Abstract
OBJECTIVES
Soluble urokinase plasminogen activator receptor (suPAR) is a biomarker that has been implicated in several cardiac pathologies and has been shown to be elevated in critically ill populations. We measured plasma suPAR in a cohort of cardiac surgical patients to evaluate its ability to predict prolonged intensive care unit (ICU) and hospital length of stay and development of complications following surgery. We compared suPAR against EuroSCORE II and C-reactive protein (CRP).
METHODS
Ninety patients undergoing cardiac surgery were recruited with samples taken preoperatively and on postoperative days 1, 2 and 3. suPAR was measured using enzyme-linked immunosorbent assay. Area under the receiver operator curve (AUROC) was used to test predictive capability of suPAR. Comparison was made with EuroSCORE II and CRP.
RESULTS
suPAR increased over time (P < 0.001) with higher levels in patients requiring prolonged ICU and hospital stay, and prolonged ventilation (P < 0.05). suPAR was predictive for prolonged ICU and hospital stay, and prolonged ventilation at all time points (AUROC 0.66–0.74). Interestingly, this association was also observed preoperatively, with preoperative suPAR predicting prolonged ICU (AUROC 0.66), and hospital stay (AUROC 0.67) and prolonged ventilation (AUROC 0.74). The predictive value of preoperative suPAR compared favourably to EuroSCORE II and CRP.
CONCLUSIONS
suPAR increases following cardiac surgery and levels are higher in those who require prolonged ICU stay, prolonged hospital stay and prolonged ventilation. Preoperative suPAR compares favourably to EuroSCORE II and CRP in the prediction of these outcomes. suPAR could be a useful biomarker in predicting outcome following cardiac surgery, helping inform clinical decision-making.
Clinical registration
West of Scotland Research Ethics Committee Reference: 12/WS/0179 (AM01).
Keywords: Biomarkers, Cardiac surgical procedures, Postoperative complications, Receptors, Urokinase plasminogen activator, Thoracic surgery
Patients undergoing cardiac surgery are at the risk of multisystem postoperative complications [1], resulting in the prolongation of intensive care unit (ICU) admission [2] and hospital stay [3].
INTRODUCTION
Patients undergoing cardiac surgery are at the risk of multisystem postoperative complications [1], resulting in the prolongation of intensive care unit (ICU) admission [2] and hospital stay [3]. The ability to predict either preoperatively or early postoperatively those patients at increased risk of complications would aid clinical decision-making. A reliable prognostic biomarker [4] would enable the identification of patients at increased risk, allowing them to receive additional monitoring and earlier intervention. Conversely, identification of patients unlikely to require extra support would allow these patients to be triaged to a fast-track recovery.
Soluble urokinase plasminogen activator receptor (suPAR) is the soluble form of the leucocyte membrane-bound urokinase plasminogen activator receptor [5] and has been linked to plasminogen activation, pericellular proteolysis and chemotaxis [5, 6]. suPAR is a novel biomarker that has been shown to have diagnostic and predictive value in cardiovascular disease [7, 8], the critically ill [9, 10] and patient’s with sepsis [11, 12].
We hypothesized that suPAR would increase following cardiac surgery and would be useful to identify patients requiring a prolonged stay in hospital and/or ICU. Furthermore, we compared the discriminative capability of suPAR against C-reactive protein (CRP) and EuroSCORE II, both of which are measured and calculated perioperatively, to assess suPAR’s potential clinical applicability against established methods.
EuroSCORE II is a scoring system used prior to cardiac surgery to provide an estimate of predicted mortality [13]. It considers various patient-dependent factors, such as cardiac and renal function, as well as surgical factors, and quantifies the overall risk of death. It is intuitive that patients at higher risk of death have higher risk of increased intensive care requirement and EuroSCORE II has been demonstrated to predict prolonged ICU stay [14]. CRP is widely measured in this patient population and is used to identify patients mounting an inflammatory response and determine those at risk of complications such as infection. For these reasons, EuroSCORE II and CRP were compared to suPAR.
Finally, we wanted to assess whether a combined model integrating commonly used clinical information with inflammatory biomarkers would have a greater value in identifying those patients requiring prolonged stays.
METHODS
Trial enrolment and ethics approval
This study is a post hoc analysis of a previous study examining acute kidney injury in patients undergoing cardiac surgery. The trial was registered in April 2012 at ClinicalTrials.Gov (Trial number NCT01573104). Ethical approval for this study (Ethic committee number: 12/WS/0179) was provided by the West of Scotland Research Ethics Service on 21 August 2012. A substantial amendment to allow the additional analyses was submitted on 26 September 2014 and approved by the same ethics committee on 27 April 2016. With informed consent, blood samples were collected from patients undergoing cardiopulmonary bypass cardiac surgery at the Golden Jubilee National Hospital between November 2011 and January 2014.
