Abstract
Background
Patients who have had COVID-19 often report persistent symptoms after resolution of their acute illness. Recent reports suggest that vaccination may be associated with improvement in post-acute symptoms. We used data from a prospective cohort to assess differences in post-acute sequelae of COVID (PASC) among vaccinated vs. unvaccinated patients.
Methods
We used data from a cohort of COVID-19 patients enrolled into a prospective registry established at a tertiary care health system in New York City. Participants underwent a baseline evaluation before COVID-19 vaccines were available and were followed 6 months later. We compared unadjusted and propensity score–adjusted baseline to 6-month change for several PASC–related symptoms and measures: anosmia, respiratory (cough, dyspnea, phlegm, wheezing), depression, anxiety, post-traumatic stress disorder (PTSD; COVID-19-related and other trauma), and quality-of-life domains among participants who received vs. those who did not receive COVID-19 vaccination.
Results
The study included 453 COVID-19 patients with PASC, of which 324 (72%) were vaccinated between the baseline and 6-month visit. Unadjusted analyses did not show significant differences in the baseline to 6-month change in anosmia, respiratory symptoms, depression, anxiety, PTSD, or quality of life (p > 0.05 for all comparisons) among vaccinated vs. unvaccinated patients. Similar results were found in propensity-adjusted comparisons and in secondary analyses based on the number of vaccine doses received.
Conclusions
Our findings suggest that COVID vaccination is not associated with improvement in PASC. Additional studies are needed to better understand the mechanisms underlying PASC and to develop effective treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07465-w.
KEY WORDS: COVID vaccination, post-acute sequelae of COVID, long COVID, symptoms
INTRODUCTION
The majority of patients with COVID-19 recover within a few weeks following their illness, but several studies suggest that a significant number of patients may continue to have persistent symptoms for several weeks to months after resolution of their acute illness.1–5 The syndrome of post-acute sequelae of COVID (PASC), sometimes termed long COVID or chronic COVID, is still not yet well defined but appears to affect a broad spectrum of patients, from those who were asymptomatic or experienced a mild case of acute COVID to those severely ill who required hospitalization and even treatment in intensive care units. Several factors, such as older age, female sex, obesity, greater number of comorbidities and more severe acute illness, increase the risk for development of PASC.6, 7 Common symptoms associated with PASC include persistent fatigue, dyspnea, anosmia, cognitive dysfunction (‘brain fog’), anxiety, depression and insomnia.4, 7, 8
Recent anecdotal reports have found that some patients with PASC describe improvement or resolution of their persistent symptoms after receiving the COVID vaccine.9–12 An early study from Britain that included 66 post-COVID patients found that those who were vaccinated showed improvement or resolution of their PASC symptoms or a decrease in worsening symptoms compared to matched participants who were unvaccinated.12 However, to date, there has been no large study to assess the effect of COVID vaccines on symptom change in patients who had COVID-19. Consequently, we undertook this study to assess whether vaccination was associated with resolution of or improvement in PASC symptoms in a prospective registry of COVID-19 patients.
METHODS
We used data from a prospective cohort of patients enrolled into an institutional Post-COVID-19 Registry at the Mount Sinai Health System (MSHS) in New York City. Study participants were recruited between July 20, 2020, and February 26, 2021, and had completed a baseline and 6-month follow-up interview. All participants were unvaccinated at the time of the baseline interview. Eligible participants were ≥ 18 years of age, had laboratory-documented infection with SARS-CoV-2, spoke English or Spanish, and received care at MSHS. Additionally, we limited the study to participants who reported at least one PASC symptom at baseline. Exclusion criteria included history of dementia. The study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai, and all participants signed informed consent.
We collected sociodemographic data at baseline including age, gender, race, ethnicity, marital status, household income and education. We also obtained information regarding the date of COVID-19 diagnosis and the site of acute COVID-19 care (outpatient, emergency room [ER], impatient or intensive care unit [ICU]). Pre-COVID-19 comorbidities (hypertension, coronary heart disease, diabetes, asthma, chronic obstructive pulmonary disease [COPD], and cancer) and smoking history were ascertained using questions from the National Health Interview Survey.13 We obtained measures of height and weight and calculated body mass index for each participant.
