Abstract
OBJECTIVES
Venoarterial extracorporeal life support (ECLS) has emerged as a potentially life-saving treatment option in therapy-refractory cardiocirculatory failure, but longer-term outcome is poorly defined. Here, we present a comprehensive follow-up analysis covering all major organ systems.
METHODS
From February 2012 to December 2016, 180 patients were treated with ECLS for therapy-refractory cardiogenic shock or cardiac arrest. The 30-day survival was 43.9%, and 30-day survivors (n = 79) underwent follow-up analysis with the assessment of medium-term survival, quality of life, neuropsychological, cardiopulmonary and end-organ status.
RESULTS
After a median of 1.9 (1.1–3.6) years (182.4 patient years), 45 of the 79 patients (57.0%) were alive, 35.4% had died and 7.6% were lost to follow-up. Follow-up survival estimates were 78.0% at 1, 61.2% at 3 and 55.1% at 5 years. NYHA class at follow-up was ≤II for 83.3%. The median creatinine was 1.1 (1.0–1.4) mg/dl, and the median bilirubin was 0.8 (0.5–1.0) mg/dl. No patient required dialysis. Overall, 94.4% were free from moderate or severe disability, although 11.1% needed care. Full re-integration into social life was reported by 58.3%, and 39.4% were working. Quality of life was favourable for mental components, but a subset showed deficits in physical aspects. While age was the only peri-implantation parameter significantly predicting medium-term survival, adverse events and functional status at discharge or 30 days were strong predictors.
CONCLUSIONS
This study demonstrates positive medium-term outcome with high rates of independence in daily life and self-care but a subset of 10–20% suffered from sustained impairments. Our results indicate that peri-implantation parameters lack predictive power but downstream morbidity and functional status at discharge or 30 days can help identify patients at risk for poor recovery.
Keywords: Extracorporeal life support, Outcome, Quality of life
INTRODUCTION
Extracorporeal life support (ECLS) has emerged as a potentially life-saving treatment in therapy-refractory cardiocirculatory failure. It can be instituted rapidly, on-site, achieves full cardiopulmonary support, restores end-organ perfusion and serves as a bridging device [1–3]. Caseloads are rising worldwide, supraregional networks have been established and short-term results are promising [2–4].
However, resource demands are tremendous while no comprehensive data on longer-term survival, downstream quality of life and functional status exist. Identifying parameters to refine patient selection, predict functional outcome and improve results is highly relevant.
Here, we present a comprehensive follow-up analysis of medium-term outcome of patients treated with ECLS for severe cardiocirculatory failure covering all major organ systems.
METHODS
Study design
We present a retrospective cohort study with cross-sectional follow-up. Since establishment of our ECLS program in February 2012 through December 2016, 180 patients were treated with femoral venoarterial ECLS for therapy-refractory cardiogenic shock (CS) or cardiac arrest with ongoing cardiopulmonary resuscitation (CPR). Therapy-refractory CS was defined as impossibility to maintain haemodynamics despite escalating doses of vasopressors or inotropes with end-organ hypoperfusion due to cardiac causes.
All patients surviving the initial 30 days after ECLS implantation were included in this study and underwent follow-up. Patients younger than 18 years, postcardiotomy cases and non-cardiac aetiologies were excluded. The primary end-point was medium-term survival. Secondary end-points were functional status characteristics at follow-up.
Retrospective baseline, discharge and 30-day data were obtained from a centre-specific database. Standardized follow-up examinations were performed from July 2017 to June 2018. Besides survival, a comprehensive status was obtained, covering all major organ systems.
A demographic questionnaire assessed adverse events since discharge, current health, work status and social life. Degree of dependence was quantified using the modified Rankin Scale (mRS), a functional outcome disability scale [range 0 (no symptoms)–6 (death)]. For health-related quality of life (HRQL) evaluation, patients completed the 36-Item Short Form Survey (SF-36, version 2, German). Thirty-six questions translate into 8 domains [physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), mental health (MH)] and 2 sum-scales [physical component summary (PCS), mental component summary (MCS); scores 0–100, normal range 40–60, higher scores indicate better HRQL].
The National Institutes of Health Stroke Scale (NIHSS), the Montreal Cognitive Assessment test (MoCA) and the Beck Depression Inventory-II (BDI-II) assessed the neurocognitive and psychological status (German versions). The NIHSS quantitatively measures neurological deficits, 0 indicates normal and higher scores indicate impairment. The MoCA evaluates cognitive function. Lower scores imply greater impairment, 30 is the maximum and 26 is the cut-off. One-point correction was applied for education ≤12 years. The BDI-II screens for and assesses the severity of depression. Scores range from 0 to 63 (0–8 no depression, higher scores more severe depression).