Data collection
Exclusion criteria for the primary study were patient/surgical refusal, preoperative renal replacement, emergency procedures, age <18 or >90 years, pregnancy, the use of ventricular assist devices, severe chronic renal failure (defined as estimated glomerular filtration rate <30 ml/min/1.73 m2) and impaired patient capacity to consent.
Baseline information was collected on admission about comorbidity status, from which EuroSCORE II [13] was calculated. Intraoperative data were collected from the recall AIMS electronic anaesthetic charting system (Informatics Clinical Information Systems Limited, Glasgow) and postoperative data from the hospitals ICU clinical information system (Centricity CIS; GE Healthcare©, Buckinghamshire, UK).
In accordance with previous studies, prolonged ICU stay was defined as over 48 h [14] and prolonged hospital stay was defined as 12 days or greater [15]. Often patients are discharged from intensive care or hospital for logistical rather than clinical reasons, at ‘set times’, such as following the morning ward round, which can confound the use of length of stay data as a continuous variable. To counter this, these variables were dichotomized to highlight patients who had deviated from normal recovery and required prolonged stays.
A composite end point of complications was used: surgical reoperation, stroke, deep sternal wound infection, postoperative renal failure, prolonged ventilation [16] and atrial fibrillation (AF). Surgical reoperation, stroke and deep sternal wound infection were included if documented in the hospital’s cardiac surgery database (Cardiac, Cardiology and Thoracic Health Information System; CaTHi, Amor Group, Renfrew, UK). In line with previous studies, renal failure was defined as acute kidney injury network [17] stage 1 or greater [18] and prolonged ventilation was defined as over 24 h [19, 20].
Blood samples were collected before induction of anaesthesia and were also collected on the morning of postoperative days 1, 2 and 3. Samples were centrifuged, frozen and stored at −80°C until analysis. suPAR was measured in duplicate using a commercially available solid phase enzyme-linked immunosorbent assay (suPARnostic®, Virogates, Denmark) according to the manufacturer’s instructions. The within-batch coefficient of variation was 6.2%, whilst the between-batch coefficient of variation was 11.8%.
CRP was determined as a routine clinical sample by an enhanced immunoturbidimetric assay run on a Roche Cobas 6000 analyser. The reference range is <10 mg/l, with a lower limit of detection of 1.0 mg/l and a coefficient of variation of 1.7%.
Statistical analysis
Analysis was undertaken using SPSS® (version 22; IBM, Armonk, NY, USA). Variables were visually inspected and tested for normality using the Shapiro–Wilk test. Categorical data are presented as frequency (%) and continuous data are presented as mean (standard deviation) or median (interquartile range) as appropriate.
Multiple comparisons across time points were performed using repeated measures ANOVA or Friedman’s test. Pairwise comparisons were performed using Wilcoxon signed rank test or a paired T-test with appropriate Bonferroni-adjusted P-values to avoid type 1 errors. Comparisons between independent groups were performed using Student’s T-test or Mann–Whitney U-test; adjustment for multiple testing was not applied. Statistical significance was determined as P-value <0.05.
The area under the receiver operator curve (AUROC) was calculated to evaluate the discriminative capability of variables for predicting patients who would require prolonged ICU or hospital stay or who would develop complications. Sensitivity, specificity and positive and negative predictive values (NPVs) were calculated according to optimum cut-off points defined as the point at which the sum of sensitivity and specificity were maximal (Youden’s Index) [21]. Multivariable logistic regression was used to develop a model incorporating preoperative suPAR and EuroSCORE II, with AUROC used to evaluate its discriminative capability.
This manuscript adheres to the STARD guidelines where appropriate.
RESULTS
Ninety patients were recruited. Of the original cohort, 2 patients had their operations cancelled after recruitment for clinical reasons, and no blood samples were obtained in a further 5 patients; these patients were excluded from analysis. The median age was 66 years. The median for EuroSCORE II was 1.2%, ventilation time was 7 h, ICU length of stay was 23 h and hospital length of stay was 7 days (Table 1).
