Abstract
OBJECTIVES
Since video-assisted thoracic surgery (VATS) was first performed in the early 1990s, there have been many developments, and the conversion rate has decreased over the years. This article highlights the specific outcomes of patients undergoing conversion to thoracotomy despite initially scheduled VATS lung resection.
METHODS
We retrospectively reviewed 501 patients who underwent thoracoscopic anatomic lung resection (i.e. lobectomy, segmentectomy or bilobectomy) between 1 January 2012 and 1 August 2017 at our institution. We explored the risk factors for surgical conversion and adverse events occurring in patients who underwent conversion to thoracotomy.
RESULTS
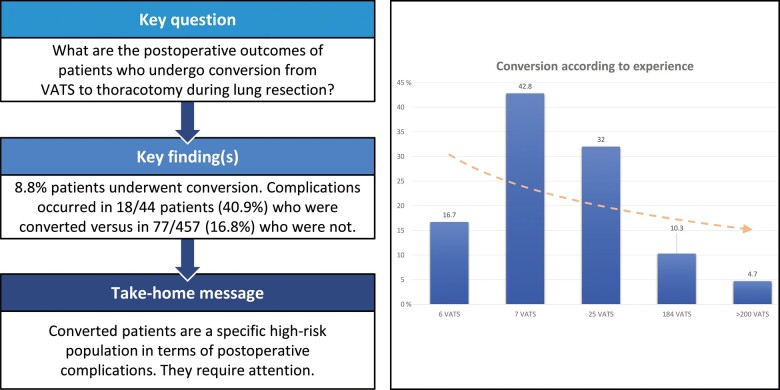
A total of 44/501 patients underwent conversion during the procedure (global rate: 8.8%). The main reasons for conversion were (i) anatomical variation, adhesions or unexpected tumour extension (37%), followed by (ii) vascular causes (30%) and (iii) unexpected lymph node invasion (20%). The least common reason for conversion was technical failure (13%). We could not identify any specific risk factors for conversion. The global complication rate was significantly higher in converted patients (40.9%) than in complete VATS patients (16.8%) (P = 0.001). Postoperative atrial fibrillation was a major complication in converted patients (18.2%) [odds ratio (OR) 5.09, 95% confidence interval (CI) 1.80–13.27; P = 0.001]. Perioperative mortality was higher in the conversion group (6.8%) than in the VATS group (0.2%) (OR 33.3, 95% CI 3.4–328; P = 0.003).
CONCLUSIONS
Through the years, the global conversion rate has dramatically decreased to <10%. Nevertheless, patients who undergo conversion represent a high-risk population in terms of complications (40.9% vs 16.8%) and perioperative mortality (6.8% vs 0.2%).
Keywords: Video-assisted thoracic surgery, Conversion, Lung resection, Perioperative complications
INTRODUCTION
Since video-assisted thoracic surgery (VATS) lobectomies were first reported in 1992 and 1993 [1–3], the proportion of minimally invasive approaches for anatomic lung resection has significantly increased. Referring to large databases, more than 60% of lobectomies are performed by VATS in the USA (STS database) [4], while only 22.9% are performed by VATS in Europe (ESTS database) [5]. These figures describe a variable degree of penetration, depending on the local economy, institutional traditions, healthcare systems and types of cancer that are encountered.
The most common minimally invasive approach is still VATS. The rapid expansion of the more recent onset of robot-assisted thoracic surgery remains limited by its additional cost and available access to equipment. While debate regarding the extent of lymph node dissection is ongoing [6], VATS is associated with similar oncological results to open thoracotomy for stage IA non-small-cell lung cancer [7]. Remarkably, VATS decreases the perioperative complication rate and length of hospital stay [8]. These results have been well established by meta-analysis [9, 10] and propensity-matched series [5, 11]. Early discharge of patients is further promoted by enhanced recovery after surgery pathways, which have been adapted for lobectomy, both open surgeries [12] and VATS [13].
Within the framework of such techniques, the preoperative environment is also evolving towards a less invasive approach: among the precepts, there is strong encouragement to avoid central lines, arterial lines, bladder catheters and epidural analgesia and a strong preference for non-opioid analgesia. Fasting is no longer required, and early mobilization is facilitated by limited operative trauma and low pain levels.
Given this minimalistic perioperative approach, we hypothesized that patients who planned to undergo VATS lobectomy, who required unpredicted conversion to open thoracotomy, would be exposed to worse outcomes and would constitute a specific high-risk population.
In this study, we reviewed a cohort of patients who intended to undergo VATS and compared the postoperative outcomes of those who successfully underwent VATS with those of patients who required conversion. We aimed to evaluate the specific complication rate, length of hospitalization and perioperative mortality in this population exhibiting unpredicted conversion to thoracotomy.