A transthoracic echocardiogram and 12-lead electrocardiogram were obtained. A 6-minute walk test (6MWT) assessed the cardiopulmonary exercise capacity.
Comprehensive blood work was obtained with focus on renal and liver function.
In case patients declined clinical examination, they were asked to complete the questionnaires via mail. For deceased patients, duration of survival and cause of death were charted. Besides comprehensively describing outcome, we correlated peri-implantation to follow-up parameters to identify the predictors of medium-term survival and beneficial functional outcome.
The study conforms to the Declaration of Helsinki and was approved by the institutional ethics committee. All patients provided informed consent.
ECLS treatment
Evaluation for ECLS implantation is performed by an interdisciplinary heart-team with special focus on general condition, comorbidities, arterial blood gas parameters and time from collapse to initiation of CPR, duration and quality of CPR, as applicable. ECLS implantation is declined in case of evidence of fatal brain damage or comorbidities significantly limiting life expectancy. The standard at our centre is percutaneous femoral cannulation using Seldinger technique, including insertion of a distal leg perfusion catheter into the superficial femoral artery. Details have been published earlier [1]. Implantation is performed on-site, including the emergency department, catheterization laboratory and intensive care unit (ICU). If patients in remote hospitals are too unstable for conventional transport, a mobile team is sent out for cannulation there and air or ground-bound patient retrieval on ECLS. We aim for a cardiac index of 2.4 l/min/m2 and titrate the blood flow according to the individual needs. Subsequent interdisciplinary care follows current guidelines [5, 6]. Preserving pulsatility is pursued to avoid pulmonary oedema, intracardiac stasis or myocardial distension. If this is not achievable by conventional means such as inotropes, left ventricular venting is considered [Impella® (Abiomed®, Danvers, USA) implantation, interventional atrioseptostomy or surgical vent-implantation]. In the setting of a recovering cardiac function while the pulmonary function still is severely compromised, we consider adding an outflow cannula to the right internal jugular vein and eventually transitioning to venovenous-ECMO. ECLS is used as a bridging device. In general, weaning is pursued. If the heart does not recover, patients are evaluated for ventricular assist device (VAD) implantation or heart transplantation (HTx) [1].
Statistics
Quantitative variables are presented as medians with interquartile ranges, and categorical variables as frequencies and percentages. Quantitative variables were screened for normal distribution using box plots and the Shapiro–Wilk test, where violations against normality were observed. Follow-up survival probabilities were estimated using Kaplan–Meier plots. Survival and censoring times were calculated from implantation to death or last known follow-up. Differences in survival were assessed using the logrank test. Cox-regression modelling was used to examine the effects of peri-implantation parameters, parameters related to ECLS therapy and status at 30 days or hospital discharge on medium-term survival. To avoid bias, survival times were calculated as the time from implantation for peri-implantation parameters, and as the time from 30 days or hospital discharge for parameters related to the course of ECLS therapy and clinical status at 30 days or discharge, respectively. In addition, regression analyses were performed to assess the influence of peri-implantation parameters on the degree of dependence (ordinal logistic regression), physical, neurocognitive and psychological status (Poisson regression). SF-36 data were tested for differences between our patients and the German reference population (PF = 2908 subjects, RP = 2900, BP = 2905, GH = 2913, VT = 2888, SF = 2911, RE = 2899, MH = 2900; PCS = 2861, MCS = 2861) using quasi-binomial regression, adjusted for age and sex [7]. Since quasi-binomial regression is only applicable if variables range between 0 and 1, values were divided by 100 prior to calculation. Statistical analyses were performed with R, version 3.5.0, and SPSS Statistics, version 25, by a biostatistician. The significance level was set to 0.05.
RESULTS
Study population
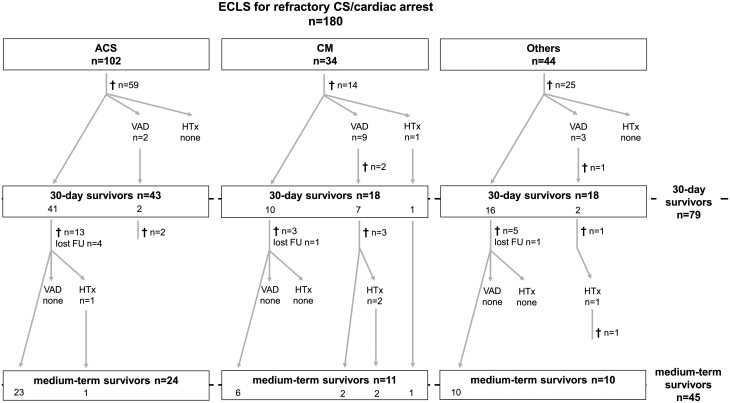
From February 2012 until December 2016, 180 patients (18.9% female) were treated with ECLS for therapy-refractory CS or cardiac arrest with ongoing CPR. The median age was 56.0 years (46.0–66.0). Aetiologies included acute coronary syndrome (ACS, 56.7%), cardiomyopathies (18.9%), pulmonary embolism (8.3%), myocarditis (6.1%) and other cardiac causes (10.0%). In total, 70.0% (n = 126) had been resuscitated, among these were 59 ECPR cases (32.8% of all patients). Seventy-nine patients (43.9%) survived the first 30 days and underwent further analysis and follow-up. Figure 1 provides a detailed flow-diagram of all patients.
