Abstract
OBJECTIVES
Redo aortic valve surgery (rAVS) is performed with increasing frequency, but operative mortality is usually higher compared to that associated with primary aortic valve surgery. We analysed our patients who had rAVS to determine the current outcomes of rAVS as a surgical benchmark in view of the growing interest in transcatheter valve techniques.
METHODS
We retrospectively reviewed 148 consecutive patients [median age 67.7 years (interquartile range 54.9–77.6); 68.2% men] who underwent rAVS following aortic valve replacement (81.6%), aortic root replacement (15%) or aortic valve repair (3.4%) between 2000 and 2018.
RESULTS
Indications for rAVS were structural valve dysfunction (42.7%), endocarditis (37.8%), non-structural valve dysfunction (17.7%) and aortic aneurysm (2.1%). Valve replacement was performed in 69.7%, and 34 new root procedures were necessary in 23%. Early mortality was 9.5% (n = 14). Female gender [odds ratio (OR) 6.16], coronary disease (OR 4.26) and lower creatinine clearance (OR 0.95) were independent predictors of early mortality. Follow-up was 98.6% complete [median 5.9 (interquartile range 1.7–10.9) years]. Survival was 74.1 ± 3.7%, 57.9 ± 5.1% and 43.8 ± 6.1% at 5, 10 and 14 years, respectively. Cox regression analysis revealed female gender [hazard ratio (HR) 1.73], diabetes (HR 1.73), coronary disease (HR 1.62) and peripheral vascular disease (HR 1.98) as independent determinants of late survival.
CONCLUSIONS
Despite many urgent situations and advanced New York Heart Association functional class at presentation, rAVS could be performed with acceptable early and late outcomes. Risk factors for survival were female gender, coronary disease and urgency. In this all-comers patient cohort needing rAVS, only a minority would eventually qualify for transcatheter valve-in-valve procedures.
Keywords: Redo surgery, Aortic valve, Valve-in-valve, Survival
Increased life expectancy after valve surgery, the ageing population in Western countries and the rising preference for bioprosthetic aortic valves lead to an increasing number of surgical patients presenting for aortic valve reinterventions [1, 2].
INTRODUCTION
Increased life expectancy after valve surgery, the ageing population in Western countries and the rising preference for bioprosthetic aortic valves lead to an increasing number of surgical patients presenting for aortic valve reinterventions [1, 2]. Currently, surgical redo aortic valve replacement remains the treatment of choice, but newer techniques such as valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) have recently emerged as an alternative, particularly in high-risk patients with degenerated bioprostheses. Four recent systematic reviews demonstrated no difference in early or 1-year mortality between ViV-TAVR and redo aortic valve replacement in patients with failed aortic bioprostheses; however, the baseline characteristics in all retrospective comparative studies included in these reviews were different in both groups, as were the postprocedural complications [3–6]. On the other hand, many patients considered for redo aortic valve surgery (rAVS) are not amenable to ViV-TAVR techniques because of paravalvular leaks, mechanical valve thrombosis or prosthetic valve endocarditis as seen in clinical practice [2, 7–10].
Surgical aortic valve reoperations can be performed with a low risk comparable to that of first-time aortic valve surgery, on the premise that the patients present electively and in good condition with a gradually failing aortic valve or valve prosthesis [11]. Most papers reporting on rAVS, however, show quite a different patient profile, with a large number of urgent procedures, endocarditis, high New York Heart Association (NYHA) functional class and reduced left ventricular function at presentation [2, 10, 12].
We sought to evaluate our experience during an 18-year period by identifying risk factors for poor outcome in reoperative aortic valve surgery in order to stratify the risk of rAVS in the light of emerging alternative strategies.
METHODS
Patient selection
Between January 2000 and December 2018, a total of 148 consecutive patients aged 18 years or older who had previously undergone aortic valve surgery had an aortic valve reoperation at the Cardiac Centre of the University Hospital Ghent, Belgium. Experienced cardiac staff surgeons performed all redo sternotomies.
Baseline patient characteristics, data from the previous aortic valve operation, perioperative data and postoperative outcome data were retrospectively collected from the patient files. Survival data at the study closing date (1 March 2020) were obtained through telephone calls to the general practitioner or the referring cardiologist. The ethics committee of the University Hospital Ghent approved the study and waived the need for patient consent due to the retrospective nature of the study (B670201836820).
The indications for surgical reintervention on the aortic valve were classified according to internationally accepted guidelines [13]. Urgent procedures were defined as interventions on patients who could not leave the hospital between diagnosis and operation; emergency procedures were interventions performed without delay on the day of the diagnosis.
The primary end point of the study was survival after the operation or operative mortality, defined as any death in the hospital or during the first 30 postoperative days. Perioperative complications such as bleeding events, pulmonary complications, new arrhythmia, low cardiac output (need for vasoactive medication), sepsis, neurological events (seizures, transient ischaemic attacks, cerebrovascular accidents), acute kidney failure (according to the Kidney Disease: Improving Global Outcomes criteria) [14], gastrointestinal complications, multiorgan failure and postoperative interventions such as revision for bleeding, new pacemaker implantation, intra-aortic balloon pump and new dialysis were recorded.
The secondary end point was freedom from all-cause late mortality.
