Abstract
OBJECTIVES
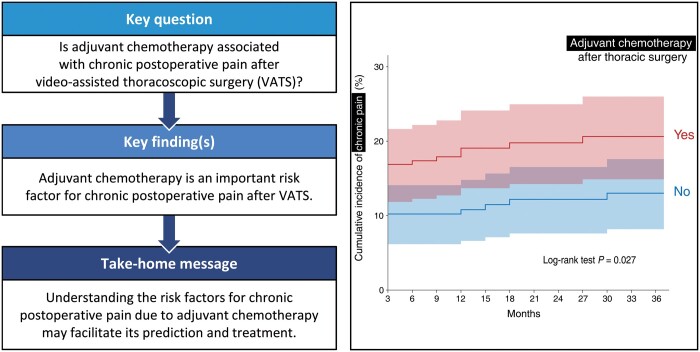
The association between adjuvant chemotherapy (AC) and chronic postoperative pain (CPP) after video-assisted thoracoscopic surgery (VATS) for lung cancer resection has not yet been reported. We, therefore, investigated the association between AC and the long-term incidence of CPP after VATS.
METHODS
We retrospectively reviewed 3015 consecutive patients who underwent VATS for lung cancer between 2007 and 2016. The patients were divided into 2 groups: those who received (AC group) and those who did not receive (non-AC group) AC within 3 months after VATS. Propensity score analysis was performed to adjust for baseline differences between the 2 groups. The cumulative incidence of CPP at the intervals of 3 months, over 36 months, was compared before and after matching. A Cox proportional hazards regression analysis was used to investigate the predictors of CPP after VATS.
RESULTS
We included and assessed 2222 patients in this study. Of these, 320 patients (14.4%) received AC within 3 months post-VATS. The cumulative incidence of CPP during 36 months post-surgery was significantly higher in the AC group than in the non-AC group, before and after matching (log-rank test; P = 0.002 and 0.027, respectively). Cox proportional hazards regression analysis also showed that AC was a significant risk factor for CPP (hazard ratio 1.62, 95% confidence interval 1.16–2.28; P = 0.005).
CONCLUSIONS
Our results indicate that AC is an important risk factor for CPP after VATS. Further understanding of the risk factors for CPP may facilitate its prediction and treatment.
Keywords: Adjuvant chemotherapy, Chronic pain, Postoperative pain, Video-assisted thoracoscopic surgery
Chronic postoperative pain (CPP) is a pain that develops after surgery and persists beyond the expected recovery period [1].
INTRODUCTION
Chronic postoperative pain (CPP) is a pain that develops after surgery and persists beyond the expected recovery period [1]. It is a relatively frequent complication after lung surgery [2]. Although the video-assisted thoracoscopic surgery (VATS) has reduced surgical stress and has facilitated postoperative recovery, CPP remains an important aftereffect in patients undergoing VATS for lung cancer resection [3]. Increased early diagnosis of lung cancer by the introduction of screening tests and early surgical resection has improved survival rates among patients with lung cancer. Therefore, CPP, which affects patients’ long-term quality of life after surgery, is an important problem [4].
The known risk factors for CPP include being female, younger age, preoperative chronic pain, psychological vulnerability, intraoperative nerve injury, longer surgical duration, genetic predisposition and intensity of acute postoperative pain [1]. Chemotherapy has also been reported to be a risk factor of CPP after breast cancer surgery [5]; however, the association of adjuvant chemotherapy (AC) with CPP after lung cancer surgery remains undetermined [6–8]. AC is recommended for patients with lung cancer with lymph node metastases or larger or locally invasive tumours [9] and is considered to increase the survival rate of patients with lung cancer [10].
Thus, it is meaningful to investigate the association between AC and the development of CPP after lung cancer surgery performed by VATS. To address the effects of confounders, which may have contributed to the inconsistent findings of previous studies, we used propensity score matching (PSM) analysis and multivariable Cox proportional hazards (PHs) regression analysis, to investigate the association between AC and the long-term incidence of CPP after lung cancer surgery.
MATERIALS AND METHODS
The institutional review board of Seoul National University Hospital approved this study (No.2004-065-1116) and exempted written informed consent due to the retrospective design. The manuscript writing followed the STROBE guidelines [11].
