Abstract
OBJECTIVES
Bovine and porcine pericardial patches are frequently used in cardiothoracic and vascular surgery. There are no guidelines recommending the usage of these patches for particular surgical approaches. However, these 2 materials supposedly possess different properties. The clinical advantage of porcine compared with bovine patches remains controversial. In this experimental study, we analysed the incorporation and vascularization of bovine and porcine pericardial patches during the initial phase after implantation.
METHODS
Bovine and porcine pericardial patches were implanted into the dorsal skinfold chamber of C57BL/6 mice (n = 8 per group) to study vascularization and inflammation at the implantation site using repetitive intravital fluorescence microscopy over a 14-day period. At the end of the in vivo experiments, CD-31-positive cells were determined to evaluate the vascularization by immunohistochemistry. Furthermore, cell proliferation and apoptosis were analysed immunohistochemically.
RESULTS
Implanted bovine patches exhibited an enhanced vascularization, as indicated by a significantly higher number of CD-31-positive cells and micro-vessels (23.2 ± 4.3 vs 16.5 ± 5.8 mm−2; P = 0.001). Furthermore, bovine patches showed a slightly but not significantly higher functional capillary density. Both patches induced a moderate leukocytic inflammatory host tissue response, and neither bovine nor porcine patches significantly affected apoptosis and cell proliferation at the implantation site.
CONCLUSIONS
Bovine and porcine pericardial patches are similarly suitable for surgery. Bovine patches exhibited an improved vascularization during the first 14 days after implantation. This may result in a quicker and improved incorporation into the surrounding tissue compared with porcine pericardial patches.
Keywords: Basic science, Animal experiment, Xenogeneic material, Angiogenesis Vascularization, Vascular grafts, Bronchial stump, Valve replacement
INTRODUCTION
Xenogeneic pericardial tissue—most commonly bovine and porcine patches—is widely used in cardiothoracic and vascular surgery [1–4]. Furthermore, bio-prosthesis heart valves are usually fabricated from those materials [5]. Since the introduction of transcatheter aortic valve implantation, the demand for steady xenogeneic pericardium has increased in recent years. Due to the mandatory crimping of the material to pass through the catheter, even exotic materials, i.e. donkey pericardium, have been tested to improve the performance [6, 7]. In addition, these materials are used in vascular surgery [3], as well as for bronchial stump coverage after lobectomy and pneumectomy in thoracic surgery [8]. Furthermore, there are multiple indications in the paediatric cardiac surgery arena. Besides closing holes at the atrial and ventricular level, pericardial patches are used for pulmonary artery plasties, aorta, aortic arch, and descending aorta enlargements and venous bridging [9]. One further application is the use as a vascular graft, whereby the patches have to be knitted firmly to fabricate a tube. In this shape, these grafts are appropriate for vascular substitution, i.e. for replacing the pulmonary arteries in major sleeve resections in oncological thoracic surgery [1, 10]. In recent years, bovine and porcine pericardial patches have been used as alloplastic grafts for the treatment of major prosthetic vascular graft infection [11].
Bovine and porcine pericardia are used with an equal distribution. In ‘real life’, the decision to utilize on or the other type of pericardial patch remains an individual surgeon-based decision. There are no clear guidelines by any scientific society thus far, suggesting the use of porcine or bovine pericardial patches in particular situations or surgical approaches. However, these 2 materials manufactured from the pericardium of 2 different mammals ultimately do not possess the very same properties. Hence, there are clinical and experimental studies comparing bovine and porcine pericardial patches for different application forms. Overall, the superiority of bovine or porcine pericardial is discussed controversially [12–14].
Some authors suggest that bovine pericardium exhibit superior incorporation and biocompatibility, resulting in better clinical long-term performance compared with porcine pericardium [5, 15, 16].
Our aim was to evaluate the quality of the incorporation and biocompatibility of decellularized bovine and porcine pericardial patches, during the initial phase after implantation.
We implanted the patches into the dorsal skinfold chambers (DSC) of C57BL/6 mice.
