Abstract
OBJECTIVES
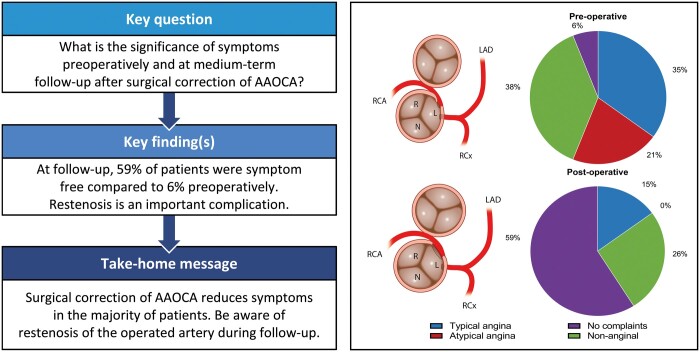
The aim of this study is to describe the significance of symptoms preoperatively and at medium-term follow-up in adolescent and adult patients who underwent surgery of anomalous aortic origin of a coronary artery (AAOCA).
METHODS
Consecutive patients who underwent surgery for AAOCA in our tertiary referral centre between 2001 and 2018 were included. Clinical characteristics and symptoms were evaluated and medium-term outcomes were recorded. Symptoms were classified according to the ‘2019 ESC guidelines on chronic coronary syndromes’.
RESULTS
A total of 53 (55% male) patients with mean age of 44 at time of surgery underwent surgical repair of AAOCA. Data on symptoms and events ˃3 months after surgery were available in 34 patients with a median follow-up of 3 years (interquartile range 1.0–5.3). Preoperatively, only 35% patients had typical anginal complaints. After surgical correction of AAOCA, 59% of the patients were free of symptoms, compared to 6% preoperatively (P < 0.001). A total of 3 (9%) patients needed a reoperation/reintervention related to the operated AAOCA. All 3 patients presented postoperatively with novel typical anginal complaints.
CONCLUSIONS
Adolescent and adult patients with AAOCA present with varying symptoms. Only 35% have typical anginal complaints. Surgical correction of AAOCA reduces the symptoms in the vast majority of patients. One should be aware of potential lesions of the operated coronary artery in patients presenting with typical anginal complaints postoperatively.
Keywords: Coronary anomalies, Medium-term follow-up, Adult congenital surgery, Complications, Symptoms
INTRODUCTION
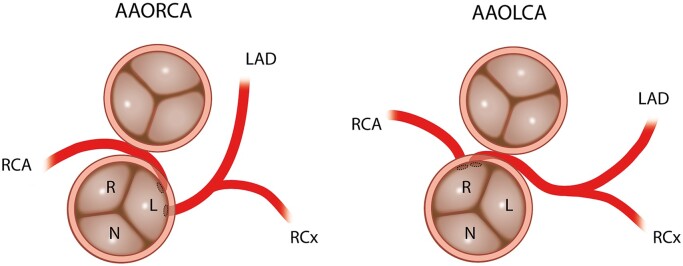
Anomalous aortic origin of the coronary arteries (AAOCAs) is a rare congenital condition with a reported incidence between 0.26% and 1.3%, [1–3]. Anomalous coronary arteries which arise from the opposite sinus of Valsalva or contralateral coronary artery are a potential cause of sudden cardiac death (SCD), especially in athletes and active young adults (Fig. 1) [1]. Presenting symptoms differ largely amongst patients [2, 4, 5]. To date, there is no consensus on indications for surgery versus conservative treatment, especially in middle-aged and older patients. Due to lack of long-term follow-up of patients after surgical treatment, indications for surgical treatment are ambiguous, especially in asymptomatic patients [6–9]. The main objective of surgery is to reduce the risk of SCD and alleviate ischaemia. The decision to operate on a patient is based on the ostial anatomy and course of the anomalous coronary artery and demonstrated ischaemia. The role of symptoms in decision-making with regard to the surgical intervention and postoperative outcomes is ambiguous. Several surgical techniques for correcting AAOCA have been used, most commonly unroofing of the intramural segment (Fig. 2), coronary reimplantation and coronary artery bypass grafting (CABG) [10, 11]. A few studies have reported persistent symptoms, restenosis of the operated anomaly after surgery, ischaemia and even cases of SCD [9, 12–15].
Figure 1:
Schematic representation of AAORCA and AAOLCA anatomy with an interarterial and intramural course of the anomalous artery (imaging view). AAOLCA: anomalous aortic origin of a left coronary; AAORCA: anomalous aortic origin of a right coronary; L: left coronary cusp; LAD: left anterior descending artery; N: non-coronary cusp; R: right coronary cusp; RCA: right coronary artery; RCx: ramus circumflex artery.