Table 1:
Baseline patient characteristics
| Characteristics | All patients (n = 88) |
|---|---|
| Age (years) | 66 (59–72) |
| Female gender | 19 (21.6) |
| Weight (kg) | 81 (SD: 15.8) |
| EuroSCORE II | 1.2 (0.71–1.57) |
| Actual mortality (%) | 1 (1.1) |
| Cardiovascular comorbidities | |
| Anya | 68 (77.3) |
| Previous MI | 28 (31.8) |
| Arterial hypertension | 59 (67.0) |
| Left main stenosis | 50/82 (61.0) |
| Triple vessel disease | 42/82 (51.2) |
| Intervention type | |
| CABG | 56 (63.6) |
| AVR | 15 (17.0) |
| MVR | 8 (9.1) |
| CABG + AVR | 4 (4.5) |
| Other | 5 (5.7) |
| CPB time (min) | 84 (66–112.5) |
| Aorta clamp time (min) | 58 (40.5–74.5) |
| Surgical time (min) | 202.5 (180–255) |
| Ventilation duration (h) | 7 (4.1–12.4) |
| Intensive care unit stay (h) | 23 (21.5–46) |
| Hospital stay (days) | 7 (6–12) |
Data are presented as median (IQR), mean (SD) or frequency (%).
Cardiovascular comorbidities refers to previous MI, hypertension, left main stenosis or triple vessel disease.
AVR: aortic valve repair; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; IQR: interquartile range; MI: myocardial infarction; MVR: mitral valve repair; Other: unspecified, MVR + CABG, MVR + foramen ovale closure, AVR + ascending aortic aneurysm repair; SD: standard deviation.
Seventeen patients (19.3%) had a prolonged ICU stay and 23 (26.1%) had a prolonged hospital stay. Those with a prolonged hospital stay were older (P < 0.001), had a longer cardiopulmonary bypass time (P = 0.004), had a longer aortic cross-clamp time (P = 0.027) and had a longer ICU stay (P = 0.002) than those who did not. There was no difference in demographics between those having a longer ICU stay and those who did not (Table 2).
Table 2:
Key group characteristics and variations
| Characteristics | Prolonged ICU stay (n = 17) | Non-prolonged ICU stay (n = 71) | Prolonged hospital stay (n = 23) | Non-prolonged hospital stay (n = 65) | Prolonged ventilation (n = 8) | Non-prolonged ventilation (n = 80) |
|---|---|---|---|---|---|---|
| Age (years) | 69 (51–78) | 66 (59–72) | 71 (68–77)†† | 63 (57–70)†† | 68 (51–71) | 66 (59–73) |
| EuroSCORE II (%) | 1.2 (0.82–1.62) | 1.1 (0.71–1.59) | 1.3 (0.97–2.09)† | 0.99 (0.68–1.47)† | 1.36 (0.81–4.55) | 1.14 (0.71–1.49) |
| CPB time (min) | 93 (69.5–128.5) | 80.5 (63.8–108.8) | 104 (81–143)†† | 78 (60.3–102.8)†† | 143 (92.3–233.8)‡ | 81 (64.5–108.5)‡ |
| Aorta clamp time (min) | 70 (39–75) | 56 (41–74) | 70 (56–81)† | 51.5 (37–72)† | 74.5 (73–122.3)‡ | 56 (39.5–72)‡ |
| Surgical time (min) | 200 (185–265) | 205 (180–251.3) | 215 (185–270) | 200 (177.5–247.5) | 280 (192.5–376.3)‡ | 200 (180–243.8)‡ |
| Ventilation duration (h) | 14.5 (7–45.8)** | 6.5 (4–9)** | 11.5 (7–32)†† | 5.5 (4–8.8)†† | 45.8 (32.3–59)‡‡ | 6.5 (4–9.8)‡‡ |
| ICU stay (h) | 71 (68.8–107.3)** | 22.5 (20–40.5)** | 46 (22–70.5)†† | 22.5 (20.5–41.5)†† | 107.3 (69.4–568.5)‡‡ | 23 (21.5–44)‡‡ |
| Hospital stay (days) | 13 (8.5–15)** | 6 (6–9)** | 14 (13–16)†† | 6 (6–7)†† | 14 (11.5–26.5)‡‡ | 7 (6–10)‡‡ |
Data are presented as median (IQR).
Symbols denote a difference between prolonged ICU stay versus non-prolonged ICU stay (Mann–Whitney U-test) **P < 0.01.
Symbols denote a difference between prolonged hospital stay versus non-prolonged hospital stay (Mann–Whitney U-test) †P < 0.05, ††P < 0.01.
Symbols denote a difference between prolonged ventilation versus non-prolonged ventilation (Mann–Whitney U-test) ‡P < 0.05, ‡‡P < 0.01.
CPB: cardiopulmonary bypass; ICU: intensive care unit; IQR: interquartile range.