This animal model allows a detailed analysis of vascularization, inflammation, cell proliferation and apoptosis in the early phase of perigraft tissue formation, using repetitive intravital fluorescence microscopy (IVM) and immunohistochemistry. We hypothesized that bovine pericardial patches show superior properties compared to porcine ones.
METHODS
Animals
Parts of the methods used in this study were previously described by Moussavian et al. [17]. For the study, we used 16 male C57BL/6 mice (8 per group), which were 8–24-week old with a body weight (BW) of 21–25 g (Charles River Laboratories GmbH, Sulzfeld, Germany).
They were housed 1 per cage and had free access to tap water and standard pellet food (Altromin, Lage, Germany). Animal care and experimental transactions were approved by the local governmental animal welfare committee and were conducted in accordance with the German legislation on the protection of animals and the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication # 85-23 Rev 1985). The animal experiments were performed in accordance with the ARRIVE guidelines with the approval number 14-3013 in the Institute for Clinical and Experimental Surgery in the Saarland University in Germany.
A total of 21 animals underwent the preparation of the DSC. 5 had to be excluded from further analysis due to infection of the DSC or due to an ischaemia of the DSC caused by tilting of the back skin of the mouse. In such cases, the mice were sacrificed before the end of the experiment in accordance with the local governmental animal welfare committee.
Preparation of the dorsal skinfold chamber
The in vivo analysis of implanted xenogeneic pericardial patches was performed using the DSC model of the mouse. The preparation of the chamber has been described in detail by others [17–19]. In brief, mice were anaesthetized by an intraperitoneal injection of 75 mg/kg BW ketamine hydrochloride (Ketavest™; Parke Davis, Freiburg, Germany) and 25 mg/kg BW dihydroxylidinothiazine hydrochloride (Rompun™; Bayer, Leverkusen, Germany).
The fur was depilated using an electrical razor and depilatory cream. Two symmetrical titanium frames were implanted on the extended dorsal skinfold of the mice. Using microsurgical instruments, 1 layer of skin including the subcutis with the panniculus carnosus muscle and 2 layers of the retractor muscle were removed within a circular area with a diameter of 15 mm. Finally, the tissue was covered with a cover glass in the observation window, providing access for direct in vivo microscopic analysis of the microcirculation (Fig. 1). After the preparation, the mice were allowed to recover from anaesthesia and surgery for 48 h. The mice tolerated the chamber and its preparation well, as indicated by normal feeding and sleeping habits (Fig. 1).
Figure 1:
(A) C5BL/6 mice after the preparation of the dorsal skinfold chambers. (B) Bovine pericardial patch in 16-fold magnification after the implantation. Scale bar: 2 mm.
Implantation of the pericardial patches
For further analysis, we implanted sterile decellularized bovine and porcine pericardial patches, which were kindly provided by LeMaitre XenoSure® Vascular (bovine) and Vascutek Terumo (porcine). Bovine patches had a 0.55-mm wall thickness whereas the porcine patches had a wall thickness of 0.32 mm. The sterile xenogeneic patches were stored in glutaraldehyde solution. Both patches were soaked for 10 min in saline solution prior to implantation. Thereafter, 2-mm2 pieces were cut for implantation into the DSC. The implantation of the bovine and porvine pericardial patches were performed 48 h after the preparation of the DSC to exclude effects on microcirculatory and inflammatory parameters referable to the surgical trauma the analysis. The animals were anaesthetized using the same protocol as for the initial preparation. Subsequently, the mice were positioned in the right lateral decubital position.
The observation window was reopened by removing the cover glass and a piece of 2 mm2 of either porcine or bovine patch was placed within the centre of the chambers. Then, the observation window was closed again (Fig. 1B).
Intravital fluorescent microscopic analysis
For the IVM, the anaesthetized mice received an intravenous injection into the retrobulbar venous plexus of 0.05 ml of 5% fluorescein isothiocyanate for the visualization of the intravascular space and 0.05 ml of 1% rhodamine 6G for the in vivo analysis of leucocyte–endothelial cell interaction (Sigma-Aldrich, Taufkirchen, Germany). Subsequently, the mice were positioned under an epi-illumination Zeiss Axiotech microscope (Zeiss, Oberkochen, Germany).