Figure 1:
Patient flow diagram. ACS: acute coronary syndrome; CM: cardiomyopathy; CS: cardiogenic shock; ECLS: extracorporeal life support; HTx: heart transplantation; lost FU: lost to follow-up; VAD: ventricular assist device.
Peri-implantation characteristics of the 30-day survivors are given in Table 1, and clinical course after ECLS implantation, status at hospital discharge and at 30 days are given in Table 2. Briefly, the median duration of ECLS was 109.8 (71.9–162.0) h. Adverse events occurred in 68.4%, and the most frequent was necessity of renal replacement therapy (RRT), neurological complications and sepsis. During the index hospitalization, 15.2% underwent VAD implantation [n = 11, 6 left (LVAD), 5 biventricular assist devices] or HTx (n = 1). The median time from ECLS to VAD implantation was 6.0 (5.0–10.0) days. Total hospital stay was 24.0 (19.0–37.0) days, whereas 5.1% died in-hospital after 30 days. A total of 44.3% were discharged to another hospital for continued care, 39.2% to rehabilitation facilities and 11.4% were discharged home. After 30 days, 54.4% had an mRS of ≥3, indicating moderate-to-severe degree of dependence. Intermittent or full ventilation was still required by 25.3% and 11.4%, respectively, and RRT by 11.5%.
Table 1:
Peri-implantation parameters as predictors of medium-term survival
| n | Median (IQR) or frequency (%) | HR | P-value | |
|---|---|---|---|---|
| Age (years) | 79 | 51.0 (43.0–60.0) | 1.06 | <0.001 |
| Female | 79 | 18 (22.8) | 1.05 | 0.91 |
| Out-of-centre implantation | 79 | 27 (34.2) | 1.12 | 0.78 |
| Awake at implantation | 79 | 11 (13.9) | 1.00 | 1.00 |
| Aetiology | ||||
| Acute coronary syndrome | 79 | 43 (54.4) | 0.88 | 0.73 |
| Cardiomyopathy/myocarditis | 79 | 24 (30.4) | 1.16 | 0.72 |
| Others | 79 | 12 (15.2) | 1.02 | 0.98 |
| INTERMACS | 79 | 1.0 (1.0–2.0) | 0.89 | 0.79 |
| CPR | 79 | 49 (62.0) | 1.04 | 0.92 |
| Implantation during CPR (ECPR) | 79 | 15 (19.0) | 0.61 | 0.36 |
| pH pre-implantation | 78 | 7.30 (7.20–7.38) | 1.55 | 0.69 |
| pH 6 h post-implantation | 78 | 7.38 (7.30–7.44) | 6.38 | 0.35 |
| Lactate pre-implantation (mmol/l) | 78 | 7.1 (3.5–12.4) | 1.00 | 0.91 |
| Lactate 6 h post-implantation (mmol/l) | 78 | 4.8 (2.4–8.7) | 0.99 | 0.83 |
| Lactate clearance (h) | 79 | 21.0 (9.0–39.0) | 1.00 | 0.96 |
| Maximum bilirubin within initial 24 h (mg/dl) | 76 | 1.8 (1.1–2.8) | 1.00 | 1.00 |
| Maximum glutamic oxaloacetic transaminase (GOT) within initial 24 h (U/l) | 76 | 455.0 (235.0–1128.5) | 1.00 | 0.61 |
| Maximum glutamic-pyruvic transaminase (GPT) within initial 24 h (U/l) | 77 | 188.0 (87.0–540.5) | 1.00 | 0.78 |
CPR: cardiopulmonary resuscitation; HR: hazard ratio; IQR: interquartile range.
