Abstract
Background
Although cervical cancer risk overall is elevated among women living with human immunodeficiency virus (HIV; WLH), it is unclear whether risks are similarly elevated across histologic subtypes.
Methods
Data from the HIV/AIDS Cancer Match Study, a linkage of 12 US HIV and cancer registries during 1996 -2016, were used. Cervical cancers were categorized as adenocarcinoma (AC), squamous cell carcinoma (SCC), or other histologic subtype. Standardized incidence ratios compared rates of AC and SCC in WLH to those in general population. For WLH, risk factors for AC and SCC were evaluated using Poisson regression. Five-year survival was estimated by HIV status and histology.
Results
Overall, 62 615 cervical cancers were identified, including 609 in WLH. Compared with the general population, incidence of AC was 1.47 times higher (95% confidence interval [CI]: 1.03–2.05) and SCC was 3.62 times higher among WLH (95% CI: 3.31–3.94). Among WLH, there was no difference in AC rates by race/ethnicity or HIV transmission group, although SCC rates were lower among White women (vs Black) and higher among women who inject drugs (vs heterosexual transmission). Among WLH, 5-year overall survival was similar for AC (46.2%) and SCC (43.8%) but notably lower than for women not living with HIV.
Conclusions
Among WLH, AC rates were modestly elevated, whereas SCC rates were greatly elevated compared with the general population. These findings suggest there may be differences in the impact of immunosuppression and HIV in the development of AC versus SCC, given their common etiology in human papillomavirus infection.
Keywords: human immunodeficiency virus, cervical cancer, adenocarcinoma, squamous cell carcinoma
Rates of cervical adenocarcinoma are only modestly increased (approximately 50%) in women living with human immunodeficiency virus compared with the general population, whereas rates of cervical squamous cell carcinoma are increased by more than 350%.
Adenocarcinoma (AC) of the uterine cervix accounts for around 25% of all cervical cancer cases in the United States [1] and continues to increase, particularly among non-Hispanic White women aged between 40 and 60 years [2]. Human papillomavirus (HPV) is the known cause of both squamous cell carcinoma (SCC) and AC of the cervix [3]; however, SCC and AC arise from different cell types (squamous and glandular, respectively). There is both epidemiologic and clinical evidence that these subtypes differ from one another, including the distribution of associated HPV types, the associated risk factors, and the response to treatment [4–9]. Specifically, the literature suggests that HPV18 is most strongly associated with cervical AC (odds ratio [OR], 105), while HPV16 is most strongly associated with SCC (OR, 30). Furthermore, both smoking and increased parity are associated with an increased risk of SCC but a decreased risk of AC [10]. These observations suggest differences in the tumor biology and host immunologic response of these cervical cancer histologic subtypes.
While it is well established that women living with human immunodeficiency virus (HIV; WLH) are at increased risk of infection with HPV and cervical cancer overall [11–13], population-based studies comparing the incidence of SCC vs AC in WLH have not been performed. The studies that have specifically addressed glandular precursors suggest that there may not be an increased prevalence of glandular lesions in WLH. One study reported that atypical glandular cells accounted for only 4% of all cervical lesions in 108 WLH [14]. Data from the Women’s Interagency HIV Study suggested that the prevalence of glandular lesions in WLH was similar to the prevalence for women not living with HIV (0.8% and 0.6%, respectively) [15]. While both of these studies suggest a similar incidence of glandular precursors regardless of HIV status, no study has compared the distribution of invasive cervical cancer histologies in WLH to that of the general population.
The current recommendations for cervical screening in WLH are for annual Pap smear screening within 1 year of onset of sexual activity or by age 29 years [16]. However, Pap smear screening is more effective in the detection of squamous cell precursors than adenocarcinoma precursors [17]. Thus, understanding the epidemiologic differences in the incidence of AC and SCC in WLH may significantly impact recommendations for use of Pap or HPV-based screening in this population. Furthermore, the immune system plays a significant role in the persistence vs regression of HPV-related disease, and many new and promising treatment strategies for cervical cancer focus on stimulation of the immune system against HPV [18]. The degree of immune suppression in WLH is known to be correlated with the risk of SCC [19], and differences in the relative risk of AC and SCC among WLH may provide insight into the role of immunosuppression for development of these different cervical cancer subtypes. Therefore, in this study, our aim was to estimate the incidence and survival of cervical AC in WLH and women in the general population and compare this to SCC.