Figure 2:
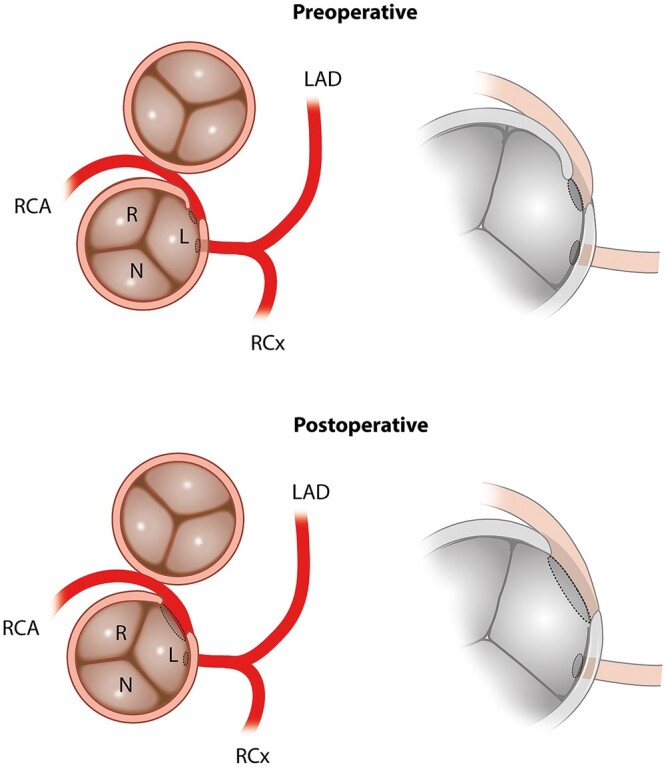
Schematic representation of AAORCA anatomy prior to and after surgical correction (imaging view). AAORCA: anomalous aortic origin of a right coronary; L: left coronary cusp; LAD: left anterior descending artery; N: non-coronary cusp; R: right coronary cusp; RCA: right coronary artery; RCx: ramus circumflex artery.
The aim of this study is to describe the significance of symptoms preoperatively and at medium-term follow-up in adolescent/adult patients who underwent surgery of AAOCA.
MATERIALS AND METHODS
Study population and data collection
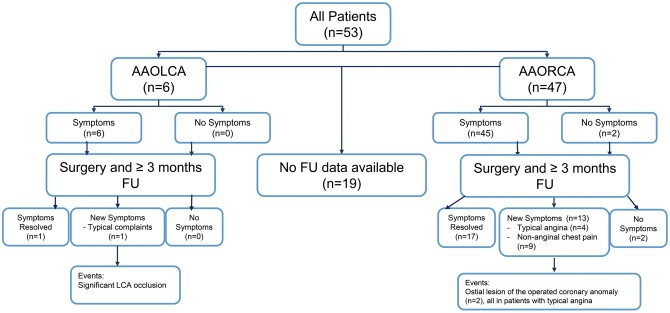
The Leiden University Medical Center serves as a national referral centre for patients with congenital heart disease. Consecutive patients who underwent surgical correction of an anomalous aortic origin of a left coronary artery (AAOLCA) or anomalous right coronary artery (AAORCA) arising from the opposite sinus of Valsalva at our centre between 2001 and 2018 were included in this study (Fig. 3). Patients with concomitant congenital heart defects (e.g. transposition of the great arteries, tetralogy of Fallot and certain forms of pulmonary atresia), and patients unable or unwilling to communicate with the research team were excluded from analysis. Patient data were collected from the electronic medical file system (EPD-Vision®, Leiden University Medical Center, Leiden, the Netherlends) and included patient demographic data, symptoms, sex, indications for surgery, anatomy of the anomalous coronary artery, surgical techniques, imaging modalities, functional tests, clinical course and outpatient visit reports. Major adverse cardiac events included sustained ventricular tachycardia or ventricular fibrillation, reoperation or percutaneous coronary intervention (PCI) on the operated coronary artery and/or (cardiac) death. The study focused on medium-term outcomes. Therefore, in-hospital events in postoperative setting (<1 month) and patients with ˂3 months follow-up were excluded.
Figure 3:
Overview of the 34 patients with follow-up. AAOLCA: anomalous aortic origin of a left coronary; AAORCA: anomalous aortic origin of a right coronary; FU: follow-up.
