Abstract
OBJECTIVES
Durability of sutureless aortic bioprosthetic valves remains a major issue. The aim of this study was to assess structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) of the Perceval bioprosthesis using the new proposed standardized definitions.
METHODS
All patients who underwent aortic valve replacement with sutureless Perceval S prostheses up to September 2016 were included. Clinical and echocardiographic follow-up was performed. New standardized definitions were used to assess the durability of sutureless bioprosthetic valves. From 2013 to 2016, 214 patients were included.
RESULTS
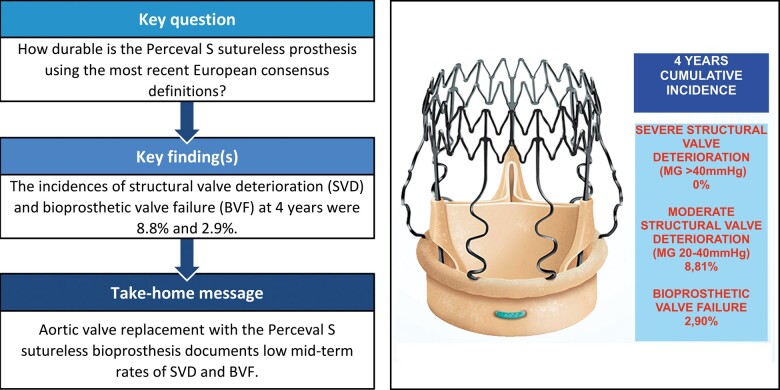
The mean age and EuroSCORE II were 79 years and 2.74. Thirty-day mortality was 0.47%. The survival rate was 96.8%, 88.1% and 85.7% at 1, 3 and 4 years, respectively. The median echocardiographic follow-up was 3.28 years. The mean pressure gradient was 11.3 mmHg. No cases showed evidence of severe SVD, 17 patients had moderate SVD with a mean pressure gradient of 24 mmHg and 8 patients had definite late BVF. The incidence of moderate SVD and BVF at 4 years was 8.8% and 2.9%, respectively.
CONCLUSIONS
Mid-term follow-up to 6.3 years after aortic valve replacement with the Perceval bioprosthesis documents favourable haemodynamic and clinical outcomes and low rates of SVD and BVF.
Keywords: Extracorporeal circulation, Minimally invasive surgery, Valve disease
INTRODUCTION
The Perceval S biological sutureless aortic prosthesis (LivaNova, London, UK) has become an attractive alternative to conventional bioprostheses for aortic valve replacement (AVR) in medium–high-risk patients due to its ease of implantation with its consequent decrease in ischaemia and extracorporeal circulation times, its excellent haemodynamic results and its design that facilitates implantation in minimally invasive surgery, among other benefits [1].
The durability of surgical biological prosthesis has been widely studied with very good results, although these studies have been analysed in very different ways. However, there are hardly any studies assessing the long-term durability of sutureless prostheses (>5 years).
Very recently, standardized European definitions of structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) have been proposed for the assessment of long-term durability of transcatheter and surgical aortic bioprosthetic valves [2]. Our objective was to assess the SVD and BVF of sutureless bioprosthetic aortic valves based on these new definitions in a consecutive series of patients operated on from October 2013 to September 2016, who subsequently underwent periodic outpatient follow-up by transthoracic echocardiography (TTE).
MATERIALS AND METHODS
Ethical statement
This study was approved by the local ethics committee of our hospital. The requirement for written informed consent was waived because of the retrospective nature of this study.