METHODS
Data Source and Study Population
In this study, we used data from the HIV/AIDS Cancer Match (HACM) Study, which has linked data from 12 US HIV and cancer registries (https://hivmatch.cancer.gov/) from Colorado (1998–2015), Connecticut (2005–2016), Georgia (2004–2012), Louisiana (1996–2016), Maryland (2008–2012), Michigan (1996–2015), New Jersey (1996–2012), New York (2001–2012), North Carolina (1996–2014), Puerto Rico (2003–2012), Texas (1999–2015), and Washington, D.C. (2007–2015). Follow-up began at the latter of 1 January of the calendar year that each registry entered the study and 4 months after the first HIV report date or AIDS diagnosis date. Participants were followed until either death or the end of registry coverage. The study was reviewed and deemed exempt by the institutional review boards at the National Cancer Institute and Johns Hopkins University, and was approved by institutional review boards at participating registries, as required.
Definitions
Invasive cervical cancers were categorized using a modified version of the Surveillance, Epidemiology, and End Results site recode, limited to International Classification of Diseases for Oncology, Third Edition, site codes C53.0–C53.9, excluding histology codes 8000–8005, 9050–9055, 9140, and 9590–9992. Cases were classified by histology as SCC (8070, 8071, 8072, 8073, 8074, 8075, 8076, 8077, 8078, 8052, 8083, 8084), AC (8140, 8141, 8143, 8147, 8260, 8261, 8262, 8263, 8310, 8323, 8384, 8480, 8481, 8482, 8570, 8571, 8572, 8573, 8574), adenosquamous (8560, 8562), and other/unknown. Only malignant cancer cases were considered, and 2009 International Federation of Gynecology and Obstetrics (FIGO) staging was used for the purpose of this analysis. For all women, the date of cancer diagnosis was obtained from the cancer registries and the date of HIV diagnosis was obtained from HIV registries. Time from HIV to cancer diagnosis was calculated according to these dates. Death dates were obtained from both cancer registries and HIV registries.
Statistical Analyses
Descriptive statistics were used to describe the characteristics of women diagnosed with invasive cervical cancer in the HACM population from 1996 to 2016 overall and separately for AC and SCC cases. The incidence rates of AC and SCC cervical cancer subtypes in WLH and the general population over the study period were calculated crudely as the number of cases over the person-years at risk. Next, to determine whether rates in WLH statistically differed from rates in the general population, standardized incidence ratios (SIRs), defined as the ratio of observed cases in WLH to the expected cancer counts, were calculated for each cervical cancer histologic subtype. The expected counts were determined using cancer rates from the general female population of the registry regions, standardized to the characteristics of the female HIV population by age, race/ethnicity, calendar year, and registry. Ninety-five percent confidence intervals (CIs) around each SIR were calculated using the exact method in SAS version 9.4 (SAS Institute, Inc.).
Among only WLH, rate ratios for AC and SCC were estimated for several covariates using multivariable Poisson models. Covariates of interest included age (<45 years and ≥45 years), race (non-Hispanic White, non-Hispanic Black, and Hispanic), calendar period (1996–2004, 2005–2010, 2011–2016), time since HIV diagnosis (<5 years, 5–10 years, and >10 years), and HIV risk group (heterosexual transmission, injection drug use, and other/unknown).
Finally, overall survival after cervical AC and SCC diagnosis for WLH and women not living with HIV in the general population was calculated from the cancer diagnosis date to date of death or last follow-up. Follow-up was performed by each cancer registry, and censoring occurred on the date of last follow-up per each registry. New York data were not included in the survival analyses because date of last follow-up was not available. The Kaplan-Meier product limit method was used to estimate 5-year survival. Multivariable Cox proportional hazards regression was used to estimate the hazard ratio for death among WLH relative to the general population, adjusting for age, race/ethnicity, year of diagnosis, and tumor stage.
RESULTS
Description of Women With Cervical Cancer by HIV Status
The study population was comprised of 62 615 women with cervical cancer from 1996 to 2016, of whom 609 (1%) were WLH. There were 54 852 women with AC or SCC subtypes, including 35 AC, 507 SCC, 10 adenosquamous, and 57 other/unknown cervical cancer cases in WLH. WLH with cervical cancer tended to be younger (43.6 years) compared with women not living with HIV in the general population (51.2), and similar patterns were seen in women with AC and SCC, likely reflecting the younger age distribution of the HIV population (Table 1). Compared with women with AC in the general population, WLH with AC were more likely to be Black (51.4% vs 11.6%) and have an unknown tumor stage (20.0% vs 9.3%) but with a similar proportion of local and distant disease. Similarly, compared with women with SCC in the general population, WLH with SCC were more likely to be Black (66.7% vs 22.4%) and have an unknown tumor stage (11.2% vs 6.4%). Compared with WLH with SCC, WLH with AC were similar in age (42.9 years vs 43.6 years) and less likely to be Black (51.4% vs 66.7%). They were more likely to have local (57.1% vs 45.4%) and less likely to have regional disease (11.4% vs 33.5%). Additional characteristics of WLH are presented in Table 1.
Table 1.