All patients were asked about recurrence of chest pain-related symptoms and reinterventions. All chest pain (related) complaints were classified according to the ‘2019 ESC guidelines on chronic coronary syndromes’ [16]: chest pain is classified as ‘typical angina’, ‘atypical angina’ and ‘non-anginal chest pain’. Typical angina is defined as (i) ‘constricting discomfort in the front of the chest or in the neck, jaw, shoulder or arm’, (ii) ‘precipitated by physical exertion’ and (iii) ‘relieved by rest or nitrates within 5 min’. Atypical angina meets 2 of these criteria and non-anginal chest pain satisfies 1 or none of the above-mentioned characteristics. Patients were also categorized into the ‘typical’ group if there were other complaints that were strongly associated with ischaemia. All chest pain-related symptoms were categorized independently into above-mentioned groups by 2 experienced cardiologists (H.W.V. and P.K.) who were blinded to the results.
Statistical analysis
Analyses were performed with SPSS Statistics (version 23, IBM Corp, Armonk, NY, USA). Descriptive statistics were used for data analysis and were expressed as mean ± standard deviation and median [interquartile range (IQR)]. Binary data were expressed in numbers with percentages. All reported P-values were 2-sided, and P-values <0.050 were considered significant.
RESULTS
Baseline patient characteristics at initial presentation
Baseline patient characteristics are described in Table 1. This study consisted of 53 patients who underwent surgery for correction of AAOCA; 47 (89%) patients had an AAORCA and 6 (11%) patients an AAOLCA. All patients had an intramural course of the anomalous coronary artery. The mean age at surgery was 44 ± 15 years (range 11–68) and 55% were male. Four patients were younger than 16 years old. Fifty-one of 53 patients (96%) had symptoms of some sort at initial presentation. The most common reason for cardiac analysis in these patients was suspicion of ischaemia (42 patients, 79%). Three (6%) patients presented with an aborted SCD (1 patient with AAOLCA and 2 patients with AAORCA, Table 2). The first patient (patient 2, AAORCA) was 17 years old and playing sports at the time of the cardiac event. No symptoms or cardiac events preceded the cardiac arrest based on ventricular fibrillation. There were no risk factors. The second patient with an AAORCA was 25 years old. This patient was resuscitated due to ventricular fibrillation during exercise; before this event, the patient had some non-specific thoracic complaints during exercise and he was a smoker. In 5 (9%) patients, AAOCA was an incidental finding and in 3 (6%) patients, it was identified through familial screening for coronary anomalies. Although no hard evidence exists regarding familial screening in coronary artery anomalies, in these patients, screening was performed by the referring centre driven by patient desire.
Table 1:
Patient characteristics
| Patient characteristics | All patients (n = 53) |
|---|---|
| Male, n (%) | 29 (55) |
| Age at surgery (years), mean ± SD | 44 (15) |
| Diabetes mellitus n (%) | 3 (6) |
| Hypertension n (%) | 10 (19) |
| Previous ischaemic coronary disease n (%) | 0 |
| Hypercholesterolemia n (%) | 13 (25) |
| TIA/CVA n (%) | |
| AAOLCA | 6 (11) |
| AAORCA | 47 (89) |
| Symptoms present, n (%) | 51 (96) |
| Primary presentation, n (%) | |
| Suspicion of ischaemia | 42 (79) |
| Aborted sudden cardiac death | 3 (6) |
| Familial screening | 3 (6) |
| Incidental finding | 5 (9) |
| Diagnostic imaging techniques, n (%) | |
| CTA | 50 (94) |
| CAG | 35 (66) |
| MRI | 8 (15) |
| Diagnostic functional test, n (%) | |
| Exercise ECG | 36 (68) |
| Positive | 8 (22) |
| Nuclear stress test | 10 (19) |
| Positive | 4 (40) |
| Adenosine stress perfusion CT | 4 (8) |
| Positive | 1 (25) |
| Dobutamine stress MRI | 2 (4) |
| Positive | 0 |
| PET-CT | 2 (4) |
| Positive | 0 |
| No test | 14 (26) |
| Surgical technique, n (%) | |
| Unroofing | 38 (72) |
| Reimplantation | 4 (8) |
| Unroofing + reimplantation | 3 (6) |
| Unroofing + CABG | 1 (2) |
| Ostioplasty | 5 (10) |
| Unroofing + ostioplasty | 1 (2) |
| CABG | 1 (2) |
| Concomitant procedure, n (%) | 15 (28) |
| Aortic valve repair | 6 |
| Tricuspid valve repair | 1 |
| Mitral- and aortic valve repair | 1 |
| Epicardial lead placement | 1 |
| Excision of cardiac lipoma | 1 |
| Pulmonary vein isolation, left atrial resection, aortic valve repair | 1 |
| CABG Ao-D-LAD | 1 |
AAOLCA: anomalous left coronary artery; AAORCA: anomalous right coronary artery; Ao: aorta; CABG: coronary artery bypass grafting; CAG: coronary angiography; CTA: computed tomographic angiography; D: diagonal branch; LAD: left anterior descending artery; MRI: magnetic resonance imaging; PET-CT: position emission tomography computed tomography; SD: standard deviation; TIA: transient ischemic attack; CVA: cerebral vascular accident.