Patient selection
From October 2013 to September 2016, 214 consecutive AVRs were performed in our centre with sutureless Perceval S prostheses. A descriptive, retrospective, non-randomized study was performed in our series. We recorded the preoperative, procedural and postoperative characteristics of the patients in the department database. The definition of renal impairment was considered for patients with a glomerular filtration rate of <60 ml/min/1.73 m2. Clinical and echocardiographic follow-up was carried out by means of a consultation 1 month after discharge in all the patients and periodically thereafter. If follow-up in our department was not possible, the data were obtained from their reference cardiologist. All patients have undergone at least 2 post-surgical echocardiograms performed during their follow-up. The post-surgical echocardiogram performed at the first consultation after surgery was used as the reference echocardiogram and the last echocardiogram performed during follow-up was used for the assessment of valve deterioration. Only 3 were lost to follow-up (all 3 were lost to both echocardiographic and clinical follow-up). The survival of all patients was assessed up to May 2020 using the regional database.
Surgery
The patients underwent isolated AVR surgery, in which a J-shaped partial sternotomy through the third intercostal space was used as a minimally invasive approach or a full sternotomy was performed (according to the criterion of the operating surgeon); alternatively, combined aortic AVR surgery with a full sternotomy approach was used. AVR was performed using the conventional technique with aortic cross-clamp, use of antegrade and/or retrograde blood cardioplegia and high transversal aortotomy.
The prosthesis was implanted using the standard technique that our group has previously described [3]. The specific annular gauges of the prosthesis were used. The prosthesis was taken down folded, guided by 3 points made in the respective nadirs with an amplitude of 2–3 mm on both sides of the valvular annulus. Without entering the outflow tract of the left ventricle (LV), the prosthesis was unfolded at the annular level and ballooning was performed at 4 atmospheric pressures for 30 s.
Definitions
Structural valve deterioration
SVD includes permanent intrinsic changes in the valve (i.e. leaflet tear, calcification, pannus deposition, flail or fibrotic leaflet) leading to degeneration and/or dysfunction, which in turn result in stenosis or intraprosthetic regurgitation. For simplicity, the Task Force specifies 2 degrees of haemodynamic SVD: (1) moderate SVD is defined as (i) mean gradient ≥20 and <40 mmHg and/or ≥10 and <20 mmHg change from baseline (before discharge or within 30 days of valve implantation) and/or (ii) moderate new or worsening (>1+/4+) intraprosthetic aortic regurgitation (AR) and (2) severe haemodynamic SVD is defined as (i) mean gradient ≥40 and/or ≥20 mmHg change from baseline (before discharge or within 30 days of valve implantation) and/or (ii) severe new or worsening (>2+/4+) intraprosthetic AR (2).
Bioprosthetic valve failure
BVF includes any of the following: (i) bioprosthetic valve dysfunction at autopsy, very likely related to the cause of death, or ‘valve-related death’, defined as any death caused by bioprosthetic valve dysfunction in the absence of confirmatory autopsy; (ii) aortic valve reintervention [i.e. valve-in-valve transcatheter aortic valve implantation (TAVI), paravalvular leak (PVL) closure or surgical AVR]; and (iii) severe haemodynamic SVD. BVF can be categorized as definite [i.e. autopsy, reintervention, severe haemodynamic (SVD)] or probable (i.e. valve-related death), and early (i.e. up to 30 days) or late (i.e. >30 days) according to the timing of onset after valve implantation [2].
Outcomes and statistics
A descriptive analysis was made of all the variables included in the study. A normality test was performed for continuous variables using the Shapiro–Wilk test. The quantitative variables were expressed as the mean (standard deviation) or median (interquartile range), as appropriate. The qualitative variables were expressed as a percentage y (absolute value n). The means were compared by Student's t-test or the Mann–Whitney test, as appropriate. The differences between group variables were analysed using Student's t-test for independent data. The association of qualitative variables was estimated by means of the chi-square or Fisher’s exact test if any variable group size was <5. A value of P < 0.05 was considered statistically significant.
The missing values were treated statistically as unknown values.
Two independent survival analyses were performed: first, using the Kaplan–Meier method for the analysis of long-term mortality and, second, using the multiple decrement model for competitive risk analysis, SVD was established as the main event and mortality during follow-up as a competitive event. It was represented by the cumulative incidence function. The same analysis was performed considering BVF as the main event. The Fine–Gray regression model was used to identify prognostic factors for the event of prosthetic degeneration in the presence of competitive risks.