METHODS
Patients
We retrospectively reviewed data from 501 consecutive patients who underwent thoracoscopic anatomic lung resection (i.e. lobectomy, bilobectomy and segmentectomy) from the inception of the programme from 1 January 2012 to 1 August 2017 at the Thoracic Surgery Department of Strasbourg University Hospital. These data were collected retrospectively on an annual basis since 2012. All thoracoscopic lung resections were included; without exclusion, 3 senior surgeons and 2 fellows in the Thoracic Surgery Department performed the various lung resections.
A request for approval from the Institutional Review Board of the French Society of Thoracic and Cardio-Vascular Surgery was submitted and approved in May 2019, according to the following reference: CERC-SFCTCV-2019-3-12-22-43-24-SeJo.
Preoperative data
Patients who had smoked more than 100 cigarettes in their lifetime and who had quit smoking at the time of diagnosis were classified as former smokers, and patients who had smoked more than 100 cigarettes in their lifetime and who were currently smoking cigarettes were classified as current smokers (defined by the Centre for Disease Control and Prevention) (Supplementary Material). Former smokers and current smokers were classified as smokers, in contrast to never smokers.
We also recorded the pulmonary history of patients with chronic obstructive pulmonary disease (COPD), overlap syndrome, sleep apnoea syndrome, a history of pulmonary embolism, tuberculosis and asthma.
In the case of cancer, histological data were registered preoperatively or postoperatively in the absence of a previously established histological diagnosis.
Operating data
A number was assigned to each of the 5 operators practising VATS during the study in order to define the conversion risk according to the operator's training.
The conversion causes were sorted according to the VALT (Vascular, Anatomy, Lymph Node, Technical) classification, which was used by Gazala et al. [14] for the first time in 2011.
To determine whether long-term surgery using VATS (including a surgical time over 4 h) or conversion in order to shorten the operating time would be better, we looked at the number of interventions completed with VATS that lasted more than 4 h (240 min) to compare the postoperative outcomes with those of converted patients.
With the idea that the morbidity associated with conversion might be due to a ‘delay’ in conversion, we also examined whether late conversion during the intervention, i.e. after prolonged dissection, or parenchymal/bronchial or vascular stapling was more damaging to the patient than early conversion. To this end, we examined all the operating reports and sorted the late conversions from the early conversions.
Postoperative data
Pathological staging followed the seventh revision of the tumour, node and metastasis (TNM) classification [15].
All patients with postoperative complications were registered. Complications were classified following the Clavien–Dindo classification [16].
We recorded in-hospital mortality and postoperative mortality.
Items collected during the postoperative follow-up of patients are presented in the Supplementary Material.
Air leak time was defined as the time during which air leaks were collected in the postoperative period. We considered only air leaks longer than 7 days. Acute respiratory failure syndrome was considered as acute respiratory failure in a hypoxic patient with a PAO2/FiO2 ratio <200.
The definition of postoperative atrial fibrillation (PAF) was a passage to atrial fibrillation, objectified by an electrocardiogram, during the postoperative period.
Postoperative pneumonia or suspected pneumonia, treated with at least 5 days of antibiotic therapy, was considered even in the absence of germs.
Postoperative death was defined as death occurring during the first postoperative month.
Revision surgery was defined as surgical reoperation during hospitalization.
The drainage time was the time between the procedure and removal of the chest tube.
Hospitalization duration was the duration from admission to discharge.
Postoperative pain was rated using the Analogue Visual Pain Scale. Per its definition, scores ranging from 0 (no pain) to 10 (maximal pain) were systematically recorded on the first postoperative day.
Study end points
The primary end point was the global perioperative complication rate in both groups, including mortality according to the need for conversion to thoracotomy.
The secondary judgement criteria were postoperative mortality, specific complication type (PAF, pneumopathy, acute respiratory failure syndrome), hospitalization length, postoperative pain assessment on D1 and specific complication rate (late conversion, VATS for more than 4 h).
Risk factors for conversion to thoracotomy
We compared the preoperative and intraoperative data to identify risk factors for conversion from initially planned VATS to thoracotomy.
Surgical technique
All procedures were performed under general anaesthesia. Single-lung ventilation was obtained with double-lumen intubation. Surgery began with a 4-cm utility incision in the fifth intercostal space at the level of the submammary line and protection with an Alexis retractor (Applied Medical Resources Corporation, CA, USA). The bronchi, fissures and vascular structures were divided with an endoscopic stapling device (endo GIA Ultra Universal Stapler, Medtronic, MN, USA). More details regarding the surgical technique, especially the lymph node dissection, have been published in a prior publication [17]. In all situations where bleeding was difficult to control by VATS and where tamponade had not resulted in controllable bleeding, conversion to thoracotomy was performed.
