years | 61.8 | 281 |
| HIV status | ||
| positive | 65.7 | 299/455 |
| negative | 27.5 | 125/455 |
| missing | 6.8 | 31/455 |
| If HIV-positive, on ARVs | ||
| Yes | 88.3 | 264/299 |
| No | 11.7 | 35/299 |
| Education | ||
| Never | 4.6 | 21/455 |
| Primary school (Grade 1–7) | 28.6 | 130/455 |
| High school (Grade 8–12) | 47.0 | 214/455 |
| University | 19.8 | 90/455 |
| Age at first sex | ||
| ≤16 years | 24.4 | 111/455 |
| 17–18 years | 31.9 | 145/455 |
| 19–20 years | 31.2 | 142/455 |
| 21–33 years | 12.5 | 57/455 |
| Lifetime sexual partners | ||
| 1–2 | 28.8 | 131/455 |
| 3–4 | 55.2 | 251/455 |
| 5–10 | 16.0 | 73/455 |
| Cervical cytology | ||
| ASC-H | 8.8 | 40/455 |
| LSIL | 5.5 | 25/455 |
| HSIL | 81.5 | 371/455 |
| Cervical cancer | 4.2 | 19/455 |
| Cervical histology | ||
| CIN1 | 2.2 | 10/455 |
| CIN2 | 19.1 | 87/455 |
| CIN3 | 38.7 | 176/455 |
| Cervical cancer | 12.3 | 56/455 |
| No dysplasia | 9.2 | 42/455 |
| No results | 17.8 | 81/455 |
| Poor quality specimens | 0.7 | 3/455 |
HPV prevalence and genotype distribution
HPV infection was detected in 84.2% (383/455) of women. The majority of participants were infected with HR-HPV type(s) (80.2%, 365/455). While only 8.1% (37/455) were infected with probable HR-HPV type(s), and 15.4% (70/455) with LR-HPV type(s). Infection with single HPV type (46.6%, 212/455) was common than multiple HPV infections (37.6%, 171/455, p = 0.007) in this population (Table 2). The overall HPV prevalence remained high in both HIV-positive women (88.3%, 264/299) and HIV-negative (80.0%, 100/125, p = 0.063). When stratified according to carcinogenicity level, the prevalence of multiple infection, HR-HPV, and LR-HPV was found to be significantly high among HIV-positives compared to HIV-negative individuals (p = 0.022, p = 0.038, and p = 0.025 respectively, Table 2).
Table 2. Prevalence of HPV infection according to HIV status among women referred to Nelson Mandela Academic Hospital Gynaecology department, Eastern Cape Province.
| Variables | All participants, N455 | HIV-negative, N = 125 | HIV-positive, N = 299 | No HIV status, N = 31 | p-value* |
|---|---|---|---|---|---|
| Any types | 84.2%, 383/455 | 80.0%, 100/125 | 88.3%, 264/299 | 61.3%, 19/31 | 0.063 |
| Single infection | 46.6%, 212/455 | 49.6%, 62/125 | 45.8%, 137/299 | 41.9%, 13/31 | 0.522 |
| Multiple infection | 37.6%, 171/455 | 30.4%, 38/125 | 42.5%, 127/299 | 19.4%, 6/31 | 0.022 |
| HR-HPV types | 80.2%, 365/455 | 76.0%, 95/125 | 84.6%, 253/299 | 54.8%, 17/31 | 0.038 |
| Probable HR-HPV types | 8.1%, 37/455 | 40.0%, 5/125 | 10.4%, 31/299 | 3.2%, 1/31 | 0.035 |
| LR-HPV | 15.4%, 70/455 | 8.8%, 11/125 | 17.7%, 53/299 | 19.4%, 6/31 | 0.025 |
*compares HIV–negative and positive prevalence. HR–HPV: High–risk human papillomavirus; LR–HPV: Low–risk human papillomavirus; HR–HPV types: HPV–16, –18, –31, –33, –35, –39, –45, –51, –52, –56, –58 and –59. Probable HR–HPV types: HPV–26, –53, –66, –67, –68, –70, –73 and –82. LR–HPV: HPV–6, –11, –40, 42, –54, –55, –61, –62, –64, –69, –71, –72, –81, –83, –84, –89 (CP6108) and–IS39.