Data regarding vaccination included vaccine type (Pfizer, Moderna, or Johnson & Johnson), date of vaccination, and number of doses received. We also linked registry data to information from the electronic medical record as an additional source of vaccination status. To ensure sufficient time for an immune response, individuals who had received at least one dose of the vaccine at least 2 weeks prior to the 6-month follow-up interview were coded as vaccinated. We also categorized participants according to the number of doses (none, one or two) received; COVID-19 patients that received one dose of the Johnson & Johnson vaccine were included in the two-dose group.
The study outcomes included multiple PASC symptoms commonly reported after recovery from acute COVID-19 infection. Presence of anosmia was evaluated with a single-item 5-point Likert question (1 = no sense of smell to 5 = excellent sense of smell) adapted from the PhenX Toolkit.14 Dyspnea with different levels of exertion was evaluated with the Modified Medical Research Council (mMRC) scale (0 = dyspnea only with strenuous exercise, 4 = dyspnea at rest), a well-validated tool used in research and clinical practice.15, 16 Other respiratory symptoms included cough, phlegm and wheezing, which were evaluated with items from St. George’s questionnaire (0 = no symptoms at all, 4 = symptoms most days of the week).17, 18
We used Patient Health Questionnaire-8 (PHQ-8) to assess for symptoms of depression.19 Anxiety symptoms were evaluated with the Generalized Anxiety Disorders-7 (GAD-7) instrument.20, 21 Presence and severity of post-traumatic stress disorder (PTSD) symptoms were evaluated using the PTSD checklist for DSM-5 (PCL-5).22 The PCL-5 was administered twice to assess for symptoms of COVID-19-related PTSD as well as PTSD related to other trauma.
We used the Patient-Reported Outcomes Measurement Information System (PROMIS)-29 v2.0 Scale to assess quality of life.23–25 This is a validated tool with well-established population norms that provides quality-of-life scores along seven health domains: physical function, anxiety, depression, fatigue, social roles, and sleep (four items per domain) and an item assessing pain (score 0–10). Responses to items in each domain were summed and scored based on population norms.23
Statistical Analyses
We compared baseline characteristics of COVID-19 patients who received or did not receive COVID vaccination using a t test or chi-square test, as appropriate. For each outcome, we compared the mean difference from baseline to 6 months between vaccinated vs. unvaccinated patients using a two-sample t test.
To adjust for differences in baseline characteristics between the two groups, we fitted a propensity score model including age, gender, race, ethnicity, marital status, income, smoking history, comorbidities, and severity of acute COVID-19. From this model, we estimated probability weights for both vaccinated and unvaccinated individuals as the inverse of the probability of group assignment. We fitted a linear regression model to compare change in symptom scores in vaccinated vs. unvaccinated participants controlling for number of days since COVID-19 diagnosis and the inverse probability weights to adjust for potential confounders. Each model was limited to participants who reported symptoms at baseline for the specific PASC outcome evaluated in the analysis. We conducted secondary analyses comparing differences in PASC symptom trajectory according to the number of doses of vaccine received.
With the cohort of patients included in this analysis, we estimated that the study would have > 80% power to identify a ≥ 1 unit difference (SD 1.25) in changes in dyspnea scores, ≥ 3 units difference (SD 5) in changes of depression scores, and ≥ 10 units difference (SD 10) in changes of quality-of-life scores among vaccinated vs. unvaccinated patients. Statistical analyses were conducted using SAS software version 9.4 (SAS, Cary, NC).
RESULTS
Of the 1189 COVID-19 patients recruited in the post-COVID-19 registry as of the time of these analyses, 464 have completed the 6-month interview as of August 23, 2021. Of these, 11 were excluded due to missing data regarding vaccination status leaving a cohort of 453 study participants (see supplemental figure). Of these 453 participants, 324 (72%) were vaccinated and 94% of these had received one of the mRNA vaccines (62% Pfizer-BioNtech and 32% Moderna). The baseline characteristics of vaccinated and unvaccinated patients are shown in Table 1. Although unvaccinated patients were slightly more likely to be never smokers (74% vs. 65%), this was not statistically significant and there were no significant differences in age, gender, smoking history and comorbidities among vaccinated vs. unvaccinated COVID-19 patients (p > 0.05 for all comparisons). However, unvaccinated patients were more likely to be unmarried (p = 0.04), report a lower annual income (p = 0.003), and have had a shorter period from COVID-19 diagnosis to the date of the baseline interview (p = 0.001).