Statistical analyses
Data were managed using a Microsoft Excel data sheet and were analysed using IBM SPSS 25.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as the median with interquartile range (IQR) unless otherwise specified. In the tables, descriptive data are presented as the mean with standard deviation (SD) or median with IQR or number of subjects [n (%)]. Comparisons between groups were made using the Student’s T-test, if applicable, or the Mann–Whitney U-test in the case of a non-normal distribution. Odds ratios (ORs) were calculated using the χ2 tests for 2 × 2 contingency tables when assumptions were met. Otherwise, a non-parametric, Fisher’s exact test was used. The normal distribution of continuous variables was verified by the Shapiro–Wilk test. Association between categorical variables were assessed using Cramer’s V. We performed the Kruskal–Wallis test to assess the relationship between several ordinal variables and 2 categorical variables. All tests were two-sided, with variables being considered significant at P-value <0.05. The multivariable analysis was using stepwise logistic regression, and the model building process was a backward variable selection approach with a significance level <0.157.
RESULTS
Cohort characteristics
We retrospectively reviewed the data from 501 patients who underwent thoracoscopic anatomical pulmonary resection (lobectomy, bilobectomy, segmentectomy) between 1 January 2012 and 1 August 2017 at the Strasbourg University Hospital's thoracic surgery department.
The median age at the time of surgery was 65 years (IQR 58–72). The median % of forced expiratory volume in 1 s (FEV1) in the study population was 86.4% (IQR 71.5–101%). Regarding smoking data, the median value for pack-years was 40 (IQR 25–50) when data were available (34.8% of smokers).
The mode of discovery of the pathology as well as the main patient histories are summarized in a table (Supplementary Material).
The proportion of smokers was 65.9%.
The demographic characteristics of all studied patients are presented in Table 1.
Table 1:
Cohort characteristics
| Variables | Population (N = 501) | VATS (N = 457) | Converted (N = 44) | P-value |
|---|---|---|---|---|
| Median age (years) | 65 | 65 | 67 | 0.06 |
| Sex | 0.80 | |||
| Men | 287 | 261 | 26 | |
| Women | 214 | 196 | 18 | |
| Type of resection | 1.00 | |||
| Lobectomy | 456 | 414 | 42 | |
| Segmentectomy | 36 | 34 | 2 | |
| Bilobectomy | 9 | 9 | 0 | |
| Smoker (current or former) | 330 | 302 | 28 | 0.11 |
| Obesity | 78 | 72 | 28 | 0.71 |
| Pulmonary history | ||||
| COPD | 90 | 82 | 8 | 0.99 |
| Overlap syndrome | 11 | 11 | 0 | 0.29 |
| Sleep apnoea | 7 | 3 | 4 | 0.02 |
| Pulmonary embolism | 9 | 9 | 0 | 0.35 |
| Asthma | 5 | 5 | 0 | 0.49 |
| FEV1 (% of predicted value) | 86.4 (IQR 71.5–101) | 86 (IQR 56.7–115.2) | 81 (IQR 51–111) | 0.129 |
| Tumour (T) | 0.72 | |||
| T1/T2 | 316 | 286 | 30 | |
| T3/T4 | 63 | 57 | 6 | |
| Nodal status (N) | 0.91 | |||
| N0 | 311 | 288 | 23 | |
| N1/N2 | 68 | 62 | 6 |
COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 s; IQR: interquartile range; VATS: video-assisted thoracic surgery.
Operator and technique
Three senior surgeons performed the majority of operations (488/501, 97%). The most experienced of them performed 279 operations (55.7%).
Lobectomy was the most common procedure performed (91.0%), followed by segmentectomy (7.2%). We found 9 unplanned superior bilobectomies (1.8%) mainly performed due to unexpected extension of the neoplastic disease to the middle lobe during right upper lobectomy. All intraoperative data are presented in Table 2.
Table 2:
Type of procedure performed and perioperative conversion
| Lung resection | Number | % | Conversion | % |
|---|---|---|---|---|
| Right upper | 144 | 28.7 | 20 | 13.8 |
| Right lower | 78 | 15.6 | 3 | 3.8 |
| Middle | 39 | 7.8 | 4 | 10.2 |
| Left upper | 116 | 23.2 | 8 | 6.9 |
| Left lower | 79 | 15.8 | 8 | 10.1 |
| Segmentectomy | 36 | 7.2 | 2 | 5.6 |
| Bilobectomy | 9 | 1.8 | 0 | 0 |
| Total | 501 | 45 | 8.9 |
The final pathological diagnosis was cancer in 445 patients (88.8%), infectious pathology in 26 patients (5.2%, including 10 cases of tuberculosis), degenerative disease in 19 patients (3.8%), benign disease in 10 patients (2%) and congenital diseases in 3 patients (lung sequestrations, 0.6%).