For the quantitative analysis of the microscopic images, we used the software CapImage (version 7.5; Zeintl, Heidelberg, Germany). Therefore, eight 0.4-mm2 microvascular regions of interest (ROIs) within the surrounding tissue at the margin of the patch were defined,for the measurement of microhemodynamics. The neovascularization of the ROIs was determined by measuring the functional capillary density, which was defined as the length of the newly formed micro-vessels per ROI in cm/cm2 [17] (Fig. 2).
Figure 2:

Standardized evaluation of the intravital fluorescence microscopy. (A) Region of interests of angiogenesis (red squares) and leucocyte-endothelial interaction (black squares) within the dorsal skinfold chambers. (B) Illustration of angiogenic sprouts by epi-illumination blue-light microscopy. (C) Illustration of leucocyte-endothelial interaction by green light epi-illumination microscopy. Scale bars: (A) = 2 mm; (B) = 200 µm; (C) = 125 µm.
Four supplementary ROIs were analysed in the proximity to the implanted material to determine the interaction of leukocytes with the microvascular endothelium. Leukocytes were classified as adherent and rolling cells depending on their interaction with the endothelium [17, 18] (Fig. 2).
Experimental protocol
Porcine (n = 8) and bovine (n = 8) pericardial patches were implanted into the DSC of 16 C57BL/6 mice. IVM measurements of angiogenesis, leucocyte-endothelial cell interaction and microhemodynamics were performed on days 3, 6, 10 and 14 after implantation. The use of the dorsal skinfold chamber model is limited to a period of 2–3 weeks because the elasticity of the dorsal skin decreases over time. This can lead to a tilting of the chamber, which can substantially affect the perfusion of the prepared tissue [18]. At the end of the in vivo studies, the anaesthetized animals were sacrificed by cervical dislocation. Subsequently, the DSC including the implants were carefully harvested and stored in formalin until further histological and immunohistochemical analyses.
Immunohistochemical analysis
Formalin-fixed specimens were embedded in paraffin and cut into 3-μm-thick sections. Immunohistochemical detection of CD-31-positive micro-vessels was conducted using a monoclonal rat anti-mouse antibody against CD-31 to detect endothelial cells. This was followed by a goat anti-rat Cy3 antibody as a secondary antibody (1:30; Dianova GmbH, Hamburg, Germany). Given that CD-31 is expressed by vascular endothelial cells, CD-31 staining is suitable to detect neovascularization in the tissue surrounding the pericardial patches [20].
Immunohistochemical detection of Ki-67-positive proliferating cells, myeloperoxidase (MPO)-positive neutrophils and cleaved cystein—aspartic acid protease 3 (caspase-3)-positive apoptotic cells was performed with avidin–biotin complex immunohistochemistry (1:100; Abcam, Cambridge, UK). For this purpose, sections were stained with a polyclonal rabbit anti-MPO antibody, a polyclonal rabbit anti-KI-67 antibody and a polyclonal rabbit anti-casp-3 antibody followed by a goat anti-rabbit immunoglobulin G biotin antibody, which was then incubated with peroxidase-labelled avidin.
MPO is primarily expressed by neutrophil granulocytes. Therefore, MPO staining is suitable for analysing inflammation and leucocyte interaction in the tissue surrounding the pericardial patch. The expression of the Ki-67 protein is fundamentally associated with cell proliferation. Ki-67 is present during all active phases of the cell cycle but is absent from resting cells [21]. Therefore, KI-67 staining is ideal for analysing the proliferation of cells surrounding the pericardial patch. Caspase-3 is a protease that plays a major role in the execution phase of cell apoptosis. The caspase-3 staining was therefore used to analyse activated caspase-3 apoptotic cells in the tissue surrounding the pericardial patches. The sections were counterstained with Meier hemalum and examined under a BX60 Olympus microscope. Quantitative analyses of the density of CD-31-positive cells and micro-vessels (mm−2), the number of MPO-positive neutrophils (mm−2) and the fraction of Ki-67-positive proliferating cells and caspase-3-positive apoptotic cells (%) in the newly formed granulation tissue adjacent to the implants were performed.
