Abstract
OBJECTIVES
Whether acute phase and immune responses are minimally affected following minimally invasive lung surgery needs further investigation. We performed a pilot study to evaluate the immune profile of patients who underwent video-assisted thoracoscopic surgery or robot-assisted thoracic surgery lobectomies for the treatment of suspicious or known stage I non-small-cell lung cancer.
METHODS
Blood samples were taken preoperatively and 3 and 24 h postoperatively were analysed for C-reactive protein, glucose, cortisol, tumour necrosis factor alpha (TNF-α), interleukin 8 (IL-8) and interleukin 10 (IL-10) levels. TNF-α, IL-8 and IL-10 were also measured in lung tissues. T (CD4, CD8), B (CD19) and natural killer (CD56, CD16) cell counts and natural killer cell functions were analysed using a flow cytometry-based assay before and after surgery.
RESULTS
Minimally invasive surgery (robot-assisted thoracic surgery + video-assisted thoracoscopic surgery) significantly decreased IL-10 (P = 0.016) levels after surgery. No significant differences were detected in TNF-α (P = 0.48) and IL-8 (P = 0.15) levels before and after surgery. C-reactive protein (P < 0.001), cortisol (P < 0.001) and glucose levels (P < 0.001) increased significantly after surgery. Lymphocyte, total T cell, CD3+CD4+ and CD3+CD8+ CD16+CD56+ cell counts were significantly lower on postoperative day 1.
CONCLUSION
There seems to be a dynamic balance between pro- and anti-inflammatory cytokines and immune cells following minimally invasive lobectomy.
Keywords: Early-stage lung cancer, Minimally invasive surgery, Natural killer, Tumour necrosis factor alpha, Interleukin 8, Interleukin 10
Along with the improvements in endoscopic video systems and endoscopic staplers, minimally invasive surgery has become more popular in the treatment of early-stage lung cancer.
INTRODUCTION
Along with the improvements in endoscopic video systems and endoscopic staplers, minimally invasive surgery has become more popular in the treatment of early-stage lung cancer. The first anatomical lung resection using video-assisted thoracoscopic surgery (VATS) was performed in 1992 [1]. Since then, many studies comparing the outcomes of open surgery and those with VATS have been published. VATS primary lung resection, including lobectomy or pneumonectomy for early-stage lung cancer, has gained acceptance because of the positive outcomes with VATS compared with those with open surgery [2]. Minor surgical stress and improved health-related quality of life after surgery expanded the use of VATS across many institutions in the world, and robotic technology in thoracic surgery has rapidly gained acceptance after positive outcomes with VATS.
The benefits associated with VATS are the reduction in the quantity of cytokines released and in cellular immunity, both of which are associated with improved long-term survival [2]. Therefore, it is possible that robot-assisted thoracic surgery (RATS) for lung cancer resection may similarly allow less suppression of the host immune system. The results of retrospective studies comparing outcomes with RATS and VATS for the treatment of non-small-cell lung cancer (NSCLC) showed no difference between the 2 groups concerning the number of dissected lymph nodes, the length of hospital stay, anaesthesia and operative times, complication rates and morbidity [3]. In a limited number of retrospective studies, long-term survival rates were also found to be similar between the 2 groups [3, 4]. These results suggest that RATS and VATS for lung cancer resection may have a similar immunosuppressive effect. Available data on this subject are limited, and further investigation is needed to determine if acute phase and immune responses are minimally affected following minimally invasive lung surgery. The goal of this pilot study was to evaluate the immune profile of patients who underwent VATS or RATS lobectomies for the treatment of suspicious or known stage I NSCLC by comparing changes in leukocytes, lymphocyte subsets, T cells, natural killer (NK) cells, and stress-related biochemical mediators and cytokines in the perioperative and early postoperative periods.
PATIENTS AND METHODS
Patients
We recruited patients who underwent VATS or RATS lobectomies for the treatment of suspicious or known stage I NSCLC between March 2019 and February 2020 in the Koç University Hospital thoracic surgery clinic. The patients met the following inclusion criteria: (i) age 40–75 years and (ii) written informed consent. Exclusion criteria included (i) prior cancer surgery; (ii) any severe chronic disease; (iii) use of systemic steroids or any other immunosuppressive drugs; and (iv) any transfusion requirements during surgery. The study was approved by the local ethics committee.
