Abstract
We report a case of resistance development toward cefiderocol in a patient with intra-abdominal and bloodstream infections caused by carbapenemase-producing Enterobacter cloacae within 21 days of cefiderocol therapy. Whole genome sequencing revealed heterogeneous mutations in the cirA gene, encoding a catecholate siderophore receptor, conferring phenotypic resistance to cefiderocol.
Keywords: antibiotic resistance, carbapenemase, Cefiderocol, cirA siderophore receptor, Enterobacter cloacae
The emergence and spread of carbapenem resistance in gram-negative bacteria is an ongoing global threat, limiting therapeutic options for severe infections [1] and are one of the leading causes of morbidity and mortality in vulnerable patients [2]. Cefiderocol is a novel synthetic conjugate siderophore cephalosporin, recently approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Cefiderocol exploits the iron transporters of the bacterial outer membrane by binding with iron molecules to form a siderophore complex, entering the periplasmic compartment and unfold its antibacterial activity by inhibiting the cell wall synthesis. Cefiderocol exhibited stability against hydrolysis by all carbapenemases, including metallo-β-lactamases (MBLs) and therefore is approved for the treatment of infections with aerobic gram-negative bacteria with limited therapy options [3].
The redundancy of the bacterial TonB-dependent iron transport system was expected to be a hurdle for rapid resistance development [4]. Nonetheless, the acquisition of cefiderocol resistance has been reported. Yet the underlying mechanisms remain unclear [5].
Here we report the in vivo development of cefiderocol resistance within 3 weeks after therapy initiation in a critically ill patient with bloodstream and intra-abdominal infection caused by carbapenem-resistant (CR) Enterobacter cloacae. Comparative genomics analysis using whole-genome sequencing (WGS) was performed to identify potential mechanisms associated with the phenotypic resistance to cefiderocol.
CLINICAL CASE
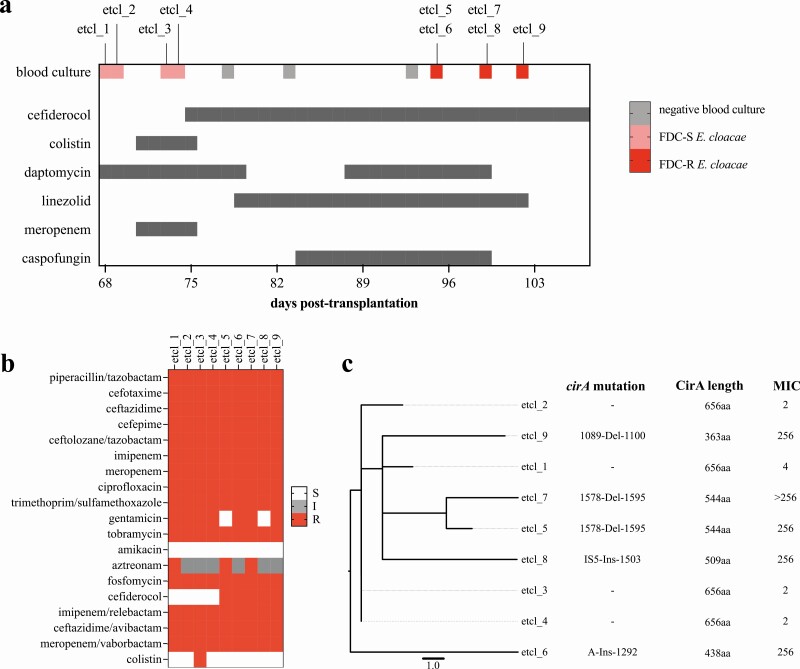
A 58-year-old male patient presented with liver cirrhosis 10 years after initial liver transplantation. Following successful othotopic liver retransplantation, he developed a complicated clinical course with leakage of the bile duct, insertion of a percutaneous transhepatic drainage, infection with vancomycin-resistant Enterococcus faecium (VREfm), and formation of biloma. On day 68 following transplantation, blood cultures were positive for Klebsiella pneumoniae and CR-Enterobacter cloacae. Two carbapenemase genes encoding for New Delhi metallo-β-lactamase (NDM) and oxacillinase, OXA-48 were detected in the CR-E. cloacae by polymerase chain reaction (PCR). Antimicrobial therapy with meropenem and colistin was initiated. CR-E. cloacae and VREfm were continuously cultured from the bile drainage. Due to severe neurological side effects, the acquisition of colistin resistance and the lack of alternatives, antimicrobial therapy was switched from meropenem and colistin to cefiderocol on day 75 (Figures 1a and 1b). The follow-up blood cultures on day 78, 83, and 93 (day 4, 9, and 19 after the first