Statistical analysis
Parameters were expressed as mean ± standard deviation of the mean and were analysed by Student’s t-test. Statistical significance was accepted for a P-value of <0.05. Statistical analysis was performed using the SPSS statistical software package (Version 26; IBM, Armonk, NY, USA). Graphics were drawn using Microsoft Excel software.
RESULTS
Vascularization of porcine and bovine patches
IVM measurements revealed that both patches induced a formation of microvascular sprouts originating from host capillaries at the margin of the patches at day 3 after implantation. During the following period until day 14, these micro-vessels increasingly grew and eventually evolved into new microvascular networks.
Overall, bovine patches compared with porcine patches tended to exhibit a slightly higher angiogenesis and vascularization, as indicated by the functional capillary density. However, this difference did not prove to be statistically significant. [day 3:5.4 ± 2.2 vs 5.8 ± 2.0 (cm/cm2), P = 0.74; day 6:9.2 ± 3.6 vs 9.0 ± 2.8 (cm/cm2), P = 0.81; day 10:13.3 ± 6.0 vs 11.9 ± 3.6 (cm/cm2), P = 0.63; day 14:16.1 ± 4.9 vs 14.7 ± 4.5 (cm/cm2), P = 0.58) (Fig. 3).
Figure 3:
Intravital fluorescence microscopy images of porcine (A) and bovine (B) pericardial patches at day 14 after implantation into dorsal skinfold chambers. *: patches; newly formed micro-vessels highlighted by arrows. (C) Functional capillary density (cm/cm2) adjacent to porcine (white bars; n = 8) and bovine patches (grey bars; n = 8), assessed using intravital fluorescence microscopy and computer-assisted image analysis. Mean ± standard deviation of the mean. Scale bars = 10 µm.
Moreover, we performed additional immunohistochemical analyses. Thereby, we detected a significantly increased density of CD31-positive micro-vessels in the granulation tissue surrounding the bovine patches compared with the porcine patches (23.2 ± 4.3 vs 16.5 ± 5.8 mm−2; P = 0.001) (Fig. 4).
Figure 4:
Immunohistochemical cross-sections at the margin of a porcine (A) and a bovine (B) pericardial patch at day 14 after implantation in the dorsal skinfold chambers of C57BL/6 mice. *: patches, note the higher micro-vessel density at the margin of the bovine compared (B) with the porcine (A) pericardial patch. (C) Micro-vessel density (mm−2) at the margin of porcine (white bar; n = 8) and bovine grafts (grey bar; n = 8) at day 14 after implantation. Mean ± standard deviation of the mean. *P = 0.001 versus porcine. Scale bars = 75 µm.
Inflammatory response
Measurements of rolling and adherent leukocytes were performed using IVM during the observation period of the experiments. We demonstrate that the numbers of rolling and adherent leukocytes in postcapillary and collecting venules ranged during the observation period between 4.2 and 7.4 leukocytes min−1 and 90 and 400 leukocytes mm−2. There were no significant differences between the 2 groups (Fig. 5). Furthermore, we performed additional MPO immunohistochemical analyses of the inflammatory response. Again, there was no significant difference in the number of MPO-positive neutrophils (395.8 ± 108.8 vs 379.3 ± 54.4 mm−2; P = 0.24) (Fig. 6).
Figure 5:
Intravital fluorescence microscopy images of porcine (A) and bovine (B) pericardial patches at day 14 after implantation into dorsal skinfold chambers. Adherent and rolling leukocytes in postcapillary and collecting venules within the margin of the patches highlighted with arrows. (C) Rolling leukocytes (mm−1) and (D) adherent leukocytes (mm−2) in porcine (white bars; n = 8) and bovine pericardial patches (grey bars; n = 8), assessed using intravital fluorescence microscopy and computer-assisted image analysis. Mean ± standard deviation of the mean. Adherent leukocytes: day 0: 392.6 ± 95.9 vs 337.3 ± 60.9 P = 0.55; day 3: 296.5 ± 119 vs 258.9 ± 74.4 P = 0.72; day 6: 282.4 ± 84.9 vs 362.2 ± 74.2 P = 0.14; day 10: 187.9 ± 80.3 vs 272.7 ± 90.4 P = 0.07; day 14: 88.9 ± 68.6 vs 118 ± 90.1 P = 0.07; rolling leukocytes: day 0: 4.2 ± 2.0 vs 5.3 ± 1.9 P = 0.42; day 3: 5.6 ± 1.7 vs 7.1 ± 2.4 P = 0.69; day 6: 6.5 ± 3.3 vs 7.4 ± 4.0 P = 0.71; day 10: 5.5 ± 1.6 vs 6.6 ± 1.0 P = 0.36; day 14: 5.1 ± 2.1 vs 6.1 ± 1.6 P = 0.13. Scale bars = 250 µm.