Patients for follow-up were statistically treated as censored data.
StataCorp software was used. StataCorp (2015) Stata Statistical Software: Release 14. College Station, TX: StataCorp LP was used for the statistical analysis.
RESULTS
Early outcomes
The baseline characteristics of the patients are presented in Table 1. The mean age was 79 years and 39.7% were men. Most patients (>90%) were in New York Heart Association Class III/IV. More than half of the patients suffered from high blood pressure, dyslipidaemia and chronic kidney disease, with a EuroSCORE II of 2.74. Aortic stenosis was severe with a mean gradient of 47 mmHg. Moderate/severe AR was present in 24 patients (11.22%), but in the rest of the AVRs performed, the indication for surgery was severe aortic stenosis.
Table 1:
Preoperative characteristics
| Preoperative characteristics (n = 214) | |||
|---|---|---|---|
| Age, mean (SD) | 79 (5) | EuroSCORE II, mean (SD) | 2.7 (2.2) |
| Sex, n (%) | NYHA, n (%) | ||
| Male | 85 (39.7) | I | 2 (0.9) |
| II | 100 (46.7) | ||
| III | 99 (46.3) | ||
| IV | 5 (2.3) | ||
| Body mass index, mean ± SD | 28.8± 4.2 | COPD, n (%) | 20 (9.4) |
| Body surface area (m2), mean ± SD | 1.84± 0.3 | Severe arteriopathy, n (%) | 5 (2.3) |
| HTN, n (%) | 167 (78) | Severe LV dysfunction, n (%) | 4 (2) |
| Smoker, n (%) | 30 (14) | Ejection fraction (%), mean (SD) | 64 (12.7) |
| Diabetes mellitus, n (%) | 56 (26.2) | Mean gradient (mmHg), mean (SD) | 47 (18.4) |
| Renal impairment, n (%) | 5 (2.34) | Peak-to-Peak Gradient (mmHg), mean (SD) | 79 (29) |
| Dyslipidaemia, n (%) | 138 (64.5) | ||
COPD: chronic obstructive pulmonary disease; HTN: hypertension; LV: left ventricle; NYHA: New York Heart Association; SD: standard deviation.
Intraoperative characteristics and 30-day outcomes are presented in Table 2. Our main surgical approach was minimally invasive (69.16%), and the majority of procedures were isolated AVRs (84.58%). A total of 1.87% underwent surgery for infective endocarditis and 2.34% were reoperations. The most used valve size was M (32.72%) and the least used was XL (14.95%). The average clamping time was 28 min. Thirty-day mortality was low (0.47%). At the beginning of our experience, in 5 cases, we had to reposition the Perceval valve to ensure that there was no periprosthetic regurgitation, and this was always successful with no postoperative complications. In 1 case, a sutureless valve was replaced by a sutured valve because of oversizing and subsequently valve dysfunction. Other major complications are presented in Table 2.
Table 2:
Intraoperative characteristics and 30-day outcomes
| Approach | 30-Day mortality, n (%) | 1 (0.47) | |
| Minimally invasive, n (%) | 148 (69.2) | ||
| Full sternotomy, n (%) | 66 (30.8) | ||
| Size, n (%) | Procedure, n (%) | ||
| S | 53 (24.8) | Isolated AVR | 181 (84.6) |
| M | 70 (32.7) | CABG + AVR | 30 (14) |
| L | 59 (27.6) | Mitral + AVR | 1 (0.5) |
| XL | 32 (14.9) | Tricuspid + AVR | 2 (0.9) |
| Clamping time (min), mean (SD) | 28 (14) | Stroke, mean (SD) | 0.9 (2) |
| ECC time (min), mean (SD) | 40 (24) | Postoperative CAVB, n (%) | 21 (9.8) |
| Infectious endocarditis, n (%) | 4 (1.9) | Reintervention, n (%) | 4 (1.9) |
| Previous surgery, n (%) | 5 (2.3) | AKI requiring haemofiltration, n (%) | 6 (2.8) |
AKI: acute kidney injury; AVR: aortic valve replacement; CABG: coronary aortic bypass grafting; CAVB: complete atrioventricular block; ECC: extracorporeal circulation; SD: standard deviation.