Characteristics of Women Diagnosed With Invasive Cervical Cancer in the HIV/AIDS Cancer Match Study from 1996 to 2016
| All Cervical Cancers | Adenocarcinoma | Squamous Cell Carcinoma | ||||
|---|---|---|---|---|---|---|
| Characteristic | WLH | Women Not Living With HIV | WLH | Women Not Living With HIV | WLH | Women Not Living With HIV |
| Number of cases | 609 (1.0%) | 62 006 (99.0%) | 35 (0.3%) | 11 332 (99.7%) | 507 (1.2%) | 42 978 (98.8%) |
| Mean age (standard deviation), years | 43.6 (8.8) | 51.2 (15.6) | 42.9 (7.6) | 50.1 (15.3) | 43.6 (8.7) | 51.1 (15.4) |
| Age categories, years | ||||||
| <35 | 94 (15.4%) | 9408 (15.2%) | 4 (11.4%) | 1785 (15.7%) | 77 (15.2%) | 6478 (15.0%) |
| 35–44 | 273 (44.8%) | 15 473 (25.0%) | 21 (60.0%) | 3238 (28.6%) | 221 (43.6%) | 10 595 (24.7%) |
| 45–54 | 188 (30.9%) | 14 269 (23.0%) | 8 (22.9%) | 2511 (22.2%) | 164 (32.3%) | 10 171 (23.7%) |
| 55+ | 54 (8.9%) | 22 856 (36.9%) | 2 (5.7%) | 3798 (33.5%) | 45 (8.9%) | 15 734 (36.6%) |
| Race | ||||||
| Non-Hispanic White | 55 (9.0%) | 36 236 (58.4%) | 8 (22.9%) | 7907 (69.8%) | 42 (8.3%) | 23 673 (55.1%) |
| Non-Hispanic Black | 398 (65.4%) | 12 495 (20.2%) | 18 (51.4%) | 1315 (11.6%) | 338 (66.7%) | 9626 (22.4%) |
| Hispanic | 156 (25.6%) | 13 275 (21.4%) | 9 (25.7%) | 2110 (18.6%) | 127 (25.0%) | 9679 (22.5%) |
| Time period of cancer diagnosis | ||||||
| 1996–2004 | 181 (29.7%) | 23 660 (38.2%) | 13 (37.1%) | 3715 (32.8%) | 142 (28.0%) | 16 951 (39.4%) |
| 2005–2010 | 272 (44.7%) | 23 810 (38.4%) | 15 (42.9%) | 4494 (39.7%) | 229 (45.2%) | 16 366 (38.1%) |
| 2011–2016 | 156 (25.6%) | 14 536 (23.4%) | 7 (20.0%) | 3123 (27.6%) | 136 (26.8%) | 9661 (22.5%) |
| Tumor stage | ||||||
| Local | 281 (46.1%) | 28 918 (46.7%) | 20 (57.1%) | 6566 (57.9%) | 230 (45.4%) | 19 292 (44.9%) |
| Regional | 185 (30.4%) | 21 079 (34.0%) | 4 (11.4%) | 2642 (23.3%) | 170 (33.5%) | 16 330 (38.0%) |
| Distant | 58 (9.5%) | 6962 (11.2%) | 4 (11.4%) | 1074 (9.5%) | 50 (9.9%) | 4596 (10.7%) |
| Unknown | 85 (14.0%) | 5047 (8.1%) | 7 (20.0%) | 1050 (9.3%) | 57 (11.2%) | 2760 (6.4%) |
| Years from HIV to cancer | N/A | N/A | N/A | |||
| Missing | 77 (12.7%) | 6 (17.1%) | 60 (11.8%) | |||
| <5 | 170 (27.9%) | 13 (37.1%) | 137 (27.0%) | |||
| 5–10 | 198 (32.5%) | 10 (28.6%) | 169 (33.3%) | |||
| >10 | 164 (26.9%) | 6 (17.1%) | 141 (27.8%) | |||
| Mode of exposure | N/A | N/A | N/A | |||
| Injection drug use | 189 (31.0%) | 7 (20.0%) | 158 (31.2%) | |||
| Other /Unknown | 149 (24.5%) | 8 (22.9%) | 221 (43.6%) | |||
| Heterosexual transmission | 271 (44.5%) | 20 (57.1%) | 128 (25.2%) | |||
Abbreviations: HIV, human immunodeficiency virus; N/A, not applicable; WLH, women living with HIV.