Table 2:
Consecutive patients with >3 months follow-up after surgical correction for anomalous aortic origin of a coronary artery with interarterial course and postoperative complaints driven catheterization (n = 5)
| Pt | Lesion | Age (years) | Clinical presentation | Ischaemia detection | Surgical repair | Preoperative symptoms | Postoperative symptoms | Δt surgery and events (months) | Postoperative events/complications + treatment |
|---|---|---|---|---|---|---|---|---|---|
| 6 | AAORCA | 47 | Suspected ischaemia | Positive | Unroofing | Non-anginal | Typical | 60 | PCI proximal LAD |
| 7 | AAOLCA | 58 | Suspected ischaemia | Positive | Reimplantation | Typical | Typical | 1 | Significant main stem stenosis, PCI main stem |
| 19 | AAORCA | 49 | Suspected ischaemia | Positive | Unroofing | Typical | Typical | 15 | No stenosis on CAG |
| 21 | AAORCA | 64 | Suspected ischaemia | Positive | Unroofing | Typical | Typical | 13 | Flattening ostium RCA, PCI proximal RCA |
| 30 | AAORCA | 44 | Suspected ischaemia | Negative | Unroofing | Atypical | Typical (near-collapse) | 10 | Stenosis ostium RCA, RIMA-RCA, clip on proximal RCA |
AAOLCA: anomalous left coronary artery; AAORCA: anomalous right coronary artery; CAG: coronary angiography; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; RCA: right coronary artery; RIMA: right internal mammary artery.
Preoperative testing
Patients were referred to our centre with different imaging modalities and functional tests, performed in the referring hospital. Of the 53 patients who were accepted for surgery by the heart-team, 50 (94%) patients underwent computed tomographic angiography (Fig. 3), 35 (66%) patients coronary angiography and 8 (15%) patients, cardiac magnetic resonance imaging (MRI). Functional ischaemia testing was performed in 74% of the patients (Table 1). In 36 patients (68%), exercise ECG testing was performed, of which 22% had an ischaemic response. Ten (19%) patients underwent a nuclear stress test, of which 40% were positive for ischaemia.
Initial surgical repair
Surgical techniques used included unroofing (72%), coronary reimplantation (8%), CABG (2%), patch augmentation (10%) or a combination of the above (8%). None of the anomalous LCA patients underwent patch augmentation of ostium and main stem (Table 1). Concomitant procedures during the surgical repair were performed in 15 cases and consisted predominantly of aortic valve resuspension in order to prevent aortic regurgitation due to manipulation after unroofing or because of pre-existing aortic regurgitation.
Clinical follow-up
One patient (1/53, 1.9%) died 1 week after LCA ostioplasty due to severe heparin-induced thrombocytopaenia causing disseminated intravascular coagulation. The central death administration was consulted, and except for the aforementioned patient, every patient (52/53, 98.1%) was still alive at follow-up [median 5 ± 16 years (IQR 2–18)].
Full follow-up data of ˃3 months were available in 34 out of 53 (64%) patients. In 19 (36%) patients, full follow-up data could not be obtained due to migration, significant language barrier or inability to contact the patient. In these 34 patients, median follow-up of 3.0 years (IQR 1.0–5.3, Fig. 1) was attained.