Figure 6:
Immunohistochemical cross-sections at the margin of a porcine (A) and a bovine (B) pericardial patch at day 14 after implantation into the dorsal skinfold chambers of C57BL/6 mice. (C) Myeloperoxidase-positive neutrophils (mm−2) at the margin of porcine (white bars; n = 8) and bovine pericardial patches (grey bars; n = 8) at day 14 after implantation. Mean ± standard deviation of the mean. Scale bars = 85 µm.
Proliferation and apoptosis of cells
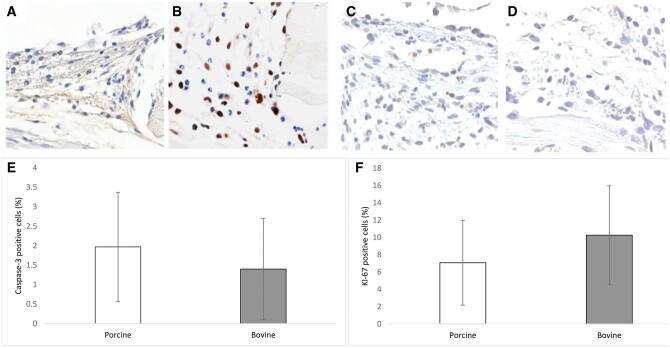
Moreover, we measured cell-proliferating activity and apoptotic cell death within the DSC. There was no significant difference in the number of Ki-67-proliferating cells in the granulation tissue surrounding the bovine patches compared with porcine patches (10.3 ± 5.7% vs 7.1 ± 4.9%; P = 0.3; Fig. 7). Furthermore, bovine pericardial patches resulted in a slightly lower number of casp-3-positive cells in the perigraft tissue with no significant differences (1.4 ± 1.3% vs 1.9 ± 1.6%; P = 0.58; Fig. 7).
Figure 7:
Immunohistochemical cross-sections at the margin of a porcine (A and C) and a bovine (B and D) pericardial patch at day 14 after implantation into dorsal skinfold chambers of C57BL/6 mice. (A and B) Ki-67-positive cells and (C and D) casp-3-positive cells. (E and F) Ki-67-positive cells (%) and casp-3-positive cells (%) in the margin of bovine (white bars; n = 8) and porcine (grey bars; n = 8) at day 14 after implantation. Mean ± standard deviation of the mean. Scale bars = 250 µm.
DISCUSSION
Xenogeneic pericardial patches are widely used in modern medicine with vastly different applications, ranging from the use as material for vascular patches [9, 10], vascular grafts [1, 11] and eventually biological valve prosthesis, in conventional replacements and for transcatheter aortic valve implantation [2, 5]. There have been many experimental and clinical studies comparing the long-term patency, durability and calcification of porcine and bovine pericardia [1, 12, 16], although the majority of such articles have concluded that bovine pericardium develops less calcification and subsequently shows fewer signs of degeneration compared with porcine pericardium [4, 5, 10]. The different incorporation of bovine and porcine decellularized pericardial patches might also be explained by the progress of decellularization itself. This particular progress is based on osmotic shock and nuclease treatment, which results in an effective cell removal and a preservation of the extracellular matrix. Although biomechanical characteristics outlive the decellularization progress, there are differences in the water content, thickness and glycosaminoglycans, which might influence their biomechanical properties and their incorporation and early host tissue response [22].