We scrutinized the electronic medical records (EMRs) of adult patients (≥18 years) who received VATS for lung cancer resection at our institution between 2007 and 2016. We collected data from 2007 as we determined that the EMR system, introduced in our hospital at the end of 2004, had not stabilized until 2006. Data of all consecutive patients that satisfied the above criteria were collected retrospectively, without a priori sample-size estimation. The cohort was divided into 2 groups: patients who received AC within 3 months after surgery (the AC group) or those who did not (non-AC group). We used a 3-month window, as we considered that it may be difficult to investigate the association between AC and CPP if the time interval between surgery and chemotherapy was longer. We did not include patients with thoracotomy to reduce the cancer-related differences between the 2 groups. We also excluded the following: patients with a history of previous thoracic surgery; patients who received reoperation at the ipsilateral site in the study period; patients who received VATS for suspected lung cancer but were confirmed not to have lung cancer; patients who had other primary cancers; patients having a VATS biopsy; patients who were not followed up for at least 3 months after surgery; and patients with inadequate medical records related to pain intensity. Patients with missing values of the covariates used in the PSM analysis were also excluded. We did not attempt to replace missing data and included all patients in the analyses, including those with missing values, using the available data.
Demographics, medical history, perioperative parameters and adjuvant therapy information were collected using EMR (Table 1). Analgesic medications prescribed for CPP during the study period were also investigated. Prolonged postoperative opioid use was defined as the use of at least 1 strong opioid for >3 months, postoperatively [6].
Table 1:
Clinical characteristics and perioperative parameters between patients with and without AC within 3 months after video-assisted thoracoscopic surgery for lung cancer resection
| AC group (n = 320) | Non-AC group (n = 1902) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 61 (55–67) | 64 (56–71) | <0.001 |
| Female gender | 140 (43.8) | 937 (49.3) | 0.068 |
| Body mass index (kg/m²) | 23.8 (22.1–25.6) | 23.8 (21.8–25.8) | 0.972 |
| Current smoker | 105 (32.8) | 546 (28.7) | 0.135 |
| Background medical status | |||
| ASA physical status I/II/III | 100 (31.2)/214 (66.9)/6 (1.9) | 553 (29.1)/1252 (65.8)/97 (5.1) | 0.036 |
| Diabetes mellitus | 34 (10.6) | 281 (14.8) | 0.049 |
| Preoperative analgesic use | 13 (4.1) | 67 (3.5) | 0.631 |
| Preoperative strong opioid usea | 1 (0.3) | 3 (0.2) | 0.546 |
| Preoperative antipsychotics use | 3 (0.9) | 44 (2.3) | 0.114 |
| Preoperative chemotherapy | 7 (2.2) | 36 (1.9) | 0.723 |
| Preoperative radiotherapy | 3 (0.9) | 38 (2.0) | 0.192 |
| Postoperative radiotherapy | 63 (19.7) | 106 (5.6) | <0.001 |
| Previous other surgeries | 184 (57.5) | 1251 (65.8) | 0.004 |
| Pathological stage | |||
| T 1/2/3/4 | 90 (28.1)/194 (60.6)/34 (10.6)/2 (0.6) | 1300 (68.3)/551 (29.0)/46 (2.4)/5 (0.3) | <0.001 |
| N 0/1/2 | 160 (50.0)/71 (22.2)/89 (27.8) | 1843 (96.9)/39 (2.1)/20 (1.1) | <0.001 |
| M 0/1 | 300 (93.8)/20 (6.2) | 1888 (99.3)/14 (0.7) | <0.001 |
| Surgical characteristics | |||
| Operation year, 2007/2008/2009/2010/2011/ 2012/2013/2014/2015/2016 | 12 (3.8)/5 (1.6)/8 (2.5)/16 (5.0)/39 (12.2)/40 (12.5)/42 (13.1)/49 (15.3)/50 (15.6)/59 (18.4) | 33 (1.7)/39 (2.1)/65 (3.4)/95 (5.0)/201 (10.6)/270 (14.2)/237 (12.5)/292 (15.4)/329 (17.3)/341 (17.9) | 0.501 |
| Operation type | <0.001 | ||
| Pneumonectomy | 3 (0.9) | 1 (0.1) | |
| Lobectomy | 297 (92.8) | 1557 (81.9) | |
| Segmentectomy | 8 (2.5) | 189 (9.9) | |
| Wedge resection | 12 (3.8) | 155 (8.1) | |
| Operation time (min) | 145 (120–185) | 140 (115–170) | 0.005 |
| Type of anaesthesia | 0.971 | ||
| Inhalation agent | 65 (20.3) | 388 (20.4) | |
| Total intravenous anaesthesia | 255 (79.7) | 1514 (79.6) | |
| Type of PCA | 0.430 | ||
| Intravenous PCA | 273 (85.3) | 1553 (81.7) | |
| Epidural PCA | 44 (13.8) | 333 (17.5) | |
| Paravertebral PCA | 1 (0.3) | 5 (0.3) | |
| None | 2 (0.6) | 11 (0.6) | |
| Intraoperative analgesic techniques | 0.949 | ||
| Intercostal nerve blockade | 65 (20.3) | 389 (20.5) | |
| Percutaneous wound injection | 33 (10.3) | 207 (10.9) | |
| Length of hospital stay (days) | 7.0 (6.0–10.0) | 7.0 (6.0–9.0) | 0.244 |
| NRS at first postoperative outpatient visit (0–10) | 2 (0–3) | 2 (0–3) | 0.730 |
The values are presented as the median (interquartile range) or n (%).