cefiderocol administration) remained negative, suggesting therapeutic success. However, cultures from the bile drainage remained positive for CR-E. cloacae and VREfm, and treatment of VREfm was switched back to linezolid. Computed tomography (CT) scan demonstrated again multiple biloma and hepatic abscesses. Caspofungin was initiated because of the detection of Candida glabrata and Candida albicans in the bile drainage. Due to persistent VREfm infection, daptomycin was again added to the regimen. On day 21 of cefiderocol therapy, cefiderocol-resistant CR-E. clocacae was detected in the blood culture. Despite clinical worsening, no potentially curative surgical option was technically possible due to the lacking arterial vascularization and consecutive bile duct necrosis as well as intrahepatic abscesses, leading to exitus letalis on day 107. Detailed information on the clinical course is given in the Supplementary materials.
Figure 1.
(a) Timeline of events and antimicrobial therapy from the first detection of carbapenem-resistant Enterobacter cloacae until exitus letalis. High-level cefiderocol resistance was first detected on day 21 after therapy initiation (day 96 after liver retransplantation). Dark gray bars indicate antimicrobial therapy duration. Dosages: daptomycin IV 700 mg every 24 hours; meropenem IV 2 g initial bolus followed by 2 g every 8 hours with prolonged infusion time; colistin IV 9 Mio loading dose followed by 2.5 Mio every 12 hours for 3 days and 4.5 Mio every 12 hours for another 3 days; cefiderocol IV 2 g initial bolus followed by 1 g every 8 hours (dose adjusted to renal clearance) for 26 days and 2 g every 8 hours for 6 days; linezolid IV 600 mg every 12 hours; caspofungin IV 70 mg on the first day and then 50 mg every 24 hours. (b) Phenotypic resistance profile of all Enterobacter cloacae blood culture isolates. (c) Phylogeny by SNPs of E. clocae isolates indicated close clonal relationship (ST96, 0 to 9 SNPs). Isolates etcl_6 to _9 exhibited high-level resistance to cefiderocol (>256 mg/L). Abbreviations: FDC-R, cefiderocol-resistant; FDC-S, cefiderocol-susceptible; I, intermediate; IV, intravenous; MIC, minimum inhibitory concentration in mg/L; MLST, multi-locus sequence typing; R, resistant; S, susceptible; SNPs, single nucleotide polymorphisms.
METHODS
Diagnostic routine microbiology was performed as described previously [6]. Antibiotic susceptibility testing (AST) was interpreted according to EUCAST clinical breakpoints v10.0. AST for cefiderocol was determined by microdilution (Sensititre, Thermo Fisher Scientific) according to the manufacturer’s protocol. Because the upper limit for the commercially available Sensititre AST is 8 mg/L for cefiderocol, a broth microdilution (range 0.5 mg/L to >256 mg/L) using iron-depleted cation-adjusted Mueller-Hinton broth was performed according to the published protocol by Hackel and colleagues [7]. For whole genome sequencing, DNA of E. cloacae isolates from blood culture was isolated and sequenced on the MiSeq Illumina platform (short-read sequencing 2x300bp), as described previously [6]. Details on data analysis and data availability (PRJNA705064) are provided in the Supplementary materials.
