Abstract
OBJECTIVES
Transvenous lead extraction using mechanical rotational- or laser sheaths is an established procedure. Lead dwell time has been recognized as a risk factor for extraction failure and procedure-related complications. We therefore investigated the safety and efficacy of transvenous extraction of leads with an implant duration of more than 10 years.
METHODS
Between January 2013 and March 2017, a total of 403 patients underwent lead extraction in 2 high-volume lead extraction centres. One hundred and fifty-four patients with extraction of at least 1 lead aged over 10 years were included in this analysis. Laser lead extraction was the primary extraction method, with additional use of mechanical rotational sheaths or femoral snares, if necessary. All procedural- and patient-based data were collected into a database and retrospectively analysed.
RESULTS
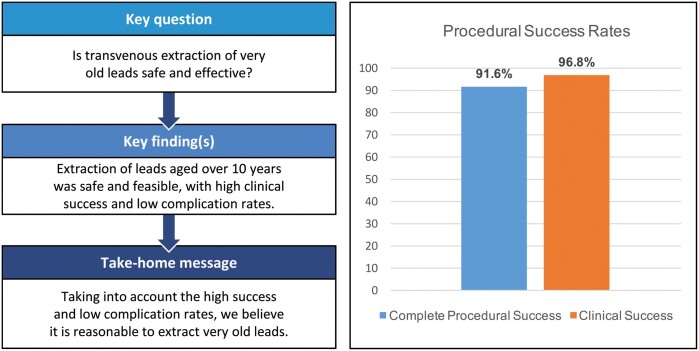
Mean patient’s age was 65.8 ± 15.8 years, 68.2% were male. Three hundred and sixty-two leads had to be extracted. The mean lead dwell time of treated leads was 14.0 ± 6.1 years. Complete procedural success was achieved in 91.6% of cases, while clinical success was achieved in 96.8%. Failure of extraction occurred in 3.2%. Leads that could not be completely removed had a significantly longer lead dwell time (18.2 vs 13.2 years; P = 0.016). Additional mechanical rotational sheaths or femoral snares were used in 26 (16.9%) patients. Overall complication rate was 4.6%, including 5 (3.3%) major and 2 (1.3%) minor complications. There was no procedure-related mortality.
CONCLUSIONS
Transvenous lead extraction in leads aged over 10 years is safe and effective when performed in specialized centres and with use of multiple tools and techniques. Leads that could not be completely extracted had a statistically significant longer lead dwell time.
Keywords: Lead extraction, Laser lead extraction, Mechanical lead extraction, Pacemaker lead extraction, Implantable cardioverter defibrillator lead extraction
The number of cardiac implantable electronic devices has been rising in recent years due to an ageing population and expansions in implantable cardioverter defibrillator (ICD)- and cardiac resynchronization therapy (CRT) indications.
INTRODUCTION
The number of cardiac implantable electronic devices has been rising in recent years due to an ageing population and expansions in implantable cardioverter defibrillator (ICD)- and cardiac resynchronization therapy (CRT) indications. With a growing number of cardiac implantable electronic device implantations and revisions, the number of device-related complications with necessity for lead extraction procedures has been rising [1–6]. More and more patients are implanted with multiple leads, and treated with cardiac implantable electronic device over long time periods. The number of leads adds to the procedural complexity of lead extraction in these patients [7]. Furthermore, the lead implant duration has been recognized as a risk factor for procedural failure and lead extraction-related complication. In recent years, several lead extraction techniques have been used and reported in the literature. In cases where manual traction does not allow for successful lead extraction, use of femoral snares, mechanical rotational- or laser sheaths have been described [7–10]. Especially, in leads with long implant duration and strong adhesions, either the use of mechanical devices or laser sheaths enables high procedural success rates [11–13]. However, extraction of chronically implanted leads remains a complex procedure with associated morbidity and mortality [12, 14, 15]. Especially in patients with very old leads, there is an increased risk of extraction failure or incomplete procedural success rates. Furthermore, the risk for procedure-associated complications is increased. In complex cases, including very old leads, the combination of multiple extraction tools might enhance procedural success rate. We here investigated success- and complication rates of lead extraction procedures in patients with leads implanted for more than 10 years.