The median follow-up was 5.9 (1.7–10.9) years. Long-term follow-up was obtained in 98.6% of patients; only 2 patients were not traceable (1.3%).
Statistical analyses
Continuous variables were expressed as mean ± standard deviation or median and interquartile range when appropriate. Continuous characteristics were compared by the t-test or by the Mann–Whitney U-test depending on the distribution. The Shapiro–Wilk test was used to explore for normality. Categorical variables were reported as numbers and percentages and were compared by means of the χ2or Fisher’s exact test. Univariable analysis using the χ2 test was performed to identify relationships between operative mortality and patient or perioperative factors. Variables with a P-value <0.25 were entered into logistic regression multivariable analysis (backward conditional) to determine independent influence on operative death. The effect of significant determinants was expressed as the odds ratio (OR) with 95% confidence intervals (CIs). The Kaplan–Meier method was used for survival analysis, with a log-rank test to compare groups. The proportional hazard assumption was verified graphically with the log-log Kaplan–Meier plot as well as by the Schoenfeld residuals. Kaplan–Meier curves were applied to up to 14 years of follow-up to maintain a meaningful minimum of 10% of the population at risk. Multivariable analysis with the Cox regression method was used to investigate independent predictors for survival. Statistical analysis was performed using SPSS 26.0 Statistics Software (IBM Corporation, Armonk, NY, USA).
RESULTS
Baseline characteristics
The median age of the 101 men and 47 women who underwent aortic valve reoperations was 68 (55–78) years. Baseline patient characteristics and comorbidities are listed in Table 1. Half of the patients presented in NYHA functional class III/IV; only 28% of patients were operated on electively. Structural valve deterioration was the most common indication for rAVS (43%), closely followed by infective endocarditis (38%). Half of the patients presented with severe aortic valve regurgitation, and one-third showed moderately to severely decreased left ventricular function on admission. Forty patients presented with a preoperative creatinine clearance <50 ml/min. The median EuroSCORE II was expectedly high at 10.6 (5.5–21.5), reflecting the important contribution of the parameters ‘redo surgery’, ‘endocarditis’, ‘pulmonary hypertension’ and ‘high NYHA class’ in this operative risk score. The EuroSCORE II was significantly higher in patients with endocarditis than in patients with other indications (15.7 vs 8.3; P = 0.007). Significantly more patients with endocarditis had rAVS on an urgent or emergency basis compared to those with other diagnoses (98% vs 57%; P < 0.001) (Table 1). The median time interval since the previous aortic valve procedure was 6.2 years but was significantly shorter for patients with endocarditis than for those with other indications (2.8 vs 7.8 years; P < 0.001).
Table 1:
Baseline patient characteristics
| Variables | Total cohort (N = 148) | Non-endocarditis (N = 92) | Endocarditis (N = 56) | P-value |
|---|---|---|---|---|
| Age (years) | 67.7 (54.9–77.6) | 66.9 (51.6–78.3) | 71.1 (59.8–76.7) | 0.75 |
| Weight (kg) | 74.1 ± 14 | 74.7 ± 14.2 | 73.2 ± 13.9 | 0.62 |
| Height (cm) | 169 ± 10 | 169 ± 11 | 170 ± 8 | 0.69 |
| BMI (kg/m²) | 25.8 ± 4.3 | 26.2 ± 4.3 | 25.2 ± 4.2 | 0.24 |
| Male gender | 101 (68.2) | 61 (66.3) | 40 (71.4) | 0.59 |
| Hypertension | 59 (40) | 40 (43.5) | 19 (33.9) | 0.25 |
| Coronary artery disease | 47 (31.8) | 28 (30.4) | 19 (33.9) | 0.66 |
| Diabetes | 17 (11.5) | 9 (9.8) | 8 (14.3) | 0.41 |
| Peripheral vascular disease | 21 (14.2) | 8 (8.7) | 13 (23.2) | 0.027 |
| COPD | 13 (8.8) | 7 (7.6) | 6 (10.7) | 0.56 |
| Creatinine clearance (ml/min) | 69 (49–89) | 67 (49.3–90) | 69 (49–85) | 0.44 |
| ≤50 ml/min | 40 (27) | |||
| NYHA functional class | 0.002 | |||
| I | 35 (23.6) | 12 (13) | 23 (41.1) | |
| II | 40 (27) | 28 (30.4) | 12 (21.4) | |
| III | 49 (33.1) | 35 (38) | 14 (25) | |
| IV | 24 (16.2) | 17 (18.5) | 7 (12.5) | |
| Degree of urgency | <0.001 | |||
| Elective | 41 (27.7) | 40 (43.5) | 1 (1.8) | |
| Urgent | 101 (68.2) | 49 (53.3) | 52 (92.9) | |
| Emergency/salvage | 6 (4.1) | 3 (3.3) | 3 (5.4) | |
| EuroSCORE II | 10.6 (5.5–21.5) | 8.3 (4.2–16.1) | 16.3 (8.2–28) | 0.007 |
| Preoperative rhythm | 0.071 | |||
| Sinus | 114 (77) | 75 (81.5) | 39 (69.6) | |
| Atrial fibrillation | 30 (20.3) | 15 (16.3) | 15 (26.8) | |
| Atrioventricular block | 2 (1.4) | 0 (0) | 2 (3.6) | |
| Pacemaker | 2 (1.4) | 2 (2.2) | 0 (0) | |
| Preoperative LV function | 0.71 | |||
| Good | 100 (67.6) | 60 (65.2) | 40 (71.4) | |
| Moderate | 41 (27.7) | 27 (29.3) | 14 (25) | |
| Poor | 7 (4.7) | 5 (5.4) | 2 (3.6) | |