Table 1.
Baseline Characteristics of Post-Covid-19 Patients
| Characteristic | Vaccinated N = 324 |
Non-vaccinated N = 129 |
p value |
|---|---|---|---|
| Age, years, mean (SD) | 50.1 (13.4) | 49.7 (14.1) | 0.79 |
| Female, no. (%) | 211 (65) | 83 (64) | 0.87 |
| Race/ethnicity, no. (%) | 0.11 | ||
| White | 200 (62) | 73 (57) | |
| Black | 46 (14) | 31 (24) | |
| Asian | 17 (5) | 2 (2) | |
| Other | 55 (17) | 22 (17) | |
| Latinx, no. (%) | 62 (19) | 26 (20) | 0.82 |
| Married, no. (%) | 160 (50) | 43 (33) | 0.04 |
| Annual income, no. (%) | 0.003 | ||
| < 25,000 | 47 (16) | 17 (15) | |
| $25,000–$60,000 | 42 (14) | 36 (32) | |
| $60,000–$150,000 | 116 (39) | 38 (34) | |
| > $150,000 | 95 (32) | 22 (20) | |
| Never smoker, no. (%) | 206 (65) | 93 (74) | 0.20 |
| Pre-Covid-19 comorbidities, no. (%) | |||
| Hypertension | 102 (32) | 44 (34) | 0.59 |
| Coronary artery disease | 15 (5) | 3 (2) | 0.51 |
| Diabetes | 38 (12) | 15 (12) | 0.82 |
| Asthma | 86 (27) | 40 (31) | 0.34 |
| Chronic obstructive pulmonary disease | 11 (3) | 4 (3) | 0.36 |
| Cancer | 41 (13) | 11 (9) | 0.36 |
| Body mass index, no. (%) | 0.92 | ||
| Normal weight | 100 (31) | 36 (28) | |
| Overweight | 94 (29) | 39 (30) | |
| Obese | 110 (34) | 44 (34) | |
| Time since Covid-19 diagnosis, days, mean (SD) | 213 (62) | 172 (56) | < 0.001 |
| Site of Covid-19 care, no. (%) | 0.11 | ||
| Outpatient | 132 (41) | 53 (41) | |
| Emergency room | 97 (30) | 28 (22) | |
| Inpatient | 73 (23) | 42 (33) | |
| Intensive care unit | 18 (6) | 6 (5) | |
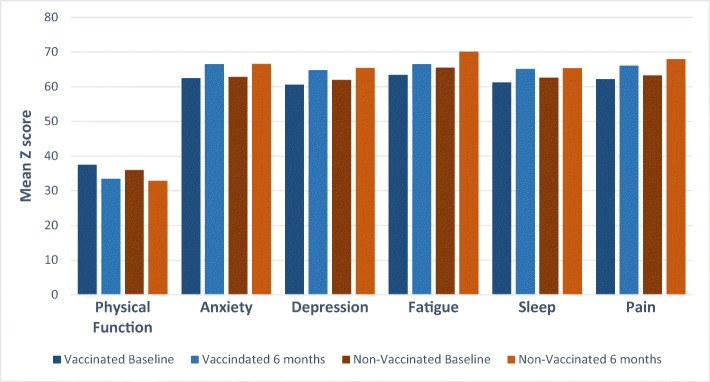
Unadjusted analyses showed no significant difference in the mean change between baseline and 6-month follow-up in anosmia (mean difference: − 0.26, 95% confidence interval [CI]: − 0.54 to 0.03, p = 0.08), or respiratory symptoms (p > 0.05 for all comparisons; Table 2). Similarly, mean changes in symptoms of depression (mean difference: 0.32, 95% CI: − 0.88 to 1.53, p = 0.60), anxiety (mean difference: 1.29, 95% CI: − 0.24 to 2.82, p = 0.10), COVID-19-related PTSD (mean difference: 3.41, 95% CI: − 1.82 to 8.63, p = 0.20) and PTSD due to other trauma (2.32, 95% CI: − 8.46 to 13.09, p = 0.66) were not significantly associated with vaccination status. There were no significant differences in the mean change of all quality-of-life domains between vaccinated and unvaccinated groups, including fatigue (mean difference: − 1.40, 95% CI: − 3.98 to 1.18, p = 0.29), sleep (mean difference: 1.16, 95% CI: − 1.10 to 3.41, p = 0.31) and pain (− 0.84, 95% CI: − 3.19 to 1.52, p = 0.49, Fig. 1).