In this context, we demonstrate for the first time that the vascularization and incorporation of bovine pericardial patches are superior during the initial 14 days after implantation compared with porcine patches. For our study, we used the DSC model, which has already been proven suitable for testing the in vivo performance of prosthetic material [17, 19, 23, 24]. By using the IVM, repetitive analysis of early inflammation, vascularization and host tissue response during 14 days after implantation was possible [18]. Overall, bovine patches showed a higher functional capillary density compared with porcine patches, albeit with no statistical significance throughout the observation period. Interestingly, the general functional capillary density was significantly lower compared with studies using Dacron grafts in the DSC model [17, 23, 24]. Dacron is a synthetic material with a cavernous surface, which is adequate for the incorporation of micro-vessels. This fast incorporation is particularly important for synthetic materials due to the higher thrombogenic potential compared with biological materials [1, 10]. Furthermore, Khorramirouz et al. [25] analysed the regeneration of tissue-engineered transcatheter valve scaffold in rats, and they showed a similar number of CD-31-positive cells for acellular porcine valves after 7 days and after 4 weeks. This stands in line with our findings after 14 days for porcine pericardial patches. Khorramirouz et al. postulated that a higher expression of CD-31 may increase the recruitment of angiogenic factors, leading to a quicker re-endothelialization.
We demonstrated a significantly higher number of CD-31-positive micro-vessels in the bovine group compared with the porcine group. There is probably an advantage for the incorporation of bovine pericardium [26], especially in vascular substitution surgery, e.g. of the pulmonary artery [1, 4]. We also assessed the numbers of rolling and adherent leukocytes. Ultimately, both pericardial patches did not induce a severe inflammation in the DSC. Apparently, severe inflammation is associated with massively increased numbers of rolling leukocytes of >30 min−1 and adherent leukocytes of >700 mm−2 [27]. In addition, the number of MPO-positive neutrophils did not differ between the groups.
Our measurements indicate that both pericardial patches only induce a slight unspecific foreign body reaction. These findings are in line with recent studies about immune response to xenogeneic pericardium [13, 26, 28, 29]. Our results indicate a low number of KI-67-positive angiogenic cells and a low number of caspase-3-positive apoptotic cells compared with studies using alloplastic materials of even bonded grafts. This leads to the conclusion that there is probably no relevant xenograft rejection of the surrounding tissue [17, 19, 23, 24, 27]. Glutaraldehyde-treated xenogeneic materials have some major advantages compared with synthetic fabrics. Usually xenogeneic pericardium is less thrombogenic, induces less inflammation and has a lower frequency of major graft infection [4, 10, 11]. A major disadvantage of xenogeneic material is the tendency for degeneration and calcification. This is a minor problem in vascular and thoracic surgery [1, 4, 10], but a major problem for xenograft bioprosthetic heart valves with an endurance of only ∼15 years [5, 12, 15, 30]. In terms of extending the persistence of the pericardium, many new materials [7, 30] and complex applications like cross-linked patches [13, 25] have been investigated in recent years.
Limitations
This present study is an animal model with mice. Most importantly there are limitations in the transferability. Eventually the implantation of a pericardial patch into the DSC of mice is different to the implantation of a pericardial patch into the human vascular system. Furthermore, this animal experiment is limited to 2–3 weeks because the elasticity of the DSC decreases over time. This can lead to a tilting of the chamber, which can substantially affect the perfusion of the prepared tissue. Therefore, we were not able to measure the long-term incorporation in this animal model. Lastly, there might also be a limitation by the small number of the groups and the similar results for both patches. Eventually only a prospective randomized trial in humans would provide the most robust data.
CONCLUSION
The present study has demonstrated that the early perigraft reaction of porcine and bovine pericardial patches were similar, without inducing severe inflammatory side effects or apoptosis. Overall, bovine patches showed a significantly higher number of CD-31 positive cells. This may result in the quicker and improved incorporation of bovine patches into the surrounding tissue compared with porcine pericardial patches.
.