The distribution of patients according to the TNM classification is also available (Table 3). The histopathological findings for patients with cancer are presented in Supplementary Material, Table S5.
Table 3:
Distribution of patients by TNM stage (primary lung cancer)
| Cancer stage | Patients, n (%) |
|---|---|
| IA | 187 (48.3) |
| IB | 74 (19.1) |
| IIA | 39 (10.1) |
| IIB | 44 (11.4) |
| IIIA | 40 (10.3) |
| IIIB | 0 (0) |
| IV | 3 (0.8) |
| Total | 387 |
TNM: tumour, node and metastasis.
Number of video-assisted thoracic surgery and conversion rate
As expected with a new technique, the number of VATS procedures performed each year increased gradually. During the first year, 66 VATS procedures were performed by 2 surgeons, and during the last year, 134 VATS procedures (+103%) were performed by 4 surgeons.
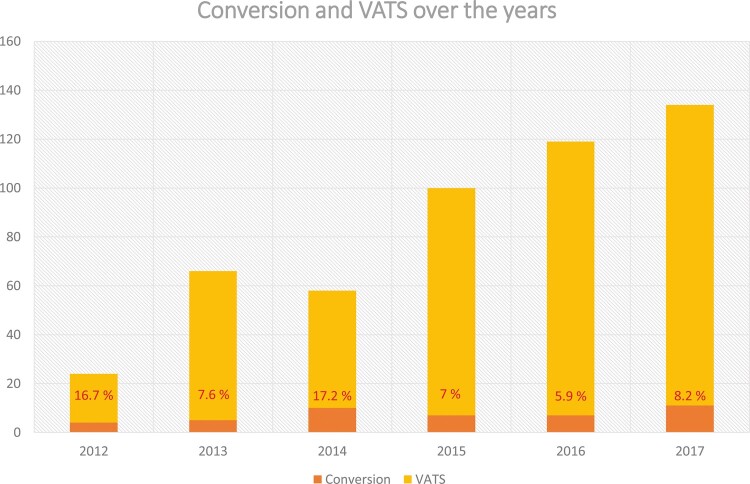
While the global conversion rate was 8.8% (44 patients, Fig. 1), there was a peak in the second year culminating at 17.2%, followed by a steep decrease down to 6.7%. There was a slight increase over the last year of study to 8.2%. This decrease over the years was not statistically significant (P = 0.194, χ2 for trend).
Figure 1:
Evolution of the conversion rate over the years. VATS: video-assisted thoracic surgery.
Conversion causes
We used the classification previously described to sort the causes of conversion [14]; 13/44 (30%) were due to vascular causes (pulmonary artery, pulmonary vein or other vessel wounds), 16/44 (37%) to anatomy (adhesions, tumour size or location), 9/44 (20%) to lymph nodes (bulky, sticky, calcified) and 6/44 (13%) to technical failure (stapler misfire, equipment failure). It should be noted that 7 out of 13 conversions (53.9%) for vascular causes were performed under emergency conditions: 4 were related to pulmonary artery injury, and 3 were related to vein injury. The amount of intraoperative blood loss ranged from 200 to 2500 ml. There were no reports of the need to clamp the central pulmonary artery during conversion.
Perioperative events
Main judgement criteria
We compared the perioperative complications in patients who underwent complete VATS with those in patients who required conversion to thoracotomy. We observed 18/44 (40.9%) complications in the conversion group and 77/457 (16.8%) in the VATS group [P = 0.0001, OR 3.41 confidence interval (CI) 95% 1.67–6.82].
According to the Clavien–Dindo classification, we observed that converted patients had more serious complications than non-converted patients (P = 0.0001, Fig. 2). Patients who underwent conversion to thoracotomy had a priori more serious complications than patients who underwent VATS.
Figure 2:
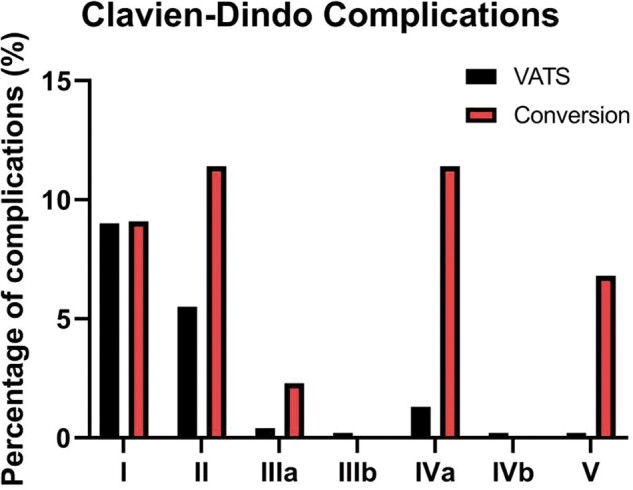
Distribution of complications according to the Clavien–Dindo classification, according to conversion status. Grade 1: any deviation from the normal postoperative course, grade 2: normal course altered, grade 3: complication that required interventions of various degrees (a: under local anaesthesia, b: general or epidural anaesthesia), grade 4: complications threatening the life of the patients (a: single-organ dysfunction, b: multi-organ dysfunction), grade 5: death of a patient. VATS: video-assisted thoracic surgery.