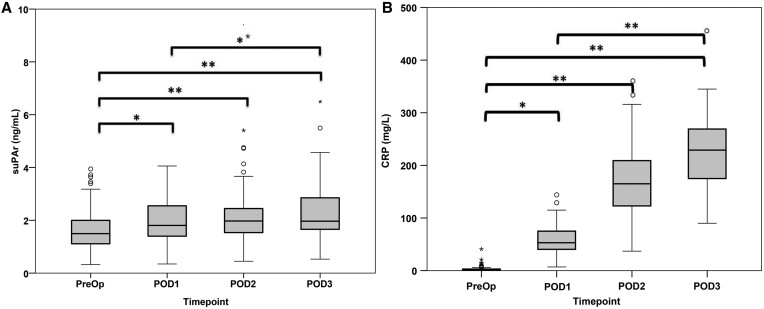
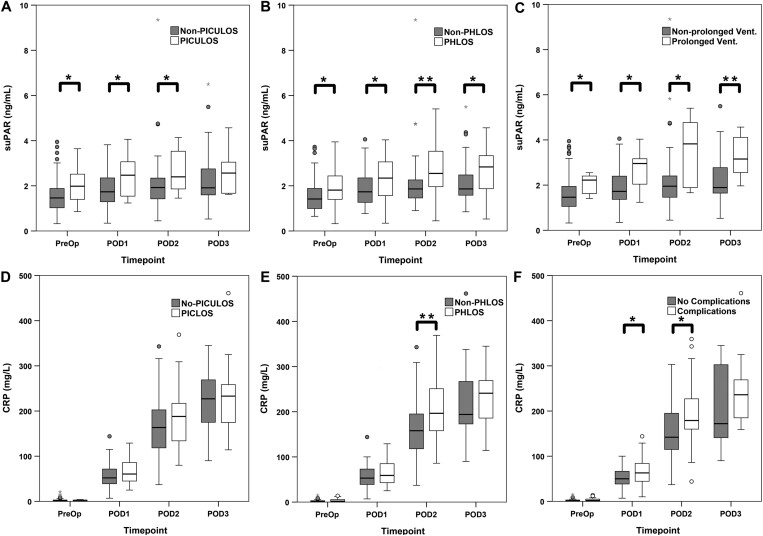
suPAR was higher at all postoperative time points compared with baseline (Fig. 1A). There were differences in suPAR levels both preoperatively and postoperative days 1 and 2 between those patients requiring a longer stay in the ICU and those who did not (Fig. 2A). There were also significant differences in suPAR levels at all time points between those patients who stayed longer in hospital and those who did not (Fig. 2B).
Figure 1:
Perioperative levels of (A) soluble urokinase plasminogen activator receptor and (B) C-reactive protein. Preoperative, postoperative day 1, postoperative day 2 and postoperative day 3. Bars demonstrate differences between two time points (Wilcoxon signed rank test with applied Bonferroni adjustment) (*P < 0.05; **P < 0.01). CRP: C-reactive protein; PreOp: preoperative baseline; POD: postoperative day; suPAR: soluble urokinase plasminogen activator receptor.
Figure 2:
Different levels of biomarkers and outcomes: soluble urokinase plasminogen activator receptor levels between (A) patients who required a Prolonged Intensive Care Unit Length of Stay and those who did not (non-Prolonged Intensive Care Unit Length of Stay), (B) patients who required a Prolonged Hospital Length of Stay and those who did not (non-Prolonged Hospital Length of Stay) and (C) patients who required a prolonged ventilation and those who did not; C-reactive protein levels between (D) patients who required a Prolonged Intensive Care Unit Length of Stay and those who did not (non-Prolonged Intensive Care Unit Length of Stay) (E) patients who required a Prolonged Hospital Length of Stay and those who did not (non-Prolonged Hospital Length of Stay) and (F) patients who developed complications and those who did not (no-complications) over time points: preoperative, postoperative day 1, postoperative day 2 and postoperative day 3. Bars demonstrate differences between groups (Mann–Whitney U-test) (*P < 0.05; **P < 0.01). CRP: C-reactive protein; PHLOS: Prolonged Hospital Length of Stay; PICULOS: Prolonged Intensive Care Unit Length of Stay; PreOp: preoperative; POD: postoperative day; suPAR: soluble urokinase plasminogen activator receptor.
CRP levels were higher at all postoperative time points compared with baseline (Fig. 1B). There was no difference in CRP levels at any time point between those patients who required prolonged ICU and those who did not (Fig. 2D). There were differences in CRP levels on postoperative day 2 between those patients requiring prolonged hospital and those who did not, but not at other time points (Fig. 2E).