The surgical method was determined according to the patient's preference after he or she was informed about the 2 surgical methods (RATS/VATS) and their risks. VATS and RATS resections were carried out whenever it was technically suitable. All operations were performed by the same surgeon at a single centre. All patients received identical anaesthesia. All patients received standardized patient-controlled analgesia and intravenous paracetamol 1 g in a 100-ml vial infused over 15 min, 2 times per day for pain control during the first and second postoperative days. Patients who underwent RATS with the da Vinci S system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) were classified as the RATS group. For a comparison group, patients who underwent VATS lobectomy were classified as the VATS group. Patient demographics, perioperative complications and lengths of hospital stays were prospectively recorded in a computerized database.
Blood and tissue sampling
Blood samples were collected before the operation and at 3 and 24 h postoperatively for C-reactive protein (CRP), glucose, cortisol, tumour necrosis factor alpha (TNF-α), interleukin 8 (IL-8) and interleukin 10 (IL-10) levels. Blood samples were collected in dry tubes and centrifuged promptly (3500 × g) for 10 min at +4°C. Sera were separated into aliquots and frozen immediately at −80°C. After surgical resection, 1 cm3 of non-neoplastic lung tissue located 1 cm from the primary tumour was removed. The lung tissue was then dissected instantly and frozen in liquid nitrogen at −80°C. Frozen lung samples (500 mg) were homogenized in 100 mmol/l phosphate buffer (pH: 7.4) containing sodium azide (0.05%) for 1 min on ice and then centrifuged at 20 000 × g at +4°C for 15 min and the supernatants were collected. CRP, glucose and cortisol levels were analysed using previously described methods [5]. TNF-α, IL-8 and IL-10 levels in serum and lung tissues were determined by Sandwich‐ELISA using commercial kits (ZhenHua, Wuhan, China). The intra‐ and intercoefficients of variability were <10% and <12%, respectively, for TNF-α, IL-8 and IL-10. For tissue homogenates, protein levels were determined by the Bradford method, and the results were reported as TNF- α, IL-8 and IL-10 per 1 mg (g) protein.
Lymphocyte subtyping
Blood samples were also collected in ethylenediaminetetra-acetic acid (2 mg/ml) before the operation and at 24 h postoperatively for flow cytometric T and B lymphocyte and NK cell subtype analysis. Cells were counted using the Sysmex automated blood counter (Roche, Germany) and adjusted to 10 000 cells/µl if necessary. The 100-µl samples were stained with mouse antihuman CD3 fluorescein isothiocyanate, CD4 peridinin-chlorophyll-protein (PerCP), CD8 phycoerythrin, CD56 phycoerythrin, CD16 PerCP and CD19 allophycocyanin (BD Biosciences, San Jose, CA, USA) and incubated for 15 min at room temperature in the dark. After incubation, erythrocytes were lysed with BD Pharmlyse erythrocyte lysing solution (BD Biosciences) for 10 min and centrifuged at 1500 × g for 5 min. The supernatant was discarded, and the cells were washed once with 1× phosphate-buffered saline (PBS) supplied with 0.5% bovine serum albumin. Samples were resuspended with 1× Attune Focusing Solution (Thermo Fisher Scientific, Waltham, MA, USA) and analysed with Attune NXT Focusing Cytometer (Thermo Fisher Scientific). A total of 100 000 events were recorded at the lymphocyte gate from the forward scatter × side scatter plot.
Peripheral blood mononuclear cell isolation and NK cell separation with magnetic-activated cell-sorting buffer
A total of 5 ml of peripheral blood was drawn from patients before and 24 h after surgery. Blood was carefully layered on the top of an equal volume of Ficcol-Paque Plus (GE Healthcare, Dornstadt, Germany) and centrifuged for 500 × g for 25 min. Plasma was discarded and a buffy coat containing peripheral blood mononuclear cells was collected. Cells were washed once with 1× PBS containing 0.5% bovine serum albumin and 2 mM ethylenediaminetetra-acetic acid [magnetic-activated cell-sorting (MACS) buffer]; they were suspended with 1 ml of the same buffer and counted with a Sysmex automated blood counter (Roche, Germany) and adjusted to 1 × 107 cells maximum. NK cells were isolated from peripheral blood mononuclear cells using a MACS NK Cells Isolation Kit (Miltenyi Biotech, Bergisch Gladbach, Germany). Briefly, cells were centrifuged at 300 × g for 10 min. The supernatant was aspirated, and the pellet was resuspended with 40 µl of MACS buffer. A total of 10 µl of NK cell biotin-antibody cocktail was added to the cells and incubated for 5 min at 40°C. After incubation, 30 µl of MACS buffer and 20 µl of NK Cell MicroBead Cocktail were added to the cells and incubated for another 10 min at 40°C. Magnet buffer (500 µl) was added, and the cells were passed through mass spectrometry columns (Miltenyi Biotech) and intact NK cells were collected. Columns were washed with another 1.5 ml of Magnet buffer to collect enriched NK cells. Purified NK cells were centrifuged at 500 × g for 5 min. The supernatant was discarded, and the cells were resuspended with 3 ml of Roswell Park Memorial Institute medium containing 1% penicillin–streptomycin solution and 10% foetal bovine serum (Gibco Laboratories, Gaithersburg, MD, USA). To stimulate NK cells, 6 µl of Cell Activation Cocktail containing phorbol 12-myristate 13-acetate, ionomycin and brefeldin A (BioLegend, San Francisco, CA, USA) was added to the medium. Cells were seeded onto 35-mm dishes and incubated in a 37°C 5% CO2 incubator for 6 h.