Indeed, ∼9% (41 non-converted and 4 converted patients) of patients in both groups had a type 1 complication according to the Clavien–Dindo classification. However, only 6 (1.3%) non-converted patients had a type 4a complication, while 5 (11.4%) converted patients had 1 complication. Similarly, according to the Clavien–Dindo classification, type 2 complications were found in 25 (5.5%) non-converted patients compared to 5 (11.4%) converted patients.
We identified 18 patients (3.6%) with complete VATS lasting more than 4 h (mean: 295 min, SD = 56), of whom 7 had complications (38.9%), and there was no difference compared to converted patients (P = 0.6).
Late conversion occurred in 21 patients, with 10 complications (47.6%), and early conversion occurred in 23 patients, with 7 complications (30.4%), but this result was not significant (P = 0.24).
Secondary judgement criteria
We focused on the different types of complications observed. PAF was observed in 8 of 44 patients (18.2%) in the conversion group versus 19 of 457 patients (4.2%) in the VATS group, and this difference was statistically significant (OR 5.09, 95% CI 1.80–13.27; P = 0.001). Postoperative pneumonia occurred in 5 out of 44 (11.4%) converted patients compared to 9/457 (2%) patients who underwent complete VATS (OR 6.34, 95% CI 1.59–22.31; P = 0.001). Acute respiratory failure syndrome was significantly more frequently observed in 4/44 patients (9.1%) in the conversion group than in 8/457 patients (1.8%) in the VATS group (OR 5.58, 95% CI 1.18–21.94; P = 0.02). Finally, we compared the duration of air leaks, but there was no significant difference, with 5/44 (11.4%) converted patients compared to 41/457 (8.9%) complete VATS patients (P = 0.56).
The length of stay was also longer in the conversion group than in the VATS group (median: 11 vs 7 days, respectively, P = 0.001). The median visual analogue pain scale value on D1 was 3 (IQR 2–5) in the VATS group and 5 (IQR 3.25–7) in the conversion group (P < 0.0001).
Finally, we also analysed perioperative mortality data: 3/44 (6.8%) patients in the conversion group and 1/457 (0.2%) patients in the VATS group died (OR 33.3, 95% CI 3.4–328; P = 0.003). These data are summarized in Table 4.
Table 4:
Perioperative complications and secondary outcomes
| Variable | Population (N = 501), n (%) | VATS (N = 457), n (%) | Converted (N = 44), n (%) | P-value |
|---|---|---|---|---|
| Perioperative complications | 95 (18.9) | 77 (16.8) | 18 (40.9) | 0.0001 |
| PAF | 27 (4.2) | 19 (4.2) | 8 (18.2) | 0.001 |
| Pneumonia | 14 (2.8) | 9 (2) | 5 (11.4) | 0.001 |
| ARDS | 12 (2.4) | 8 (1.8) | 4 (9.1) | 0.02 |
| Air leak | 46 (9.2) | 41 (9) | 5 (11.4) | 0.56 |
| Length of stay | 7 | 7 | 11 | 0.001 |
| VAS at D1 | 4 | 3 | 5 | 0.0001 |
| Perioperative mortality | 4 (0.8) | 1 (0.2) | 3 (6.8) | 0.003 |
ARDS: acute respiratory failure syndrome; PAF: postoperative atrial fibrillation; VAS: visual analogue scale; VATS: video-assisted thoracic surgery.
Conversion risk factors
We analysed the preoperative data to suggest or identify the risk factors for conversion.
There was no sex difference in our study (8.41% vs 9.06%, P = 0.80). However, younger patients experienced conversion marginally less frequently [median age of 65 years (IQR 51.75–78.25) vs 67 years (IQR 54–80) in the conversion group] (P = 0.06).
There was no significant difference in COPD, asthma, overlap syndrome or pulmonary embolism between the 2 groups (Table 1).
There was no difference between smokers and non-smokers (P = 0.74).