RESULTS
The phenotypic resistance profile is displayed in Figure 1b. All E. cloacae isolates belonged to ST96 and harbored blaNDM-5 and blaOXA-48. Minimum inhibitory concentrations (MIC) of cefiderocol for blood culture isolates etcl_1 was 4 mg/L, and 2 mg/L for etcl_2, _3, and _4. These isolates are considered susceptible based on the CLSI breakpoints, but etcl_1 is considered resistant according to the EUCAST clinical breakpoints. AST of subsequent isolates, etcl_5 to _9, revealed a considerably higher MIC of ≥ 256 mg/L (Figure 1c). Etcl_3 was phenotypically colistin resistant, but neither mutation nor acquisition of genes associated with colistin resistance was detected.
Blood culture isolates were cryopreserved, and we performed WGS on all 9 isolates detected in the blood cultures of this patient before and during cefiderocol therapy. Alignment of the core genome (4811 genes, 4 575 108 nucleotides) revealed close genetic relationship with single nucleotide polymorphism (SNP) ranging between 0 and 9 among the patient’s isolates, indicating a common clonal origin (Figure 1c).
Resistance to cefiderocol has been reported to be associated with alterations of the intrinsic AmpC and siderophore receptors [8]. In the presented case, the chromosomal AmpC of all isolates were identical, so AmpC-mediated resistance was unlikely. To find a potential resistance mechanism, we analyzed the SNPs and deletion distribution among the genes of the core-genome and compared them between cefiderocol-susceptible and cefiderocol-resistant isolates. Comparison of the draft genomes between the cefiderocol-susceptible (etcl_1 to _4) and resistant (etcl_5 to _9) isolates revealed alterations in only one gene, cirA, which encodes a TonB-dependent catecholate siderophore receptor (Supplementary Figure 1).
In isolates etcl_5, _6, _7 and _9, we detected various mutations in the cirA gene between 1089 and 1595 bp, which were absent in all isolates prior to cefiderocol exposure (Figure 1c). In silico protein translation revealed truncation of the CirA protein in the isolates with the high-level cefiderocol resistance (Figure 1c, Supplementary materials). In isolate etcl_8, we detected an insertion of an IS5-like Transposon, which also resulted in truncation of the CirA protein (Figure 1c and Supplementary Figures 2 and 3).
DISCUSSION
Cefiderocol is a promising agent for the treatment of gram-negative bacteria with limited therapeutic options [3, 9]. Although the development of cefiderocol resistance has been described, reduced susceptibility toward cefiderocol in Enterobacterales is not a common encounter [10]. To the best of our knowledge, this case is the first report on the development of high-level cefiderocol resistance during cefiderocol therapy. In the present case, cefiderocol resistance in E. cloacae developed rapidly within 21 days of therapy. WGS identified heterogeneous functional alterations in the catecholate siderophore receptor gene, cirA. Indeed, the C-3 side chain of cefiderocol consists of a catechol group, which binds to iron molecules to enter the periplasmic compartment through a catecholate iron transporter, demonstrating the importance of the catecholate siderophore receptor as the entry mechanism for cefiderocol in E. cloacae. Furthermore, in the annotated draft genome of E. cloacae isolates in the present case, we identified cirA as the only catecholate receptor present, explaining the impact of the mutation of this gene.
Furthermore, the mutation observed in this case were heterogeneous but located in a narrow window from 1089 to 1595 bp of the genes, indicating a potential mutational hot spot leading to a highly truncated protein and nonfunctional receptor. The potential significance of CirA in cefiderocol’s mode of entry has been previously investigated for Escherichia coli by Ito and colleagues [4]. In contrast to our case, a deletion of the cirA gene had little impact on the cefiderocol MIC, whereas an additional deletion of the ferrichrome receptor gene fiuA led to a significant increase of the MIC toward cefiderocol. Based on this observation and other published studies [4, 11], a redundancy of the TonB-dependent ferric transport system in the outer membrane of gram-negative bacteria such as Pseudomonas aeruginosa or E. coli may prevent rapid development of resistance to cefiderocol. The rapid development of resistance in the presented case might have been favored by elevated initial cefiderocol MICs, caused by the production of metallo-β-lactamase. Generally, the cefiderocol MIC in isolates producing metallo-β-lactamases remain below the threshold for resistance [12, 13]; therefore, cefiderocol can still be considered as a viable option to treat infections with carbapenemase-producing gram-negatives. In E. cloacae complex, the development of cefiderocol resistance following cefepime exposure has been reported as a result of an amino acid deletion in the R2-loop of AmpC beta-lactamase [14]. The alteration of the chromosomal AmpC beta-lactamase is a general response directed to the beta-lactam antibiotic class following beta-lactam exposure and hence it is not a mutation directly interfering with cefiderocol or its delivery mode so that cefiderocol resistance in this case can be considered as a collateral effect.