| Transaortic gradient (mmHg) | ||||
| Peak | 27 (15–54) | 36 (11–67) | 26 (17–38) | 0.003 |
| Mean | 15 (7–32) | 20 (5–40) | 12 (8–17) | 0.006 |
| Aortic valve regurgitation | <0.001 | |||
| None | 29 (19.6) | 13 (14.1) | 16 (28.6) | |
| Mild/moderate | 45 (30.4) | 18 (19.6) | 27 (48.2) | |
| Severe | 74 (50) | 61 (66.3) | 13 (23.2) | |
| Pulmonary hypertension (>50 mmHg) | 41 (27.7) | 32 (34.8) | 9 (16.1) | 0.014 |
| Indication for rAVS | <0.001 | |||
| Endocarditis | 56 (37.8) | 56 (100) | ||
| Structural valve dysfunction | 63 (42.7) | 63 (68.5) | ||
| Prosthetic | 44 (29.8) | |||
| Native (AoS, AR) | 19 (12.9) | |||
| Non-structural valve dysfunction | 26 (17.7) | 26 (28.2) | ||
| Paravalvular leak | 10 (6.8) | |||
| Pannus | 4 (2.7) | |||
| Valve thrombosis | 9 (6.1) | |||
| Patient-prosthesis mismatch | 1 (0.7) | |||
| Subaortic stenosis | 2 (1.4) | |||
| Aortic root/ascending aneurysm | 3 (2.1) | 3 (3.3) | ||
| Interval from previous aortic valve surgical procedure (years) | 6.2 (1.8–11.4) | 7.8 (3.5–12.9) | 2.8 (0.8–6.5) | 0.001 |
Values are presented as median (interquartile range), mean ± SD or n (%).
AoS: aortic valve stenosis; AR: aortic regurgitation; BMI: body mass index; COPD: chronic obstructive pulmonary disease; LV: left ventricle; NYHA: New York Heart Association; rAVS: redo aortic valve surgery; SD: standard deviation.
The majority of the patients underwent rAVS after a previous aortic valve replacement procedure (81.8%); 22 (15%) patients had an earlier root procedure. The details of the previous interventions can be found in Table 2. In about half of the patients, a variety of combined procedures had been associated at the time of the previous surgery. Patients with a previous valve-sparing procedure were reoperated on mainly for progressive aortic valve regurgitation, some for calcified bicuspid valve stenosis. Reoperation after the Ross procedure was mainly for an autograft aneurysm, some with accompanying valve regurgitation. Most patients had undergone only 1 previous aortic valve procedure (84.5%), but for 22 patients and 1 patient, this was their third or fourth aortic valve procedure, respectively.
Table 2:
Details of previous aortic valve procedures
| Variables | Result (N = 148) |
|---|---|
| Type of previous procedure | |
| Aortic valve replacement | 134 (90.5) |
| Aortic valve repair | 14 (9.5) |
| Details of previous aortic procedure | |
| AVR | 116 (78.4) |
| Redo AVR | 5 (3.4) |
| Bentall | 9 (6.1) |
| David | 9 (6.1) |
| Ross | 4 (2.7) |
| Isolated AVP | 5 (3.4) |
| Type of previous aortic valve prosthesis | |
| None (aortic valve sparing) | 14 (9.5) |
| Xenograft | 67 (45.3) |
| Mechanical | 48 (32.4) |
| Homograft | 15 (10.1) |
| Autograft | 4 (2.7) |
| Combined procedures in previous operative procedurea | |
| None | 76 (51.3) |
| Aortic root replacement | 22 (14.9) |
| Supracoronary ascending aortic graft | 2 (1.4) |
| Aortic root patch | 2 (1.4) |
| CABG | 33 (22.3) |
| MVP | 7 (4.8) |
| MVR | 5 (3.4) |
| PVR | 4 (2.7) |
| TVP | 1 (0.7) |
| Number of previous procedures | |
| 1 | 125 (84.5) |
| 2 | 22 (14.9) |
| 3 | 1 (0.7) |
Values are presented as median (interquartile range), mean ± SD or n (%).
More than 1 combined procedure is possible.
AVP: aortic valve repair; AVR: aortic valve replacement; CABG: coronary artery bypass graft; MVP: mitral valve repair; MVR: mitral valve replacement; PVR: pulmonary valve replacement; SD: standard deviation; TVP: tricuspid valve repair.
Perioperative results
The intraoperative details concerning the procedures and prostheses, cardiopulmonary bypass (CPB) and aortic cross-clamp times, time in the intensive care unit (ICU) and total hospital length of stay for the entire cohort can be found in Table 3. Median CPB and aortic cross-clamp times were significantly different between patients with isolated aortic valve replacement (n = 70) compared with those with combined procedures (n = 76) [CPB: 114 (97–131) vs 154 (123–195) min; P < 0.001; aortic cross-clamp: 72 (62–87) vs 109 (80–129) min; P < 0.001]. In 60% of the patients, a previously implanted aortic valve prosthesis was replaced; 9.6% of patients with recurrent native valve problems had a first-time aortic valve replacement. In addition to 9 patients who had redo Bentall procedures, 34 new root replacements were performed, often to repair a root severely damaged by endocarditis, or for heavily calcified roots after previous homografts. A variety of other concomitant operations were needed, mainly mitral procedures (16.9%), a coronary artery bypass graft (12.2%) and tricuspid valve repairs (6.7%). No re-entry damage to important cardiac structures was noted during reoperation. The median ICU stay was 3 (1–6) and 2 (1–3) days (P = 0.023) in patients with and without endocarditis, respectively.