Incidence of SCC and AC by HIV Status
The crude incidence rate of SCC in WLH was 39.19 cases/100 000 person-years (95% CI: 35.77–42.60) compared with 5.87 (95% CI: 5.82–5.93) in the general population. The standardized incidence ratio, comparing observed counts of SCC in WLH to expected counts in the general population after standardizing to the characteristics of the female HIV population, was 3.62 (95% CI: 3.31–3.94; Table 2). Differences between WLH and the general population were less pronounced for AC. Specifically, the crude incidence rate in WLH was 2.71 cases per 100 000 women (95% CI: 1.81–3.60) for SCC compared with 1.53 cases (95% CI: 1.50–1.56) for AC. The SIR for AC was 1.47 (1.03, 2.05). SIRs for adenosquamous carcinoma and other/unknown histologies are presented in Table 2.
Table 2.
Standardized Incidence Ratios for Cervical Cancer Histologic Subtypes in Women Living With Human Immunodeficiency Virus from 1996 to 2016 Compared With the General Population
| Histologic Group | Observed | Expected | Standardized Incidence Ratio (95% Confidence Interval) |
|---|---|---|---|
| Adenocarcinoma | 35 | 24 | 1.47 (1.03–2.05) |
| Squamous cell carcinoma | 507 | 140 | 3.62 (3.31–3.94) |
| Adenosquamous carcinoma | 10 | 6 | 1.83 (.88–3.36) |
| Other /Unknown | 57 | 15 | 3.89 (2.95–5.05) |
Risk Factors Associated With SCC and AC Among WLH
Among WLH, White women had a lower rate of SCC compared with Black women (adjusted rate ratio [aRR], 0.53; 95% CI: 0.38–0.73; Table 3); while no significant differences by race were observed for AC, estimates trended in the opposite direction (aRR, 2.05; 95% CI: .89–4.75). Women who acquired HIV through injection drug use had a higher rate of SCC compared with women with heterosexual HIV acquisition (aRR, 1.44; 95% CI: 1.17–1.78), but no differences were observed between these 2 HIV risk groups for AC.
Table 3.
Risk Factors for Adenocarcinoma and Squamous Cell Carcinoma Incidence Among Women Living With Human Immunodeficiency Virus
| Adenocarcinoma | Squamous Cell Carcinoma | |||
|---|---|---|---|---|
| Univariate | Multivariatea | Univariate | Multivariatea | |
| Age, years | ||||
| <45 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| ≥45 | 0.52 (0.25–1.09) | 0.58 (0.27–1.24) | 0.92 (0.77–1.10) | 0.89 (0.74–1.07) |
| Race | ||||
| NH Black | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| NH White | 2.00 (0.87–4.62) | 2.05 (0.89–4.75) | 0.56 (0.41–0.77) | 0.53 (0.38–0.73) |
| Hispanic | 1.42 (0.64–3.18) | 1.39 (0.62–3.11) | 1.07 (0.88–1.32) | 1.04 (0.85–1.28) |
| Time period of cancer diagnosis | ||||
| 1996–2004 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 2005–2010 | 0.62 (0.30–1.31) | 0.74 (0.34–1.58) | 0.87 (0.71–1.07) | 0.89 (0.72–1.11) |
| 2011–2016 | 0.45 (0.18–1.12) | 0.59 (0.22–1.57) | 0.80 (0.63–1.01) | 0.83 (0.65–1.08) |
| Years since HIV diagnosis | ||||
| <5 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 5–10 | 0.63 (0.28–1.43) | 0.67 (0.29–1.55) | 1.01 (0.80–1.26) | 1.00 (0.80–1.27) |
| >10 | 0.48 (0.18–1.27) | 0.61 (0.22–1.70) | 1.07 (0.85–1.36) | 1.11 (0.86–1.43) |
| HIV risk group | ||||
| Heterosexual | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Injection drug use | 0.69 (0.29–1.63) | 0.64 (0.27–1.55) | 1.41 (1.15–1.72) | 1.44 (1.17–1.78) |
| Other | 0.59 (0.26–1.33) | 0.59 (0.26–1.35) | 0.85 (0.68–1.06) | 0.85 (0.68–1.05) |
Abbreviations: HIV, human immunodeficiency virus; NH, Non-Hispanic.
aModels are fully adjusted for all variables in the table: age, race, time period of cancer diagnosis, years since HIV diagnosis, and HIV risk group.