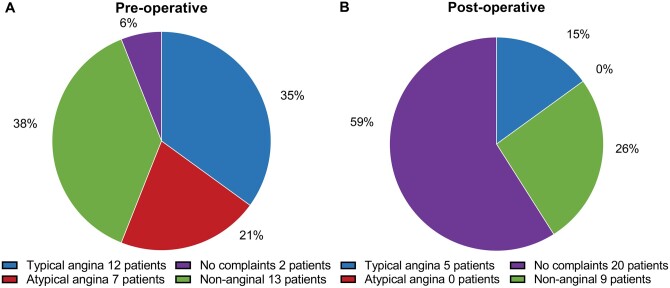
The pre- and postoperative symptoms in the 34 patients with ˃3 months follow-up are shown in Fig. 4. Preoperatively, 35% (12/34) of patients presented with typical angina, 21% (7/34) with atypical angina and 38% (13/34) had non-anginal chest pain. Only 6% of the patients (2/34) were asymptomatic before surgery. After surgical correction, 59% (20/34) of the patients reported to be free of symptoms, this being a significant reduction in the total burden (P < 0001). In 15% (5/34) of the patients, a postoperative catheterization was performed due to typical complaints after surgery (Table 2). In 3 of these patients (9%, 3/34), lesions of the operated AAOCA were diagnosed, detailed in Table 2. Patient 6 presented with typical complaints 5 years after surgery; on coronary angiography, there was an occlusion of the left anterior descending artery, and thus not associated with the unroofed right coronary artery (RCA). Patient 7 (reimplantation of AAOLCA) had a significant left main stenosis for which a successful PCI was performed. Patient 19, also presented with typical complaints; however, on catheterization, no stenosis was seen and no additional treatment was performed. Patient 21 (unroofing of AAORCA) had a flattened ostium of the RCA for which a PCI was performed. Patient 30 (unroofing of AAORCA) presented with a near-collapse and angiography revealed ostial stenosis of the RCA for which a CABG was performed (right internal mammary artery graft on the RCA, clip proximal RCA).
Figure 4:
Preoperative and postoperative symptoms of 34 patients with follow-up classified according to the ‘2019 ESC guidelines on chronic coronary syndromes’.
Table 3 presents an overview of the remaining 27 patients and their clinical and anatomical characteristics, surgical course and follow-up. All patients with atypical angina at presentation were free of symptoms after surgery. Two patients were asymptomatic prior to surgery and remained asymptomatic postoperatively and during follow-up. In these patients, AAOCA was diagnosed through familial screening and was judged to be a malignant variant and, therefore, these patients underwent surgical correction.
Table 3:
Consecutive patients with >3 months follow-up after surgical correction for anomalous aortic origin of a coronary artery with interarterial course and no postoperative events (n = 29)
| Pt | Lesion | Age | Clinical presentation | Ischaemia detection | Surgical repair | Preoperative symptoms | Postoperative symptoms |
|---|---|---|---|---|---|---|---|
| 1 | AAORCA | 25 | Screening | Negative | Unroofing and reimplantation | Typical | No complaints |
| 2 | AAORCA | 17 | Aborted SCD | Not conclusive | Reimplantation | Typical | No complaints |
| 3 | AAORCA | 53 | Suspected ischaemia | Negative | Reimplantation | Atypical | No complaints |
| 4 | AAORCA | 46 | Suspected ischaemia | Negative | Unroofing and reimplantation | Typical | No complaints |
| 5 | AAORCA | 34 | Screening | Positive | Reimplantation | No complaints | No complaints |
| 8 | AAORCA | 66 | Suspected ischaemia | Negative | Unroofing and CABG non-anomalous vessel | Atypical | Non-anginal: chest discomfort |
| 9 | AAORCA | 66 | Suspected ischaemia | Negative | CABG of anomalous vessel | Typical | No complaints |
| 10 | AAORCA | 25 | Suspected ischaemia | Negative | Unroofing | Non-anginal | No complaints |
| 11 | AAORCA | 45 | Suspected ischaemia | Positive | Unroofing | Atypical | No complaints |
| 12 | AAORCA | 56 | Suspected ischaemia | Negative | Unroofing | Atypical | Non-anginal: tiredness/loss of condition |
| 13 | AAORCA | 20 | Suspected ischaemia | Positive | Unroofing | Atypical | No complaints |
| 14 | AAORCA | 50 | Suspected ischaemia | Positive | Unroofing | non-anginal | no complaints |
| 15 | AAORCA | 46 | Suspected ischaemia | Negative | Unroofing | Typical | Non-anginal: tiredness/loss of condition |
| 16 | AAORCA | 13 | Suspected ischaemia | Negative | Unroofing | Atypical | Non-anginal: tiredness/loss of condition |
| 17 | AAOLCA | 15 | Aborted SCD | Negative | Ostiumplasty | Typical | No complaints |
| 18 | AAORCA | 29 | Screening | Positive | Unroofing | Non-anginal | Non-anginal: palpitations |
| 20 | AAORCA | 51 | Screening | Positive | Unroofing | Atypical | Non-anginal: sharp chest pain |
| 22 | AAORCA | 53 | Suspected ischaemia | Positive | Unroofing | Non-anginal | Non-anginal: tiredness/loss of condition |
| 23 | AAORCA | 48 | Suspected ischaemia | Positive | Unroofing | Non-anginal | Non-anginal: sharp chest pain |
| 24 | AAORCA | 59 | Suspected ischaemia | Positive | Unroofing | Non-anginal | Non-anginal: tiredness/loss of condition |
| 25 | AAORCA | 67 | Suspected ischaemia | Positive | Unroofing | Non-anginal | No complaints |
| 26 | AAORCA | 42 | Suspected ischaemia | Positive | Unroofing | Non-anginal | No complaints |
| 27 | AAORCA | 15 | Screening | Positive | Unroofing | No complaints | No complaints |