Intracellular cytokine staining of NK cells
Incubated NK cells were collected and centrifuged at 500 × g for 5 min. Supernatants were discarded; the cells were fixed and permeabilized with 150 µl of Cytofast Fix Perm Solution (BioLegend) for 20 min at room temperature. A total of 2 ml of 1× PermWash solution (Biolegend) was added to the cells and centrifuged for 5 min at 500 × g. The supernatant was discarded. A total of 5 µl of mouse antihuman interferon-gamma (IFN-ɣ) fluorescein isothiocyanate and IL-22 PerCP (Biolegend) were added to the cells and incubated for 20 min at room temperature in the dark. After incubation, the cells were washed twice with the first 1× PermWash solution and 1× PBS with 0.5% PBS. The pellet was resuspended with 500 µl of Attune NXT Focusing Solution, and the samples were analysed with Attune NXT Focusing Cytometer. Fifty thousand cells were gated in forward scatter × side scatter plots.
Statistical analyses
Descriptive statistics (mean, median, standard deviation, minimum, maximum) were used to express continuous variables. Normality of parameters was assessed using the Shapiro–Wilk test. The Student’s t-test was used to compare 2 normally distributed groups. Non-parametric statistical methods were used for values with skewed distribution. The Mann–Whitney U-test was used to compare 2 non-normally distributed groups. Evaluation of the change between time points was performed with the Wilcoxon signed-rank test. The Friedman test was used to compare >2 related groups. The Bonferroni-corrected Wilcoxon signed-rank test was used as a post hoc test. The χ2 (Fisher’s exact test where available) test was used for categorical variables and expressed as observation counts (and percentages). Statistical significance was accepted when the two-sided P-value was <0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 25.0, 2017; IBM Corp, Armonk, NY, USA).
RESULTS
Study population
A total of 22 patients (mean age 61.9 ± 8.9 years; 73% men) were included. Clinical data of the patients are given in Table 1. Sex distribution among the groups showed no significant difference. There were no significant differences between the 2 groups regarding KCO, duration of anaesthesia and surgery, length of hospital stay, tumour diameter and number of dissected lymph nodes. The patients in the RATS group were older than the patients in the VATS group (P = 0.016). Forced vital capacity (%) (P = 0.034) and forced expiratory volume in 1 s values (%) (P = 0.016) were lower in the RATS group. The VATS group (8/11) had more adenocarcinomas compared to the RATS group (5/11). Complication rates were similar in both groups, but the postoperative infection rate was high in the VATS group (Table 2).