Hydrocodone, hydromorphone, morphine, oxycodone and transdermal fentanyl patch.
AC: adjuvant chemotherapy; ASA: American Society of Anesthesiologist physical status classification; PCA: patient-controlled analgesia; NRS: numeric rating scale.
The type of procedure (VATS versus thoracotomy) was determined by the surgeon based on the patient’s condition and the surgeon’s experience. Thoracic surgeons performed three-port VATS with rib sparing. A 4-cm working port and a 5-mm camera port were created at the fifth and seventh intercostal space on the anterior axillary line, respectively, and a 10-mm instrument port was created at the sixth intercostal space on the posterior axillary line. The entire course of surgery was conducted by experienced thoracic surgeons. Anaesthesia was induced and maintained with short-acting volatile agents or propofol with remifentanil. Intravenous patient-controlled analgesia consisted of a combination of fentanyl (10–20 μg/ml) and morphine (0.4–0.7 mg/ml) at a basal infusion rate of 0.5 ml/h and a bolus of 1 ml with a lockout interval of 10 min. When patients tolerated oral feeding, oral analgesics were initiated.
The oncologists determined the AC implementation following the National Comprehensive Cancer Network guidelines, at the time of surgery [12], on an individual basis. The AC regimen was mainly cisplatin- or carboplatin based (Supplementary Material, Table S1), decided by agreement between the doctor and patient, after considering the patient’s status.
CPP was defined as a consecutive 11-point numeric rating scale (NRS) score of ≥3 in 2 measurements, at least 3 months apart [6]. Based on the EMRs, we used the surgical-site pain intensity, as evaluated by thoracic surgeons using 11-point NRS, in the outpatient clinic. CPP at 3 months after surgery was defined using the NRS scores measured at the first postoperative outpatient visit, performed ∼2 weeks after surgery and at the 3-month follow-up.
Statistical analyses were conducted using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses, a two-sided P-value of <0.05 was considered statistically significant. The normal distribution of continuous variables was tested by the Shapiro–Wilk test. Continuous data are presented as the mean (standard deviation) or the median [interquartile range (IQR)] and were analysed by the independent t-test or the Mann‒Whitney U-test, respectively. Categorical data are presented as frequency or percentage and were analysed by the χ2 test or Fisher’s exact test, as appropriate. The following main analyses were performed.
First, a PSM analysis was performed to reduce the potential confounding effect of the covariates of the baseline characteristics and perioperative parameters. The propensity score was defined as the probability of receiving AC within 3 months after surgery, determined by the logistic regression analysis. The following variables were used as contributors to the propensity score: age, sex, American Society of Anesthesiologists physical status, current smoking, T and N stage, preoperative chemotherapy, pre- and postoperative radiotherapy, history of other surgeries, operation type, duration of surgery (min), intraoperative analgesia (none or percutaneous bupivacaine injection or intercostal nerve blockade), NRS at the first postoperative outpatient visit and year of surgery. The year of surgery, as a categorical variable, was included, given the recent decrease in the incidence of CPP at our institution [6]. We matched patients at a ratio of 1:1, using the nearest neighbour method, with a calliper width of 0.1 of the pooled standard deviation of the logit of the propensity score. The balance of the matched patients was assessed using the standardized mean difference for each contributor. Before and after matching, Kaplan–Meier survival curve analyses for the development of CPP during the study period were performed to evaluate the effect of AC on the long-term incidence of CPP.
Second, we performed Cox PH regression analysis for CPP during the 36 months after surgery to assess the robustness of the PSM methods and results. Visual inspection of log-minus-log survival plots for categorical variables and restricted cubic splines for continuous variables were used to test the PH assumptions of the Cox regression analysis. The following variables were included in the analysis, without the variable selection process: age, female sex, body mass index, pathological staging (stage I versus stage II or more), postoperative radiotherapy, operation type (pneumonectomy or lobectomy versus segmentectomy or wedge resection), duration of surgery (h), intraoperative analgesia, NRS score at the first postoperative outpatient visit, year of surgery and AC within 3 months after surgery. The following variables were excluded from the analysis, due to the violation of PH assumptions: American Society of Anesthesiologists physical status, current smoker, preoperative antipsychotics use, preoperative chemotherapy, preoperative radiotherapy and four-staged pathological staging.