| 28 | AAORCA | 63 | Suspected ischaemia | Positive | Unroofing | Non-anginal | No complaints |
| 29 | AAORCA | 11 | Suspected ischaemia | Negative | Unroofing | Non-anginal | No complaints |
| 31 | AAORCA | 66 | Suspected ischaemia | Positive | Unroofing | Non-anginal | No complaints |
| 32 | AAORCA | 52 | Screening | Negative | Unroofing | Typical | No complaints |
| 33 | AAORCA | 43 | Suspected ischaemia | Negative | Unroofing | Non-anginal | No complaints |
| 34 | AAORCA | 47 | Suspected ischaemia | Not conclusive | Unroofing | Atypical | No complaints |
AAORCA: anomalous right coronary artery; AAOLCA: anomalous left coronary artery; aSCD: aborted sudden cardiac death; CABG: coronary artery bypass graft; FU: follow-up; Ischaemic detection: outcome of ischaemic detection preoperatively; PCI: percutaneous coronary intervention; Pt: consecutive patient number; RDA: right descending artery; RIMA: right internal mammary artery; VF: ventricular fibrillation; Δt time between surgery and event in months.
DISCUSSION
In this study, we report on the medium-term outcomes (median of 3 years IQR 1.0–5.3) of 34 patients who underwent surgical correction for AAOCA. Our main findings are the following:
Of patients who were referred to our centre with AAOCA, 94% initially present with symptoms: 35% have typical complaints, 21% atypical complaints, 38% non-anginal complaints and 6% have no complaints at all.
After surgical correction of AAOCA, 59% of the patients are free of symptoms. Compared to 6% preoperatively (P <0.001).
Patients who had significant lesions of the operated coronary artery during medium-term follow-up (3/34, 9%), all presented with novel typical anginal complaints in the outpatient clinic.
The clinical presentation of adults with an AAOCA varies. In our study, 35% of patients presented with typical angina which is comparable to previous reports [4, 13, 17]. Consequently, the indication for intervention is based on other clinical factors [18, 19]. Guidelines of the American College of Cardiology, American Heart Association and Thoracic Surgery suggest that surgical intervention may be warranted in younger patients with evidence of ischaemia [18]. Palmieri et al. [19] reported good clinical outcomes after conservative treatment strategy (exercise restriction) in 23 young athletes.
To our knowledge, we are the first group to report specifically on an adolescent and adult group, with mean age of 44 years at time of surgery. Particularly in older patients with AAORCA without signs of ischaemia, indication for intervention is currently not clearly defined. In previous studies, the risk of SCD appears highest in young patients and particularly in interarterial AAOLCA; therefore, the indication for surgical correction in this group is not up for debate [5, 20]. The current guidelines recommend revascularization for interarterial AAOLCA regardless of ischaemia of symptoms [21]. In patients with AAORCA without signs of ischaemia, the indication for intervention relies on numerous factors to guide management. Clinical presentation, anatomical and functional characteristics of the AAOCA as well as patient-specific factors all have to be taken into account [18, 21].
Perioperative mortality in our study was 1.9% (1/53), which is in line with previously reported postoperative mortality rates of AAOCA correction in children and young adults [9, 22–24].
According to the literature, in the majority of the patients, AAOCA is an incidental finding, probably due to a vast increase in the use of computed tomography (CT) and MRI in our current clinical practice. In our study, only 9% of the patients were diagnosed with AAOCA as an incidental finding, reflecting the subselection of patients who were operated. In current clinical practice, therefore, numerous anatomical, (patho)physiological factors and the individual operative risk are considered when evaluating an AAOCA patient. Our results show a low discriminative value of the type of complaints, as over 60% of all AAOCA patients did not have typical complaints at initial evaluation.
After ˃3 months following the surgical correction of AAOCA, 59% of the patients were free of symptoms. This was a significant improvement compared to the preoperative situation and was unrelated to the type of preoperative complaints. Interestingly, out of the 5 patients having typical complaints at follow-up, 3 (60%) needed reintervention due to a significant lesion of the operated artery. This is in line with previous literature [15, 25]. In our series, 9% (3/34) of the operated patients needed reintervention due to a significant lesion in the operated artery. The rate of reintervention is relatively high in relation to the literature which varies between 1.7% and 3.3% [9, 13, 14]. This may be a reflection of the older age of the study population compared to most series reporting on paediatric patients [7, 26].