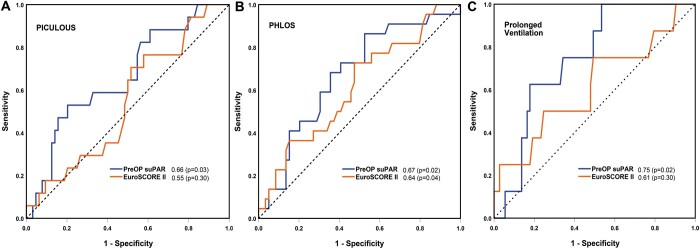
Plasma suPAR levels preoperatively and postoperative day 2 were significant predictors of increased length of ICU and hospital stay, respectively. The predictive value of preoperative suPAR compared favourably to EuroSCORE II and CRP (Table 3 and Fig. 3A and B).
Table 3:
Area under the receiver operator curve of suPAR and logistic EuroSCORE II for each outcome
| Prolonged ICU stay | Prolonged hospital stay | Prolonged ventilation | |
|---|---|---|---|
| suPAR levelsa | |||
| PreOp | 0.66 (0.52–0.81) | 0.67 (0.54–0.80) | 0.75 (0.61–0.88) |
| POD1 | 0.68 (0.53–0.82) | 0.66 (0.52–0.79) | 0.74 (0.53–0.95) |
| POD2 | 0.71 (0.57–0.86) | ||
| POD3 | 0.68 (0.52–0.84) | ||
| EuroSCORE II | |||
| PreOp | 0.55 (0.41–0.69) | 0.64 (0.51–0.77) | 0.61 (0.39–0.84) |
| suPAR and EuroSCORE II | |||
| PreOp | 0.67 (0.53–0.81) | 0.68 (0.55–0.81) | 0.74 (0.58–0.90) |
| CRP levelsa | |||
| PreOp | 0.43 (0.27–0.59) | 0.59 (0.44–0.73) | 0.56 (0.32–0.79) |
| POD1 | 0.62 (0.48–0.76) | 0.59 (0.45–0.73) | 0.59 (0.42–0.76) |
| POD2 | 0.70 (0.57–0.82) | ||
| POD3 | 0.62 (0.42–0.82) | ||
Values presented are area under the receiver operator curve with (95% confidence intervals). Values highlighted in bold are statistically significant P < 0.05.
suPAR and CRP beyond POD1 were not used to predict prolonged ICU stay or prolonged ventilation as those patients still in ICU or still ventilated on POD2 automatically qualified in those categories.
CRP: C-reactive protein; ICU: intensive care unit; PreOp: preoperative; POD1, 2 or 3: postoperative days 1, 2 or 3; suPAR: soluble urokinase plasminogen activator receptor.
Figure 3:
Receiver operator characteristic curves demonstrating the ability of preoperative soluble urokinase plasminogen activator receptor, labelled ‘PreOp suPAR’ and EuroSCORE II to predict patients who will require (A) Prolonged Intensive Care Unit Length of Stay (B) Prolonged Hospital Length of Stay and (C) prolonged ventilation. Area under the receiver operator curve is shown with a corresponding P-value in parentheses. PHLOS: Prolonged Hospital Length of Stay; PICULOS: Prolonged Intensive Care Unit Length of Stay; PreOp: preoperative; suPAR: soluble urokinase plasminogen activator receptor.
For predicting increased time in ICU, the optimum cut-off point was for preoperative suPAR as identified by ROC curve analysis with a concentration of 1.96 ng/ml, giving a sensitivity of 52.9% and a specificity of 79.7%. This corresponded to a positive predictive value (PPV) of 30.8% and an NPV of 90.7%. For predicting a prolonged hospital stay, the optimum cut-off point was for postoperative day 2 suPAR with a concentration of 2.37 ng/ml, sensitivity 63.2%, specificity 81.5%, PPV 54.5% and NPV 86.3%.
Complications
At least one of the composite complications developed in 40 (45.5%) of the 88 patients: 31 (35.2%) developed new onset AF, 16 (18.2%) developed postoperative renal failure with 5 (5.6%) patients requiring renal replacement therapy, 8 (9.1%) required prolonged ventilation, 6 (6.8%) required reoperation, 3 (3.4%) had a deep sternal wound infection and 1 patient had prolonged neurological dysfunction. One patient died, equating to a mortality of 1.1%—for the purposes of analysis, this patient was treated as having a prolonged ICU and hospital stay. There was no difference in EuroSCORE II or suPAR levels at any time point between those patients who developed a composite complication and those who did not. Median CRP levels were higher in patients who went onto develop complications on postoperative days 1 and 2 (Fig. 2F).