With our available sample size of 2222 patients for Cox PH regression analysis, we had 92.6% power to detect a priori hazard ratio (HR), which we deemed clinically important with a significance criterion of 0.05. We hypothesized that the HR of AC within 3 months after surgery was 1.7 and the probability of events was assumed to be 0.15.
RESULTS
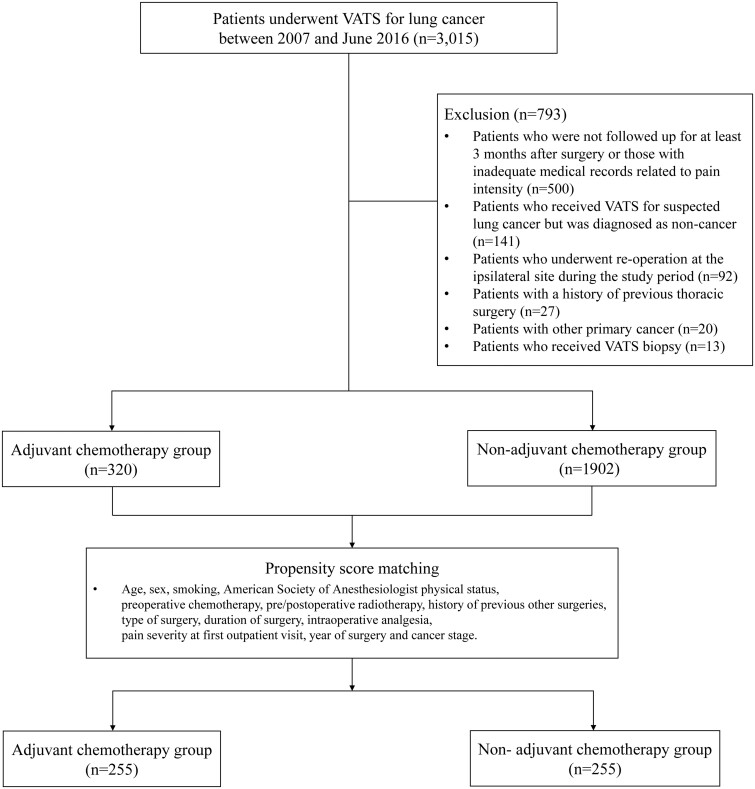
During the study period, 3015 adult patients underwent VATS for lung cancer resection at our hospital. After 793 patients were excluded following the exclusion criteria, the remaining 2222 patients were finally analysed (Fig. 1). Of the excluded patients, 210 (26.5%) patients underwent AC.
Figure 1:
Flow diagram of the study. VATS: video-assisted thoracoscopic surgery.
During the 36 months of follow-up, 417 (18.8%) patients received AC. The median number of AC cycles in these patients was 4 (IQR 4–5) and the median interval between the surgery and first chemotherapy was 33 days (IQR 23–54 days). Among them, 320 (76.7%) patients received AC within 3 months after surgery and 97 (23.3%) patients received AC 3 months after surgery. The median number of AC cycles in these patient groups was 4 (IQR 4–5) and 3 (IQR 1–6), respectively, and the median interval between the surgery and first chemotherapy was 29 days (IQR 21–35 days) and 638 days (IQR 342–1160 days), respectively.
Patient’s clinical characteristics and perioperative parameters were compared between the 2 groups (Table 1). The AC group participants were younger, less likely to have diabetes mellitus, less likely to receive other surgery before VATS, more likely to receive postoperative radiotherapy and have a longer duration of surgery. There were also significant differences between the 2 groups in pathological staging and operation type. Mortality within 36 months after surgery was significantly higher in the AC group than in the non-AC group (n = 43, 13.4% vs n = 96, 5.0%; P < 0.001).