Overall Survival and Risk Factors for Mortality by HIV Status and Histology
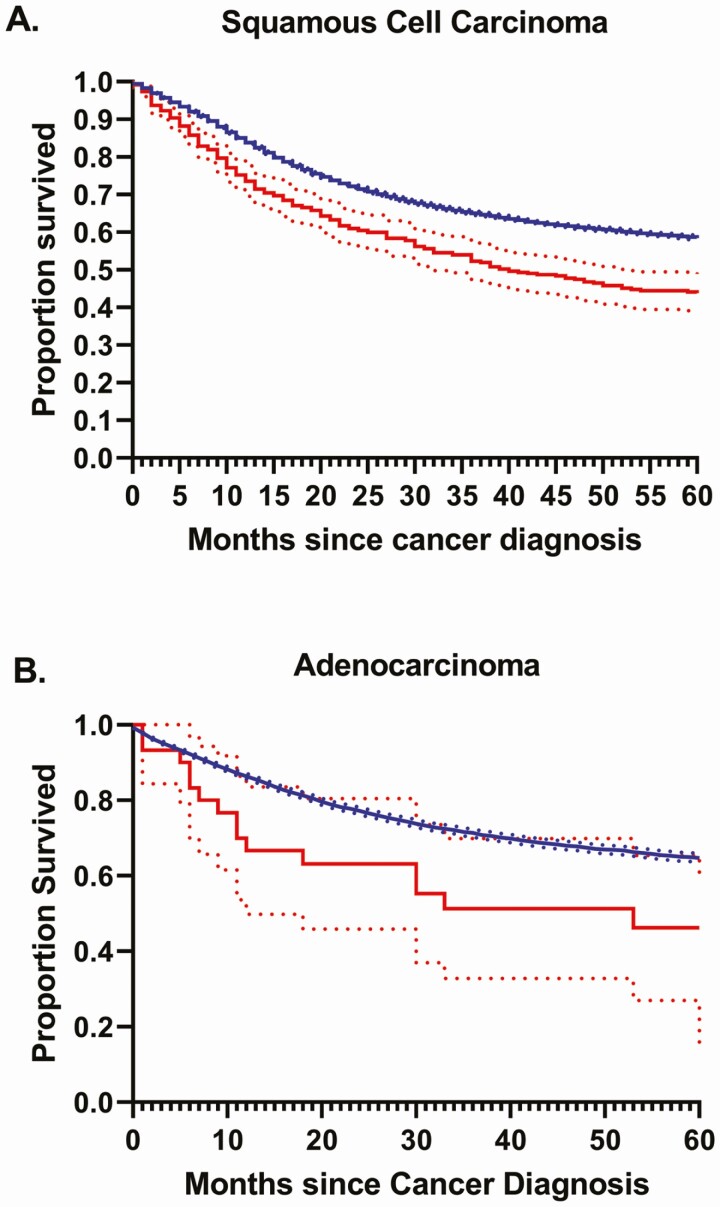
Overall survival differed by histologic subtype and HIV status (Figure 1). Specifically, 5-year survival was highest among women not living with HIV with AC at 64.7% (95% CI: 63.5%–65.9%) followed by women with SCC at 58.5% (95% CI: 57.9%–59.1%). Five-year overall survival was significantly lower among WLH with SCC and was qualitatively but not statistically lower among WLH with AC compared with women not living with HIV. Among WLH, survival was not significantly different between women with AC (46.2%; 95% CI: 27.0%–65.4%) and SCC (43.8%; 95% CI: 38.7%–48.8%).
Figure 1.
Overall survival for women living with human immunodeficiency virus (HIV) (red) compared with women not living with HIV (blue) diagnosed with cervical (A) squamous cell carcinoma and (B) adenocarcinoma.
Independent risk factors for AC and SCC mortality were similar (Table 4). The adjusted risk of death among women with AC (hazard ratio [HR], 2.52; 95% CI: 1.53–4.12) and SCC (HR, 2.02; 95% CI: 1.79–2.29) was greater for WLH compared with women not living with HIV in the general population. Furthermore, for both AC and SCC, risks of death increased with each year increase in age, were lower for non-Hispanic White and Hispanic women compared with non-Hispanic Black women, and were greatly increased for women with regional/distant and unknown tumor stages compared with women with local disease.
Table 4.
Hazard of Death for Adenocarcinoma and Squamous Cell Carcinoma in Women Living With Human Immunodeficiency Virus and Women in the General Population
| Risk Factors | Adenocarcinoma, HR (95% CI)a | Squamous Cell Carcinoma, HR (95% CI)a |
|---|---|---|
| HIV status | ||
| General population | Ref | Ref |
| Women living with HIV | 2.52 (1.53–4.12) | 2.02 (1.79–2.29) |
| Age (per year increase) | 1.05 (1.04–1.05) | 1.03 (1.03–1.03) |
| Race | ||
| NH Black | Ref | Ref |
| NH White | 0.69 (.62–.75) | 0.88 (.85–.92) |
| Hispanic | 0.74 (.65–.84) | 0.73 (.69–.76) |
| Year of cancer diagnosis (per year increase) | 0.99 (.98–.99) | 1.00 (1.00–1.00) |
| Tumor stage | ||
| Local | Ref | Ref |
| Regional and distant | 4.33 (3.97–4.72) | 3.15 (3.02–3.27) |
| Unknown | 2.73 (2.39–3.11) | 2.28 (2.12–2.44) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; NH, Non-Hispanic.
aModels are fully adjusted for all variables in the table: HIV status, age, race, year of diagnosis, and tumor stage.