MATERIALS AND METHODS
Between January 2013 and March 2017, a total of 403 patients underwent lead extraction in 2 high-volume extraction centres. All patients with at least 1 treated lead implanted for more than 10 years (n = 154) were included in this study. IRB approval was obtained for the study (ÄK Hamburg WF-026/17).
Laser lead extraction with 80-Hz high-frequency laser sheaths was the primary extraction method. If necessary, additional tools like mechanical rotational sheaths (Evolution R/l Cook Medical; TightRail Spectranetics/Philips Medical), femoral snares (Needle Eye Snare, Cook Medical) or lassos (ev3 Amplatz Goose Neck Snare, Covidien) were used. The patients were referred from external hospitals or from the outpatient clinic of the 2 centres. Data were collected into a computerized database at the time of the procedure and retrospectively analysed.
Lead extraction and reimplantation technique
All procedures were performed under fluoroscopic guidance in a conventional cardiac surgical- or hybrid operating room under general anaesthesia as previously described [16, 17]. Invasive arterial blood pressure monitoring was performed. Transoesophageal echocardiography was used throughout the procedure. All patients were prepared for sternotomy with heart lung machine standby. In cases that were classified as high-risk procedures, 6-Fr sheaths were placed in the femoral artery and vein. A 6-Fr pigtail was introduced through the venous sheath and brought into SVC level. This pigtail was used to perform a venography if necessary.
Risk classification was left due to operator’s discretion. Factors like lead age, number of leads, young patient age and dual coil ICD leads were taken into consideration for decision whether to place femoral sheaths.
Leads were dissected using electrocautery and the sleeves were removed. Lead locking devices (LLD™—Philips Healthcare) were placed in the inner lumen of the leads. Laser lead extraction was performed using 80-Hz laser sheaths (GlideLight™, Philips Healthcare). Sheath sizes included 12-, 14-, or 16-Fr sheaths. Laser lead extraction was performed as previously described [16]. If necessary, 11- or 13-Fr mechanical rotational sheaths (TightRail™, Philips Healthcare or Evolution®, Cook Medical Inc., Bloomington, IN, USA) were additionally employed. In case of lead fracture or residual lead fragments, femoral snares One Snare, EN Snare (both Merit Medical, South Jordan, UT, USA), Needle’s Eye Snare (Cook Medical Inc., Bloomington, IN, USA) or lassos (ev3 Amplatz Goose Neck Snare, Covidion, Ireland) were utilized (Fig. 1).
Figure 1:
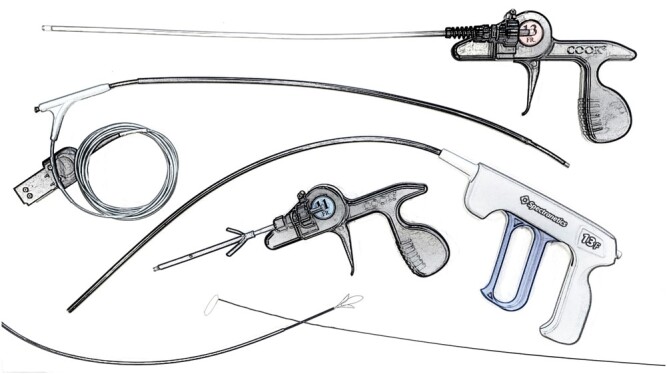
Armentarium used for extraction of old leads. From top to bottom: Evolution Cook Medical, GlideLight Spectranetics/Philips, TightRail Spectranetics/Philips, Evolution Shorty Cook Medical, Amplatz Goose Neck Snare Covidien (left), Needle’s eye snare Cook Medical (right).
Device reimplantation was performed complementary to lead extraction as a one-step procedure from the contralateral side in patients without systemic infection. In patients with pocket infection, local surgical debridement of the pacemaker/ICD pocket was conducted. Antibiotic prophylaxis was administered for reimplantation. In patients with systemic infection, either a 2-step approach with transvenous device reimplantation in an infection-free interval was conducted or epicardial leads were implanted
In patients with pacemaker dependency, a new active-fixation right ventricular (RV) pacing lead was inserted at the ipsilateral side, subcutaneously tunnelled and connected to an externalized pacemaker device for temporary pacing, as previously described [16, 18]. Device reimplantation of the permanent device and removal of the temporal RV lead were then performed in an infection-free interval. In some CRT patients, alternatively, an epicardial left ventricular (LV)-lead was placed through a left-lateral mini-thoracotomy and connected to a VVI pacemaker. In an infection-free interval, an additional transvenous RV-defibrillation and RA lead were implanted and the device was upgraded to a CRT-D device.