Table 2:
Clinical course after ECLS implantation, status at hospital discharge and at 30 days as predictors of medium-term survival
| n | Median (IQR) or frequency (%) | HR | P-value | |
|---|---|---|---|---|
| Course of hospitalization | ||||
| Duration of support (h) | 79 | 109.8 (71.9–162.0) | 1.00 | 0.46 |
| VAD implantation | 79 | 11 (13.9) | 2.57 | 0.03 |
| Heart transplantation | 79 | 1 (1.3) | <0.001 | 1.00 |
| Patients with adverse events | 79 | 54 (68.4) | 2.50 | 0.06 |
| ECLS-related bleeding | 79 | 6 (7.6) | 0.42 | 0.40 |
| Distal leg ischaemia | 79 | 4 (5.1) | <0.001 | 1.00 |
| Cerebral complication | 79 | 24 (30.4) | 2.38 | 0.02 |
| RRT | 78a | 33 (42.3) | 2.25 | 0.04 |
| Sepsis | 79 | 19 (24.1) | 1.93 | 0.10 |
| Bowel ischaemia | 79 | 2 (2.5) | 1.44 | 0.72 |
| Duration of ventilation (days) | 79 | 13.0 (8.0–22.0) | 1.04 | 0.002 |
| Tracheostomy | 79 | 43 (54.4) | 2.46 | 0.04 |
| ICU stay (days) | 79 | 20.0 (15.0–32.0) | 1.02 | 0.05 |
| Hospital stay (days) | 79 | 24.0 (19.0–37.0) | 1.01 | 0.41 |
| Discharge | ||||
| Home | 79 | 9 (11.4) | 0.35 | 0.30 |
| To rehab | 79 | 31 (39.2) | 0.25 | 0.01 |
| To other hospital | 79 | 35 (44.3) | 4.75 | 0.002 |
| Died in-hospital after 30 days | 79 | 4 (5.1) | na | na |
| Status at 30 days | ||||
| Pulmonary status at 30 days | ||||
| Not ventilated | 79 | 50 (63.3) | 0.27 | <0.001 |
| Intermittently ventilated | 79 | 20 (25.3) | 1.68 | 0.20 |
| Fully ventilated | 79 | 9 (11.4) | 5.07 | <0.001 |
| Renal status at 30 days | ||||
| No treatment | 78a | 11 (14.1) | 0.52 | 0.37 |
| Oral diuretics | 78a | 58 (74.4) | 1.15 | 0.77 |
| IV diuretics | 78a | 0 | na | na |
| RRT | 78a | 9 (11.5) | 1.37 | 0.56 |
| Liver status at 30 days | ||||
| Unremarkable | 79 | 23 (29.1) | 0.26 | 0.03 |
| Elevated bilirubin/GOT/GPT | 79 | 51 (64.6) | 1.22 | 0.63 |
| Complicationb | 79 | 5 (6.3) | 59.79 | <0.001 |
| Degree of dependence at 30 days | ||||
| Rankin Scale ≤3 | 79 | 36 (45.6) | 0.07 | <0.001 |
| Rankin Scale ≥3 | 79 | 43 (54.4) | 15.20 | <0.001 |
One patient with chronic dialysis pre-ECLS was excluded.
Includes any complication related to the hepatobiliary system, e.g. non-cardiogenic shock-related liver failure or secondary sclerosing cholangitis.
HR: hazard ratio; ICU: intensive care unit; IQR: interquartile range; na: not applicable; RRT: renal replacement therapy; VAD: ventricular assist device.
Medium-term survival
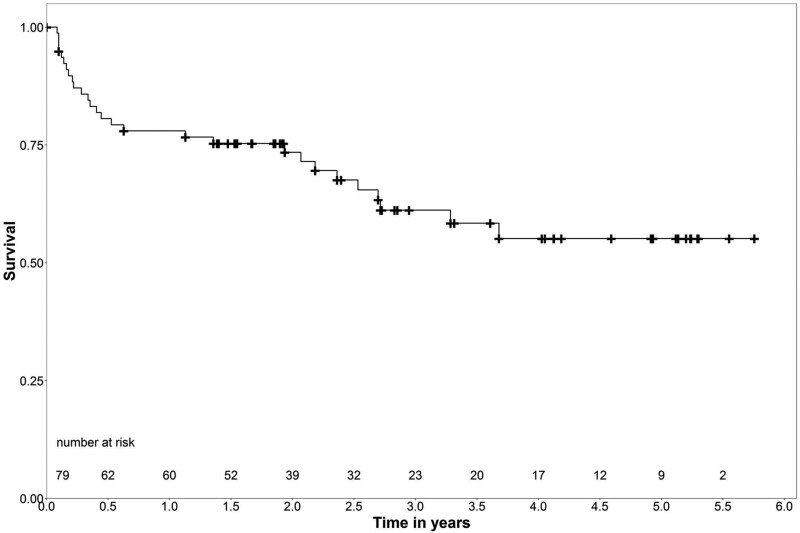
Medium-term survival of the 30-day survivors is shown in Fig. 2. The median follow-up was 1.9 (1.1–3.6, range 0.1–5.8) years, with an overall follow-up of 182.4 patient years. The logrank test showed no significant differences in survival between the aetiologies ACS, cardiomyopathies and others (P = 1.0). The median survival free from VAD implantation or HTx was 1.7 (0.1–2.9) years (range 2 days to 5.3 years).