Clinical outcome and structural valve deterioration assessment
The median echocardiographic duration of follow-up was 3.28 (interquartile range 1.4–2.6) years.
The survival rate was 96.8% [95% confidence interval (CI): 92.9–98.5], 88.1% (95% CI: 82.1–92.2) and 85.7% (95% CI: 79.1–90.3) at 1, 3 and 4 years, respectively (Fig. 1).
Figure 1:
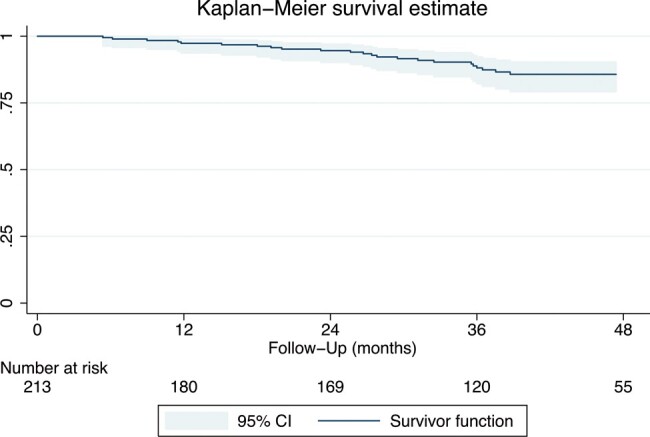
Kaplan–Meier actuarial survival analysis.
All patients (except 3 lost to follow-up) underwent at least 2 echocardiograms during their follow-up. In the overall population, mean aortic gradient remained unchanged during follow-up. The mean gradient of the last echocardiographic follow-up was 11.3 mmHg. There were no cases of moderate/severe intraprosthetic AR. There were also no cases of severe SVD. Seventeen (7.9%) patients had moderate SVD, all of them as a consequence of meeting mean gradient criteria between 20 and 40 mmHg. No patients developed mean gradients >40 mmHg or severe intraprosthetic AR; therefore, there were no cases of severe SVD over time in this series. Figure 2 shows the mean gradients measured during the preoperative period, first postoperative TTE and last postoperative TTE in patients without SVD and with moderate SVD. No statistically significant differences between the groups were obtained for the preoperative gradients, but there were significant differences for the gradients at 30 days (10.7 vs 15.5 mmHg, P = 0.0008) and in the last echocardiogram (10 vs 24 mmHg, P = 0.0001).
Figure 2:
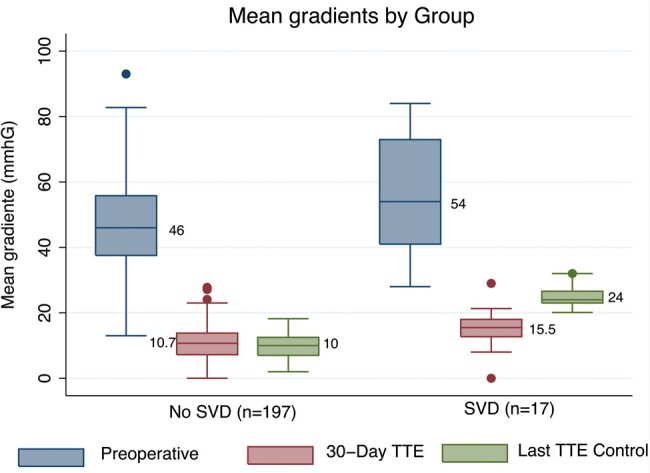
Mean gradient in no structural valve deterioration group and moderate structural valve deterioration group.