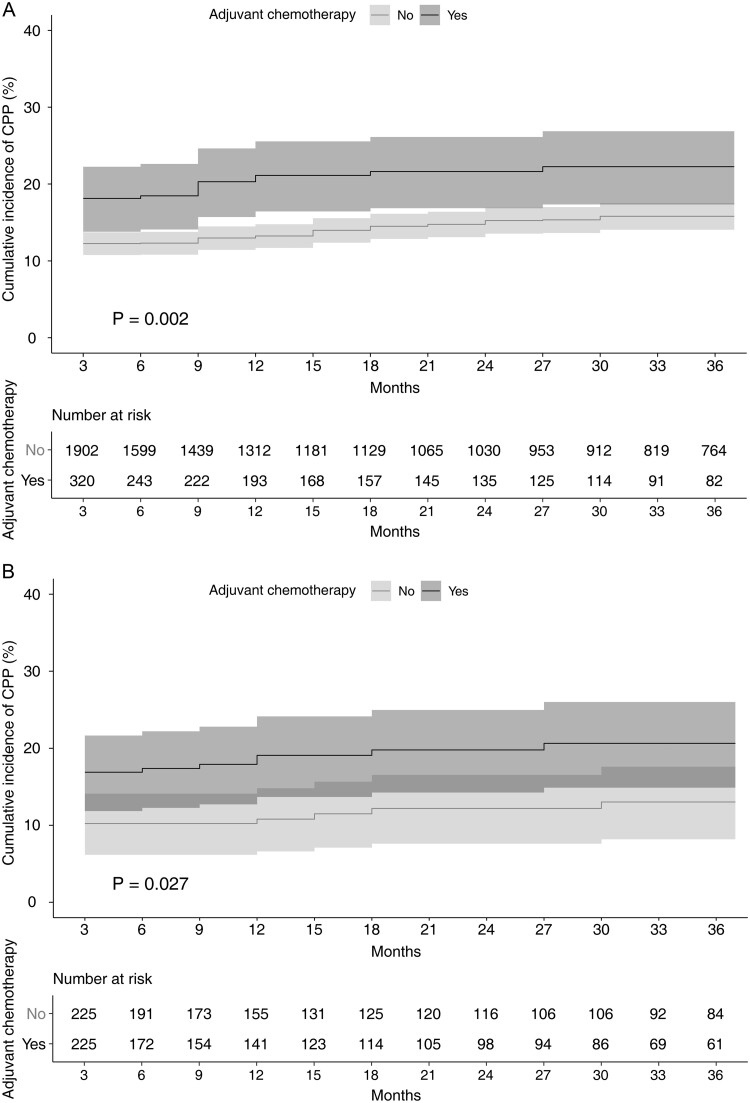
In total, 349 (15.7%) patients were diagnosed with CPP during the study period. PSM yielded 225 pairs (Supplementary Material, Fig. S1 and Supplementary Material, Table S2). The incidence of CPP at 3 months after surgery was significantly higher in the AC group than in the non-AC group before and after PSM (before: AC group, 18.1% vs non-AC group, 12.3%, P = 0.004; after: AC group 16.9% vs non-AC group 10.2%, P = 0.039). Detailed information on the CPP incidence at each point is shown in Supplementary Material, Table S3. Kaplan‒Meier curve analyses showed significant differences in the development of CPP between the 2 groups, before and after PSM (before: log-rank test P = 0.002; after: log-rank test P = 0.027, Fig. 2).
Figure 2.
Kaplan–Meier survival curve analysis of chronic postoperative pain after video-assisted thoracoscopic surgery according to the patients with (black) and without (grey) adjuvant chemotherapy before (A) and after (B) propensity score matching. The results of log-rank test between the groups are shown on the figure. The 95% confidence interval of each group appears as a shaded area. CPP: chronic postoperative pain.
Among the 349 patients with CPP, 278 (79.7%) were prescribed analgesics for CPP after VATS for lung cancer resection (Table 2). The most commonly prescribed analgesics were weak opioids (76.2%). The proportion of strong opioid prescription was significantly higher in the AC group than in the non-AC group (52.9% vs 23.5%, P < 0.001), and the proportion of prolonged strong opioid use was also significantly higher in the AC group (19.1% vs 8.2%, P = 0.008).
Table 2:
Analgesics that the patients with lung cancer received for chronic postoperative pain after video-assisted thoracoscopic surgery between patients with and without AC within 3 months after surgery during the study period
| AC group (n = 68) | Non-AC group (n = 281) | P-value | |
|---|---|---|---|
| No medication | 8 (11.8) | 63 (22.4) | 0.051 |
| Acetaminophen | 20 (35.7) | 42 (20.0) | 0.014 |
| Anticonvulsantsa | 16 (23.5) | 20 (7.1) | 0.001 |
| Antidepressantsb | 6 (8.8) | 14 (5.0) | 0.222 |
| Lidocaine transdermal patch | 4 (5.9) | 12 (4.3) | 0.569 |
| Nonsteroidal anti-inflammatory drugs | 24 (35.3) | 82 (29.2) | 0.326 |
| Weak opioidsc | 56 (82.4) | 210 (74.7) | 0.186 |
| Strong opioidsd | 36 (52.9) | 66 (23.5) | <0.001 |
| Prolonged strong opioid use (≥3 months) | 13 (19.1) | 23 (8.2) | 0.008 |
The values are presented as the median (interquartile range) or n (%).