Figure 2:
Medium-term survival of the 30-day survivors.
Six patients (7.6%) were lost to follow-up after 1.5 (0.1–2.4) years, and 28 (35.4%) had died 0.4 (0.2–2.2) years after ECLS implantation (4 during the index hospitalization after 30 days). Causes of death were available for 21 patients; for 7, the cause remained unclear. Two patients died of cardiac causes. Seven died of multiorgan failure and another 2 of multiorgan failure after having declined VAD implantation or transplantation. Four patients died secondary to infection or sepsis, and 3 due to neurological causes. One patient died from liver failure, 1 from ARDS and another of acute rejection after HTx.
Forty-five patients (57.0%) were alive and contacted 2.9 (1.9–4.8) years after ECLS implantation (the total follow-up time for these patients was 143.4 patient years). The follow-up survival estimates were 78.0% at 1 year, 61.2% at 3, and 55.1% at 5 years.
Peri-implantation parameters, data on ECLS therapy and status at hospital discharge and at 30 days were analysed as predictors of survival beyond 30 days. Older age detrimentally affected the medium-term survival [hazard ratio (HR) 1.06, p < 0.001]. Other peri-implantation parameters had no significant influence on medium-term survival (Table 1), however, the clinical course during ECLS therapy until discharge from the primary hospital and the status at 30 days did (Table 2). Adverse events were strong predictors of non-survival, especially cerebral complications (HR 2.38, P = 0.02) and requirement of RRT (HR 2.25, P = 0.04). Increased duration of ventilation had a negative influence (HR 1.04, P = 0.002), and so did necessity of tracheostomy (HR 2.46, P = 0.04). Requirement of VAD implantation also negatively influenced survival (HR 2.57, P = 0.03). Besides, discharge disposition and organ function at 30 days strongly predicted the medium-term survival.
Comprehensive status at follow-up
Thirty-six of the 45 patients (80.0%) alive at follow-up consented to comprehensive follow-up analyses. Among the 9 patients alive at follow-up but declining study participation, CS leading to ECLS implantation was caused by ACS in 5 and cardiomyopathies in 4. None of these patients had undergone VAD implantation or HTx. Four had required CPR (44.4%), 1 of them (11.1%) was an ECPR case at the time of ECLS implantation.
For the 36 patients participating in the study, CS had been caused by ACS in 52.8% (n = 19), by cardiomyopathies in 19.4% (n = 7), by pulmonary embolism in 13.9% (n = 5), by myocarditis in 8.3% (n = 3) and by other cardiac reasons in 5.6% (n = 2). Twenty-four (66.7%) had been resuscitated and 10 of them (27.8%) were ECPR cases. While 31 patients presented for examination, 5 declined clinical examination but completed the questionnaires (general questionnaire, SF-36, BDI-II). Thus, unless otherwise specified, for general and questionnaire data, the sample size was 36, and for other data, it was 31. Only 2 patients (5.6%) were actively listed for HTx or considered for transplantation or VAD implantation at the time of follow-up. Cross-sectional follow-up analyses were performed 2.6 (1.7–4.0) years after ECLS implantation (102.0 patient years) (for details see Table 3). At follow-up, the median age was 53.5 (42.0–58.0) years. Of all patients, 36.1% were New York Heart Association class (NYHA class) I and 47.2% class II. At the time of follow-up, 2 of the 36 patients were on LVAD support (both implanted during the index hospitalization). Four were status post-HTx, 1 had undergone transplantation during the index hospitalization, 3 during the follow-up period, and 2 of them did have VADs implanted during the index hospitalization (Fig. 1). Most of these were patients with cardiomyopathy. The median time since VAD implantation or HTx (i.e. the last major procedure) was 3.4 (2.4–5.1, minimum 1.8) years. Overall, 94.4% were free from moderate or severe disability (mRS ≤2). To need any kind of care was reported by 11.1%. When compared to prior to ECLS, 58.3% felt fully re-integrated into their social life. Of those younger than 65 years, 39.4% were working full or part time, 21.2% were not working for any reason and 39.4% were retired.