Of the 17 patients with moderate SVD, only 1 met BVF criteria for reoperation due to late prosthetic valve endocarditis. It is noteworthy that 11 patients (5.1%) already met moderate SVD criteria for mean gradient in the first post-surgical echocardiogram, and in 9 of these 11 patients, the mean elevated gradient decreased over time to values <20 mmHg in the last follow-up TTE performed.
In our series, 8 patients (3.7%) presented definite late BVF. Only 1 patient was from the moderate SVD group. The causes of BVF were: 3 deaths from prosthetic endocarditis in unoperated patients, 4 reinterventions (3 due to severe periprosthetic AR and 1 due to Enterococcus faecalis recurrent aortic prosthetic valve endocarditis) and 1 valve-in-valve TAVI due to severe periprosthetic AR.
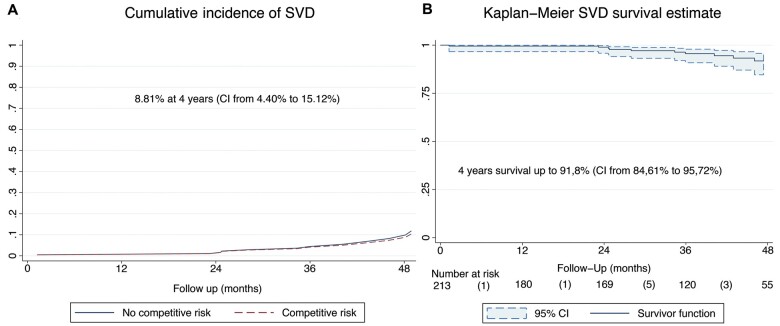
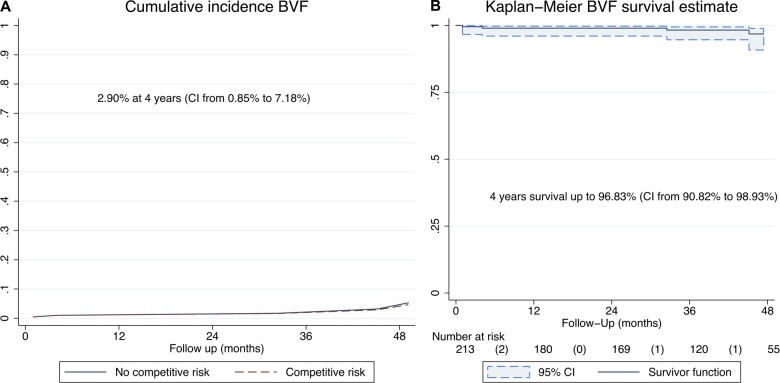
Incidence (competing risk analysis) of moderate SVD and freedom from moderate SVD (Kaplan–Meier analysis) are shown in Fig. 3A and B, respectively. The incidence of SVD was 8.8% (95% CI: 4.4–15.1) and freedom from SVD was 91.8% (95% CI: 84.6–95.7) at 4 years. Incidence (competing risk analysis) of BVF and freedom from BVF (Kaplan–Meier analysis) are shown in Fig. 4A and B, respectively. The incidence of BVF was 2.9% (95% CI: 0.8–7.2) and freedom from BVF was 96.8% (95% CI: 90.8–98.9) at 4 years.
Figure 3:
Incidence of moderate structural valve deterioration and freedom from moderate structural valve deterioration. (A) Incidence of moderate structural valve deterioration (death-competing risk analysis). (B) Freedom from structural valve deterioration (Kaplan–Meier analysis).
Figure 4:
Incidence of bioprosthetic valve failure and freedom from bioprosthetic valve failure. (A) Incidence of bioprosthetic valve failure (death-competing risk analysis). (B) Freedom from bioprosthetic valve failure (Kaplan–Meier analysis).
Using the Fine–Gray regression model taking into account the competitive risk of death, we found that implantation of an XL size prosthesis is a highly significant protective factor for the development of SVD.