Gabapentin, pregabalin.
Serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants.
Tramadol, tramadol/acetaminophen combination.
Hydrocodone, hydromorphone, morphine, oxycodone, transdermal fentanyl patch.
AC: adjuvant chemotherapy.
Table 3 presents the results of the Cox PH regression analysis for CPP during the first 36 months after surgery. AC within 3 months after surgery was identified as a significant predictor of CPP during the first 36 months after surgery (HR 1.62, 95% confidence interval 1.16–2.28; P = 0.005).
Table 3:
Cox proportional hazards regression analysis to predict chronic postoperative pain during the first 36 months after video-assisted thoracoscopic surgery for lung cancer resection
| Variables | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Adjuvant chemotherapy within 3 months after surgery | 1.62 (1.16–2.28) | 0.005 |
| Age (years) | 1.00 (0.99–1.01) | 0.851 |
| Female (versus male) | 1.24 (0.99–1.54) | 0.058 |
| Body mass index (kg/m²) | 1.03 (1.00–1.07) | 0.084 |
| Pathological staging (versus stage I) | ||
| Stage II or more | 1.05 (0.75–1.48) | 0.765 |
| Operation time (h) | 1.12 (1.00–1.25) | 0.046 |
| Operation type (versus pneumonectomy or lobectomy) | ||
| Segmentectomy | 0.75 (0.48–1.15) | 0.183 |
| Wedge resection | 1.08 (0.73–1.61) | 0.688 |
| Intraoperative analgesia (versus none) | ||
| Intercostal nerve blockade | 0.71 (0.52–0.96) | 0.027 |
| Percutaneous bupivacaine injection | 1.14 (0.81–1.62) | 0.449 |
| NRS at first postoperative outpatient visit | 1.42 (1.36–1.49) | <0.001 |
| Postoperative radiotherapy | 0.99 (0.68–1.45) | 0.975 |
| Year of surgery (versus 2007) | ||
| 2008 | 0.74 (0.31–1.76) | 0.496 |
| 2009 | 0.42 (0.17–1.02) | 0.054 |
| 2010 | 0.55 (0.25–1.20) | 0.132 |
| 2011 | 0.93 (0.47–1.84) | 0.825 |
| 2012 | 0.57 (0.28–1.16) | 0.118 |
| 2013 | 0.45 (0.22–0.91) | 0.027 |
| 2014 | 0.57 (0.28–1.12) | 0.103 |
| 2015 | 0.51 (0.25–1.01) | 0.052 |
| 2016 | 0.40 (0.20–0.81) | 0.011 |
CI: confidence interval; NRS, numeric rating scale.
DISCUSSION
In this study, we investigated the effect of AC on the long-term incidence of CPP after VATS for lung cancer resection, after adjusting for possible confounding factors by PSM. Survival analysis with PSM showed that the incidence of CPP during the first 36 months after surgery was significantly different between the matched groups, with and without AC. The multivariable adjustment also showed that AC was a significant risk factor for CPP. Therefore, physicians should endeavour to prevent CPP in patients with lung cancer with VATS scheduled for AC.
Previous surgical studies have demonstrated an association between AC and CPP [13, 14]. Chemotherapy is reportedly associated with the incidence of phantom limb pain in paediatric patients [13]. In breast cancer surgery, there are conflicting results regarding the effect of AC on the development of CPP [14, 15]. Unlike our previous study [6], some previous studies have reported that AC is not associated with CPP after lung cancer surgery [7, 8]. However, recent studies suggest that AC is a strong risk factor for chronic opioid use after various curative cancer surgeries [16] or curative lung cancer surgeries [17]. Furthermore, AC is still a significant risk factor for CPP, even after adjusting for the variables related to disease progression in this study.