Table 2.
Unadjusted Differences in Post-Covid Symptom Scores Among Vaccinated Compared to Unvaccinated Patients
| Domain | Vaccinated | Non-vaccinated | Difference change vaccinated vs. non-vaccinated (95% CI) |
p value | ||
|---|---|---|---|---|---|---|
| Baseline Mean (SD) |
Change | Baseline Mean (SD) |
Change | |||
| Anosmia (N = 163) | 2.44 (0.67) | − 0.58 | 2.40 (0.74) | − 0.33 | − 0.26 (− 0.54 to 0.03) | 0.08 |
| Respiratory symptoms | ||||||
| Dyspnea (N = 286) | 0.57 (0.50) | − 0.09 | 0.58 (0.50) | − 0.12 | 0.02 (− 0.19 to 0.23) | 0.83 |
| Cough (N = 231) | 2.33 (0.81) | − 0.56 | 2.26 (0.85) | − 0.57 | 0.003 (− 0.39 to 0.39) | 0.99 |
| Phlegm (N = 146) | 2.30 (0.85) | − 0.82 | 2.37 (0.85) | − 0.53 | − 0.28 (− 0.76 to 0.20) | 0.25 |
| Wheezing (N = 101) | 2.41 (0.78) | − 0.82 | 2.46 (0.69) | − 1.23 | 0.41 (− 0.27 to 1.09) | 0.23 |
| Depression symptoms (N = 288) | 10.70 (5.02) | 1.86 | 10.20 (4.05) | 1.53 | 0.32 (− 0.88 to 1.53) | 0.60 |
| Anxiety symptoms (N = 212) | 9.88 (4.08) | 2.50 | 9.97 (5.03) | 1.21 | 1.29 (− 0.24 to 2.82) | 0.10 |
| Covid PTSD symptoms (N = 127) | 40.16 (12.16) | 9.99 | 38.26 (11.64) | 6.58 | 3.41 (− 1.82 to 8.63) | 0.20 |
| Non-Covid PTSD symptoms (N = 42) | 34.00 (8.59) | 14.38 | 44.38 (12.47) | 12.07 | 2.32 (− 8.46 to 13.09) | 0.66 |
| Quality of life | ||||||
| Physical function (N = 218) | 37.41 (4.65) | − 4.02 | 35.92 (4.76) | − 3.07 | − 0.95 (− 2.96 to 1.05) | 0.35 |
| Anxiety (N = 244) | 62.42 (5.41) | 4.07 | 62.83 (6.84) | 3.73 | 0.33 (− 1.98 to 2.65) | 0.78 |
| Depression (N = 171) | 60.56 (5.05) | 4.18 | 61.94 (5.49) | 3.44 | 0.74 (− 1.81 to 3.29) | 0.57 |
| Fatigue (N = 261) | 63.36 (6.06) | 3.14 | 65.51 (6.39) | 4.54 | − 1.40 (− 3.98 to 1.18) | 0.29 |
| Social roles (N = 135) | 58.78 (7.26) | 3.49 | 56.39 (9.47) | 5.81 | − 2.32 (− 5.51 to 0.87) | 0.15 |
| Sleep (N = 171) | 61.20 (4.60) | 3.92 | 62.56 (5.59) | 2.76 | 1.16 (− 1.10 to 3.41) | 0.31 |
| Pain (N = 232) | 62.14 (5.59) | 3.86 | 63.22 (5.58) | 4.70 | − 0.84 (− 3.19 to 1.52) | 0.49 |
SD standard deviation, CI confidence interval
Figure 1.
Comparison of changes in PASC symptoms on PROMIS scale between baseline and 6 months for vaccinated vs. non-vaccinated participants.