Our findings indicated that high-level resistance could be acquired rapidly through mutations of the CirA siderophore receptor during cefiderocol therapy. However, it remains unclear whether the rapid development of resistance is a feature of Enterobacter spp. or if the presence of one or both carbapenemases increases the propensity for resistance acquisition. As a limitation, we did not perform molecular validation to confirm the direct effect of cirA deletion on cefiderocol susceptibility. Further investigations are needed to study the role and diversity of the siderophore receptor repertoire in Gram-negative bacteria. The acquisition of cefiderocol resistance in E. cloacae during therapy is alarming and should be closely monitored.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Selina Hassel, Nicole Henny, Suzan Leccese, and Delal Sahin for their excellent technical assistance. Bacterial isolates were collected and sequenced as part of the MDRO surveillance program at the authors’ university hospital. Individual informed consent was waived after consulting the local ethics committee for the scientific use of anonymized patient and clinical data, along with bacterial genomic data (S474/2018).
Potential conflict of interest. S. Z. reports nonfinancial support from Shionogi Germany, is a member of the cefiderocol advisory board of Shionogi Germany, and received honorarium for Shionogi board meeting in October 2020. M. A. W. provided presentations for, is an advisory board member of and received speaker honorarium and non-financial support from Shionogi, Pfizer, MSD, Eumedica, Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kelly AM, Mathema B, Larson EL. Carbapenem-resistant Enterobacteriaceae in the community: a scoping review. Int J Antimicrob Agents 2017; 50:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis 2018; 5:ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sato T, Yamawaki K. Cefiderocol: discovery, chemistry, and in vivo profiles of a novel siderophore cephalosporin. Clin Infect Dis 2019; 69:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ito A, Sato T, Ota M, et al. . In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob Agents Chemother 2018; 62:e01454–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naseer S, Weinstein EA, Rubin DB, et al. . US Food and Drug Administration (FDA): benefit-risk considerations for cefiderocol (Fetroja(R)). Clin Infect Dis 2020; 12:e1103–11. [DOI] [PubMed] [Google Scholar]
- 6. Kocer K, Boutin S, Dalpke AH, Heeg K, Mutters NT, Nurjadi D. Comparative genomic analysis reveals a high prevalence of inter-species in vivo transfer of carbapenem-resistance plasmids in patients with haematological malignancies. Clin Microbiol Infect 2020; 26:780.e1–e8. [DOI] [PubMed] [Google Scholar]
- 7. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis 2019; 94:321–5. [DOI] [PubMed] [Google Scholar]
- 8. Shields RK, Iovleva A, Kline EG, Kawai A, McElheny CL, Doi Y. Clinical evolution of AmpC-mediated ceftazidime-avibactam and cefiderocol resistance in enterobacter cloacae complex following exposure to cefepime. Clin Infect Dis 2020; 71:2713–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonomo RA. Cefiderocol: a novel siderophore cephalosporin defeating carbapenem-resistant pathogens. Clin Infect Dis 2019; 69:519–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant gram-negative bacteria. Antimicrob Agents Chemother 2020; 64:e01582–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikaido H, Rosenberg EY. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J Bacteriol 1990; 172:1361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kazmierczak KM, Tsuji M, Wise MG, et al. . In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-beta-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents 2019; 53:177–84. [DOI] [PubMed] [Google Scholar]
- 13. Delgado-Valverde M, Conejo MDC, Serrano L, Fernández-Cuenca F, Pascual Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother 2020; 75:1840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawai A, McElheny CL, Iovleva A, et al. . Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother 2020; 64:e00198–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.