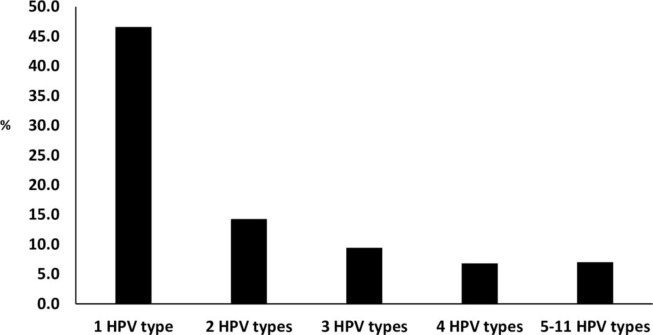
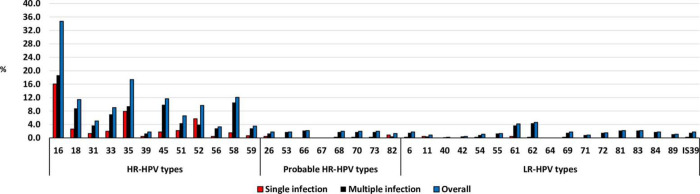
A proportion of 46.6% (212/455) were infected with one HPV type, 14.3% (65/455) were infected with two different HPV types, 9.5% (43/455) were infected with three different HPV types, 6.8% (31/455) were infected with four different HPV types, and 7.0% (32/455) were infected with five to eleven different HPV types (Fig 1). The distribution of HPV types detected among participants is presented in Fig 2. The six most dominant HPV types were HPV-16 (34.7%), followed by HPV-35 (17.4%), HPV-58 (12.1%), HPV-45 (11.6%), HPV-18 (11.4%) and HPV-52 (9.7%, Fig 2). When focusing on dominant HPV types that were detected as a single infection, HPV-16 remains the most dominant type, followed by HPV-35 and HPV-52 (Fig 2).
Fig 1. Human papillomavirus infection among women referred to Nelson Mandela Academic Hospital Gynaecology department, Eastern Cape Province.
Fig 2. Human papillomavirus genotype distribution among women referred to Nelson Mandela Academic Hospital Gynaecology department, Eastern Cape Province.
Single HPV infection is defined as infection with one HPV type, while multiple HPV infections as the detection of two or more HPV types in the same sample.
HPV prevalence according to cervical histology data
A proportion of 98.2% (55/56) women with cervical cancer were HPV infected, and infection with a single HPV type was more common than multiple infections (58.9% 33/56; 39.3% 22/56, p = 0.058). Of women with CIN-3, 89.7% (157/175) were HPV infected, 85.1% (74/87) CIN-2 women, and 50.0% (5/10) CIN-1 (Table 3). HR-HPV types were common, and their prevalence was increasing with disease severity, CIN2 (79.3%, 69/87), CIN3 (86.9%, 153/176), and cervical cancer (96.4%, 54/56, p = 0.003). The HPV prevalence was found to be higher among the HIV-positive women with CIN2 (92.2%, 59/64) compared to HIV-negatives (65.2%, 15/23, p = 0.004, Table 3).
Table 3. Human papillomavirus prevalence according to cervical histology data among women referred to Nelson Mandela Academic Hospital Gynaecology department, Eastern Cape Province.