In post hoc analyses, association between individual complications and suPAR and CRP levels were analysed. Patients requiring prolonged ventilation had higher levels of suPAR preoperatively and at all postoperative time points (Fig. 2C). Preoperative suPAR was predictive of prolonged ventilation with an AUROC of 0.74 (Table 3). The optimum cut-off point for preoperative suPAR as identified by AUROC analysis was a concentration of 1.40 ng/ml, sensitivity 100%, specificity 46.6%, PPV 17.1% and NPV 100%. There was no difference in suPAR levels between patients developing any of the other individual complications compared with those who did not.
When CRP was analysed, levels were higher preoperatively in patients who developed AF (3 mg/l compared with 1 mg/l; P = 0.002). This difference was present on postoperative day 1 (69 mg/l compared with 47 mg/l; P = 0.001) and postoperative day 2 (179 mg/l compared with 145 mg/l; P = 0.021). There was no difference in CRP levels between those patients developing any of the other individual complications compared with those who did not.
Surgical procedure
suPAR levels were compared between the 56 patients who had coronary artery bypass grafting (CABG) and the 32 patients who had more complex cardiac surgeries (Table 1). Those patients who had more complex procedures had higher levels of suPAR on postoperative day 1 (2.37 ng/ml compared with 1.57 ng/ml; P = 0.002), postoperative day 2 (2.42 ng/ml compared with 1.86 ng/ml; P = 0.004) and postoperative day 3 (2.77 ng/ml compared with 1.85 ng/ml; P = 0.009), but not at baseline (1.81 ng/ml compared with 1.44 ng/ml; P = 0.18). There was no difference in CRP levels at any time point.
Combined model
A combined model of EuroSCORE II and preoperative suPAR levels produced similar AUROC to preoperative suPAR levels alone (Table 3).
DISCUSSION
suPAR increases following cardiac surgery and is higher in patients requiring longer stays in the ICU and hospital and in those ventilated for >24 h. This difference in suPAR concentrations was present preoperatively and compared favourably to EuroSCORE II and CRP.
We found suPAR was elevated from baseline at all postoperative time points. A study by Gozdzik et al. [22] did not find elevated suPAR levels following cardiac surgery. This discrepancy in results could be explained by the difference in time frames over which suPAR was investigated, and the patient populations. Gozdzik et al. studied 60 patients undergoing isolated CABG surgery, whilst we included patients undergoing a variety of cardiac surgery procedures. We found that those patients having CABG surgery only had lower suPAR postoperatively compared with those undergoing more complex procedures. Our study demonstrated a sustained rise in suPAR that was apparent on postoperative day 1, and beyond. This may not have been seen in Gozdzik et al.’s study [22] which looked at levels up to 24 h only.
suPAR was higher in patients requiring prolonged ICU and hospital stay and prolonged ventilation compared with those who did not; unexpectedly this difference was demonstrated preoperatively. Increases in suPAR are associated with immune system activation [6], suggesting that these patients had higher levels of inflammation at baseline. Some patients may have underlying comorbidities, which contribute to higher suPAR levels preoperatively and predispose to a more complicated postoperative course. For example, suPAR has been shown to be higher in those with coronary artery disease with levels increasing in parallel with the severity of disease [23]; it is plausible that those patients requiring prolonged stay and ventilation could have more severe disease at baseline, explaining the higher suPAR and poorer outcome.
A recent study by Hodges et al. [24] demonstrated that preoperative suPAR levels predicted complications and mortality following aortic valve replacement. Our study provides further evidence of the value of preoperative suPAR levels in predicting outcomes following cardiac surgery.
Interestingly, CRP levels were not elevated in those who went on to require prolonged stays preoperatively or on postoperative days 1 and 3. CRP can take 2–3 days for levels to peak after a surgical insult [25] and this delay can make it difficult to differentiate between patients developing complications and those demonstrating a ‘normal’ response. It is possible that suPAR is a faster-reacting inflammatory biomarker, and therefore a better early discriminator, compared to CRP with values closer to peak on postoperative day 1.
In the current study, EuroSCORE II was higher in patients who had prolonged hospital stay but performed poorly in predicting prolonged ICU stay (AUROC 0.55) (Table 3 and Fig. 3). A combined model, using preoperative suPAR and EuroSCORE II, was better at predicting these outcomes than EuroSCORE alone. However, the predictive capability of the combined model was driven by suPAR (see Supplementary Material, Table S1).