Definitions
The removal of leads was classified as lead extraction when the use of advanced extraction tools like Excimer laser- and/or mechanical rotational sheath was necessary. Laser treatment time is defined as the device-determined operating time during which laser pulses are emitted and the impulses delivered characterize the number of actually applicated laser impulses. Procedural success- and complications rates were determined according to the 2017 Heart Rhythm Society Expert Consensus paper on transvenous lead extraction [19].
Statistical analysis
Categorical variables are displayed as frequencies and percentages. Continuous values are expressed as mean ± standard deviation and were compared using Student’s t-test. A P-value <0.05 was considered statistically significant. Statistical analysis was conducted using SPSS 21.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Lead extraction indications
Indications for lead extraction included pocket infection in 43 (27.9%) patients and systemic infection in 55 (35.7%) patients. Lead dysfunction was the indication for lead extraction in 36 (23.4%) patients, system upgrade in 9 (5.8%) patients, venous stenosis or occlusion in 2 (1.3%) patients, chronic pain in 2 (1.3%) patients and other indications in 7 (4.6%) patients (Table 1).
Table 1:
Patient demographics
| Patients | n = 154 |
|---|---|
| Demographics | |
| Age (years) | 65.8 ± 15.9 |
| Male gender | 105 (68.2) |
| Ejection fraction <30% | 40 (26.5) |
| Previous cardiac surgery | 39 (25.8) |
| Pacemaker dependency | 55 (35.7) |
| Chronic kidney disease | 51 (33.1) |
| Coronary artery disease | 58 (37.7) |
| Indication for lead removal | |
| Pocket infection | 43 (27.9) |
| Systemic infection | 55 (35.7) |
| Lead dysfunction | 36 (23.4) |
| System upgrade | 9 (5.8) |
| Chronic pain | 2 (1.3) |
| Venous stenosis/occlusion | 2 (1.3) |
| Other indications | 7 (4.6) |
Continuous variables are expressed as mean ± standard deviation and categorical are expressed by values and percentages.
Patient demographics
Mean patient age was 65.8 ± 15.9 years, and 68.2% were male. Mean body mass index was 26.3 ± 4.5. Arterial hypertension was present in 110 patients (71.4%), while 38 (24.7%) had diabetes mellitus. Chronic kidney disease was diagnosed in 51 (33.1%) patients and 40 (26.05) patients had a severely reduced left ventricular ejection fraction (LVEF) (<30%). Thirty-nine patients (25.3%) had a previous cardiac surgical procedure. Detailed patient characteristics are shown in Table 1.
Lead characteristics
A total of 362 leads were treated, including 128 (35.4%) atrial, 209 (57.7%) RV and 25 (6.9%) coronary sinus leads. Eighty-five ICD leads were treated including 30 (14.4%) single-coil and 55 (26.3%) dual-coil leads. The mean lead dwell time of treated leads was 14.0 ± 6.1 years. Two hundred and fourteen (59.1%) leads were implanted from the left side while 148 (40.9%) were implanted from the right side. An active lead tip fixation was present in 245 leads (68.8%) and passive fixation in 117 leads (32.3%). The mean number of leads per patient was 2.6, and the mean number of treated leads was 2.4. Lead demographics are shown in Table 2.
Table 2:
Lead demographics
| Number of treated leads | n = 362 |
|---|---|
| Time from implantation (years) | 14.0 ± 6.1 |
| Lead type | |
| Atrial lead | 128 (35.4) |
| Ventricular lead | 209 (57.7) |
| PM | 124 (59.3) |
| ICD single coil | 30 (14.4) |
| ICD dual coil | 55 (26.3) |
| Coronary sinus lead | 25 (6.9) |
| Lead fixation | |
| Active | 245 (67.7) |
| Passive | 117 (32.3) |
| Side of implantation | |
| Left | 214 (59.1) |
| Right | 148 (40.9) |
Continuous variables are expressed as mean ± standard deviation and categorical are expressed by values and percentages.