| All | HIV-positive | HIV-negative | p-value | ||||
|---|---|---|---|---|---|---|---|
| Variable | % | n/N | % | n/N | % | n/N | |
| CIN2, N = 87 | |||||||
| Any HPV infection | 85.1 | 74/87 | 92.2 | 59/64 | 65.2 | 15/23 | 0.004 |
| Single HPV infection | 47.1 | 41/87 | 50.0 | 32/64 | 39.1 | 9/23 | 0.467 |
| Multiple HPV infection | 37.9 | 33/87 | 42.2 | 27/64 | 26.1 | 6/23 | 0.215 |
| HR-HPV types | 79.3 | 69/87 | 87.5 | 56/64 | 56.5 | 13/23 | 0.005 |
| 1HR-HPV type | 53.6 | 37/69 | 51.8 | 29/56 | 61.5 | 8/13 | 0.556 |
| ≥2HR-HPV types | 17.4 | 12/69 | 21.4 | 12/56 | 0.0 | 0/13 | 0.104 |
| 1HR-HPV & other types | 11.6 | 8/69 | 10.7 | 6/56 | 15.4 | 2/13 | 0.639 |
| ≥2HR-HPV & other types | 17.4 | 12/69 | 16.1 | 9/56 | 23.1 | 3/13 | 0.685 |
| CIN3, N = 176 | |||||||
| Any HPV infection | 89.8 | 158/176 | 90.7 | 116/129 | 87.2 | 41/47 | 0.592 |
| Single HPV infection | 48.3 | 85/176 | 45.7 | 58/129 | 55.3 | 26/47 | 0.401 |
| Multiple HPV infection | 41.5 | 73/176 | 45.0 | 58/129 | 31.9 | 15/47 | 0.237 |
| HR-HPV types | 86.9 | 153/176 | 86.8 | 111/129 | 87.2 | 41/47 | 1.000 |
| 1HR-HPV type | 53.6 | 82/153 | 50.0 | 55/111 | 63.4 | 26/41 | 0.146 |
| ≥2HR-HPV types | 22.9 | 35/153 | 22.3 | 25/111 | 24.4 | 10/41 | 0.830 |
| 1HR-HPV & other types | 9.8 | 15/153 | 13.4 | 15/111 | 0.0 | 0/41 | 0.012 |
| ≥2HR-HPV & other types | 13.7 | 21/153 | 14.3 | 16/111 | 12.2 | 5/41 | 1.000 |
| Cervical cancer, N = 56 | |||||||
| Any HPV infection | 98.2 | 55/56 | 100.0 | 33/33 | 95.5 | 21/22 | 0.400 |
| Single HPV infection | 58.9 | 33/56 | 54.5 | 18/33 | 63.6 | 14/22 | 0.583 |
| Multiple HPV infection | 39.3 | 22/56 | 45.5 | 15/33 | 31.8 | 7/22 | 0.403 |
| HR-HPV types | 96.4 | 54/56 | 97.0 | 32/33 | 90.9 | 21/22 | 1.000 |
| 1HR-HPV type | 59.3 | 32/54 | 53.1 | 17/32 | 66.7 | 14/21 | 0.300 |
| ≥2HR-HPV types | 33.3 | 18/54 | 34.4 | 11/32 | 33.3 | 7/21 | 1.000 |
| 1HR-HPV & other types | 1.9 | 1/54 | 3.1 | 1/32 | 0.0 | 0/21 | 1.000 |
| ≥2HR-HPV & other types | 5.6 | 3/54 | 9.4 | 3/32 | 0.0 | 0/21 | 0.269 |
*compares HIV–negative and positive prevalence. Single HPV infection is defined as infection with one HPV type, while multiple HPV infections as the detection of two or more HPV types in the same sample. HR–HPV: High–risk human papillomavirus; LR–HPV: Low–risk human papillomavirus; HR–HPV types: HPV–16, –18, –31, –33, –35, –39, –45, –51, –52, –56, –58 and –59. Probable HR–HPV types: HPV–26, –53, –66, –67, –68, –70, –73 and –82. LR–HPV: HPV–6, –11, –40, 42, –54, –55, –61, –62, –64, –69, –71, –72, –81, –83, –84, –89 (CP6108) and–IS39.
HIV–positive and HIV–negative participants do not always add up to all women because there were participants with unknown HIV status.
Among women with cervical cancer, HPV-16 (64.3%) was the most dominant type, followed by HPV-45 (21.4%), HPV-18 (19.6%), and HPV-35 (12.5%). Among women with CIN3, HPV-16 (35.2%) was the most dominant type, followed by HPV-35 (22.2%), HPV-58 (15.3%), and HPV-45 (12.5%). Among women with CIN2, HPV-16 (33.3%) remain the most dominant type, followed by HPV-58 (13.8%), HPV-35 (12.6%), and HPV-45 (12.6%, Fig 3).
Fig 3.
High–risk human papillomavirus genotype distribution among women with CIN2 (A), CIN3 (B) or cervical cancer (C) referred to Nelson Mandela Academic Hospital Gynaecology department, Eastern Cape Province according to cervical histology data.