Table 1:
Clinical data
| Variables | All patients mean ±SD or n | VATS (n = 11) | RATS (n = 11) | P-value |
|---|---|---|---|---|
| Age | 61.9 ± 8.9 | 57.5 ± 8.5 | 66.4 ± 7.2 | 0.016 |
| Gender, male/female | 16/6 | 9/2 | 7/4 | 0.34 |
| CCI | 1.6 ± 2.0 | 1.2 ± 1.7 | 2.1 ± 2.3 | 0.33 |
| KCO (ml/min/mmHg/l) | 84.1 ± 15.7 | 82.6 ± 17.5 | 85.6 ± 14.5 | 0.70 |
| FVC (%) | 95.0 ± 16.9 | 102.0 ± 15.0 | 88.0 ± 16.5 | 0.034 |
| FEV1 (%) | 89.2 ± 16.7 | 96.9 ± 14.3 | 81.5 ± 15.6 | 0.016 |
| Length of anaesthesia (min) | 246.2 ± 70.1 | 229.3 ± 67.9 | 263.2 ± 71.3 | 0.15 |
| Length of surgery (min) | 183.6 ± 67.8 | 167.7 ± 64.5 | 199.4 ± 70.4 | 0.27 |
| Length of hospital stay (days) | 7.3 ± 3.2 | 7.36 ± 3.2 | 7.2 ± 3.4 | 0.95 |
| Tumour diameter (mm) | 19.2 ± 10.1 | 18.7 ± 9.8 | 19.7 ± 10.9 | 0.90 |
| Dissected LN | 4.3 ± 2.5 | 4.3 ± 2.7 | 4.3 ± 2.5 | 0.90 |
| Tumour histological diagnosis, Adenoca/SCC/others | 13/7/2 | 8/2/1 | 5/5/1 | 0.37 |
Adenoca: adenocarcinoma; CCI: Charlson Comorbidity Index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; KCO: carbon monoxide transfer coefficient; LN: lymph node; RATS: robot-assisted thoracic surgery; SCC: squamous cell cancer; VATS: video-assisted thoracoscopic surgery.
Table 2:
Complications
| n (%) | VATS (n = 11) | RATS (n = 11) | |
|---|---|---|---|
| Arrhythmia | 2 (9) | – | 2 (18) |
| Pneumonia | 2 (9) | 2 (18) | – |
| Reintubation | 1 (5) | – | 1 (9) |
| Mesentery ischaemia | 1 (5) | – | 1 (9) |
| Urinary tract infection | 1 (5) | 1 (9) | – |
| Prolonged air leak | 1 (5) | 1 (9) | – |
| Wound infection | 1 (5) | 1 (9) | – |
RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracoscopic surgery.
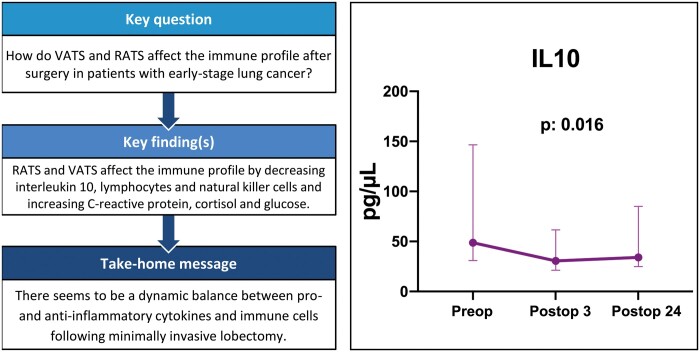
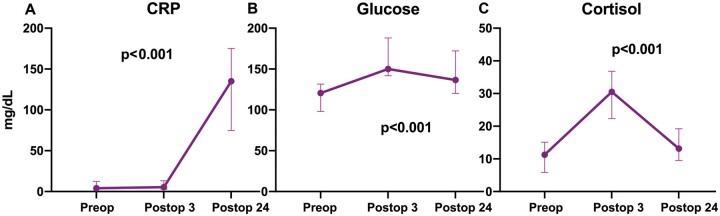
Minimally invasive surgery (RAT + VATS) significantly decreased the IL-10 (P = 0.016) level 24 h postoperatively compared to the preoperative baseline level (Fig. 1). No significant differences were detected in TNF-α (P = 0.48) and IL-8 (P = 0.15) levels before and after surgery (Fig. 1). CRP (P < 0.001), cortisol (P < 0.001) and glucose levels (P < 0.001) increased significantly postoperatively (Fig. 2). Compared to the baseline, a significant increase in the total white cell count on postoperative day 1 was detected in all patients (P < 0.01). Lymphocyte, total T cell, CD3+CD4+ and CD3+CD8+ CD16+CD56+cell counts were significantly lower on postoperative day 1 (Fig. 3). The presurgical IFN-ɣ and IL-22 cytokine production of NK cells was ∼20% above the postoperative level (Fig. 4).
Figure 1:
The change in interleukin 10, tumour necrosis factor alpha and interleukin 8 levels before and after surgery. Values are expressed as median and interquartile range (25th–75th percentiles). IL-8: interleukin 8; IL-10: interleukin 10; postop3: 3 h postoperatively; postop24: 24 h postoperatively; preop: before the operation; TNF-α: tumour necrosis factor alpha.
Figure 2:
The change in C-reactive protein, cortisol and glucose levels before and after surgery. Values are expressed as median and interquartile range (25th–75th percentiles). CRP: C-reactive protein; postop3: 3 h postoperatively; postop24: 24 h postoperatively; preop: before the operation.