DISCUSSION
In this US population–based study to evaluate the epidemiology of AC in WLH, we demonstrate that the crude incidence rate of AC is almost 7 times less than the incidence of SCC in WLH. When comparing WLH to the general population, we found that AC incidence was increased 50% whereas SCC incidence was several times higher. Overall 5-year survival among WLH with AC was less than 50% compared with nearly 65% for women in the general population. Taken together, these findings highlight the significant epidemiologic variations between SCC and AC in WLH, despite their common etiology, human papillomavirus.
While the incidence of SCC in WLH was 3.5 times higher than the general population, rates of AC were only increased by 50%. This finding is somewhat surprising, particularly given the findings that the HPV18 subtype, which is most commonly associated with cervical AC, is also more common in WLH. In fact, WLH with high-grade cervical dysplasia are 50% more likely to have HPV18 than women with high-grade cervical dysplasia in the general population [20]. Given increased prevalence of HPV18 as well as the association of HPV18 with AC, a more profound increase in the incidence of AC among WLH, perhaps even greater than that of SCC, might be expected; however, our findings indicate that AC does not increase to the same degree as SCC in WLH. This suggests that there is a significant difference in these 2 subtypes, which may be related to the impact of HIV, HPV-related changes in glandular vs squamous cells of the cervix, differences in screening, the impact of HPV subtypes, or differences in the immune microenvironment. Given that the incidence of AC overall is lower than that of SCC, it is not surprising that the incidence of AC in WLH is also lower than that of SCC. However, the incidence of AC in WLH was lower than might be expected given the increased risk of cervical cancer overall in this population and the increased risk of SCC in WLH. Furthermore, in this study, only 18% of cervical cancer cases in the general population were AC, which is lower than in other studies [1]. This is noteworthy because the SIR for WLH may be overestimated if AC in the general population is under-ascertained. The finding that AC is not increased in WLH to the same extent as SCC is important in understanding how best to prioritize and screen for cervical cancer in WLH based on their risk of underlying squamous and glandular cancers. Specifically, when using Pap smear alone, as per recommendations for WLH, it is important to recognize that AC may be commonly missed [17]. HPV testing, on the other hand, is equally and highly effective at detecting both squamous and glandular lesions [21].
Five-year survival for AC was similar to that of SCC among WLH and was clinically lower than that of women not living with HIV with AC. Consistent with our findings, while some studies, have shown increased mortality among WLH with cervical cancer [22], others suggest similar survival between WLH and those in the general population with cervical cancer [23]. Variations in these findings may be due to other associated factors, such as CD4 count [24] or the impact of AIDS on overall mortality [25, 26], which could not be evaluated directly in this study. For both WLH with AC and WLH with SCC compared with the general population, increased risk of death was associated with older age, Black race, and regional/distant disease and unknown stage of disease. These findings indicate age, race, and stage-related disparities in WLH that go beyond those already documented in women in the general population, regardless of histologic subtype [27, 28]. In the general population, studies suggest age-related declines in mortality are at least partially due to older women being less likely to receive appropriate treatment [29]. Given the additional comorbidity and potential for drug interactions, it is plausible that lack of treatment is even more likely in older WLH compared with the general population, as has been observed for other cancers [30]. The relatively high SIR for other/unknown histologies also suggests that WLH with cervical cancer may not be receiving the same work-up as women in the general population. Nearly 20% of WLH with AC have unstaged disease. These findings, combined with the higher hazard of death among the group of unstaged cancer patients living with HIV, raise important questions with regards to healthcare equity.
Black WLH and those who acquired HIV through injection drug use were noted to have increased risk of SCC, but risk factors were not as clear for AC. This may be due to the smaller sample size of AC in WLH, limiting the ability to identify such associations. An interesting pattern, however, was that non-Hispanic Black women had a higher risk of SCC and a lower risk of AC compared with non-Hispanic White women. In addition, mortality was significantly higher among non-Hispanic Black women with SCC and AC compared with all other races in our population of WLH. Compared with findings from epidemiologic studies in the general population, our study findings highlight the intersectional nature of race-, age-, and HIV-related disparities on cervical cancer incidence and mortality. Future research should focus on the implementation of interventions to mitigate racial disparities and the impact of racism. Such interventions are critical to improving outcomes in this diverse population.