Author contributions
Georg Schlachtenberger: Investigation; Writing—original draft. Fabian Doerr: Writing—review & editing. Annamaria Brezina: Investigation; Methodology. Hruy Menghesha: Writing—review & editing. Matthias B. Heldwein: Writing—review & editing. Gerardus Bennink: Writing—review & editing; Contribution of the part about paediatric cardiac surgery. Michael D. Menger: Methodology; Resources; Writing—review & editing. Mohammed Moussavian: Conceptualization; Data curation; Methodology. Khosro Hekmat: Project administration; Supervision; Writing—review & editing. Thorsten Wahlers: Project administration; Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Claudia Heilmann and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- BW
Body weight
- Caspase-3
Cystein—aspartic acid protease 3
- DSC
Dorsal skinfold chambers
- IVM
Intravital fluorescence microscopy
- MPO
Myeloperoxidase
- ROI
Region of interest
REFERENCES
- 1. D’Andrilli A, Maurizi G, Ciccone AM, Andreetti C, Ibrahim M, Menna C. et al. Long-segment pulmonary artery resection to avoid pneumonectomy: long-term results after prosthetic replacement. Eur J Cardiothorac Surg 2018;53:331–5. [DOI] [PubMed] [Google Scholar]
- 2. Convelbo C, El Hafci H, Petite H, Zegdi R.. Traumatic leaflet injury: comparison of porcine leaflet self-expandable and bovine leaflet balloon-expandable prostheses. Eur J Cardiothorac Surg 2018;53:1062–7. [DOI] [PubMed] [Google Scholar]
- 3. Dorweiler B, Kayser C, Zipp F, Groschel K, Vahl CF.. Long-term performance of the bovine pericardium patch in conventional carotid endarterectomy. Thorac Cardiovasc Surg 2015;63:168–74. [DOI] [PubMed] [Google Scholar]
- 4. Maurizi G, D'Andrilli A, Venuta F, Rendina EA.. Reconstruction of the bronchus and pulmonary artery. J Thorac Dis 2016;8:S168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liao K, Seifter E, Hoffman D, Yellin EL, Frater RW.. Bovine pericardium versus porcine aortic valve: comparison of tissue biological properties as prosthetic valves. Artif Organs 2008;16:361–5. [DOI] [PubMed] [Google Scholar]
- 6. Kim MS, Jeong S, Lim HG, Kim YJ.. Differences in xenoreactive immune response and patterns of calcification of porcine and bovine tissues in alpha-Gal knock-out and wild-type mouse implantation models. Eur J Cardiothorac Surg 2015;48:392–9. [DOI] [PubMed] [Google Scholar]
- 7. Mao J, Rassoli A, Tong Y, Rouse EN, Le-Bel G, How D. et al. Donkey pericardium compares favorably with commercial xenopericardia used in the manufacture of transcatheter heart valves. Artif Organs 2019;43:976–87. [DOI] [PubMed] [Google Scholar]
- 8. Di Maio M, Perrone F, Deschamps C, Rocco G.. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196–200. [DOI] [PubMed] [Google Scholar]
- 9. Bell D, Betts K, Justo R, Forde N, Venugopal P, Corno AF. et al. Multicenter experience with 500 CardioCel implants used for the repair of congenital heart defects. Ann Thorac Surg 2019;108:1883–8. [DOI] [PubMed] [Google Scholar]
- 10. Texakalidis P, Giannopoulos S, Charisis N, Giannopoulos S, Karasavvidis T, Koullias G. et al. A meta-analysis of randomized trials comparing bovine pericardium and other patch materials for carotid endarterectomy. J Vasc Surg 2018;68:1241–56. [DOI] [PubMed] [Google Scholar]
- 11. Lutz B, Reeps C, Biro G, Knappich C, Zimmermann A, Eckstein HH.. Bovine pericardium as new technical option for in situ reconstruction of aortic graft infection. Ann Vasc Surg 2017;41:118–26. [DOI] [PubMed] [Google Scholar]
- 12. Yap KH, Murphy R, Devbhandari M, Venkateswaran R.. Aortic valve replacement: is porcine or bovine valve better? Interact CardioVasc Thorac Surg 2013;16:361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Umashankar PR, Sabareeswaran A, Shenoy SJ.. Long-term healing of mildly cross-linked decellularized bovine pericardial aortic patch. J Biomed Mater Res B Res 2017;105:2145–52. [DOI] [PubMed] [Google Scholar]