Table 3:
Redo procedure details
| Variables | Results (N = 148) |
|---|---|
| Cardiopulmonary bypass time (min) | 132 (109–167) |
| Aortic clamping time (min) | 84 (69–116) |
| Type of redo procedure | |
| Redo AVR | 89 (60.1) |
| AVR | 14 (9.6) |
| Redo Bentall | 9 (6.1) |
| Bentall | 34 (23) |
| Ross | 1 (0.7) |
| AVP | 1 (0.7) |
| Type of aortic valve prosthesis used | |
| Mechanical | 84 (56.8) |
| Xenograft | 57 (38.5) |
| Homograft | 5 (3.4) |
| Autograft | 1 (0.7) |
| None | 1 (0.7) |
| Concomitant proceduresa | |
| None | 70 (47.3) |
| Aortic root replacement | 44 (29.7) |
| CABG | 18 (12.2) |
| MVP | 15 (10.2) |
| MVR | 10 (6.7) |
| TVP | 10 (6.7) |
| PVR | 3 (2) |
| Annular abscess debridement | 10 (6.7) |
| Ascending aortic replacement | 2 (1.4) |
| Aortic root patch | 2 (1.4) |
| Aortic arch replacement | 1 (0.7) |
| Ventricular septal defect closure | 1 (0.7) |
| Septal myectomy | 1 (0.7) |
| Re-entry trauma | 0 (0) |
Values are expressed as median (interquartile range) or n (%).
More than 1 concomitant procedure is possible.
AVP: aortic valve repair; AVR: aortic valve replacement; CABG: coronary artery bypass graft; MVP: mitral valve repair; MVR: mitral valve replacement; PVR: pulmonary valve replacement; TVP: tricuspid valve repair.
Early outcomes
Postoperative events occurred in 61.5% (n = 91) of patients (Table 4). The majority of patients (93.2%) were extubated within 24 h after redo surgery. Acute kidney failure occurred frequently (26.4%), leading to the need for temporary dialysis in 12 patients (8.1%). Revision for bleeding was needed in 12.8% of patients. New arrhythmias occurred in nearly a third of the patients (n = 44, 29.7%), mainly transient atrial fibrillation (n = 36) and a new third-degree atrioventricular block (n = 7), resulting in new permanent implantation of pacemakers in 9 (6.6%) patients.
Table 4:
Postoperative complications, hospital stay and mortality
| Variables | Results (N = 148) |
|---|---|
| Postoperative eventsa | 91 (61.5) |
| Ventilation >24 h | 10 (6.8) |
| Pulmonary complication | 23 (15.5) |
| New arrhythmia | 44 (29.7) |
| Bleeding | 22 (14.9) |
| Low cardiac output | 13 (8.8) |
| Sepsis | 9 (6.1) |
| Neurological event | 5 (3.4) |
| Acute kidney failure | 39 (26.4) |
| Gastrointestinal complication | 4 (2.7) |
| Multiorgan failure | 7 (4.7) |
| Postoperative interventionsa | 40 (27.2) |
| Revision for bleeding | 19 (12.8) |
| Intra-aortic balloon pump | 13 (8.8) |
| New dialysis | 12 (8.1) |
| New pacemaker implant | 9 (6.6) |
| Intensive care stay (days) | 2 (1–4) |
| Hospital stay (days) | 16 (10–36) |
| Mortality 30-day/in-hospital | 14 (9.5) |
| Combined postoperative events (morbidity + mortality) | 91 (61.5) |
| Cardiac rhythm at discharge (n = 136) | |
| Sinus | 107 (78.7) |
| Atrial fibrillation | 18 (13.2) |
| Pacemaker | 11 (8.1) |
Values are expressed as median (interquartile range) or n (%).
Combinations are possible.
The hospital stay was significantly longer in patients with endocarditis compared to those with other indications [40 (22–47) vs 12 (9–15) days; P < 0.001], mainly due to the scheduled 4- to 6-week intravenous antibiotic treatment.
Operative mortality (30-day and in-hospital combined) was 9.5% (n = 14). The most common cause of death was low cardiac output (n = 6); 4 patients died of multiorgan failure; 1 died of persistent sepsis; 2 died of severe respiratory complications; and 1 died of intractable surgical bleeding. Only 2 of the 41 elective patients died (4.9%) compared to 11.2% (n = 12) of the cases with urgent presentation; however, this difference did not reach statistical significance (P = 0.25).