ICD: implantable cardioverter defibrillator; PM: pacemaker.
Procedural data
The mean procedural time was 119.5 ± 61.1 min, and the mean fluoroscopy time was 14.8 ± 9.7 min; 12-, 14- or 16-Fr 80-Hz GlideLight Laser sheaths were used in all patients. The mean laser treatment time was 71.7 ± 69.3 s, and the mean number of laser impulses was 5515.4. Additionally, mechanical rotational sheaths were also used in 25 patients, while femoral snares were additionally used in 3 patients (in 2 patients in combination with mechanical rotational sheaths and laser).
Success and complication rates
Complete procedural success was observed in 141/154 patients (91.6%), while clinical success was achieved in 149/154 (96.8%) cases. A failure of extraction was seen in 5 (3.3%) patients. Three hundred and forty-four of 362 (95.0%) leads were completely extracted, whereas partial removal was observed in 10 leads (2.8%). Complete failure of lead extraction was seen in 8 (2.2%) leads in 5 patients (3.2%). Leads that could not be completely removed were significantly older than leads where complete extraction success was achieved (18.2 vs 13.2 years; P = 0.016). The overall complication rate was 4.6%. Five major (3.3%) and 2 (1.3%) minor complications occurred. The major complications included 1 perforation of the lateral side of the superior vena cava, 1 laceration of the right atrium at SVC level, 1 perforation of the right atrial appendage, 1 pericardial effusion requiring a pigtail catheter, and damage of the tricuspid valve requiring tricuspid valve surgery. This complication occurred in a patient in whom besides laser sheaths, a mechanical rotational sheaths was used. Emergency sternotomy was necessary in 3 patients and cardiopulmonary bypass was used in 2 of these patients. In the patient with right atrial appendage perforation, the perforation was sutured without cardiopulmonary bypass. All patients with major complications could be discharged from hospital without further neurological complications. Two minor complications including 1 pocket haematoma, that had to be surgically revised, and 1 hematothorax with necessity for a pleura drain, were observed. Complications are displayed in Table 3.
Table 3:
Outcome and complications
| Number of treated leads | n = 362 |
|---|---|
| Extraction | |
| Complete | 344 (95.0) |
| Partial | 10 (2.8) |
| Failure | 8 (2.2) |
| Number of treated patients | n = 154 |
| Outcome | |
| Complete procedural success | 141 (91.6) |
| Clinical success | 149 (96.8) |
| Failure | 5 (3.2) |
| Complications | |
| Minor | 2 (1.3) |
| Major | 5 (3.3) |
| SVC laceration | 2 (1.3) |
| RA perforation | 1 (0.7) |
| Pericardial effusion | 1 (0.7) |
| Tricuspid valve damage | 1 (0.7) |
| Overall | 7 (4.6) |
Continuous variables are expressed as mean ± standard deviation and categorical are expressed by values and percentages.
No procedure-related death occurred in any of the patients in the study group. In-hospital mortality was 1.3%. One patient with systemic infection died during in-hospital stay due to sepsis-related multi-organ failure. A further patient with systemic infection experienced a progressive RV failure the day after lead extraction. The patient received a VA extracorporeal membrane oxygenation for haemodynamic stabilization. Unfortunately, the patient experienced a fulminant stroke at postoperative day 4 and died.
DISCUSSION
In this study including leads with a mean lead dwell time of 14 years, we have shown a high rate of complete lead extraction (95.0%) and high rates of procedural (91.6%) and clinical success (96.8%). Furthermore, we did not observe any procedure-related death, and the minor- and major complication rates were 1.3% and 3.3%, respectively.