Propensity score–adjusted analyses (Table 3) showed no differences in change in anosmia (mean difference: − 0.02, 95% CI: − 0.35 to 0.31), dyspnea (mean difference: 0.05, 95% CI: − 0.15 to 0.25), cough (mean difference: − 0.17, 95% CI: − 0.55 to 0.22), phlegm (mean difference: − 0.47, 95% CI: − 0.87 to 0.10) or wheezing (mean difference: − 0.16, 95% CI: − 0.83 to 0.50) among vaccinated vs. unvaccinated patients. Mean change in depression (mean difference: 0.02, 95% CI: − 1.18 to 1.22), anxiety (mean difference: 0.51, 95% CI: − 0.93 to 0.04), COVID-19-related (mean difference: 2.53, 95% CI: − 3.06 to 8.12) or other trauma-related (mean difference: − 2.53, 95% CI: − 12.11 to 7.04) PTSD symptoms were not associated with vaccination status (Table 3). Change in quality-of-life domain scores was also not significantly different among vaccinated vs. unvaccinated patients (mean difference in physical function: − 1.16, 95% CI: − 3.35 to 1.02; mean difference in anxiety: − 0.29, 95% CI: − 2.84 to 2.27; mean difference in depression: − 1.12, 95% CI: − 3.80 to 1.56; mean difference in fatigue: − 1.42, 95% CI: − 4.15 to 1.32; mean difference in social roles: − 0.17, 95% CI: − 3.18 to 2.83; mean difference in sleep: 1.51, 95% CI: − 0.86 to 3.87; and mean difference in pain: − 0.02, 95% CI: − 2.74 to 2.70).
Table 3.
Adjusted Associations Between Vaccination and Post-Covid-19 Symptoms According to the Number of Doses
| Domain | Mean difference vaccinated vs. non-vaccinated (95% CI) | ||
|---|---|---|---|
| Primary analyses | Secondary analyses | ||
| One or two doses | One dose | Two doses | |
| Anosmia | − 0.02 (− 0.35 to 0.31) | − 0.17 (− 0.62 to 0.28) | − 0.13 (− 0.42 to 0.15) |
| Respiratory symptoms | |||
| Dyspnea | 0.05 (− 0.15 to 0.25) | 0.10 (− 0.20 to 0.39) | − 0.06 (− 0.29 to 0.16) |
| Cough | − 0.17 (− 0.55 to 0.22) | 0.22 (− 0.27 to 0.72) | − 0.32 (− 0.73 to 0.08) |
| Phlegm | − 0.47 (− 0.87 to 0.10) | − 0.28 (− 0.92to 0.36) | − 0.27 (− 0.76 to 0.21) |
| Wheezing | − 0.16 (− 0.83 to 0.50) | − 0.30 (− 1.17 to 0.56) | − 0.28 (− 0.98 to 0.42) |
| Depression symptoms | 0.02 (− 1.18 to 1.22) | 0.39 (− 1.15 to1.95) | 0.31 (− 0.95 to 1.56) |
| Anxiety symptoms | 0.51 (− 0.93 to 0.04) | 1.87 (− 0.01 to 3.76) | 0.62 (− 0.71 to 1.95) |
| Covid PTSD symptoms | 2.53 (− 3.06 to 8.12) | 7.58 (− 0.21 to 15.37) | 2.90 (− 2.13 to 7.93) |
| Non − Covid PTSD symptoms | − 2.53 (− 12.11 to 7.04) | − 11.62 (− 26.04 to 2.80) | − 1.75 (− 11.85 to 8.35) |
| Quality of life | |||
| Physical function | − 1.16 (− 3.35 to 1.02) | − 0.30 (− 2.95 to 2.36) | − 0.87 (− 3.04 to 1.30) |
| Anxiety | − 0.29 (− 2.84 to 2.27) | − 0.26 (− 3.24 to 2.72) | − 0.69 (− 3.06 to 1.68) |
| Depression | − 1.12 (− 3.80 to 1.56) | − 1.53 (− 5.09 to 2.04) | 1.53 (− 1.22 to 4.28) |
| Fatigue | − 1.42 (− 4.15 to 1.32) | − 1.15 (− 4.29 to 2.00) | − 1.39 (− 3.93 to 1.15) |
| Social roles | − 0.17 (− 3.18 to 2.83) | − 0.23 (− 3.67 to 4.13) | − 1.99 (− 5.11 to 1.14) |
| Sleep | 1.51 (− 0.86 to 3.87) | 1.00 (− 1.72 to 3.74) | 1.95 (− 0.21 to 4.10) |
| Pain | − 0.02 (− 2.74 to 2.70) | − 1.91 (− 4.74 to 0.92) | − 0.40 (− 2.69 to 1.89) |
CI confidence interval
Similarly, adjusted analyses showed no significant difference in changes in any of the outcomes when comparing patients who received one or two doses compared to those who were unvaccinated (Table 3).