Risk factors related to early outcomes
Univariable analysis to define which baseline patient covariates were associated with operative mortality demonstrated that older age (P = 0.020), female gender (P = 0.003), associated coronary artery disease (P = 0.010), lower creatinine clearance (P < 0.001) and NYHA functional class III/IV at presentation (P = 0.032), as well as the occurrence of postoperative acute kidney failure (P = 0.002), were significantly associated with higher 30-day and hospital mortality at rAVS (Table 5). Neither preoperative left ventricular function, pulmonary hypertension, urgency of the procedure, the indication for redo surgery nor the presence of endocarditis had a significant influence on postoperative mortality.
Table 5:
Risk factor analysis for operative mortality
| Variables | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| P-value | OR | 95% CI | P-value | OR | 95% CI | |
| Age at redo (years) | 0.020 | 1.07 | 1.011–1.139 | |||
| Female gender | 0.003 | 6.55 | 1.935–22.196 | 0.009 | 6.16 | 1.578–24.043 |
| PVD | 0.12 | 2.75 | 0.776–9.766 | |||
| Diabetes | 0.59 | 1.70 | 0.216–14.393 | |||
| Hypertension | 0.81 | 0.87 | 0.286–2.657 | |||
| COPD | 0.45 | 0.54 | 0.106–2.709 | |||
| Coronary disease | 0.010 | 4.55 | 1.431–14.447 | 0.032 | 4.26 | 1.132–16.050 |
| Creatinine clearance (ml/min) | <0.001 | 0.95 | 0.916–0.975 | 0.003 | 0.95 | 0.912–0.982 |
| NYHA III–IV | 0.032 | 4.26 | 1.136–15.9 | |||
| LV function | 0.78 | 0.85 | 0.269–2.691 | |||
| PHT | 0.19 | 2.12 | 0.688–6.544 | |||
| Urgent/emergency | 0.25 | 2.46 | 0.527–11.520 | |||
| Indication for redo | 0.99 | 1.17 | 0.269–5.054 | |||
| Endocarditis | 0.86 | 1.11 | 0.351–3.484 | |||
| Postoperative AKI | 0.002 | 6.21 | 1.144–20.030 | |||
AKI: acute kidney injury; CI: confidence interval; COPD: chronic obstructive pulmonary disease; LV: left ventricular; NYHA: New York Heart Association; OR: odds ratio; PHT: pulmonary hypertension; PVD: peripheral vascular disease.
Female gender (OR 6.16, 95% CI 1.58–24.04; P = 0.009), coronary artery disease (OR 4.26, 95% CI 1.13–16.05; P = 0.032) and lower preoperative creatinine clearance (OR 0.95, 95% CI 0.91–0.98; P = 0.003) were the only independent variables found to be significant predictors for early mortality by multivariable analysis (Table 5).
Postoperative events and interventions occurred in 61.5% of patients. In univariable analysis, older age at presentation (OR 1.05, 95% CI 1.025–1.070; P < 0.001), lower creatinine clearance (OR 0.97, 95% CI 0.954–0.988; P = 0.001) and NYHA functional class III/IV (OR 2.29, 95% CI 1.158–4.510; P = 0.017) were significant predictors of combined postoperative morbidity, whereas urgent or emergency presentation failed to show significance (OR 2.07, 95% CI 0.996–4.306; P = 0.051). At multivariable analysis, only older age at operation (OR 1.05, 95% CI 1.023–1.061; P < 0.001) was independently associated with the occurrence of postoperative events.
Late outcomes
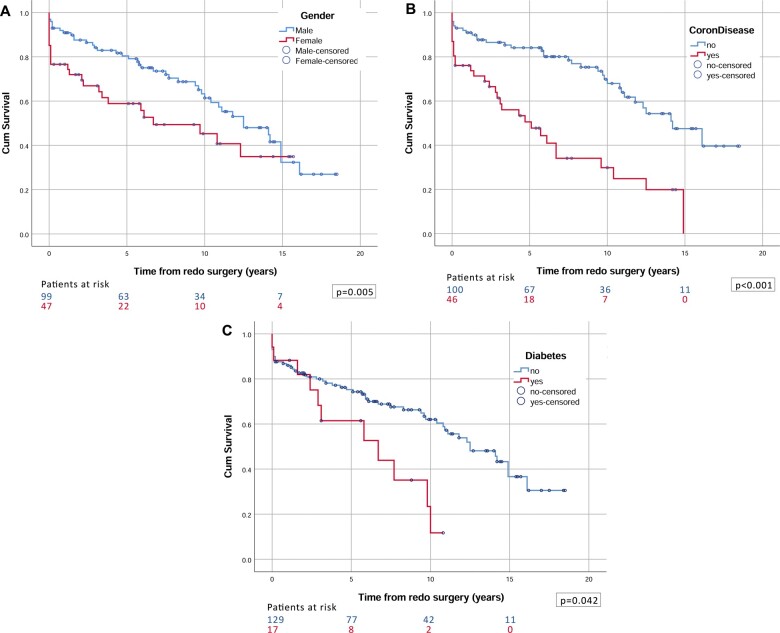
Follow-up was 98.6% complete and comprised 980 patient years. Median (interquartile range) duration of follow-up was 5.9 (1.7–10.9) years, for a maximum of 18.5 years. Overall survival was 74.1 ± 3.7% at 5 years, 57.9 ± 5.1% at 10 years and 43.8 ± 6.1% at 14 years (Fig. 1). The same risk factors as those for early mortality, supplemented with postoperative acute renal insufficiency and rhythm at discharge, were compared using log-rank tests. In Cox multivariable regression analysis, female gender, associated coronary disease, peripheral vascular disease, diabetes and postoperative acute renal insufficiency were identified as independent negative predictors affecting long-term survival (Table 6) (Fig. 2).