Table 3:
Status at follow-up
| n | Median (IQR) or frequency (%) | |
|---|---|---|
| General condition | ||
| Age (years) | 36 | 53.5 (42.0–58.0) |
| Female | 36 | 9 (25.0) |
| Body mass index (BMI) (kg/m2) | 36 | 26.7 (23.2–30.0) |
| NYHA class | 36 | 2.0 (1.0–2.0) |
| Number of drugs currently taken | 36 | 8.0 (5.0–10.0) |
| Been to rehab | 36 | 35 (97.2) |
| Hospitalizations since ECLS | 36 | 2.0 (1.0–4.0) |
| Downstream adverse events | ||
| Myocardial infarction | 36 | 2 (5.6) |
| Stroke | 36 | 1 (2.8) |
| Downstream procedures | ||
| PCI | 36 | 5 (13.9) |
| Mitraclip | 36 | 0 |
| VAD implantation | 36 | 0 |
| Heart transplantation | 36 | 3 (8.3) |
| In need of care | 36 | 4 (11.1) |
| Degree of dependence (mRS) | 36 | 1.0 (1.0–2.0) |
| Health-related quality of life | ||
| SF-36 | ||
| Physical functioning | 36 | 72.5 (50.0–88.8) |
| Role physical | 36 | 62.5 (0.0–100.0) |
| Bodily pain | 36 | 80.0 (51.3–100.0) |
| General health | 36 | 52.0 (35.0–67.0) |
| Vitality | 36 | 50.0 (35.0–68.8) |
| Social functioning | 36 | 87.5 (62.5–100.0) |
| Role emotional | 36 | 100.0 (33.3–100.0) |
| Mental health | 36 | 76.0 (56.0–84.0) |
| Physical component summary | 36 | 45.2 (33.3–52.0) |
| Mental component summary | 36 | 52.9 (41.8–55.2) |
| Neurocognitive and psychological status | ||
| BDI-II score | 36 | 8.5 (2.3–18.8) |
| MoCA score | 29 | 26.0 (22.5–28.0) |
| NIHSS score | 27 | 1.0 (1.0–2.0) |
| Cardiopulmonary function | ||
| EKG | ||
| Sinus rhythm | 26 | 25 (96.2) |
| Atrial fibrillation | 26 | 1 (3.8) |
| LBBB | 26 | 3 (11.5) |
| Echocardiography | ||
| Left ventricular enddiastolic diameter (LVEDD) (mm) | 26 | 53.0 (46.8–63.0) |
| Ejection fraction (EF) (%) | 26 | 49.0 (37.3–55.3) |
| TAPSE (mm) | 26 | 20.0 (18.0–23.0) |
| Percentage of predicted 6MWD (%) | 21 | 68.8 (61.7–91.6) |
| End-organ function | ||
| Creatinine (mg/dl) | 31 | 1.1 (1.0–1.4) |
| Urea (mg/dl) | 31 | 43.0 (33.0–60.0) |
| Cystatin C (mg/l) | 31 | 1.2 (0.9–1.4) |
| GFR (ml/min) | 31 | 91.0 (69.0–99.0) |
| Bilirubin (mg/dl) | 31 | 0.8 (0.5–1.0) |
| GOT(U/l) | 31 | 28.0 (25.0–34.0) |
| GPT (U/l) | 31 | 26.0 (19.0–40.0) |
BDI-II: Beck Depression Inventory-II; IQR: interquartile range; LBBB: left bundle branch block; 6MWD: 6-minute walk distance; MoCA: Montreal Cognitive Assessment test; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; PCI: percutaneous coronary intervention; SF-36: 36-Item Short Form Survey; VAD: ventricular assist device.