Mainwaring et al. [13] report on a significantly younger group with 115 AAOCA patients with a follow-up of 6 years, and the median age at surgery was 16 years. In this study, 2 patients had recurrent symptoms of chest pain and underwent reoperation (1 had revision of the initial repair and 1 had repair of a myocardial bridge) [13]. Nees et al. [14] reported on 2 patients with AAOLCA that needed reoperation due to restenosis of the anomalous coronary artery, 2 months and 6 years after surgery, respectively. One patient, aged 68, had recurrent chest pain, and showed an abnormal electrocardiogram and was treated with a bypass graft because of significant stenosis of the operated artery. The other patient, aged 10, survived an aborted SCD 6 years postoperatively, and CT and interoperative examination showed ostial narrowing due to fibrous tissue around the left coronary orifice [14]. In the study of Padalino et al. [9], 3 patients needed reintervention of the operated artery. These cases, together with our data, indicate that restenosis of the corrected anomalous artery is a complication that can be observed during medium- and long-term follow-up of adult patients. It therefore seems justified to perform lifetime follow-up in patients after surgical correction of AAOCA.
Limitations
Despite our role as a national referral centre, the sample size is small, reflecting the rarity of the condition. The nature of the data is largely descriptive, and symptoms may be subjective, particularly when evaluated retrospectively. However, the complaints were judged by 2 independent cardiologist who were blinded to the results. Given our role as a referral centre, patients are typically sent back to the referring cardiologist for lifelong follow-up at the local hospital. This contributed to the high rate of loss to follow-up.
CONCLUSIONS
Our data show the varying symptoms at presentation in adolescent and adult patients with AAOCA. Only 35% have typical anginal complaints. Surgical correction of AAOCA reduces the symptoms in the vast majority of patients. One should be aware of potential lesions of the operated coronary artery in patients presenting with typical anginal complaints postoperatively.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Ronald Slagter (Department of Anatomy and Embryology, Leiden University Medical Center, Netherlands) for his assistance with Figs 1 and 2.
Conflict of interest: none declared.
Author contributions
Fleur M.M. Meijer: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft. Anastasia D. Egorova: Supervision; Validation; Writing—review & editing. Monique R.M. Jongbloed: Conceptualization; Writing—review & editing. Claire Koppel: Writing—review & editing. Gracia Habib: Data curation; Formal analysis; Writing—review & editing. Mark G. Hazekamp: Conceptualization; Writing—review & editing. Hubert W.Vliegen: Conceptualization; Supervision; Writing—review & editing. Philippine Kies: Conceptualization; Project administration; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Krishnasamy Arunkumar, Hitendu Hasmukhlal Dave, Gaetano D. Gargiulo, Massimo A. Padalino and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
Abbreviations
- AAOCA
Anomalous aortic origin of a coronary artery
- AAOLCA
Anomalous aortic origin of a left coronary artery
- AAORCA
Anomalous aortic origin of a right coronary artery
- CABG
Coronary artery bypass grafting
- CT
Computed tomography
- IQR
Interquartile range
- MRI
Magnetic resonance imaging
- PCI
Percutaneous coronary intervention
- RCA
Right coronary artery
- SCD
Sudden cardiac death
REFERENCES
- 1. Gatzoulis M, Webb G, Daubeney P. Part X: Coronary Anomalies of the Coronary Arteries. Diagnosis and Management of Adult Congenital Heart Disease. Philadelphia, PA: Elsevier, 2018, 588. [Google Scholar]
- 2. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 1990;21:28–40. [DOI] [PubMed] [Google Scholar]
- 3. Labombarda F, Coutance G, Pellissier A, Mery-Alexandre C, Roule V, Maragnes P et al. Major congenital coronary artery anomalies in a paediatric and adult population: a prospective echocardiographic study. Eur Heart J Cardiovasc Imaging 2014;15:761–8. [DOI] [PubMed] [Google Scholar]
- 4. Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000;35:1493–501. [DOI] [PubMed] [Google Scholar]
- 5. Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 1992;20:640–7. [DOI] [PubMed] [Google Scholar]
- 6. Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD, Tweddell JS. Jr, Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg 2017;153:1440–57. [DOI] [PubMed] [Google Scholar]