Infection with a single HPV type (47.1%, 41/87) was more common than infection with multiple HPV types (37.9%, 33/87, p = 0.283) among women with CIN2 (Table 3). When focusing on the frequency of each HPV type detected in multiple infection, it was found to be between 1.1% and 11.5% among the HR-HPV types; and 0.0% - 9.2% for the LR/probable-HPV types. While among single infection, it was found to range between 0.0% and 16.1% among the HR-HPV types; and 0.0% and 2.3% for the LR/probable-HPV types (Fig 3A).
Infection with a single HPV type (48.3%, 85/176) was more common than multiple HPV infections (41.5%, 73/176, p = 0.238) among women with CIN3 (Table 3). When focusing on the frequency of each HPV type detected in multiple infection, it was found to be between 2.8% and 18.8% among the HR-HPV types; and 0.0% - 4.5% for the LR/probable-HPV types. While among single infection, it was found to range between 0.0% and 16.5% among the HR-HPV types; and 0.0% and 0.6% for the LR/probable-HPV types (Fig 3B).
Infection with a single HPV type (58.9%, 33/56) was more common than infection with multiple HPV types (39.3%, 22/56, p = 0.058) among women with cervical cancer (Table 3). When focusing on the frequency of each HPV type detected in multiple infections, it was found to be between 0.0% and 33.9% among the HR-HPV types; and 0.0% and 3.6% for the LR/probable-HPV types. While among single infection, it was found to range between 0.0% and 30.4% among the HR-HPV types; and 0.0% and 1.8% for the LR/probable-HPV types (Fig 3C).
HPV type distribution and prevalence of HPV types targeted by HPV vaccines
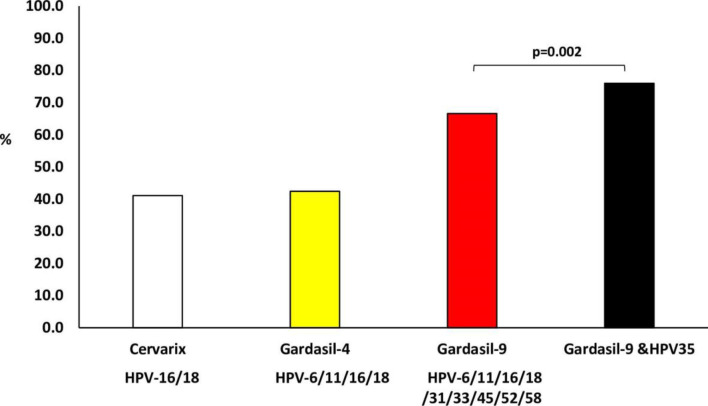
HPV type(s) targeted by the Cervarix® HPV vaccine (HPV-16 and/or 18), currently used in the South African school-based HPV vaccination program, were detected in 41.1% (187/455), those targeted by Gardasil®4 (HPV-6, -11, -16 and/or -18) were detected in 42.4% (193/455), and those targeted by Gardasil®9 (HPV-6, -11, -16, -18, -31, -33, -45, -52 and/or -58) were detected in 66.6% (303/455, Fig 4).
Fig 4. Prevalence of Human papillomavirus (HPV) types targeted by current commercial HPV vaccines among women referred to Nelson Mandela Academic Hospital Gynaecology Department, Eastern Cape.
(Cervarix vaccine targets HPV–16/18; Gardasil–4 vaccine targets HPV–6/11/16/18 and Gardasil–9 vaccine targets HPV–6/11/16/18/31/33/45/52/58).
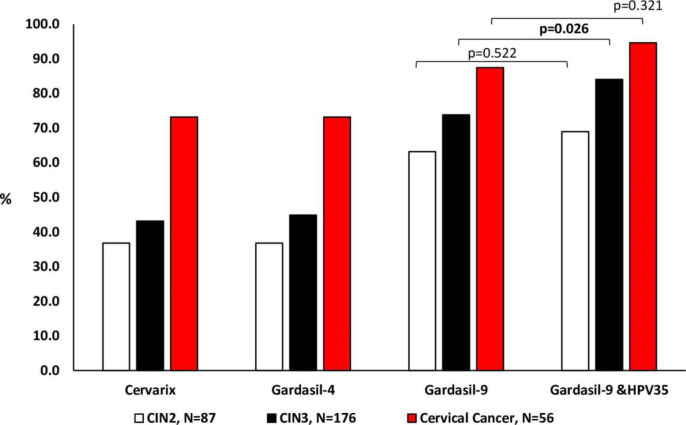
When stratified according to cervical disease status, types targeted by the Cervarix® HPV vaccine were detected in 36.8% (32/87) women with CIN2, 43.2% (76.176) women with CIN3, and 73.2% (41/56) women with cervical cancer. Types targeted by the Gardasil®4 HPV vaccine were detected in 36.8% (32/87) women with CIN2, 44.9% (79/176) women with CIN3, and 73.2% (41/56) women with cervical cancer. Types targeted by Gardasil®9 HPV vaccine were detected in 63.2% (55/87) women with CIN2, 73.9% (130/176) women with CIN3, and 87.5% (49/56) women with cervical cancer (Fig 5).