In our study, leads that could not be completely removed had a significantly longer lead dwell time compared with leads that were completely lead extracted. However, the high complete lead extraction rate of 95% despite long lead dwell time could only be achieved by use of multiple tools. In our study, in 26 patients, besides laser sheaths, mechanical rotational sheaths, femoral snares, or both were used. In patients with calcified adhesions, for example, use of mechanical rotational sheaths was necessary in order to free the leads from these adhesions. Furthermore, leads with previous extraction attempts and lead fracturing, or intraoperative lead breakage sometimes necessitate the use of femoral snares. The necessity for multiple extraction tools in older leads is in line with a previously published study by Rinaldi et al. [20]. Here, in the group of patients with longer lead dwell time (11.6 vs 6.6 years), more patients needed additional femoral lead extraction techniques. The use of different techniques and tools allows for a patient-tailored individual extraction strategy, resulting in a higher procedural success rate. Especially, when treating those leads with very long lead dwell time, physicians performing extractions should be familiar with different extraction tools and techniques.
Regarding complication rates, our study shows a slightly higher rate of major complications (3.3%) in comparison to other previously published transvenous lead extraction studies. Previous laser lead extraction studies have reported major complication rates of 0.9–2.5%, however, with lead dwell times ranging between 5.4 and 6.8 years [11, 15–17, 21]. Studies using mechanical rotational sheaths have shown major complication rates between 0% and 1.5%. The lead dwell times in these studies were between 7.1 and 9.1 years [22–25]. Therefore, the significantly shorter lead dwell time in these studies might have contributed to a reduced rate of major complications. In a study by Malecka et al. [26], including 43 patients with lead implant duration >20 years, the complication rate was 4.6% (2.3% major complications and 2.3% minor complications), which is comparable to our results. The use of additional tools like mechanical rotational sheaths or snares was not associated with an increased rate of major complications in our study. One major complication (Tricuspid valve damage) was seen in a patient in whom besides laser lead extraction, a mechanical sheath was used. No vascular lacerations were seen in any patient, when besides laser sheaths, mechanical rotational sheaths or snares were used. However, the number of used additional tools (mechanical sheaths, snares), as well as the absolute number of major complications, was too low to adequately address this question and larger studies are needed to finally answer the role of several additional tools on complications in complex extraction cases.
Although 5 patients in our series experienced major complications, and 3 needed emergency sternotomy, none of the patients died or suffered a persisting neurological disability. This shows that in reasonable operative settings, and with careful preoperative preparation, an experienced team can usually deal well with those major complications. However, it is essential to mention that all of our patients were treated in general anaesthesia under advanced haemodynamic monitoring including invasive arterial blood pressure monitoring and surveilled by transoesophageal echocardiography. All of them were also prepared for emergency sternotomy and all interventions were performed with ‘true’ stand-by option for extracorporeal circulation. Furthermore, in high-risk cases, at the beginning of the procedure, venous and arterial sheaths were placed in the femoral vessels for safety reasons. These may crucially accelerate the establishment of cardiopulmonary bypass in case of haemodynamic deterioration due to severe vascular injury during extraction procedure. Those careful pre- and intraoperative arrangements together with the experience of operators were the decisive points for patient survival in all our cases with major complications.
Regarding the procedural success rates, laser lead extraction studies have shown success rates ranging between 90% and 100% [11, 15–17, 21], while studies using mechanical rotational sheaths have reported procedural success rates between 80% and 98.3% [22–25]. With a complete procedural success rate of 91.6%, our results are well in line with those previously published, although our study includes leads with much longer lead dwell time.