In this study, we were able to estimate the incidence of AC among WLH and use SIRs to account for differences in the distribution of factors such as age and race in WLH compared with the general population. In addition, since AC is a relatively rare subtype, we were able to use this large population-based study to estimate and compare 5-year survival by histologic subtype and HIV status. However, despite this large dataset, the absolute number of AC cases in WLH remains low overall. Additionally, since not every state contributes data to this dataset, it is possible that the observed incidence, mortality, and risk factors are not fully representative of the broader US population of WLH. Although this study did include a large sample of WLH, there are other limitations that must be considered when interpreting the data. This linked registry-based dataset has incomplete data on CD4 count and viral load, limiting our ability to better explore the association between level of immune suppression and incidence of AC. However, it is interesting to note that although data by time period was limited, nonsignificant declines in incidence of AC were observed after the 1996–2004 time period of widespread uptake of highly active antiretroviral therapy in the United States. Furthermore, additional factors may impact epidemiologic differences in cervical cancer histologic subtypes that were not accounted for in this study. Despite these limitations, this is, to our knowledge, the largest study of WLH comparing histologic subtypes in cervical cancer.
In conclusion, this study helps to describe the epidemiology of AC in WLH and demonstrates the differential association of HIV (and related immunosuppression or population characteristics) on these various cervical cancer subtypes. These data also further contribute to the growing literature that suggest that AC and SCC are distinct disease subtypes and that differential treatment strategies may be necessary to improve outcomes. The differences in the elevated rates of AC and SCC among WLH are hypothesis-generating, and further investigation is needed to better understand why these epidemiologic and potentially immunologic differences exist despite the underlying common etiology of high-risk HPV infection. These findings could help guide screening and diagnostic recommendations in this population and could have critical implications for treatment of these distinct disease subtypes in the future.
Notes
Acknowledgments. The authors gratefully acknowledge the support and assistance provided by individuals at the following state human immunodeficiency virus (HIV)/AIDS and cancer registries: Colorado, Connecticut, District of Columbia, Georgia, Louisiana, Maryland, Michigan, New Jersey, New York, North Carolina, Puerto Rico, and Texas. We also thank Timothy McNeel at Information Management Services for programming support.
Disclaimer. The views expressed in this article are those of the authors and should not be interpreted to reflect the views or official policies of the National Cancer Institute (NCI), Centers for Disease Control and Prevention (CDC), or the Department of Health and Human Services, HIV/AIDS or cancer registries, or their contractors, nor does the mention of trade names, commercial practices, or organizations imply endorsement by the US government.
Financial support. This research was supported, in part, by the Intramural Research Program of the NCI. The following cancer registries were supported by the cooperative agreement funded by the Center for Disease Control (CDC) National Program of Cancer Registries: Colorado (NU58DP006347-01), Georgia (5U58DP003875-01), Louisiana (NU58DP006332-03-00), Maryland (NU58DP006333), Michigan (17NU58DP006334), New Jersey (NU58/DP003931-05-00), New York (6NU58/DP006309), North Carolina (1NU58DP006281), and Texas (1NU58DP006308). The District of Columbia is supported by CDC cooperative agreement DP006302. The following cancer registries were supported by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI): Connecticut (HHSN261201800002I), Louisiana (HHSN261201800007I/ HHSN26100002), New York (HHSN261201800009I), and New Jersey (HHSN261201300021I, N01-PC-2013-00021). The New Jersey State Cancer Registry was also supported by the State of New Jersey, the Maryland Cancer Registry was supported by the State of Maryland and the Maryland Cigarette Restitution Fund, the Louisiana Tumor Registry was also supported by the State of Louisiana (0587200015), and the New York State Cancer Registry was also supported by the State of New York. The following HIV registries were supported by the HIV Incidence and Case Surveillance Branch of the CDC National HIV Surveillance Systems: Colorado (NU62PS003960), Connecticut (5U62PS001005-05), Louisiana (NU62PS924522-02-00), Michigan (U62PS004011-02), New Jersey (U62PS004001-2), New York (NU62PS924546-02-00; PS18-1802: Integrated HIV Surveillance and Prevention Programs for Health Departments, National Center for HIV, Viral Hepatitis, STD, and TB Prevention). A. F. R. was supported, in part, by the National Institutes of Health/NCI-funded Johns Hopkins Specialized Programs of Research Excellence in Cervical Cancer (P50CA098252-16).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Howlader N, Noone NA, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2012. Bethesda, MD: National Cancer Institute, 2015. [Google Scholar]