Fig 5. Prevalence of Human papillomavirus (HPV) types targeted by current commercial HPV vaccines among women according to cervical disease status (Cervarix vaccine targets HPV–16/18; Gardasil–4 vaccine targets HPV–6/11/16/18 and Gardasil–9 vaccine targets HPV–6/11/16/18/31/33/45/52/58).
HPV-35 prevalence and distribution according to cervical histology diagnosis
HPV-35 was the second most common HPV type detected (17.4%), single infection was observed in 7.9% and multiple infection in 9.5% (Fig 2). Among women with CIN2, HPV-35 was the third most dominant type (12.6%), single infection was observed in 5.7% and multiple infection in 6.9%. While among women with CIN3, HPV-35 was the second most dominant type (22.2%), single infection was in 8.0% and multiple infection in 14.2%. HPV-35 was the fourth most common type among women with cervical cancer (12.5%), single infection was observed in 7.1% and multiple infection in 5.4% (Fig 3).
There was a statistically significant increase when the prevalence of women infected with HPV-35 only or with other HPV types other than those targeted by Gardasil®9 was included among those infected with Gardasil®9 HPV types (66.6%, 303/455 increase to 76.0%, 346/455, p = 0.002) in the overall population. When participants were grouped according to histology, this increase was also observed among women with CIN3 (73.9%, 130/176 increase to 84.1%, 148/176, p = 0.026). However, there was no significant increase among CIN2 (63.2%, 55/87; 69.0%, 60/87, p = 0.522) and cervical cancer (87.5%, 49/56; 94.6%, 53/56, p = 0.321, Fig 5).
Discussion
This study investigated the cervical HPV prevalence and distribution among women referred to the hospital with cervical disease. As expected, the overall HPV (84.2%) was high in the study population. However, the overall HPV prevalence among women with CIN2 (85% compared to 93%) and CIN3 (89% compared to 97%) was lower than the study recently reported by Taku et al. in the same district as the current report [31]. The observed low overall HPV prevalence and that of individual types in the current study could be affected by specimen type and collection. The cervical biopsy was used in the current study for HPV detection, while the cervical swab/brush collected from the whole cervix was used in the study by Taku et al. [31]. When the cervical biopsy was collected for histopathology analysis, an adjacent piece was provided for HPV detection; it is possible that the biopsy for HPV detection was not part of the lesion. Two cervical cancer cases were negative for HR-HPV infection and could be an indication of false-negative result due to the integration of HPV which resulted in disruption or loss of the primer targeted sequence. It is also possible that the HPV DNA was absent in the collected cervical biopsy [32].
The majority of the observed single infections appeared as HR-HPV types. Few LR-HPV types were detected as a single infection and their prevalence was low (0.4%). It is possible that the probable HR or HR-HPV type(s) responsible for the observed cervical lesion was mis-detected [32]. Even though infection with a single HPV type was common in this study the prevalence of multiple infection was high considering the fact that the cervical biopsy specimen was used, and this was more commonly observed among HR-HPV than in probable/LR-HPV types. HPV multiple infections is reported to be higher when cervical cells are used as the specimen than when the biopsies/tissue is used [33]. According to Guan et al. (2012) using cervical biopsies tends to reduce HPV multiple infection prevalence and increase the focus on HPV types causally associated with the observed lesion [33]. However, in this study, the high prevalence of multiple HPV types even when biopsies were used could be due to cross-contaminated during specimen collection because of the high prevalence of multiple HPV types in the South African population [34,35].