Figure 1:

Kaplan–Meier survival analysis for the total cohort.
Table 6:
Multivariable predictors for reduced long-term survival
| Variables | Log-rank analysis | Cox regression analysis | HR | 95% CI |
|---|---|---|---|---|
| P-value | P-value | |||
| Age at redo (years) | <0.001 | |||
| Female gender | 0.039 | 0.005 | 1.73 | 1.181–2.546 |
| PVD | 0.016 | 0.008 | 1.98 | 1.197–3.280 |
| Diabetes | 0.029 | 0.042 | 1.73 | 1.019–2.944 |
| Hypertension | 0.025 | |||
| COPD | 0.42 | |||
| Coronary disease | <0.001 | 0.016 | 1.62 | 1.096–2.397 |
| Creatinine clearance (ml/min) | <0.001 | |||
| NYHA functional III–IV | 0.44 | |||
| LV function | 0.47 | |||
| PHT | 0.25 | |||
| Urgent/emergency | 0.30 | |||
| Endocarditis | 0.15 | |||
| Postoperative AKI | <0.001 | 0.001 | 2.06 | 1.360–3.132 |
| Non-sinus rhythm at discharge | <0.001 |
AKI: acute kidney injury; CI: confidence intervals; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LV: left ventricular; NYHA: New York Heart Association; PHT: pulmonary hypertension; PVD: peripheral vascular disease.
Figure 2:
Log-rank comparisons for late survival according to (A) gender, (B) associated coronary disease and (C) diabetes.
DISCUSSION
With the increasing numbers of choices for bioprosthetic valves, the longer life expectancy in Western countries and the more widespread application of valve-sparing techniques in younger patients, redo valve surgery is becoming increasingly common in cardiac surgery practices. Where primary aortic valve surgery can be performed with an operative mortality between 1.8% and 3.7% [15–17], valvular reoperations have usually been associated with higher mortality, varying largely between 1.4% and 18%, mainly due to the different patient profiles, the various indications for repeat surgery and associated procedures, making comparisons harder [2, 10–12, 17–19].
Our retrospective review of rAVS after previous aortic valve surgery demonstrated that outcomes were acceptable and comparable to those in previously published series. In a relatively similar patient group, also comprising a fair number of endocarditis cases, Leontyev et al. and Joshi et al. found an early mortality of 5.8% and 7.3%, respectively, albeit in a younger patient cohort with a median age of 58 years, whereas the patients in our study were on average 10 years older [2, 10].
In small series as well as in large registry publications, the preoperative profiles of patients scheduled for rAVS are usually worse than those of patients having their first aortic valve surgery, and these patients are also younger; however, these patients typically present with more renal failure, peripheral vascular disease, coronary disease, arrhythmia and congestive heart failure, as was demonstrated in the Society of Thoracic Surgeons database study [17]. In this large registry study, 72% of patients were admitted for rAVS in NYHA functional class III or IV and in severe cardiac failure; urgent surgery was necessary in 41%. In our experience, 50% of patients were in NYHA functional class III/IV preoperatively, and 72% needed an urgent operation, perhaps partially explained by the higher incidence of endocarditis in our series [17]. An equivalent profile was found in the Leipzig study, which reported 41% of endocarditis cases in a comparable cohort presenting with NYHA functional class III/IV in 41% and needing urgent surgery in 62% [2]. But even without including endocarditis as an indication for rAVS, this high-risk profile on admission was also encountered in publications on rAVS for degenerative aortic valve prostheses only [10, 11, 18, 19]. These findings suggest that the timing of referral for redo surgery may be a major issue, often influenced by a false fear of the redo operation by itself, and that an earlier operation might prevent some of the postoperative morbidity.
The postoperative complications were mainly related to the bad overall condition of the patients rather than to the technical problems occurring during the redo procedure, such as re-entry injury to cardiac structures, which we did not encounter in our cohort [20]. The high incidences of reopening for bleeding, postoperative acute renal failure and new dialysis are frequent findings also reported in other studies [1, 2, 9–11, 21]. The incidence of new pacemaker implants is also clearly higher than in primary aortic valve operations, and pacemaker implantation rates of up to 25% have been described, a number to which our new pacemaker need of 6.6% still compares favourably [2, 7, 9, 11, 17, 22, 23]. Redo AVR often requires extensive debridement of the aortic annulus close to the ventricular septum for annular abscess in endocarditis or due to extensive, deep decalcification after previous prosthesis or homograft implants. In our study, the need for a new pacemaker could not be related to a particular indication for rAVS.