Although the mechanism by which AC affects CPP is not yet known, we assume that the following processes are involved. First, AC may have acted as a factor contributing to CPP, similar to the double crush syndrome [18]. Neuropathic pain caused by intraoperative nerve injury accounts for a large portion of CPP after thoracic surgery [19]. Intraoperatively damaged nerves may be more susceptible to neurotoxic effects of chemotherapeutics [20]. This concern has been raised in 1 case report of severe brachial plexopathy after peripheral nerve blockade in a patient who previously received chemotherapy [21]. The combination of 2 minor insults (cisplatin and local anaesthetic/epinephrine exposure) could result in a pharmacological double crush syndrome [21]. Based on this report, the American Society of Regional Anesthesia and Pain Medicine practice advisory described chemotherapy-induced neuropathy (CIN) as a risk factor for neurological complications associated with regional anaesthesia [22]. However, it is difficult to explain the development of CPP by intraoperative nerve injury alone [23]. Second, the chemotherapeutics not only cause changes in nociceptive processing of sensory neurons but also alter the nociceptive processing at the spinal dorsal horn level, resulting in hyperalgesia [24]. Third, for patients scheduled for AC, the psychological distress could have been higher than in patients without planned AC, which would have had a significant impact on the development of CPP. Anxiety plays an important role in the development of CPP [25] and AC may cause psychological stress, including anxiety, during the perioperative period in patients with cancer [26]. Although we were unable to assess the psychological status of patients in this study, we consider that the greater mental burden caused by the more advanced cancer stage and the need for further postoperative cancer therapy in patients scheduled for AC may have played an important role in the development of CPP. Taken together, AC may be an important risk factor for CPP after VATS for lung cancer resection.
Despite the retrospective design of our study, our results (after rigorous adjustment for the potential confounders associated with CPP) provide further evidence on the effect of AC on the development of CPP in surgical cancer patients. Therefore, physicians should pay more attention to prevent CPP in patients scheduled for AC after lung cancer surgery. In addition to the previously known methods of CPP prevention [1], the following methods should be considered for patients scheduled for AC after the VATS. First, the administration of perioperative duloxetine, which is a treatment and preventive medication for CIN [27], should be considered for preventing CPP in these patients. Perioperative duloxetine has been reported to reduce postoperative opioid requirements [28]. Although there was no statistically significant difference in the incidence of CPP in that study, which had a small sample size, the incidence of CPP at 3 and 6 months after surgery was lower in the duloxetine group [28]. Second, AC can cause anxiety in patients with cancer and perioperative anxiety is an important risk factor for CPP; thus, various methods should be used to manage the psychological distress in these patients [29].
Limitations
There are several limitations to this study. First, some potential risk factors of CPP, such as preoperative chronic pain, psychological conditions and duration of chest tube drainage [1, 8], could not be included due to the lack of detailed information. Second, patients who received chemotherapy 3 months after surgery were not included in the AC group. In addition, we could not compare the effect of preoperative and postoperative chemotherapy on the development of CPP due to the small number of patients who had received preoperative chemotherapy. Third, we were not able to investigate the cumulative dose of the administered chemotherapeutics. The cumulative dose of chemotherapeutics has been reported to be the main risk factor associated with the persistence of neurotoxicity [30]. Fourth, we could not investigate CIN in our study population due to the imprecision of the CIN diagnostic code. However, we considered that CPP in this study differed from CIN caused by chemotherapy as we investigated pain intensity at the surgical site. Fifth, we considered only the pain intensity at the incisional site based on EMRs. Not only the pain at the incisional site but also pain beyond this site and even other symptoms such as numbness and paraesthesia can occur after thoracic surgery. Sixth, the cancer staging, which was a fundamental difference between the 2 groups, could affect the development of CPP. To reduce the impact of this important confounder, we included the cancer staging in the PSM and Cox PH regression analysis. Last, a substantial number of patients were excluded due to inadequate medical records or lack of follow-up. This likely excluded some patients with risk factors for CPP reported in our previous study [6].
CONCLUSION
In conclusion, our study showed that AC is an important risk factor for CPP after VATS for lung cancer resection. AC is an important treatment associated with survival rate in patients with lung cancer after resection surgery. Given that many patients undergo such treatment, the effect of AC on the development of CPP presents a significant issue. Identification of all risk factors for CPP after VATS for lung cancer resection may facilitate its prediction and treatment.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to thank Editage for English language editing.
Conflict of interest: none declared.