HR-HPV prevalence was high and increasing with increasing cervical disease [21,36,37]. Even though LR-HPV types were detected, they were more commonly detected as multiple HPV infections with other HR, probable and/or LR-HPV types. It is important to note that among the types not targeted by current commercial HPV vaccines but commonly detected in cervical cancer cases among women of African ancestry origin [22], HPV-35 was detected in 17.4% of the study population and appeared as a single infection in 7.9%. The prevalence of HPV-35 among women with CIN3 was similar to the one reported by Taku et al. (2021) in the same Province. Rad et al. (2017) reported HPV-35 as the fourth most common HPV type among South African women with cervical cancer [36]. Similarly, Denny et al. reported HPV-35 (9.7%) as the fourth most dominant type among women from South Africa, Ghana, and Nigeria with invasive cervical cancer [21]. In a meta-analysis study, Clifford et al. (2016) also report HPV-35 as the fourth most dominant type among African women with invasive cervical cancer regardless of HIV status [38]. Among Botswana women with CIN2 or CIN3, an HPV-35 prevalence of 40.0% has been reported [39]. The current HPV vaccines do not target HPV-35; the addition of HPV-35 to the Gardasil®9 types would increase the protection against HPV-associated diseases among women of African ancestry [22].
HPV-52 was found to be the sixth most dominant type in this population; however, it could be underestimated because, in cases of co-infection with HPV-33, -35, or -58, the Roche HPV genotyping assay used in the study cannot determine if HPV-52 is also present. It is therefore important to further investigate HPV-52 prevalence in this population. It is acknowledged that the study population does not represent the population of Eastern Cape Province and cannot be generalised. Despite these limitations, the information reported remains important for this province and South Africa as there is currently limited HPV information on this population.
Conclusion
High overall HPV and HR-HPV prevalence were observed. HR-HPV prevalence was significantly increasing with increasing cervical intraepithelial lesion grades. HPV-35 was among the most commonly detected HPV types. The current HPV vaccines do not target HPV-35; the addition of HPV-35 to the Gardasil®9 types would increase the protection against HPV-associated diseases among women of African ancestry. This data will provide the National Department of Health with crucial HPV prevalence and distribution of HPV genotypes data among non-HPV vaccinated women in the Eastern Cape Province of South Africa.
Supporting information
(XLSX)
Acknowledgments
We thank the study participants who kindly participated in the study, Nelson Mandela Academic Hospital Department of Gynaecology staff members and the research team.
Data Availability
Data will be available after the manuscript is accepted.
Funding Statement
This work was funded by the Cancer Association of South Africa and the University of Cape Town Research Fund (ZZAM). ALW salary supported by the National Research Foundation of South Africa (Grant Number: 64815). Opinions, findings, and conclusions or recommendations are those of the authors alone and do not reflect the Cancer Association of South Africa, National Research Foundation and University of Cape Town Research Fund's opinions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in South Africa. Summary Report. 2019. [Accessed 10 June 2021]. Available from https://hpvcentrenet/statistics/reports/ZAFpdf. [Google Scholar]
- 2.National Cancer Registry South Africa. Cancer Incidence in South Africa, 2014. National Institute of Communicable Disease, National Cancer Registry of South Africa. [Accessed 06 June 2021]. Available from: https://www.nicd.ac.za/wp-content/uploads/2019/12/2014-NCR-tables.pdf.
- 3.Somdyala NI, Bradshaw D, Dhansay MA, Stefan DC. Increasing Cervical Cancer Incidence in Rural Eastern Cape Province of South Africa From 1998 to 2012: A Population-Based Cancer Registry Study. JCO Global Oncology. 2020;6:1–8. doi: 10.1200/JGO.19.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNAIDS. Global HIV statistics. 2021. [Accessed 10 June 2021]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica.