- 14. Umashankar PR, Arun T, Kumary TV.. Effect of chronic inflammation and immune response on regeneration induced by decellularized bovine pericardium. J Biomed Mater Res A 2013;101A: 2202–9. [DOI] [PubMed] [Google Scholar]
- 15. Aguiari P, Fiorese M, Iop L, Gerosa G, Bagno A.. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interact CardioVasc Thorac Surg 2016;22:72–84. [DOI] [PubMed] [Google Scholar]
- 16. Gauvin R, Marinov G, Mehri Y, Klein J, Li B, Larouche D. et al. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J Biomater Appl 2013;28:552–65. [DOI] [PubMed] [Google Scholar]
- 17. Moussavian MR, Laschke MW, Schlachtenberger G, von Heesen M, Wagner M, Glanemann M. et al. Perigraft vascularization and incorporation of implanted Dacron prostheses are affected by rifampicin coating. J Vasc Surg 2016;64:1815–24. [DOI] [PubMed] [Google Scholar]
- 18. Laschke MW, Vollmar B, Menger MD.. The dorsal skinfold chamber: window into the dynamic interaction of biomaterials with their surrounding host tissue. Eur Cell Mater 2011;22:147–64. [DOI] [PubMed] [Google Scholar]
- 19. Menger MD, Hammersen F, Walter P, Messmer K.. Neovascularization of prosthetic vascular grafts. Quantitative analysis of angiogenesis and microhemodynamics by means of intravital microscopy. Thorac Cardiovasc Surg 1990;38:139–45. [DOI] [PubMed] [Google Scholar]
- 20. Newman PJ, Berndt MC, Gorski J, White GC 2nd, Lyman S, Paddock C. et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990;247:1219–22. [DOI] [PubMed] [Google Scholar]
- 21. Scholzen T, Gerdes J.. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311–22. [DOI] [PubMed] [Google Scholar]
- 22. Zouhair S, Sasso ED, Tuladhar SR, Fidalgo C, Vedovelli L, Filippi A. et al. A comprehensive comparison of bovine and porcine decellularized pericardia: new insights for surgical applications. Biomolecules 2020;10:371–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeanmonod P, Laschke MW, Gola N, von Heesen M, Glanemann M, Dold S. et al. Silver acetate coating promotes early vascularization of Dacron vascular grafts without inducing host tissue inflammation. J Vasc Surg 2013;58:1637–43. [DOI] [PubMed] [Google Scholar]
- 24. Jeanmonod P, Laschke MW, Gola N, von Heesen M, Glanemann M, Menger MD. et al. Early host tissue response to different types of vascular prostheses coated with silver acetate or vaporized metallic silver. Eur J Vasc Endovasc Surg 2014;47:680–8. [DOI] [PubMed] [Google Scholar]
- 25. Khorramirouz R, Go JL, Noble C, Morse D, Lerman A, Young MD.. In vivo response of acellular porcine pericardial for tissue engineered transcatheter aortic valves. Sci Rep 2019;9:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu X, Han L, Golts E, Baradarian S, Kassab GS.. Homologous and heterologous assessment of a novel biomaterial for venous patch. J Vasc Surg Venous Lymphat Disord 2020;8:458–69 e1. [DOI] [PubMed] [Google Scholar]
- 27. Rucker M, Laschke MW, Junker D, Carvalho C, Schramm A, Mulhaupt R. et al. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials 2006;27:5027–38. [DOI] [PubMed] [Google Scholar]
- 28. Boni L, Chalajour F, Sasaki T, Snyder RL, Boyd WD, Riemer RK. et al. Reconstruction of pulmonary artery with porcine small intestinal submucosa in a lamb surgical model: viability and growth potential. J Thorac Cardiovasc Surg 2012;144:963–9 e1;discussion 69. [DOI] [PubMed] [Google Scholar]
- 29. Wilhelmi M, Giere B, Harder M.. Interaction of cells with decellularized biological materials. Adv Biochem Eng Biotechnol 2012;126:105–16. [DOI] [PubMed] [Google Scholar]
- 30. Manji RA, Lee W, Cooper DKC.. Xenograft bioprosthetic heart valves: past, present and future. Int J Surg 2015;23:280–4. [DOI] [PubMed] [Google Scholar]