Patient risk factors for early mortality that were reported in most studies are often similar to those in our analysis, such as high NYHA functional class, older age, prior CABG or coronary disease and higher overall risk scores [1, 7, 9–11, 19]. Urgency by itself has rarely been identified as an early risk factor, suggesting that patients who undergo urgent rAVS have outcomes similar to those of their counterparts who have elective procedures, if they survive the critical perioperative period [8]. Coronary artery disease has, however, often been identified as a risk factor that impacts early and late mortality, which we also have noticed [1, 11]. Similar to the results of other researchers, we did not identify endocarditis as a risk factor for early demise in our patient group, but Leontyev et al. [2, 9] found that endocarditis negatively influenced late mortality. In concordance with others, we found that reduced renal function was significantly related to postoperative morbidity, because it resulted in more acute kidney failure and a need for haemodialysis but was also directly related to mortality, compromising both early and late survival [2, 9]. Women represented only 32% of our study population, but female gender was a significant negative predictive factor for early and late mortality. This result cannot be completely explained by their risk profile, which was mainly comparable to that of the male patients from our study, apart from a slightly higher incidence of postoperative atrial fibrillation.
New techniques such as ViV-TAVR have recently emerged as a technical alternative for some reoperative aortic valve indications. Recently published systematic reviews demonstrated comparable early and 1-year mortality between ViV-TAVR and redo aortic valve replacement in patients with structural valve degeneration of aortic bioprostheses [3–6]. These studies inherently represent a significant treatment allocation bias, because no randomized controlled trials on this topic exist so far. Patients treated with ViV were generally older and at higher predicted surgical risk than those undergoing redo surgery [3–6]. Patients undergoing ViV-TAVR were reported to have shorter ICU and hospital stays, fewer bleeding events, less need for dialysis and fewer pacemaker implants. On the other hand, surgical redo valves had lower transvalvular gradients and fewer chances for patient-prosthesis mismatch, paravalvular leak or vascular complications. Moreover, many patients considered for rAVS have problems that are not amenable to ViV-TAVR techniques, such as valve dehiscence, mechanical valve thrombosis or the increasing number of prosthetic valve endocarditis cases that we see in our practice [2, 7–10]. If we estimated the number of possible candidates for ViV-TAVR in our study, excluding cases with endocarditis, mechanical valves, non-structural valve dysfunction, aortic aneurysms and age ˂65 years, and did not consider prosthesis size, we were left with 35 out of 148 patients (23.6%). Looking at the patient profiles of these 35 possible ViV-TAVR candidates, we found significantly more patients of female gender (P = 0.004), NYHA functional class III/IV (P = 0.026), older age (P < 0.001) and higher EuroSCORE II (P = 0.003) compared to the other patients who did not have indications for ViV-TAVR. We acknowledge that our study describes a historical cohort. In view of the recent general trend towards a larger number of bioprosthetic valves implanted in younger individuals, the indications for transcatheter intervention are still profoundly changing, and, based on comorbidities and high preoperative risk scores, ViV-TAVR might also be offered to younger patients with degenerative aortic valves in the future. Based on the results from this study, our heart team might consider some of the high-risk patients, such as elderly women with high NYHA functional class scores, for ViV-TAVR, even if it is at the cost of a haemodynamically inferior result because of the potential to induce patient-prosthesis mismatch.
Limitations
The retrospective design and the analysis of observational data are obvious limitations of this study. Therefore, generalizing our findings to other centres and other populations should be done with caution. The precise timing of the operation and the choice of the aortic valve procedure were left to the discretion of the surgeon, which might induce some bias in the type of aortic valve prosthesis used or the associated procedure performed. The early postoperative data are almost complete; however, the data related to the patients who died during follow-up and the data about late left ventricular and aortic valve function, NYHA functional class, and cause of death were often incomplete and therefore were not analysed.
CONCLUSION
Despite a high rate of urgent conditions and advanced NYHA functional class at first presentation, suggesting a major issue with the timing of patient referral, reoperative aortic valve surgery can be performed with acceptable early and late outcomes. A degenerate bioprosthesis and prosthetic endocarditis were the main indications. Valve replacement for a failed bioprosthesis or a native aortic valve after prior repair was the most common procedure; however, root replacement was necessary in a considerable number of cases due to annular or root destruction. Late survival was adversely influenced by female gender and patient comorbidity, such as associated coronary or peripheral vascular disease or diabetes. If we excluded patients with endocarditis, mechanical valves, non-structural valve dysfunction, aortic aneurysms and age younger than 65 years, about 24% of all cases in need of aortic valve reintervention, particularly high-risk elderly patients, might have benefitted from less invasive transcatheter options.
Conflict of interest: none declared.