Health-related quality of life
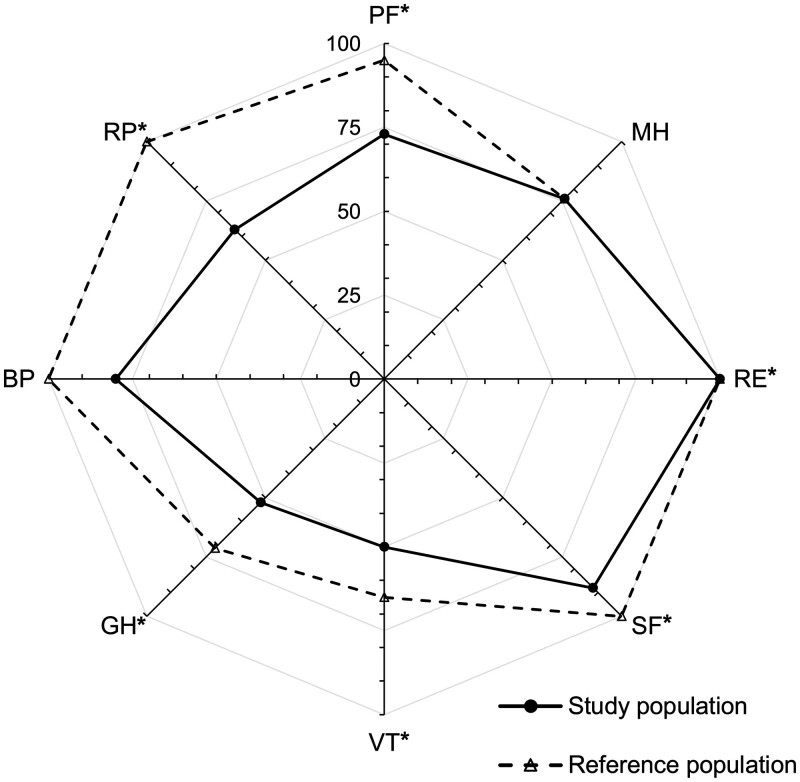
The medians of all domains and sum-scales were within or higher than the normal range (Table 3). The percentages of individual patients scoring below the normal range were 13.9% for PF, 44.4% for RP, 11.1% for BP, 30.6% for GH, 30.6% for VT, 19.4% for SF, 27.8% for RE, 11.1% for MH, 38.9% for PCS and 19.4% for MCS. For comparison, Fig. 3 provides age- and gender-matched German reference population data with expected significant differences for all components except BP and MH.
Figure 3:
SF-36 domains. *Statistically significant differences (P < 0.05). BP: bodily pain; GH: general health; MH: mental health; PF: physical functioning; RE: role emotional; RP: role physical; SF: social functioning; VT: vitality.
Neurocognitive and psychological status
Per BDI-II, 50.0% showed no signs of depression, 27.8% minimal or slight depression, 11.1% moderate and 11.1% signs of severe depression. We obtained NIHSS and MoCA data for 27 and 29 patients with the scores of 1.0 (1.0–2.0) and 26.0 (22.5–28.0), respectively. On the NIHSS, 55.6% had ≤1 point, 22.2% 2 points and 22.2% >2 points.
Cardiopulmonary function
Patients on VAD support or after HTx were excluded from this analysis (n = 26 included). Elektrocardiogram (EKG) analysis revealed sinus rhythm in 96.2%, atrial fibrillation in 3.8% and 11.5% had complete left bundle branch block. The median left ventricular ejection fraction was 49.0 (37.3–55.3)% and tricuspid annular plane systolic excursion (TAPSE) 20.0 (18.0–23.0) mm. Assessment of functional exercise capacity (6MWT) was unfeasible for musculoskeletal reasons in 5 cases. Twenty-one patients underwent the 6MWT, and 2 had to terminate early due to dizziness or dyspnoea. Percentages of predicted 6MW distances were 68.8 (61.7–91.6)% for all patients and 68.9 (65.7–93.9)% when excluding those with early termination.
Renal and liver function
No patient required dialysis. The median creatinine was 1.1 (1.0–1.4) mg/dl, glomerular filtration rate (GFR) 91.0 (69.0–99.0) ml/min and bilirubin 0.8 (0.5–1.0) mg/dl.
Predictive value
We analysed age at ECLS implantation, gender, INTERMACS level and prior or ongoing CPR as predictors of mRS, BDI-II, PCS and MCS. P-values were adjusted for multiple testing (Bonferroni–Holm procedure). INTERMACS level, extracorporeal cardiopulmonary resuscitation (ECPR) and gender correlated with BDI-II results (adjusted P = 0.003, P = 0.03 and P < 0.001, respectively). Besides, no significant results were observed.
DISCUSSION
ECLS has become a standard of care for critically ill patients in severe cardiocirculatory failure [2]. Short-term outcome and its predictors have been described [1–4]. However, little is known about survival beyond 30 days, functional status, or HRQL. All these are acknowledged, highly relevant outcome measures. Here, we present a comprehensive follow-up analysis and demonstrate favourable medium-term outcome, despite high rates of early functional impairments.
In our cohort, the 30-day survival was 43.9%. Despite high early hospital mortality, the medium-term survival of the 30-day survivors was favourable, with the survival estimates of 78.0% at 1 year, 61.2% at 3 years and 55.1% at 5 years. Most deaths occurred within the initial 6 months with stable survival thereafter. Presumably, several of these early deaths are related to the index event, adverse events and prolonged recovery. This highlights the importance of intensified care and follow-up within the initial months as a vulnerable period after CS and ECLS treatment. When interpreting these survival rates, 2 aspects are essential. First, patients in CS refractory to best conventional therapy or under CPR without return of spontaneous circulation almost always face certain death; thus, outcome is to be interpreted in the light of an otherwise dismal prognosis. Second, many suffer from chronic heart failure or typical comorbidities limiting life expectancy per se, and some will require VAD implantation or HTx with associated complications and mortality. Survival especially after 6 months is good, demonstrating that early mortality is the Achilles-heel in ECLS [8].