- 5.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. The Lancet Infectious diseases. 2007;7(7):453–9. doi: 10.1016/S1473-3099(07)70158-5 . [DOI] [PubMed] [Google Scholar]
- 6.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. International journal of cancer Journal international du cancer. 2017;141(4):664–70. Epub 2017/04/04. doi: 10.1002/ijc.30716 ; PubMed Central PMCID: PMC5520228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lekoane KMB, Kuupiel D, Mashamba-Thompson TP, Ginindza TG. The interplay of HIV and human papillomavirus-related cancers in sub-Saharan Africa: scoping review. Syst Rev. 2020;9(1):88. Epub 2020/04/24. doi: 10.1186/s13643-020-01354-1 ; PubMed Central PMCID: PMC7178989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. New England Journal of Medicine. 2005;353(20):2101–4. doi: 10.1056/NEJMp058171 [DOI] [PubMed] [Google Scholar]
- 9.Schiffman M, Castle PE. Human papillomavirus—Epidemiology and public health. Archives of Pathology & Laboratory Medicine. 2003;127(8):930–4. doi: 10.5858/2003-127-930-HPEAPH [DOI] [PubMed] [Google Scholar]
- 10.Winer RL, Feng Q, Hughes JP, O’Reilly S, Kiviat NB, Koutsky LA. Risk of female human papillomavirus acquisition associated with first male sex partner. The Journal of infectious diseases. 2008;197(2):279–82. Epub 2008/01/09. doi: 10.1086/524875 ; PubMed Central PMCID: PMC2875685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbulawa ZZ, Johnson LF, Marais DJ, Coetzee D, Williamson AL. Risk factors for oral human papillomavirus in heterosexual couples in an African setting. The Journal of infection. 2014;68(2):185–9. doi: 10.1016/j.jinf.2013.10.012 . [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. The Journal of adolescent health: official publication of the Society for Adolescent Medicine. 2008;43(4 Suppl):S5–25, S.e1-41. Epub 2008/10/01. doi: 10.1016/j.jadohealth.2008.07.009 . [DOI] [PubMed] [Google Scholar]
- 13.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infectious Diseases. 2007;7(7):453–9. doi: 10.1016/S1473-3099(07)70158-5 [DOI] [PubMed] [Google Scholar]
- 14.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. Epub 2007/09/11. doi: 10.1016/S0140-6736(07)61416-0 . [DOI] [PubMed] [Google Scholar]
- 15.Mbulawa ZZ, Coetzee D, Marais DJ, Kamupira M, Zwane E, Allan B, et al. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. The Journal of infectious diseases. 2009;199(10):1514–24. doi: 10.1086/598220 . [DOI] [PubMed] [Google Scholar]
- 16.Dreyer G. Clinical implications of the interaction between HPV and HIV infections. Best practice & research Clinical obstetrics & gynaecology. 2018;47:95–106. Epub 2017/09/30. doi: 10.1016/j.bpobgyn.2017.08.011 . [DOI] [PubMed] [Google Scholar]
- 17.Williamson A-L. The Interaction between Human Immunodeficiency Virus and Human Papillomaviruses in Heterosexuals in Africa. Journal of Clinical Medicine. 2015;4(4):579–92. doi: 10.3390/jcm4040579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald AC, Tergas AI, Kuhn L, Denny L, Wright TC Jr., Distribution of Human Papillomavirus Genotypes among HIV-Positive and HIV-Negative Women in Cape Town, South Africa. Frontiers in oncology. 2014;4:48. Epub 2014/03/29. doi: 10.3389/fonc.2014.00048 ; PubMed Central PMCID: PMC3953716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Franceschi S, Howell‐Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. International journal of cancer. 2011;128(4):927–35. doi: 10.1002/ijc.25396 [DOI] [PubMed] [Google Scholar]
- 20.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high‐grade cervical lesions: a meta‐analysis update. International journal of cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527 [DOI] [PubMed] [Google Scholar]
- 21.Denny L, Adewole I, Anorlu R, Dreyer G, Moodley M, Smith T, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub‐Saharan Africa. International journal of cancer. 2014;134(6):1389–98. doi: 10.1002/ijc.28425 [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro M, Gage JC, Clifford GM, Demarco M, Cheung LC, Chen Z, et al. Association of HPV35 with cervical carcinogenesis among women of African ancestry: Evidence of viral‐host interaction with implications for disease intervention. International Journal of Cancer. 2020. doi: 10.1002/ijc.33033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–63. Epub 2016/06/25. doi: 10.1016/S2214-109X(16)30099-7 . [DOI] [PubMed] [Google Scholar]