The composite of complications used in our study was based upon a list of serious complications following cardiac surgery as defined by the Society of Thoracic Surgeons [16], with the addition of AF, which has been shown to significantly affect mortality and morbidity [26]. To further explore the apparent paradox that suPAR is predictive of prolonged intensive care and hospital stay, but not associated with postoperative complications, whilst CRP is not predictive of prolonged stay but is associated with complications, we conducted a post hoc analysis of suPAR and CRP against individual complications.
Elevated suPAR was predictive only for prolonged ventilation. Geboers et al. [27] examined the ability of suPAR to predict the outcomes of patients admitted to ICU with acute respiratory distress syndrome, observing higher levels in those with more severe disease. It is plausible, therefore, that the association between suPAR and prolonged ventilation reflects the development of lung injury. As this relationship between suPAR and duration of mechanical ventilation was also apparent preoperatively, we suggest that suPAR may also serve as a predictor of susceptibility to lung injury rather than simply a measure of disease severity. Although the PPV of suPAR in identifying patients who go on to require prolonged mechanical ventilation was poor (17.1%), the high NPV (100%) was such that preoperative measurement of suPAR could help identify those patients unlikely to require prolonged ventilation. These patients could therefore be suitable for triage to fast-track recovery programmes, an area of growing interest and study in the elective cardiac surgery population [28].
When assessing CRP, we found that higher levels postoperatively were associated with the development of AF. Although a common complication (35% of patients in this study), AF following cardiac surgery often responds promptly to medical management and therefore the presence of this complications would not necessarily prolong intensive care or hospital stay, explaining the lack of association observed.
To our knowledge, this is one of the largest studies to examine suPAR in patients undergoing various types of cardiac surgeries and to describe the use of suPAR to predict outcomes. Given its retrospective nature, and the number of comparisons made the results of this study must be considered ‘hypothesis generating’ to support planning of subsequent, prospective studies. Furthermore, the relatively small sample size of 90 patients and moderate predictive capability of suPAR make it difficult to come to concrete conclusions on the ability of this biomarker to predict prolonged stay and complications. It would be therefore informative to examine any additional predictive value of plasma suPAR in combination with other potential clinical predictors enabling robust multivariable analysis and greater predictive capability.
CONCLUSION
We found that suPAR levels increased after cardiac surgery and that high suPAR levels, both pre- and postoperatively, were associated with prolonged ICU stay, prolonged hospital stay, and prolonged duration of ventilation. In addition, suPAR compared favourably to EuroSCORE II and CRP in predicting these outcomes. The next step is to explore the applicability and effectiveness of suPAR as a predictive biomarker in conjunction with other currently utilized clinical prediction scores in patients undergoing cardiac surgery in a larger study. The aim of this would be to assess whether suPAR could improve the prediction of outcomes in combination with other biomarkers and clinical predictors.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lisa Jolly from the Institute of Infection, Immunity and Inflammation at the University of Glasgow who performed all laboratory analysis. They also thank John Kinsella for his contributions to this research.
Funding
This work was supported by the National Institute of Academic Anaesthesia through the Royal College of Anaesthetists Research, Education and Travel grant via the Ernest Leach Fund to P.M. The funding body had no role in design of the study, collection, analysis and interpretation of data or writing of the manuscript.
Conflict of interest: none declared.
Author contributions
Chase T. Schultz-Swarthfigure: Investigation; Methodology; Writing—original draft; Writing—review & editing. Philip McCall: Funding acquisition; Supervision; Writing—review & editing. Robert Docking: Investigation; Methodology; Writing—review & editing. Helen F. Galley: Supervision; Writing—review & editing. Benjamin Shelley: Conceptualization; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Alexander Kogan, Masamichi Ono and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AF
Atrial fibrillation
- AUROC
Area under the receiver operator curve
- CABG
Coronary artery bypass grafting
- CRP
C-reactive protein
- ICU
Intensive care unit
- NPV
Negative predictive value
- PPV
Positive predictive value
- suPAR
Soluble urokinase plasminogen activator receptor
Presented at the BJA Research Forum, 12–13 November 2015, York at The Principal Hotel.