In the field of lead extractions, there is an ongoing debate concerning the aggressivity of lead management. In cases with local or systemic infection, there is a clear evidence with class I indication in the guidelines [19] for complete lead and device removal. The question whether to extract or abandon a superfluous lead in non-infectious cases is, despite a certain evidence, still not finally resolved. Pokorney et al. analysed a cohort of 6859 patients with non-infected leads, where 16.2% (1113) of patients underwent lead extraction procedure, whereas in all other patients, the malfunctioning leads were capped and left in place, for short- and long-term outcome. They found no difference in survival between the groups in short-term and in 5-year follow-up. However, the group with elective lead extraction was associated with lower risk of long-term device-related infections relative to lead abandonment group. Similar results were published by Kutarski et al. [27]. In their study assessing late consequences of abandoned leads, they found a higher risk of device infections, technical problems during subsequent lead extractions and worse long-term outcome in patients with abandoned leads. Although the available data are indicating that more electrodes lead to more intravascular adhesions, more thrombosis, vascular occlusions and have a higher risk of device infections in the long term, the dramatic consequences of vascular injuries with fulminant bleeding and the consequences of cardiovascular arrest and eventual neurological complications with hypoxic brain damage or even a fatal outcome keep the enthusiasm for ‘all-extracting strategy’ under control. This is strengthened by the assumption that the longer lead dwell time will influence the success and increase substantially the complication rate and mortality while performing the lead extraction. This raises the question whether different strategies should be applied depending on the lead dwell time and should electrodes with a long implant time only be removed in the case of proven device infection or lead endocarditis? With regard to this, our study with a mean lead dwell time of 14 years showed a slightly higher complication rate in comparison to transvenous lead extraction studies with significantly shorter dwell times (between 5.4 and 6.8 years [11, 15–17, 21] and 7.1 and 9.1 years [22–25], respectively). Despite the fact that older leads are more difficult to extract, we were able to achieve high rates of procedural- as well as clinical success. Notably, this was in a substantial number of cases possible only when combining different extraction techniques (laser with mechanical rotational sheaths and snares).
However, despite our encouraging data, the patients with very long lead dwell time often tend to possess serious comorbidities, which may influence the individual risk of lead extraction. Therefore, in all cases where both lead extraction and abandoning of leads can be considered, all risks and benefits of each strategy should be discussed in a heart team and with the patient and afterwards an individualized, patient-tailored strategy should be applied.
CONCLUSION
When taking into account the possible late consequences of abandoned leads and the results from our study, showing high clinical success and low complication rates, we believe that it is reasonable to perform lead extractions of very old leads in experienced centres. However, the more frequent necessity for utilization of multiple extraction tools requires more experience and versatility of the operator and increases the costs of surgery.
Conflict of interest: Samer Hakmi, Heiko Burger and Simon Pecha are Proctors for Spectranetics (Philips Healthcarel), Samer Hakmi, Heiko Burger and Simon Pecha received speaker honoraria from Spectranetics (Philips Healthcare).
Author contributions
Simon Pecha: Conceptualization; Methodology; Writing—original draft. Tibor Ziegelhoeffer: Data curation; Investigation; Writing—original draft. Yalin Yildirim: Data curation; Methodology. Yeong-Hoon Choi: Supervision; Validation. Stephan Willems: Supervision; Validation. Hermann Reichenspurner: Supervision; Validation. Heiko Burger: Conceptualization; Data curation; Writing—original draft. Samer Hakmi: Data curation; Supervision; Validation; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Ulrich Otto von Oppell, Luca Paolo Weltert and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
Abbreviations
- ICD
Implantable cardioverter defibrillator
- RV
Right ventricular/ventricle
REFERENCES
- 1. Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch D. et al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol 2010;33:705–11. [DOI] [PubMed] [Google Scholar]
- 2. Mond HG, Irwin M, Ector H, Proclemer A.. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005 an international cardiac pacing and electrophysiology society (ICPES) project. Pacing Clin Electrophysiol 2008;31:1202–12. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 5. Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458–77. [DOI] [PubMed] [Google Scholar]