- 7. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Circulation 2008;118:e714. [DOI] [PubMed] [Google Scholar]
- 8. King N-M, Tian DD, Munkholm-Larsen S, Buttar SN, Chow V, Yan T. The aberrant coronary artery–the management approach. Heart Lung Circ 2018;27:702–7. [DOI] [PubMed] [Google Scholar]
- 9. Padalino MA, Franchetti N, Sarris GE, Hazekamp M, Carrel T, Frigiola A et al. Anomalous aortic origin of coronary arteries: early results on clinical management from an international multicenter study. Int J Cardiol 2019;291:189–93. [DOI] [PubMed] [Google Scholar]
- 10. Poynter JA, Williams WG, McIntyre S, Brothers JA, Jacobs ML, Overman D et al. Anomalous aortic origin of a coronary artery a report from the congenital heart surgeons society registry. World J Pediatr Congenit Heart Surg 2014;5:22–30. [DOI] [PubMed] [Google Scholar]
- 11. Mainwaring RD, Reddy VM, Reinhartz O, Petrossian E, Punn R, Hanley FL. Surgical repair of anomalous aortic origin of a coronary artery. Eur J Cardiothorac Surg 2014;46:20–6. [DOI] [PubMed] [Google Scholar]
- 12. Wittlieb-Weber CA, Paridon SM, Gaynor JW, Spray TL, Weber DR, Brothers JA. Medium-term outcome after anomalous aortic origin of a coronary artery repair in a pediatric cohort. J Thorac Cardiovasc Surg 2014;147:1580–6. [DOI] [PubMed] [Google Scholar]
- 13. Mainwaring RD, Murphy DJ, Rogers IS, Chan FP, Petrossian E, Palmon M et al. Surgical repair of 115 patients with anomalous aortic origin of a coronary artery from a single institution. World J Pediatr Congenit Heart Surg 2016;7:353–9. [DOI] [PubMed] [Google Scholar]
- 14. Nees SN, Flyer JN, Chelliah A, Dayton JD, Touchette L, Kalfa D et al. Patients with anomalous aortic origin of the coronary artery remain at risk after surgical repair. J Thorac Cardiovasc Surg 2018;155:2554–64.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brothers JA, McBride MG, Seliem MA, Marino BS, Tomlinson RS, Pampaloni MH et al. Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol 2007;50:2078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knuuti J Wijns W Saraste A Capodanno D Barbato E Funck-BrentanoC et al . 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The task force for the diagnosis and management of chronic coronary syndromes of the european society of cardiology (ESC). Eur Heart J 2019;3:407. [DOI] [PubMed] [Google Scholar]
- 17. Angelini P, Velasco JA, Flamm S. Coronary anomalies incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449–54. [DOI] [PubMed] [Google Scholar]
- 18. Brothers JA, Frommelt MA, Jaquiss RD, Myerburg RJ, Fraser CD, Tweddell JS. Expert consensus guidelines: anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg 2017;153:1440–57. [DOI] [PubMed] [Google Scholar]
- 19. Palmieri V, Gervasi S, Bianco M, Cogliani R, Poscolieri B, Cuccaro F et al. Anomalous origin of coronary arteries from the “wrong” sinus in athletes: diagnosis and management strategies. Int J Cardiol 2018;252:13–20. [DOI] [PubMed] [Google Scholar]
- 20. Cheitlin MD, De Castro CM, Mcallister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva: a not-so-minor congenital anomaly. Circulation 1974;50:780–7. [DOI] [PubMed] [Google Scholar]
- 21. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons . J Am Coll Cardiol 2008;52:e143–263. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen A, Haas F, Evens J, Breur J. Sudden cardiac death after repair of anomalous origin of left coronary artery from right sinus of valsalva with an interarterial course. Neth Heart J 2012;20:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies JE, Burkhart HM, Dearani JA, Suri RM, Phillips SD, Warnes CA et al. Surgical management of anomalous aortic origin of a coronary artery. Ann Thorac Surg 2009;88:844–8. [DOI] [PubMed] [Google Scholar]
- 24. Brothers J, Gaynor JW, Paridon S, Lorber R, Jacobs M. Anomalous aortic origin of a coronary artery with an interarterial course: understanding current management strategies in children and young adults. Pediatr Cardiol 2009;30:911–21. [DOI] [PubMed] [Google Scholar]
- 25. Sachdeva S, Frommelt MA, Mitchell ME, Tweddell JS, Frommelt PC. Surgical unroofing of intramural anomalous aortic origin of a coronary artery in pediatric patients: single-center perspective. J Thorac Cardiovasc Surg 2018;155:1760–8. [DOI] [PubMed] [Google Scholar]
- 26. Mery CM, De Leon LE, Molossi S, Sexson-Tejtel SK, Agrawal H, Krishnamurthy R et al. Outcomes of surgical intervention for anomalous aortic origin of a coronary artery: a large contemporary prospective cohort study. J Thorac Cardiovasc Surg 2018;155:305–19.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.