Author contributions
Katrien François: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing—original draft; Writing—review & editing. Laurent De Backer: Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Thomas Martens: Writing—review & editing. Tine Philipsen: Writing—review & editing. Yves Van Belleghem: Writing—review & editing. Thierry Bové: Formal analysis; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Akira Marui, Stefano Schena and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Abbreviations
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- HR
Hazard ratio
- ICU
Intensive care unit
- NYHA
New York Heart Association
- OR
Odds ratio
- rAVS
Redo aortic valve surgery
- ViV-TAVR
Valve-in-valve transcatheter aortic valve replacement
REFERENCES
- 1. Balsam LB, Grossi EA, Greenhouse DG, Ursomanno P, Deanda A, Ribakove GH et al. Reoperative valve surgery in the elderly: predictors of risk and long-term survival. Ann Thorac Surg 2010;90:1195–201. [DOI] [PubMed] [Google Scholar]
- 2. Leontyev S, Borger M, Davierwala P, Walther T, Lehmann S, Kempfert J et al. Redo aortic valve surgery: early and late outcomes. Ann Thorac Surg 2011;91:1120–6. [DOI] [PubMed] [Google Scholar]
- 3. Gozdek M, Raffa GM, Suwalski P, Kołodziejczak M, Anisimowicz L, Kubica J et al. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: systematic review and meta-analysis. Eur J Cardiothorac Surg 2018;53:495–504. [DOI] [PubMed] [Google Scholar]
- 4. Nalluri N, Atti V, Munir AB, Karam B, Patel NJ, Kumar V et al. Valve in valve transcatheter aortic valve implantation (ViV-TAVI) versus redo-Surgical aortic valve replacement (redo-SAVR): a systematic review and meta-analysis. J Interv Cardiol 2018;31:661–71. [DOI] [PubMed] [Google Scholar]
- 5. Takagi H, Mitta S, Ando T. Meta-analysis of valve-in-valve transcatheter versus redo surgical aortic valve replacement. Thorac Cardiovasc Surg 2019;67:243–50. [DOI] [PubMed] [Google Scholar]
- 6. Tam DY, Vo TX, Wijeysundera HC, Dvir D, Friedrich JO, Fremes SE. Transcatheter valve-in-valve versus redo surgical aortic valve replacement for the treatment of degenerated bioprosthetic aortic valve: a systematic review and meta-analysis. Catheter Cardiovasc Interv 2018;92:1404–11. [DOI] [PubMed] [Google Scholar]
- 7. Garrido-Olivares L, Maganti M, Armstrong S, David TE. Clinical outcomes of aortic root replacement after previous aortic root replacement. J Thorac Cardiovasc Surg 2013;146:611–15. [DOI] [PubMed] [Google Scholar]
- 8. Chong BK, Jung SH, Choo SJ, Chung CH, Lee JW, Kim JB. Reoperative aortic root replacement in patients with previous aortic root or aortic valve procedures. Korean J Thorac Cardiovasc Surg 2016;49:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szeto WY, Bavaria JE, Bowen FW, Geirsson A, Cornelius K, Hargrove WC et al. Reoperative aortic root replacement in patients with previous aortic surgery. Ann Thorac Surg 2007;84:1592–9. [DOI] [PubMed] [Google Scholar]
- 10. Joshi Y, Achouh P, Menasché P, Fabiani J-N, Berrebi A, Carpentier A et al. Multiple reoperations on the aortic valve: outcomes and implications for future potential valve-in-valve strategy. Eur J Cardiothorac Surg 2018;53:1251–7. [DOI] [PubMed] [Google Scholar]
- 11. Stulak JM, Tchantchaleishvili V, Daly RC, Eleid MF, Greason KL, Dearani JA et al. Conventional redo biological valve replacement over 20 years: surgical benchmarks should guide patient selection for transcatheter valve-in-valve therapy. J Thorac Cardiovasc Surg 2018;156:1380–90. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko T, Loberman D, Gosev I, Rassam F, McGurk S, Leacche M et al. Reoperative aortic valve replacement in the octogenarians—minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg 2014;147:155–62. [DOI] [PubMed] [Google Scholar]
- 13. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732–8. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. Am J Kidney Dis 2013;61:686–8. [DOI] [PubMed] [Google Scholar]
- 15. Langanay T, Rouzé S, Tomasi J, Aymami M, Rehman SM, Anselmi A et al. Conventional aortic valve replacement in 2005 elderly patients: a 32-year experience. Eur J Cardiothorac Surg 2018;54:446–52. [DOI] [PubMed] [Google Scholar]
- 16. Di Eusanio M, Fortuna D, De Palma R, Dell’Amore A, Lamarra M, Contini GA et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011;141:940–7. [DOI] [PubMed] [Google Scholar]
- 17. Kaneko T, Vassileva CM, Englum B, Kim S, Yammine M, Brennan M et al. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg 2015;100:1298–304. [DOI] [PubMed] [Google Scholar]
- 18. Finch J, Roussin I, Pepper J. Failing stentless aortic valves: redo aortic root replacement or valve in a valve? Eur J Cardiothorac Surg 2013;43:495–504. [DOI] [PubMed] [Google Scholar]
- 19. Naji P, Griffin BP, Sabik JF, Kusunose K, Asfahan F, Popovic ZB et al. Characteristics and outcomes of patients with severe bioprosthetic aortic valve stenosis undergoing redo surgical aortic valve replacement. Circulation 2015;132:1953–60. [DOI] [PubMed] [Google Scholar]
- 20. Morales D, Williams E, John R. Is resternotomy in cardiac surgery still a problem? Int Cardiovasc Thorac Surg 2010;11:277–86. [DOI] [PubMed] [Google Scholar]
- 21. Woitek FJ, Stachel G, Kiefer P, Haussig S, Leontyev S, Schlotter F et al. Treatment of failed aortic bioprostheses: an evaluation of conventional redo surgery and transfemoral transcathere aortic valve-in-valve implantation. Int J Cardiol 2020;300:80–6. [DOI] [PubMed] [Google Scholar]
- 22. Erlebach M, Wottke M, Deutsch M, Krane M, Piazza N, Lange R et al. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprothetic valves: consecutive patients in a single-center setting. J Thorac Dis 2015;7:1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silaschi M, Wendler O, Seiffert M, Castro L, Lubos E, Schirmer J et al. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact CardioVasc Thorac Surg 2017;24:63–70. [DOI] [PubMed] [Google Scholar]