DISCUSSION
In this study of 453 post-COVID patients, we did not find a significant difference in change in PASC symptoms from baseline to 6 months between vaccinated and unvaccinated patients. Our study is the first prospective cohort study to assess whether COVID vaccination may affect PASC symptoms. Thus, these findings suggest that while vaccination is important in providing additional immunological protection against reinfection, it does not appear to be associated with changes in PASC symptoms. Additional research is needed to understand the mechanisms underlying PASC and to identify effective treatments for these patients.
Although PASC is still not well defined, the National Institutes of Health convened a workshop in December 2020 to discuss current literature about this syndrome and identify knowledge gaps.1 Studies from China, Italy and France have found that between 15 and 84% of COVID-19 patients may experience long-term symptoms.1–5, 8, 26 These PASC symptoms,4, 7, 8, 26 including anosmia, respiratory issues such as cough or dyspnea, depression, anxiety, cognitive dysfunction, and/or fatigue, may persist for several weeks to months after resolution of acute COVID-19. While more severe acute COVID-19 illness appears to be associated with higher risk for PASC, a few other risk factors such as being female, older, or obese, or having more comorbidities also seem to have a relationship with PASC development. 6, 7
Several mechanisms have been proposed to explain persistence of symptoms in PASC including viral reservoirs, continued inflammation, development of autoantibodies, and/or sequelae of organ damage during acute infection.1, 26–30 SARS-CoV-2 virus particles have been found in gut enterocytes for up to 4–7 months after initial diagnosis.28, 31 This viral reservoir may result in persistent inflammation that can then lead to the development of PASC symptoms. Persistent markers of inflammation have been found in patients up to several months after the acute illness.32, 33 Inflammation may also induce endothelial damage and micro-thrombi formation in multiple organs which may then result in many of the observed PASC symptoms, such as dyspnea as well as cognitive dysfunction and fatigue. Finally, autoantibodies may be implicated in the development of PASC.29, 34, 35
As such, COVID vaccination may help reduce or eliminate PASC symptoms by eradicating the viral reservoir or by resetting a dysregulated immune response to the primary infection. Several anecdotal reports have suggested that those suffering from PASC symptoms improve after receiving the COVID vaccine. However, in this larger prospective study of post-COVID patients, we did not find a significant difference in change in PASC symptoms or other objective measures between vaccinated and unvaccinated patients. Thus, while some may report significant improvement in PASC symptoms after vaccination, these findings were not borne out in our study population.
Our study has some strengths and limitations. This is a prospective observational cohort study from one large urban city and not a randomized study to assess the effect of COVID vaccination on symptom change. Thus, we cannot exclude systematic differences among vaccinated vs. unvaccinated patients. However, our two groups were similar at baseline, and we used propensity score adjustment to account for baseline characteristics that might affect vaccination status. Differences in vaccine type may be a limitation in determining effect of vaccination on changes in PASC symptoms but the majority of our participants received one of the two mRNA vaccines. It is also possible that biases in reporting symptoms may have affected our results, but this appears unlikely as we used objective measures to assess symptoms at each time point and did not ask participants to report change in symptoms. Those who completed the 6-month interview may also have had more severe symptoms than those who dropped out so there is a possibility of selection bias. Finally, our study may not have sufficient power to identify small differences in some PASC symptoms among the two study groups, and because our cohort consisted of COVID-19 patients who experienced the first wave of COVID, our results may not be generalizable to others who may have been infected in later waves or different strains of the coronavirus. Strengths of this study include the prospective nature of the cohort where symptoms were collected with standardized and validated instruments. We also assessed multiple domains pertaining to commonly reported PASC symptoms.