- 2. Dahlström LA, Ylitalo N, Sundström K, et al. Prospective study of human papillomavirus and risk of cervical adenocarcinoma. Int J Cancer 2010; 127:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berrington de González A, Sweetland S, Green J. Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis. Br J Cancer 2004; 90:1787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riethdorf S, Riethdorf L, Milde-Langosch K, Park TW, Löning T. Differences in HPV 16- and HPV 18 E6/E7 oncogene expression between in situ and invasive adenocarcinomas of the cervix uteri. Virchows Arch 2000; 437:491–500. [DOI] [PubMed] [Google Scholar]
- 5. Rose PG, Java JJ, Whitney CW, Stehman FB, Lanciano R, Thomas GM. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol 2014; 135:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai CH, Hsueh S, Hong JH, et al. Are adenocarcinomas and adenosquamous carcinomas different from squamous carcinomas in stage IB and II cervical cancer patients undergoing primary radical surgery? Int J Gynecol Cancer 1999; 9:28–36. [DOI] [PubMed] [Google Scholar]
- 7. Tornesello ML, Losito S, Benincasa G, et al. Human papillomavirus (HPV) genotypes and HPV16 variants and risk of adenocarcinoma and squamous cell carcinoma of the cervix. Gynecol Oncol 2011; 121:32–42. [DOI] [PubMed] [Google Scholar]
- 8. Cancer ICoESoC. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13 541 women with carcinoma of the cervix and 23 017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer 2006; 118:1481–95. [DOI] [PubMed] [Google Scholar]
- 9. Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–10. [DOI] [PubMed] [Google Scholar]
- 10. Altekruse SF, Lacey JV Jr, Brinton LA, et al. Comparison of human papillomavirus genotypes, sexual, and reproductive risk factors of cervical adenocarcinoma and squamous cell carcinoma: northeastern United States. Am J Obstet Gynecol 2003; 188:657–63. [DOI] [PubMed] [Google Scholar]
- 11. Denny L, Boa R, Williamson AL, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol 2008; 111:1380–7. [DOI] [PubMed] [Google Scholar]
- 12. Lehtovirta P, Finne P, Nieminen P, et al. Prevalence and risk factors of squamous intraepithelial lesions of the cervix among HIV-infected women—a long-term follow-up study in a low-prevalence population. Int J STD AIDS 2006; 17:831–4. [DOI] [PubMed] [Google Scholar]
- 13. Denslow SA, Rositch AF, Firnhaber C, Ting J, Smith JS. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS 2014; 25:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massad LS, Xie X, Darragh TM, et al. Histologic correlates of glandular abnormalities in cervical cytology among women with human immunodeficiency virus. Obstet Gynecol 2009; 114:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Delmas MC, Larsen C, van Benthem B, et al. Cervical squamous intraepithelial lesions in HIV-infected women: prevalence, incidence and regression. European Study Group on Natural History of HIV Infection in Women. AIDS 2000; 14:1775–84. [DOI] [PubMed] [Google Scholar]
- 16. Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Genit Tract Dis 2019; 23:87–101. [DOI] [PubMed] [Google Scholar]
- 17. Zhao C, Li Z, Austin RM. Cervical screening test results associated with 265 histopathologic diagnoses of cervical glandular neoplasia. Am J Clin Pathol 2013; 140:47–54. [DOI] [PubMed] [Google Scholar]
- 18. Dyer BA, Feng CH, Eskander R, et al. Current status of clinical trials for cervical and uterine cancer using immunotherapy combined with radiation. Int J Radiat Oncol Biol Phys 2021; 109:396–412. [DOI] [PubMed] [Google Scholar]
- 19. Seresini S, Origoni M, Caputo L, et al. CD4+ T cells against human papillomavirus-18 E7 in patients with high-grade cervical lesions associate with the absence of the virus in the cervix. Immunology 2010; 131:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clifford GM, Gonçalves MA, Franceschi S; HPV and HIV Study Group . Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20:2337–44. [DOI] [PubMed] [Google Scholar]
- 21. Bruehl FK, Dyhdalo KS, Hou Y, Clapacs E, Przybycin CG, Reynolds JP. Cytology and curetting diagnosis of endocervical adenocarcinoma. J Am Soc Cytopathol 2020; 9:556–62. [DOI] [PubMed] [Google Scholar]
- 22. Wu ES, Urban RR, Krantz EM, et al. The association between HIV infection and cervical cancer presentation and survival in Uganda. Gynecol Oncol Rep 2020; 31:100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys 2018; 101:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grover S, Mehta P, Wang Q, et al. Association between CD4 count and chemoradiation therapy outcomes among cervical cancer patients with HIV. J Acquir Immune Defic Syndr 2020; 85:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis. Public Health 2016; 139:3–12. [DOI] [PubMed] [Google Scholar]
- 26. Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017; 26:1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gopalani SV, Janitz AE, Campbell JE. Cervical cancer incidence and mortality among non-Hispanic African American and white women, United States, 1999–2015. J Natl Med Assoc 2020; 112:632–8. [DOI] [PubMed] [Google Scholar]
- 28. Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017; 123:1044–50. [DOI] [PubMed] [Google Scholar]
- 29. Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy 2019; 18:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rositch AF, Jiang S, Coghill AE, et al. Disparities and determinants of cancer treatment in elderly Americans living with human immunodeficiency virus/AIDS. Clin Infect Dis 2018; 67:1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]