DISCUSSION
In the absence of long-term studies (>5 years) on the durability of sutureless Perceval S, we evaluated the results and durability of this sutureless prosthesis in a series of 214 patients who underwent consecutive surgeries in our hospital with a 6.3-year follow-up and using the recent SVD standardized definitions. Our study shows that the Perceval S sutureless bioprosthesis maintains a very good haemodynamic profile over time and a low incidence of SVD and BVF. In fact, no patient had severe SVD, 17 patients had moderate SVD (at the expense of gradients between 20 and 40 mmHg) and 8 patients had BVF (only 1 of them with moderate SVD).
It is noteworthy that our study is the only series (i) of patients with implantation of a Perceval S sutureless prosthesis in which there was a high number of patients with the longest median echocardiographic follow-up and (ii) in which the new European definitions are used for the first time to define SVD.
The long-term results of ‘conventional surgical bioprostheses’ have been analysed in many large series with great heterogeneity in terms of the variables studied and the results obtained. In general, the mean age of the patients included in these studies is lower than that of the patients included in the TAVI series, which in itself constitutes an important bias when comparing the long-term durability of these valves. Some surgical series evaluate durability as a function of survival or survival without reoperation; others analyse SVD based on haemodynamic progression criteria. In one of the large series studying Carpentier-Edwards bioprostheses, survival without reoperation was 98 ± 0.2%, 96 ± 1% and 67 ± 4% at 5, 10 and 20 years, respectively [4]. Another group [5] studied the long-term results of this same valve using clinical and echocardiographic criteria, finding an overall actuarial freedom from SVD at 10 and 20 years of 94.2 ± 0.8% and 48.5 ± 4.6%, respectively. The series studied by Johnston et al. [6] evaluate SVD in 12 569 patients (81 706 patient-years), in whom explant due to SVD at 10 and 20 years was 1.9% and 15%, respectively. Porcine bioprostheses (Hancock II) demonstrated good long-term durability in patients ≥60 years [7]; however, Mitroflow prostheses in some series showed early dysfunction (freedom from SVD of 85.7 ± 2% at 5 years and 33.5 ± 4% at 10 years) [8]. In none of these studies were unified criteria used to assess the durability of the bioprostheses. In any case, we can conclude that almost all the classic bioprostheses used in the last 30 years have shown good results in the very long term. In our series, the mean age is higher (79 years) than in most studies of sutured bioprostheses, but even so, mean survival at 4 years is high and comparable to that of these other studies when similar follow-up times are compared. The durability of the Perceval S sutureless prosthesis is difficult to compare since the same standardized criteria are not available, but if we analyse the reoperation rate, the results are similar to those of conventional bioprostheses.
Most of the studies investigating the Perceval sutureless prosthesis are series describing their experience in terms of morbidity and mortality and intrahospital transvalvular gradients [9]. Very few other registries, in addition to evaluating the short-term results of the Perceval prosthesis, provide information on the durability of these sutureless prostheses in the medium term [10, 11]. Zannis et al. [10], after a mean follow-up of 13 months in their series, obtained a survival of 85.5%, a reoperation rate of 4.9% (3 patients due to PVL, 3 due to intraprosthetic insufficiency and 1 due to pannus growth). Still, the authors conclude that there were no cases of SVD. The mean gradient did not increase over time and was 9.0 mmHg at 12 months, but the mean follow-up was little >1 year, so it cannot be considered a long-term study. Concistrè et al. [11], with a series of 617 patients and mean follow-up times of up to 16 months, showed that Perceval has an excellent haemodynamic performance (11.9 mmHg) and a survival rate of 91% and there were only 4 reoperations (2 due to prosthetic endocarditis, 1 due to severe aortic stenosis and the other due to PVL).