In conclusion, our study found that COVID vaccination does not appear to improve or change PASC symptoms in this large prospective cohort of patients who have recovered from COVID. While COVID vaccination remains important for additional immunological protection against reinfection, it does not appear to improve PASC symptoms or related measures in COVID-19 patients. Further studies are needed to better define the trajectory of PASC as well as to understand the pathophysiology underlying its development so that effective treatments can be developed to treat the long-term complications associated with COVID infection.
Supplementary Information
(DOCX 26 kb)
Declarations
Conflict of Interest
Dr. Wisnivesky received consulting honorarium from Atea, Sanofi, PPD, and Banook and grants from Sanofi, Arnold Consulting, and Regeneron. Dr. Aberg reports grants from Atea, Emergent Biosolutions, Frontier Technologies, Gilead Sciences, Glaxo Smith Kline, Janssen, Merck, Pfizer, Regeneron, and Viiv Healthcare and consulting honorarium from Glaxo Smith Kline and Merck. The other authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lerner AM, Robinson DA, Yang L, et al. Toward Understanding COVID-19 Recovery: National Institutes of Health Workshop on Postacute COVID-19. Ann Intern Med. Published online March 30, 2021. 10.7326/M21-1043 [DOI] [PMC free article] [PubMed]
- 2.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in Adults at 6 Months After COVID-19 Infection. JAMA Netw Open. 2021;4(2):e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havervall S, Rosell A, Phillipson M, et al. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA. 2021;325(19):2015–2016. doi: 10.1001/jama.2021.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Lond Engl. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein L, Guarino B. Some long-haul covid-19 patients say their symptoms are subsiding after getting vaccines. Washington Post. https://www.washingtonpost.com/health/long-haul-covid-vaccine/2021/03/16/6effcb28-859e-11eb-82bc-e58213caa38e_story.html. Published March 16, 2021. Accessed June 21, 2021.
- 10.Stone W. Mysterious Ailment, Mysterious Relief: Vaccines Help Some COVID Long-Haulers. NPR.org. Published March 31, 2021. Accessed June 21, 2021. https://www.npr.org/sections/health-shots/2021/03/31/982799452/mysterious-ailment-mysterious-relief-vaccines-help-some-covid-long-haulers
- 11.Lovelace Jr B. Some people with “long Covid” say their symptoms ease after getting vaccine. CNBC. Published April 7, 2021. Accessed June 21, 2021. https://www.cnbc.com/2021/04/07/covid-vaccine-long-haulers-report-symptoms-easing-after-getting-shot.html
- 12.Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv. Published online March 14, 2021:2021.03.11.21253225. 10.1101/2021.03.11.21253225
- 13.National Health Interview Survey (NHIS). Published June 2, 2021. Accessed June 21, 2021. https://www.cdc.gov/nchs/nhis/index.htm
- 14.PhenX Toolkit: Covid19. Published February 22, 2021. Accessed June 21, 2021. https://www.phenxtoolkit.org/covid19
- 15.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med. 2010;10:32. doi: 10.1186/1471-2466-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther. 2000;22(9):1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CB, Jones PW, O’Leary CJ, Cole PJ, Wilson R. Validation of the St. George’s Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997;156(2 Pt 1):536–541. doi: 10.1164/ajrccm.156.2.9607083. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Löwe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 22.Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 23.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®-29 v2.0 profile physical and mental health summary scores. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2018;27(7):1885–1891. doi: 10.1007/s11136-018-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scordo KA, Richmond MM, Munro N. Post-COVID-19 Syndrome: Theoretical Basis, Identification, and Management. AACN Adv Crit Care. Published online May 4, 2021:e1-e8. 10.4037/aacnacc2021492 [DOI] [PubMed]
- 28.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses. 2021;13(4):700. doi: 10.3390/v13040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokuyama M, Ladinsky MS, Jha D, et al. 785 SARS CoV-2 infects enterocytes in vivo and can persist up to 7 months following resolution of symptoms. Gastroenterology. 2021;160(6):S-159. doi: 10.1016/S0016-5085(21)01130-6. [DOI] [Google Scholar]
- 32.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. “The long tail of Covid-19” - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2020;9:1349. doi: 10.12688/f1000research.27287.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Townsend L, Fogarty H, Dyer A, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost JTH. 2021;19(4):1064–1070. doi: 10.1111/jth.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20(4):102792. doi: 10.1016/j.autrev.2021.102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)