- 24.Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. 2019;2019(11). Epub 2019/11/23. doi: 10.1002/14651858.CD013479 ; PubMed Central PMCID: PMC6873216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Sanjose S, Brotons M, LaMontagne DS, Bruni L. Human papillomavirus vaccine disease impact beyond expectations. Curr Opin Virol. 2019;39:16–22. Epub 2019/08/06. doi: 10.1016/j.coviro.2019.06.006 . [DOI] [PubMed] [Google Scholar]
- 26.Carlander C, Lagheden C, Eklund C, Kleppe SN, Dzabic M, Wagner P, et al. HPV types in cervical precancer by HIV status and birth region: a population-based register study. Cancer Epidemiology and Prevention Biomarkers. 2020;29(12):2662–8. doi: 10.1158/1055-9965.EPI-20-0969 [DOI] [PubMed] [Google Scholar]
- 27.Botha M, Dreyer G. Guidelines for cervical cancer screening in South Africa. Southern African Journal of Gynaecological Oncology. 2017;9(1):8–12. [Google Scholar]
- 28.Jordaan S, Michelow P, Richter K, Simoens C, Bogers J. A review of cervical cancer in South Africa: previous, current and future. Health Care Current Reviews. 2016;4(180):2. [Google Scholar]
- 29.Simms KT, Steinberg J, Caruana M, Smith MA, Lew J-B, Soerjomataram I, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. The lancet oncology. 2019;20(3):394–407. doi: 10.1016/S1470-2045(18)30836-2 [DOI] [PubMed] [Google Scholar]
- 30.Brotherton JM, Kaldor JM, Garland SM. Monitoring the control of human papillomavirus (HPV) infection and related diseases in Australia: towards a national HPV surveillance strategy. Sexual Health. 2010;7(3):310–9. doi: 10.1071/SH09137 [DOI] [PubMed] [Google Scholar]
- 31.Taku O, Mbulawa ZZ, Phohlo K, Garcia-Jardon M, Businge CB, Williamson A-L. Distribution of Human Papillomavirus (HPV) Genotypes in HIV-Negative and HIV-Positive Women with Cervical Intraepithelial Lesions in the Eastern Cape Province, South Africa. Viruses. 2021;13(2):280. doi: 10.3390/v13020280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpers C, Streeck RE. Genome organization and nucleotide sequence of human papillomavirus type 39. Virology. 1991;181(1):419–23. doi: 10.1016/0042-6822(91)90518-g [DOI] [PubMed] [Google Scholar]
- 33.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. International journal of cancer Journal international du cancer. 2012;131(10):2349–59. doi: 10.1002/ijc.27485 . [DOI] [PubMed] [Google Scholar]
- 34.Mbulawa ZZ, Coetzee D, Williamson AL. Human papillomavirus prevalence in South African women and men according to age and human immunodeficiency virus status. BMC infectious diseases. 2015;15:459. Epub 2015/10/28. doi: 10.1186/s12879-015-1181-8 ; PubMed Central PMCID: PMC4624185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter K, Becker P, Horton A, Dreyer G. Age-specific prevalence of cervical human papillomavirus infection and cytological abnormalities in women in Gauteng Province, South Africa. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2013;103(5):313–7. doi: 10.7196/samj.6514 . [DOI] [PubMed] [Google Scholar]
- 36.Rad A, Sørbye SW, Dreyer G, Hovland S, Falang BM, Louw M, et al. HPV types in cervical cancer tissue in South Africa: A head-to-head comparison by mRNA and DNA tests. Medicine. 2017;96(47):e8752. Epub 2018/02/01. doi: 10.1097/MD.0000000000008752 ; PubMed Central PMCID: PMC5708968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. Journal of acquired immune deficiency syndromes (1999). 2016;73(3):332. doi: 10.1097/QAI.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramogola-Masire D, McGrath CM, Barnhart KT, Friedman HM, Zetola NM. Subtype distribution of human papillomavirus in HIV-infected women with cervical intraepithelial neoplasia stages 2 and 3 in Botswana. International journal of gynecological pathology: official journal of the International Society of Gynecological Pathologists. 2011;30(6):591. doi: 10.1097/PGP.0b013e31821bf2a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Aardt MC, Dreyer G, Pienaar HF, Karlsen F, Hovland S, Richter KL, Becker P. Unique Human Papillomavirus–Type Distribution in South African Women With Invasive Cervical Cancer and the Effect of Human Immunodeficiency Virus Infection. International Journal of Gynecologic Cancer. 2015. Jun 1;25(5). doi: 10.1097/IGC.0000000000000422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data will be available after the manuscript is accepted.