REFERENCES
- 1. Nearman H, Klick JC, Eisenberg P, Pesa N. Perioperative complications of cardiac surgery and postoperative care. Crit Care Clin 2014;30:527–55. [DOI] [PubMed] [Google Scholar]
- 2. Azarfarin R, Ashouri A, Totonchi Z, Bakhshandeh H, Yaghoubi A. Factors influencing prolonged ICU stay after open heart surgery. Res Cardiovasc Med 2014;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliveira EK, Turquetto ALR, Tauil PL, Junqueira LF, Luiz Porto GG. Risk factors for prolonged hospital stay after isolated coronary artery bypass grafting. Rev Bras Cir Cardiovasc 2013;28:353–63. [DOI] [PubMed] [Google Scholar]
- 4. Preeshagul I, Gharbaran R, Jeong K, Abdel-Razek A, Lee L, Elman E et al. Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J Cardiothorac Surg 2013;8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huai Q, Mazar AP, Kuo A, Parry CP, Shaw D, Callahan J et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science 2006;311:656–9. [DOI] [PubMed] [Google Scholar]
- 6. Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers 2009;27:157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyngbæk S, Marott JL, Sehestedt T, Hansen TW, Olsen MH, Andersen O et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol 2013;167:2904–11. [DOI] [PubMed] [Google Scholar]
- 8. Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppensen JL. suPAR: a new biomarker for cardiovascular disease? Can J Cardiol 2015;31:1293–302. [DOI] [PubMed] [Google Scholar]
- 9. Donadello K, Scolletta S, Taccone FS, Covajes C, Santonocito C, Cortes DO et al. Soluble urokinase-type plasminogen activator receptor as a prognostic biomarker in critically ill patients. Crit Care 2014;29:144–9. [DOI] [PubMed] [Google Scholar]
- 10. Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 2011;15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suberviola B, Castellanos-Ortega A, Ruiz A, Lopez-Hoyos M, Santibanez M. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med 2013;39:1945–52. [DOI] [PubMed] [Google Scholar]
- 12. Zeng M, Chang M, Zheng H, Li B, Chen Y, He W et al. Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med 2016;34:375–80. [DOI] [PubMed] [Google Scholar]
- 13. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–45. [DOI] [PubMed] [Google Scholar]
- 14. Ettema RGA, Peelen LM, Schuurmans MJ, Nierich AP, Kalkman CJ, Moons KGM. Prediction models for prolonged intensive care unit stay after cardiac surgery systematic review and validation study. Circulation 2010;122:682–9. [DOI] [PubMed] [Google Scholar]
- 15. Toumpoulis IK, Anagnostopoulos CE, Swistel DG, DeRose JJ Jr. Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery? Eur J Cardiothorac Surg 2005;27:128–33. [DOI] [PubMed] [Google Scholar]
- 16.The Society of Thoracic Surgeons. Quality Performance Measures: Adult Cardiac Surgery Composite Measures. 2014. http://www.sts.org/quality-research-patient-safety/quality/quality-performance-measures (18 April 2018, date last accessed).
- 17. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg 2010;90:1939–43. [DOI] [PubMed] [Google Scholar]
- 19. Yende S, Wunderink R. Causes of prolonged mechanical ventilation after coronary artery bypass surgery. Chest 2002;122:245–52. [DOI] [PubMed] [Google Scholar]
- 20. Siddiqui MA, Paras I, Jalal A. Risk factors of prolonged mechanical ventilation following open heart surgery: what has changed over the last decade? Cardiovasc Diagn Ther 2012;2:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Youden WJ. An index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 22. Gozdzik W, Adamik B, Gozdzik A, Rachwalik M, Kustrzycki W, Kubler A. Unchanged plasma levels of the soluble urokinase plasminogen activator receptor in elective coronary artery bypass graft surgery patients and cardiopulmonary bypass use. PLoS One 2014;9:e98923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc 2014;3:e001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges GW, Bang CN, Eugen-Olsen J, Olsen MH, Boman K, Ray S et al. suPAR predicts postoperative complications and mortality in patients with asymptomatic aortic stenosis. Open Heart 2018;5:e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole DS, Watts A, Scott-Coombes D, Avades T. Clinical utility of peri-operative C-reactive protein testing in general surgery. Ann R Coll Surg Engl 2008;90:317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lapar DJ, Speir AM, Crosby IK, Fonner E, Brown M, Rich JB et al. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg 2014;98:527–33. [DOI] [PubMed] [Google Scholar]
- 27. Geboers DG, de Beer FM, Boer AM, Poll T, Horn J, Cremer OL et al. Plasma suPAR as a prognostic biological marker for ICU mortality in ARDS patients. Intensive Care Med 2015;41:1281–90. [DOI] [PubMed] [Google Scholar]
- 28. Salhiyyah K, Elsobky S, Raja S, Attia R, Brazier J, Cooper GJ. A clinical and economic evaluation of fast-track recovery after cardiac surgery. Heart Surg Forum 2011;14:E330–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.