- 6. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 2012;60:1540–5. [DOI] [PubMed] [Google Scholar]
- 7. Farooqi FM, Talsania S, Hamid S, Rinaldi CA.. Extraction of cardiac rhythm devices: indications, techniques and outcomes for the removal of pacemaker and defibrillator leads. Int J Clin Pract 2010;64:1140–7. [DOI] [PubMed] [Google Scholar]
- 8. Byrd CL, Schwartz SJ, Hedin NB, Goode LB, Fearnot NE, Smith HJ.. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol 1990;13:1871–5. [DOI] [PubMed] [Google Scholar]
- 9. Arujuna A, Williams S, Whittaker J, Shetty A, Roy D, Bostock J. et al. Trends, indications and outcomes of cardiac implantable device system extraction: a single UK centre experience over the last decade. Int J Clin Pract 2012;66:218–25. [DOI] [PubMed] [Google Scholar]
- 10. Bordachar P, Defaye P, Peyrouse E, Boveda S, Mokrani B, Marquie C. et al. Extraction of old pacemaker or cardioverter-defibrillator leads by laser sheath versus femoral approach. Circ Arrhythm Electrophysiol 2010;3:319–23. [DOI] [PubMed] [Google Scholar]
- 11. Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C.. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol 2002;25:804–8. [DOI] [PubMed] [Google Scholar]
- 12. Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R. et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol 1999;33:1671–6. [DOI] [PubMed] [Google Scholar]
- 13. Starck CT, Rodriguez H, Hurlimann D, Grunenfelder J, Steffel J, Salzberg SP. et al. Transvenous lead extractions: comparison of laser vs. mechanical approach. Europace 2013;15:1636–41. [DOI] [PubMed] [Google Scholar]
- 14. Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, Crossley GH 3rd. et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009;6:1085–104. [DOI] [PubMed] [Google Scholar]
- 15. Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW. et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol 2010;55:579–86. [DOI] [PubMed] [Google Scholar]
- 16. Pecha S, Linder M, Gosau N, Castro L, Vogler J, Willems S. et al. Lead extraction with high frequency laser sheaths: a single-centre experience. Eur J Cardiothorac Surg 2017;51:902–5. [DOI] [PubMed] [Google Scholar]
- 17. Tanawuttiwat T, Gallego D, Carrillo RG.. Lead extraction experience with high frequency excimer laser. Pacing Clin Electrophysiol 2014;37:1120–8. [DOI] [PubMed] [Google Scholar]
- 18. Pecha S, Aydin MA, Yildirim Y, Sill B, Reiter B, Wilke I. et al. Transcutaneous lead implantation connected to an externalized pacemaker in patients with implantable cardiac defibrillator/pacemaker infection and pacemaker dependency. Europace 2013;15:1205–9. [DOI] [PubMed] [Google Scholar]
- 19. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R. et al. HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e503–51. [DOI] [PubMed] [Google Scholar]
- 20. Gould J, Sidhu BS, Porter B, Sieniewicz BJ, Teall T, Williams SE. et al. Prolonged lead dwell time and lead burden predict bailout transfemoral lead extraction. Pacing Clin Electrophysiol 2019;42:1355–64. [DOI] [PubMed] [Google Scholar]
- 21. Kennergren C, Bucknall CA, Butter C, Charles R, Fuhrer J, Grosfeld M. et al. Laser-assisted lead extraction: the European experience. Europace 2007;9:651–6. [DOI] [PubMed] [Google Scholar]
- 22. Starck CT, Gonzalez E, Al-Razzo O, Mazzone P, Delnoy PP, Breitenstein A. et al. Results of the patient-related outcomes of mechanical lead extraction techniques (PROMET) study: a multicentre retrospective study on advanced mechanical lead extraction techniques. Europace 2020;22:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzone P, Melillo F, Radinovic A, Marzi A, Paglino G, Della Bella P. et al. Use of the new rotating dilator sheath TightRail for lead extraction: a bicentric experience. J Arrhythmia 2020;36:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Witte OA, Adiyaman A, Smit JJJ, Ramdat Misier AR, Elvan A, Ghani A. et al. Success and complication rates of lead extraction with the first- vs. the second-generation Evolution mechanical sheath. Europace 2017;19:1717–22. [DOI] [PubMed] [Google Scholar]
- 25. Oto ALI, Aytemir K, Canpolat UĞUR, Yorgun H, Şahiner L, Kaya EB. et al. Evolution in transvenous extraction of pacemaker and implantable cardioverter defibrillator leads using a mechanical dilator sheath. Pacing Clin Electrophysiol 2012;35:834–40. [DOI] [PubMed] [Google Scholar]
- 26. Ząbek A, Boczar K, Dębski M, Matusik PT, Pfitzner R, Ulman M. et al. Transvenous extraction of very old (over 20-year-old) pacemaker leads using mechanical systems: effectiveness and safety. Pacing Clin Electrophysiol 2019;42:998–1005. [DOI] [PubMed] [Google Scholar]
- 27. Jachec W, Polewczyk A, Segreti L, Bongiorni MG, Kutarski A.. To abandon or not to abandon: late consequences of pacing and ICD lead abandonment. Pacing Clin Electrophysiol 2019;42:1006–17. [DOI] [PubMed] [Google Scholar]