In terms of the few studies evaluating the Perceval prosthesis over time, in the Shrestha et al. [12] study with the follow-up of up to 5 years for 731 patients, their median echocardiographic follow-up was under 2 years, as reflected in their Kaplan–Meier curve; the cumulative survival at 3 years is only 27 patients at risk. In the study we present, we have a median echocardiographic follow-up of 3.2 years, with 120 patients at risk at 3 years on the Kaplan–Meier curve. The mean gradients are also consistent with our study, with these being lower than those of conventional prostheses. Shrestha et al., on the other hand, report 7 patients (1%) with late major PVL, and valve explants occurring in 11 patients (1.5% per late patient-year). Eight explants were due to endocarditis, 1 was related to a left-shunt between the aorta and the right ventricle, 1 to fibrous pannus overgrowth and 1 to a pseudo-aneurysm of the non-coronary sinus resulting in paravalvular regurgitation. Nevertheless, the study by Shrestha et al. [12] did not use the new European definitions to assess the durability of sutureless bioprosthetic valves [2].
The only study examining the durability of the Sutureless Perceval bioprosthesis with a mean follow-up beyond 3 years is the study derived from the Pilot clinical trial [13] with a sample of only 30 patients (mean age 80.4 ± 3.8 years, mean logistic EuroSCORE 13.2 ± 7.3). With a mean follow-up of 4.2 years, 71.3% were alive and had excellent haemodynamic values (the mean gradient was 9.3 mmHg). There were no cases that required reoperation, but it is noteworthy that 1 patient died of prosthetic endocarditis and another presented complete resolution of endocarditis after completing antibiotic treatment. Unlike this study, our series has a much greater number of patients, with an average follow-up of >3 years, and it uses the new European criteria for SVD and BVF. In any case, in comparison with these Perceval prosthesis follow-up studies, in our surgical series, if we analyse, the results in terms of haemodynamic profile, mortality and reoperation rate are quite consistent with previous publications with shorter follow-ups.
After surgery with Perceval, the mean gradient of our patients was low, as in most known studies, and during long-term follow-up this mean gradient hardly changed over time (11.3 mmHg). The same result was seen in the few studies that carried out long-term assessments [13]. All this confirms the hypothesis that the initial advantage of this prosthesis of obtaining such a good haemodynamic profile seems to be maintained over time. It is worth noting that at the 30-day echocardiogram, 11 patients (5.2%) presented gradients between 20 and 40 mmHg, and throughout their follow-up 9 of these patients improved their mean gradient to <20 mmHg; that is, if we followed the standardized definition of SVD, we would initially have to wrongly classify this group of patients in the moderate SVD group, when these high gradients might be the consequence of a hyperdynamic state due to anaemia or other post-surgical conditions. On the other hand, throughout their follow-up, 17 patients (7.9%, a slightly higher percentage than during the immediate postoperative period) presented gradients between 20 and 40 mmHg (average gradient of 24 mmHg), and we classified them in the moderate SVD group, but only 1 patient with moderate SVD had to undergo reoperation for prosthetic endocarditis (BVF group). Therefore, the clinical impact of presenting moderate SVD (meeting only high gradient criteria) appears to be negligible and classifying a prosthesis as SVD in the first months after implantation, based solely on gradients, could be misleading.
Limitations
Our report has some limitations. This is a retrospective study. Follow-up echocardiograms were not performed systematically once a year, although all patients had at least 2 post-surgical echocardiograms, the last echocardiogram being the one used for long-term evaluation. The results of our study are limited by the sample size from a single centre. Although the mean gradients of patients with moderate SVD were not especially high, none of them underwent multislice computed tomography to rule out subclinical thrombosis, so the incidence of SVD could have been overestimated.
CONCLUSION
During our experience with mid-term follow-up Perceval sutureless, we found favourable clinical and haemodynamic results and low rates of SVD and BVF. Despite these encouraging results, additional studies are required to evaluate SVD further in larger and longer series using standardized follow-ups and definitions.
Conflict of interest: J.C.C. and F.E.-C. have received lecture fees (modest) from Palex Medical. The other authors have no conflicts of interest to declare.