Figure 3:
Leucocyte, lymphocyte and natural killer cell counts after surgery. Values are expressed as median and interquartile range (25th–75th percentiles). postop: 24 h postoperatively; preop: before the operation.
Figure 4:
Presurgical and post-surgical interferon-gamma and interleukin 22 cytokine production in natural killer cells. Values are expressed as median and interquartile range (25th–75th percentiles). IFN-ɣ: interferon-gamma; IL-22: interleukin 22; postop: 24 h postoperatively; preop: before the operation.
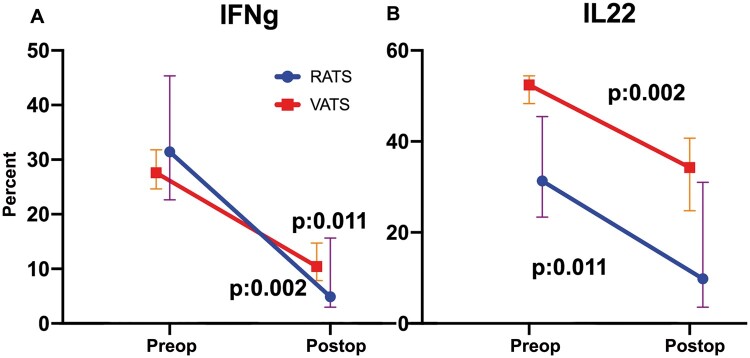
The amounts of TNF-α and IL-10 in the lung tissue sample taken during RATS compared to those in the lung tissue taken during VATS were not statistically significant (P = 0.09). No significant intragroup differences were seen with total cells, CD3+CD4+ and CD3+CD8+ CD16+CD56+ cells. Even though it was not significant, a greater reduction in IFN-ɣ- and IL-22-producing NK cells on postoperative day 1 was observed in the RATS group (Fig. 5).
Figure 5:
A reduction in interferon-gamma- and interleukin 22-producing natural killer cells on postoperative day 1 in robot-assisted thoracic surgery and video-assisted thoracoscopic surgery groups. Values are expressed as median and interquartile range (25th–75th percentiles). IFN-ɣ: interferon-gamma; IL-22: interleukin 22; postop: 24 h postoperatively; preop: before the operation; RATS: robot-assisted thoracic surgery; VATS: video-assisted thoracoscopic surgery.
DISCUSSION
We found (i) an increase in CRP, glucose and cortisol levels and neutrophil count after surgery in both groups; (ii) a decrease in IL-10 levels and in lymphocyte, total T cell, CD3+CD4+ and CD3+CD8+ CD16+CD56+cell counts after surgery in both groups; and (iii) no significant differences in acute phase and immune responses between the RATS and VATS groups.
Surgical trauma can cause a systemic inflammatory response by increasing cytokines such as TNF-α, IL-6 and IL-1 and by suppressing lymphocytes, CD8+ T cells, CD4+ T cells and NK cells in the early postoperative period [6]. Based on our study results, surgical trauma in minimally invasive surgery, including both RATS and VATS, seems mainly to affect immune cells, which correlated with decreased IL-10 level cytokine release. Acute phase and stress-related mediators, including CRP, glucose and cortisol, were also found significantly increased after surgery. Currently, less is known about the possible link between the acute phase response in minimally invasive thoracic surgery and NK- and T-cell suppression. Our study results indicate that the increase in acute phase mediators may result in lymphocyte and NK-cell suppression in the early postoperative period following minimally invasive surgery.
These findings could be crucial because IL-10 is an anti-inflammatory cytokine and mostly suppresses T-cell-mediated immunity [7]. Suppression of cell-mediated immunity leads to a decrease in the NK-cell-mediated cytotoxic antitumour response [7]. The cytolytic potential of NK cells is decreased in lung cancer [8]. An increase in IL-10 levels leads to a further decrease in the cytolytic activity of NK cells. In particular, IFN-ɣ NK cells play an essential role in the inhibition of lung cancer metastases [8]. The noticeable change in IL-10 and decrease in the percentage of IFN-ɣ NK cells following minimally invasive surgery may have a long-term effect on lung cancer survival, which needs further investigation. Elevated IL-6 and IL-8 levels are associated with an increased risk of complications [9]. The increased levels of proinflammatory cytokines may contributed to the increased levels of IL-10. Yim et al. [5] investigated the proinflammatory and anti-inflammatory cytokine effects of VATS lobectomy versus open surgery in 36 patients with stage I NSCLC. They showed significantly reduced levels of IL-10, IL-6 and IL-8 in the VATS group postoperatively. Interestingly, we detected non-significant changes in TNF-α and IL-8 cytokine levels before and after surgery that may be related to decreased IL-10 levels following surgery. Reduced proinflammatory cytokine levels following surgery may also be related to positive outcomes of minimally invasive surgery shown in previous reports such as shorter hospital stay, improved tolerance to subsequent adjuvant chemotherapy and a low complication rate [2].