More surprisingly, we observed no increase in conversion when the T or N extension status increased. Indeed, there was no difference in tumour size between the converted and non-converted groups (T1–T2 vs T3–T4; 9.5% vs 8.3%, respectively, P = 0.72), nor was there any difference in the lymph node extension status (N0 vs N1–N2; 9.26% vs 8.8%, respectively, P = 0.91).
In our study, the preoperative FEV1 was not predictive of conversion (P = 0.718).
In this study, there was no difference between VATS resection due to cancer or VATS resection due to a benign cause (9.0% and 7.1%, respectively, P = 0.65).
Surgeon's experience and conversion
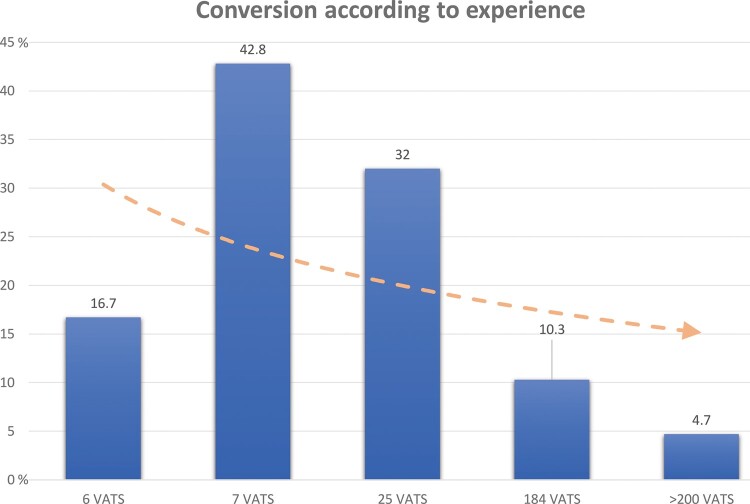
The analysis of the data concerning the operator showed that his or her volume (or experience) of VATS seems to have an influence on his or her conversion percentage. Indeed, we observed a decreasing trend in the conversion rate based on the surgeons' experience. However, due to a small number of interventions for some operators, we were not able to do statistical analyses.
The results are presented in Fig. 3 and Table 5.
Figure 3:
Conversion rate in VATS lung resection according to each operator's experience in 1 centre. The pink arrow is a logarithmic representation of the decrease in conversion rate. VATS: video-assisted thoracic surgery.
Table 5:
Conversion rate according to the operator's experience in videothoracoscopy
| Operator | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|
| VATS, n (%) | 5 (83.3) | 4 (57.2) | 17 (68) | 165 (89.7) | 266 (95.3) | 457 |
| Converted, n (%) | 1 (16.7) | 3 (42.8) | 8 (32) | 19 (10.3) | 13 (4.7) | 44 |
| Total | 6 | 7 | 25 | 184 | 279 | 501 |
VATS: video-assisted thoracic surgery.
Multivariable analysis
In the multivariable analysis model for conversion, we included age, year of procedure, FEV1, COPD, gender, pulmonary disease and obesity. After backward variable selection, there was no variable to keep in our model (P > 0.157).
In the multivariable analysis model for perioperative complications, we included FEV1, age, COPD, year of procedure, conversion, pulmonary disease, obesity and gender (Supplementary Material, Table S6). After backward variable selection, we kept 4 variables in our multivariable model. All 4 variables were statistically associated with perioperative complications: age (OR 1.02, 95% CI 1.00–1.05; P = 0.034), year of procedure (OR 1.56, 95% CI 1.3–1.86; P = 0.0001), conversion (OR 4.47, 95% CI 2.21–9.05; P = 0.0001) and obesity (OR 0.34, 95% CI 0.15–0.75; P = 0.008) (Supplementary Material, Table S7).
DISCUSSION
The main objective of this study was to describe the specific characteristics of a population of patients who underwent conversion from VATS to thoracotomy. Indeed, over the past 2 decades, VATS has been established as a modern and safe and surgical approach with limited pain. Eventually, a specific population appeared that was expected to experience short-term hospitalization, painless surgery and some scarring but that did not benefit from surgery; these patients underwent conversion to thoracotomy.
In the early 2000s, many studies evaluated the conversion rate and the various conversion risk factors. However, only a few focused on the complication rate in this population and found a rate ranging from 34.2% to 46% [18, 19].
To the best of our knowledge, the largest cohort of 1227 selected patients compared 604 patients who benefitted from VATS lung resection to 623 patients undergoing open thoracotomy and was published by Puri et al. [18]; however, this study compared patients with VATS resection with the same number of patients with planned thoracotomy. The conversion rate and complication rate in the converted patient subgroup were comparable to those in our study.