Author contributions
José Manuel Martínez-Comendador: Conceptualization; Data curation; Investigation; Writing—original draft. Francisco Estevez-Cid: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—review. Miguel González Barbeito: Data curation; Formal analysis; Validation; Writing—review. Carlos Velasco García De Sierra: Data curation; Formal analysis. Alberto Bouzas Mosquera: Data curation; Formal analysis. Cayetana Barbeito: Data curation; Formal analysis. José Cuenca Castillo: Supervision; Conceptualization. José Herrera-Noreña: Supervision; Conceptualization.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- AVR
Aortic valve replacement
- AR
Aortic regurgitation
- BVF
Bioprosthetic valve failure
- CI
Confidence interval
- PVL
Paravalvular leak
- SVD
Structural valve deterioration
- TAVI
Transcatheter aortic valve implantation
- TTE
Transthoracic echocardiography
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
REFERENCES
- 1. Martínez-Comendador J, Castaño M, Gualis J, Martín E, Maiorano P, Otero J.. Sutureless aortic bioprosthesis. Interact CardioVasc Thorac Surg 2017;25:114–21. [DOI] [PubMed] [Google Scholar]
- 2. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T. et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2017;52:408–17. [DOI] [PubMed] [Google Scholar]
- 3. González Barbeito M, Estévez-Cid F, Pardo Martínez P, Velasco García de Sierra C, Iglesias Gil C, Quiñones Laguillo C. et al. Surgical technique modifies the postoperative atrioventricular block rate in sutureless prostheses. J Thorac Dis 2019;11:2945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forcillo J, Pellerin M, Perrault LP, Cartier R, Bouchard D, Demers P. et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg 2013;96:486–93. [DOI] [PubMed] [Google Scholar]
- 5. Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, Mirza A, Loardi C, May MA. et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015;99:831–7. [DOI] [PubMed] [Google Scholar]
- 6. Johnston DR, Soltesz EG, Vakil N, Rajeswaran J, Roselli EE, Sabik JF. et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg 2015;99:1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. David TE, Armstrong S, Maganti M.. Hancock II bioprosthesis for aortic valve replacement: the gold standard of bioprosthetic valves durability? Ann Thorac Surg 2010;90:775–81. [DOI] [PubMed] [Google Scholar]
- 8. Alvarez JR, Sierra J, Vega M, Adrio B, Martinez-Comendador J, Gude F. et al. Early calcification of the aortic Mitroflow pericardial bioprosthesis in the elderly. Interact CardioVasc Thorac Surg 2009;9:842–6. [DOI] [PubMed] [Google Scholar]
- 9. D'Onofrio A, Salizzoni S, Filippini C, Tessari C, Bagozzi L, Messina A. et al. Surgical aortic valve replacement with new-generation bioprostheses: sutureless versus rapid-deployment. J Thorac Cardiovasc Surg 2020;159:432–442. [DOI] [PubMed] [Google Scholar]
- 10. Zannis K, Joffre J, Czitrom D, Folliguet T, Noghin M, Lansac MN. et al. Aortic valve replacement with the perceval S bioprosthesis: single-center experience in 143 patients. J Heart Valve Dis 2014;23:795–802. [PubMed] [Google Scholar]
- 11. Concistrè G, Chiaramonti F, Bianchi G, Cerillo A, Murzi M, Margaryan R. et al. Aortic valve replacement with perceval bioprosthesis: single-center experience with 617 implants. Ann Thorac Surg 2018;105:40–6. [DOI] [PubMed] [Google Scholar]
- 12. Shrestha M, Fischlein T, Meuris B, Flameng W, Carrel T, Madonna F. et al. European multicentre experience with the sutureless Perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg 2016;49:234–41. [DOI] [PubMed] [Google Scholar]
- 13. Meuris B, Flameng WJ, Laborde F, Folliguet TA, Haverich A, Shrestha M.. Five-year results of the pilot trial of a sutureless valve. J Thorac Cardiovasc Surg 2015;150:84–8. [DOI] [PubMed] [Google Scholar]