Comorbidity indices, findings such as the dimensions of the tumour, clinical and pathological staging and complication rate may influence the degree of the immunosuppression. A reduction in IFN-ɣ- and IL-22-producing NK cells on postoperative day 1 was observed more frequently in the RATS group. However, the members of the RATS group were older and their pulmonary function test results were lower compared to those in the VATS group; a reduction in IFN-ɣ and IL-22-producing NK cells may be due to the differences detected in the RATS group.
Our study has several limitations. First, the sample size was small, and our results only reflect the experience of a single centre. Second, it is not a randomized trial, so the outcomes may be affected by selection bias. A short follow-up period was the other limitation. This is a pilot study, and it is too early to make any precise conclusions. However, there seems to be a dynamic balance between pro- and anti-inflammatory cytokines and immune cells following a minimally invasive lobectomy. If minimally invasive surgical modalities (both RATS and VATS) make a positive difference in host immunity responses, it may affect the complication rate in the short term and relapse and survival in the long term. Therefore, the selection of the appropriate surgical technique from among the available surgical modalities may play a role in the clinical response to the treatment of early-stage lung cancer.
Funding
This work was supported by research grants from the Scientific and Technological Research Council of Turkey (TUBITAK) (Grant no: 118S779).
Conflict of interest: none declared.
Author contributions
Suat Erus: Conceptualization; Writing—original draft. Ayşe Bilge Öztürk: Writing—original draft. Özgür Albayrak: Lab work. Said İncir: Lab work. Murat Hüseyin Kapdağlı: Formal analysis. Ekin Ezgi Cesur: Resources. Ömer Yavuz: Methodology. Serhan Tanju: Conceptualization; Writing—review & editing. Şükrü Dilege: Conceptualization; Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Giuseppe Cardillo, Alper Toker and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- CRP
C-reactive protein
- IL-10
Interleukin 10
- IL-8
Interleukin 8
- IFN-ɣ
Interferon-gamma
- MACS
Magnetic-activated cell-sorting
- NK
Natural killer
- NSLC
Non-small-cell lung cancer
- PBS
Phosphate-buffered saline
- RATS
Robot-assisted thoracic surgery
- TNF-α
Tumour necrosis factor alpha
- VATS
Video-assisted thoracoscopic surgery
REFERENCES
- 1. Mack MJ, Hazelrigg SR, Landreneau RJ, Naunheim KS. The first international symposium on thoracoscopic surgery. Ann Thorac Surg 1993;56:605–806. [Google Scholar]
- 2. Ng CS, Wan IY, Yim AP. Impact of video-assisted thoracoscopic major lung resection on immune function. Asian Cardiovasc Thorac Ann 2009;17:426–32. [DOI] [PubMed] [Google Scholar]
- 3.Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: a meta-analysis. Medicine (Baltimore) 2019;98:e17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li C, Hu Y, Huang J, Li J, Jiang L, Lin H et al. Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB-IIIA non-small cell lung cancer. Transl Lung Cancer Res 2019;8:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yim AP, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243–7. [DOI] [PubMed] [Google Scholar]
- 6. Craig SR, Leaver HA, Yap PL, Pugh GC, Walker WS. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455–63. [DOI] [PubMed] [Google Scholar]
- 7. Tsuruma T, Yagihashi A, Torigoe T, Sato N, Kikuchi K, Watanabe N et al. Interleukin-10 reduces natural killer sensitivity and downregulates MHC class I expression on H-ras-transformed cells. Cell Immunol 1998;184:121–8. [DOI] [PubMed] [Google Scholar]
- 8. Aktaş ON, Öztürk AB, Erman B, Erus S, Tanju S, Dilege Ş. Role of natural killer cells in lung cancer. J Cancer Res Clin Oncol 2018;144:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wan S, LeClerc JL, Vincent JL. Cytokine responses to cardiopulmonary bypass: lessons learned from cardiac transplantation. Ann Thorac Surg 1997;63:269–76. [DOI] [PubMed] [Google Scholar]