However, we consider the comparison between VATS conversion and planned thoracotomy as unfair. First, the anaesthetic management is not the same for planned thoracotomy and VATS, which implies more pain in the immediate postoperative days for converted patients. Second, emergency thoracotomy deteriorates respiratory function more significantly due to muscle sectioning and costal spacing. Third, conversion can be experienced as a psychological trauma in patients who are not expecting it. Furthermore, in our centre, it is estimated that 60–70% of major lung resections are performed by VATS. The planned thoracotomies mostly concern large tumour adhering to chest wall or vascular/bronchial structures.
In other studies, authors found that patients who underwent conversion during VATS had more PAF than patients with planned thoracotomy. In a meta-analysis that included more than 3000 patients in a VATS group and planned thoracotomy group, the prevalence of PAF was 19% in the conversion group compared to 9% in the planned thoracotomy group [20]. Our results, with an 18.2% prevalence of PAF in the converted group, were consistent with the results of this study, and we believe that this complication explains in part the prolonged duration of hospital stay.
To date, many factors have been described to be involved in the occurrence of atrial fibrillation after thoracic surgery. In the case of conversion from VATS to thoracotomy, with respect to our data, we hypothesized that the pain (and the resulting tachycardia) that was significantly higher in converted patients than in non-converted patients could partly explain the difference observed. Indeed, anaesthetic conditioning is different for planned VATS and planned thoracotomy: patients who require conversion therefore do not have epidural analgesia and are not ‘prepared’ for thoracotomy pain.
We can also compare the results of Whitson's study in terms of the incidence of pneumonia. Indeed, we observed ∼2% incidence of postoperative pneumonia in our VATS group and 11% in converted patients. In Whitson's study, the authors found a 2.7% incidence of pneumonia after VATS, a higher percentage that we can explain by the population studied (any VATS without distinguishing converted from non-converted patients). The incidence of pneumonia after open thoracotomy in this meta-analysis was 6%, which is consistent with our initial hypothesis: the population of patients who undergo conversion from VATS to thoracotomy is at higher risk (11.4% in our study) than the population of patients who undergo planned thoracotomy. In our multivariable model analysis, conversion during VATS remained strongly associated with perioperative complications (OR = 4.47). Interestingly, obesity could also be highlighted as an independent negative predictor of perioperative complication (OR = 0.34); however, this analysis was based on limited data (n = 8).
A recent study also showed that a prolonged intervention (>150 min) would increase mortality within 3 months postoperatively [21]. In our study, we did not observe any statistically significant difference in terms of complications between non-converted patients who underwent surgery for more than 4 h (38.9%) and converted patients (40.9%). It therefore seems difficult to conclude that conversion is necessary to shorten VATS.
Similarly, we did not observe any difference between complications in patients who underwent early conversion and those who underwent late conversion. We therefore believe that the loss of opportunity comes from the conversion often carried out under emergency conditions and not from the moment of occurrence.
We wanted to update the conversion rate of VATS to thoracotomy for several reasons: 1 study described the learning curve of a thoracic surgeon during VATS [17], but at least 2 factors can explain the variation in the conversion rate over the years. First, at the beginning of our VATS experience, only 2 thoracic surgeons started VATS, and the conversion rate decreased in subsequent years, but less experienced surgeons started VATS later with higher conversion rates, which moderated the overall decrease in the conversion rate. Second, surgeons started by selecting favourable cases, most often T1 or T2, without lymph node invasion, and then gradually expanded the criteria for VATS. Overall, regarding the conversion rate, we can conclude that the experience accumulated by the surgical team allows us to reduce the conversion rate. Indeed, over a 5-year study period, we see that the conversion rate has been more than halved (16.7–8.2%). Several recent developments are likely to lower the conversion rate as well as the complication rate: preoperative planning, which makes it easier to understand complex segmentectomies with VATS, and postoperative rehabilitation, which reduces postoperative complications by preparing the patient and speeding up the return home.
Limitations
One limitations of our study is its retrospective design, which increases the risk of selection bias but, more importantly, classification bias, as we focused more on the records of converted patients than those of non-converted patients in search of a perioperative complication, for example. Another limitation is the single institution study design, which makes the results more complicated to generalize.
CONCLUSION
In conclusion regarding the limitations, we must quote the recent and very interesting article by Fourdrain et al. [22], who found essentially the same results in terms of cardiac and respiratory complications in patients who required conversion. However, in this article, the authors compared the converted population to a population of patients who underwent planned thoracotomy and only oncological surgeries: the authors then observed no significant difference in postoperative mortality, either at 30 days or at 90 days.