Abbreviations
- AC
Adjuvant chemotherapy
- CIN
Chemotherapy-induced neuropathy
- CPP
Chronic postoperative pain
- EMR
Electronic medical record
- HR
Hazard ratio
- IQR
Interquartile range
- NRS
Numeric rating scale
- PH
Proportional hazards
- PSM
Propensity score matching
- STROBE
Strengthening the reporting of observational studies in epidemiology
- VATS
Video-assisted thoracoscopic surgery
Author contributions
Susie Yoon: Methodology; Formal analysis; Visualization; Writing—original draft; Writing—review & editing. Won-Pyo Hong: Data curation. Hyundeok Joo: Methodology; Data curation; Writing—original draft. Dongyeon Jang: Data curation. Samina Park: Writing—review & editing. Ho-Jin Lee: Conceptualization; Methodology; Formal analysis; Investigation; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Haruhisa Matsuguma, Meinoshin Okumura, Noriyoshi Sawabata and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain 2018;31:155–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet 2019;393:1537–46. [DOI] [PubMed] [Google Scholar]
- 3. Steegers MAH, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OHG. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955–61. [DOI] [PubMed] [Google Scholar]
- 4. Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain 2014;8:139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macrae W. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- 6. Yoon S, Hong WP, Joo H, Kim H, Park S, Bahk JH et al. Long-term incidence of chronic postsurgical pain after thoracic surgery for lung cancer: a 10-year single-center retrospective study. Reg Anesth Pain Med 2020;45:331–6. [DOI] [PubMed] [Google Scholar]
- 7. Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology 2017;126:938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peng Z, Li H, Zhang C, Qian X, Feng Z, Zhu S. A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on quality of life. PLoS One 2014;9:e90014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S–313S. [DOI] [PubMed] [Google Scholar]
- 10. Pirker R, Filipits M. Adjuvant therapy in patients with completely resected non–small-cell lung cancer: current status and perspectives. Clin Lung Cancer 2019;20:1–6. [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzche PC, Vandenbroucke JP et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645–53. [Google Scholar]
- 13. Smith J, Thompson JM. Phantom limb pain and chemotherapy in pediatric amputees. Mayo Clin Proc 1995;70:357–64. [DOI] [PubMed] [Google Scholar]
- 14. Meretoja TJ, Leidenius MHK, Tasmuth T, Sipilä R, Kalso E. Pain at 12 months after surgery for breast cancer. JAMA 2014;311:90–2. [DOI] [PubMed] [Google Scholar]
- 15. Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain 2006;7:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JS, Hu HM, Edelman AL, Brummett CM, Englesbe MJ, Waljee JF et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol 2017;35:4042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brescia AA, Harrington CA, Mazurek AA, Ward ST, Lee JSJ, Hu HM et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg 2019;107:363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Upton AM, Mccomas A. The double crush in nerve-entrapment syndromes. Lancet 1973;302:359–62. [DOI] [PubMed] [Google Scholar]
- 19. Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain 2013;154:95–102. [DOI] [PubMed] [Google Scholar]
- 20. Windebank AJ, Grisold W. Chemotherapy‐induced neuropathy. J Peripher Nerv Syst 2008;13:27–46. [DOI] [PubMed] [Google Scholar]
- 21. Hebl JR, Horlocker TT, Pritchard DJ. Diffuse brachial plexopathy after interscalene blockade in a patient receiving cisplatin chemotherapy: the pharmacologic double crush syndrome. Anesth Analg 2001;92:249–51. [DOI] [PubMed] [Google Scholar]
- 22. Neal JM, Barrington MJ, Brull R, Hadzic A, Hebl JR, Horlocker TT et al. The second ASRA practice advisory on neurologic complications associated with regional anesthesia and pain medicine: executive summary 2015. Reg Anesth Pain Med 2015;40:401–30. [DOI] [PubMed] [Google Scholar]
- 23. Wildgaard K, Ringsted TK, Hansen HJ, Petersen RH, Werner MU, Kehlet H. Quantitative sensory testing of persistent pain after video-assisted thoracic surgery lobectomy. Br J Anaesth 2012;108:126–33. [DOI] [PubMed] [Google Scholar]
- 24. Weng H-R, Cordella J, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain 2003;103:131–8. [DOI] [PubMed] [Google Scholar]
- 25. Theunissen M, Peters ML, Bruce J, Gramke HF, Marcus MA. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 2012;28:819–41. [DOI] [PubMed] [Google Scholar]
- 26. So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs 2010;14:17–22. [DOI] [PubMed] [Google Scholar]
- 27. Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy‐induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther 2011;90:377. [DOI] [PubMed] [Google Scholar]
- 28. Ho K-Y, Tay W, Yeo M-C, Liu H, Yeo S-J, Chia S-L et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth 2010;105:371–6. [DOI] [PubMed] [Google Scholar]
- 29. Charalambous A, Giannakopoulou M, Bozas E, Paikousis L. A randomized controlled trial for the effectiveness of progressive muscle relaxation and guided imagery as anxiety reducing interventions in breast and prostate cancer patients undergoing chemotherapy. Evid Based Complement Alternat Med 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long‐term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 2010;116:2322–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.