The strength of our study was the limitation of the problem of VATS attempts, without including the thoracotomy group planned from the outset to artificially increase its power. This study allowed us to update the rate of conversion to thoracotomy during videothoracoscopy for lung resection in order to highlight the high rate of global and specific complications (PAF, pneumonia) as well as perioperative mortality. Finally, it allowed us to observe the important reduction of the conversion rate over the years, according to the experience of the surgical team.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Supplementary Material
ABBREVIATIONS
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- FEV1
Forced expiratory volume in 1 s
- IQR
Interquartile range
- OR
Odds ratio
- PAF
Postoperative atrial fibrillation
- SD
Standard deviation
- TNM
Tumour, node and metastasis
- VATS
Video-assisted thoracic surgery
Author contributions
Joseph Seitlinger: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Writing—original draft; Writing—review & editing. Anne Olland: Methodology; Writing—original draft; Writing—review & editing. Sophie Guinard: Data curation; Investigation. Gilbert Massard: Conceptualization; Supervision; Validation; Writing—original draft. Pierre-Emmanuel Falcoz: Conceptualization; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Tanel Laisaar and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
REFERENCES
- 1. Hazelrigg SR, Nunchuck SK, LoCicero J. Video assisted thoracic surgery study group data. Ann Thorac Surg 1993;56:1039–43; discussion 1043–4. [DOI] [PubMed] [Google Scholar]
- 2. Roviaro G, Rebuffat C, Varoli F, Vergani C, Mariani C, Maciocco M. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244–7. [PubMed] [Google Scholar]
- 3. Walker WS, Carnochan FM, Tin M. Thoracoscopy assisted pulmonary lobectomy. Thorax 1993;48:921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Detterbeck F, Molins L. Video-assisted thoracic surgery and open chest surgery in lung cancer treatment: present and future. J Vis Surg 2016;2:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falcoz P-E, Puyraveau M, Thomas P-A, Decaluwe H, Hürtgen M, Petersen RH et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhang W, Wei Y, Jiang H, Xu J, Yu D. Thoracotomy is better than thoracoscopic lobectomy in the lymph node dissection of lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2016;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higuchi M, Yaginuma H, Yonechi A, Kanno R, Ohishi A, Suzuki H et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg 2014;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Long H, Tan Q, Luo Q, Wang Z, Jiang G, Situ D et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg 2018;105:386–92. [DOI] [PubMed] [Google Scholar]
- 9. Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957–63. [DOI] [PubMed] [Google Scholar]
- 10. Cheng D, Downey RJ, Kernstine K, Stanbridge R, Shennib H, Wolf R et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261–92. [DOI] [PubMed] [Google Scholar]
- 11. Paul S, Sedrakyan A, Chiu Y-L, Nasar A, Port JL, Lee PC et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813–17. [DOI] [PubMed] [Google Scholar]
- 12. Madani A, Fiore JF, Wang Y, Bejjani J, Sivakumaran L, Mata J et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899–908; discussion 908–10. [DOI] [PubMed] [Google Scholar]
- 13. Gonfiotti A, Viggiano D, Voltolini L, Bertani A, Bertolaccini L, Crisci R et al. Enhanced recovery after surgery and video-assisted thoracic surgery lobectomy: the Italian VATS group surgical protocol. J Thorac Dis 2018;10:S564–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gazala S, Hunt I, Valji A, Stewart K, Bédard ER. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact CardioVasc Thorac Surg 2011;12:962–4. [DOI] [PubMed] [Google Scholar]
- 15. Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther 2009;9:413–23. [DOI] [PubMed] [Google Scholar]
- 16. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazzella A, Olland A, Falcoz PE, Renaud S, Santelmo N, Massard G. Video-assisted thoracoscopic lobectomy: which is the learning curve of an experienced consultant? J Thorac Dis 2016;8:2444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puri V, Patel A, Majumder K, Bell JM, Crabtree TD, Krupnick AS et al. Intraoperative conversion from video-assisted thoracoscopic surgery lobectomy to open thoracotomy: a study of causes and implications. J Thorac Cardiovasc Surg 2015;149:55–62.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim S-W, Hong J-M, Kim D. What is difficult about doing video-assisted thoracic surgery (VATS)? A retrospective study comparing VATS anatomical resection and conversion to thoracotomy for lung cancer in a university-based hospital. J Thorac Dis 2017;9:3825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008–16; discussion 2016–8. [DOI] [PubMed] [Google Scholar]
- 21. Brunelli A, Dinesh P, Woodcock-Shaw J, Littlechild D, Pompili C. Ninety-day mortality after video-assisted thoracoscopic lobectomy: incidence and risk factors. Ann Thorac Surg 2017;104:1020–6. [DOI] [PubMed] [Google Scholar]
- 22. Fourdrain A, De Dominicis F, Iquille J, Lafitte S, Merlusca G, Witte-Pfister A et al. Intraoperative conversion during video-assisted thoracoscopy does not constitute a treatment failure. Eur J Cardiothorac Surg 2019;55:660–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.