Objective measures to predict survival beyond 30 days and favourable functional outcome are crucial to guide treatment. While 30-day survival is the first milestone, long-term survival free from functional impairment is what remains to be achieved. In earlier studies, peri-implantation parameters predicted short-term outcome, and especially age, CPR, end-organ failure and metabolic parameters correlated with early mortality [1, 3, 4, 9, 10].
In this analysis focusing on survival beyond 30 days, age at implantation was the only baseline parameter significantly influencing medium-term survival. As age will always impact survival, this cannot necessarily be considered a true ECLS-related finding, although de Waha et al. [11] demonstrated inferior outcome for patients older than 60 years. Elderly patients exhibit poorer organ reserve and reduced capability of functional improvement, with lower likelihoods of permanently recovering from CS [11]. Interestingly, in our study, parameters indicating the severity of cardiocirculatory failure such as INTERMACS level, CPR and parameters describing the process of surmounting it, e.g. lactate clearance, did not predict medium-term survival. The SAVE score was created to predict in-hospital survival and is the most commonly used tool [3]. It is not validated for ECPR, which applied to ∼20% of our cohort. We tested several of its components but except for age none of the tested items predicted the medium-term survival. The ENCOURAGE score predicts the ICU survival of patients treated with ECLS for acute myocardial infarction and CS [10]. Survival probabilities are also provided for 6 months, but according to our findings, this still comprises early mortality. Presumably, peri-implantation factors impact early survival, but after stabilization is achieved and the hyper-acute phase is overcome, their effects lose and downstream events gain impact. Adverse events during and after ECLS are common and strongly correlate with outcome as they cause relevant morbidity and mortality. While these findings cannot assist in triaging the patients for or against ECLS implantation, the parameters can assist in identifying patients at risk for poor recovery and trigger intensified rehabilitation. In addition, this underscores the necessity of highly trained interdisciplinary teams, permanent availability of full-spectrum diagnostic and therapeutic facilities to prevent or manage adverse events and raises a call for the definition of competence centres, minimum institutional infrastructure standards and caseloads [2, 12].
About 23% of the ACS and ‘other’ aetiologies cohort patients were alive at follow-up without having received HTx or VAD implantation. For the cardiomyopathy cohort, 14.7% of the medium-term survivors had needed transplantation or VAD. This could indicate that, for some ACS patients, ECLS on the one hand allowed for high-risk PCI, and on the other hand that controlled reperfusion using ECLS after revascularization may have contributed to a recovery in function sufficient for persistent weaning. A similar conclusion could be true for ‘other’ aetiologies. This category includes patients with myocarditis or pulmonary embolism, which may have more potential for sustained recovery. However, since several patients deceased and were not investigated further, prospective, larger studies are necessary for definite conclusions.
Our patients’ overall status at follow-up was positive. The median degree of dependence was an mRS level of 1, equalling no significant disability and being able to carry out all usual daily activities despite some symptoms. Altogether, 94.4% were free from moderate or severe disability and only 11.1% required care. The majority was in NYHA class I–II. Left ventricular ejection fraction was robust with good right ventricular function. Although 26.9% were unable to perform the 6MWT or had to terminate early, functional capacity of the other patients was satisfactory. End-organ functions had largely recovered.
All median HRQL scores were within or higher than the normal range. As expected, results were inferior to matched controls. However, results compared favourable to similar cohorts, patients with chronic heart failure or after recovery from severe and life-threatening conditions [13–16]. In comparison to a heart failure population, scores of our patients were higher and ranged between those of patients in NYHA class I and II [14]. The highest number of patients with scores below normal was seen for RP and thus PCS. This corresponds to the patients unable to perform the 6MWT or terminating early, and those requiring care in daily life. As the SF-36 is not disease specific, non-ECLS-related conditions could have affected the results.
Mental HRQL was satisfactory. Concurrently, 77.8% showed no or only minimal to slight depression. The median MoCA score was 26.0 (22.5–28.0). The original cut-off is 26, but derivations have been reported frequently in normative studies, and it has been considered too conservative [17, 18]. In a US study, mean scores were 23.4 ± 4.0 and 66% fell below 26 points [17]. In a German-speaking sample, scores were 26.1 ± 2.5 while 31.1% were below 26 [18]. Our results compare similar, with overall favourable results. However, 10.3% of our patients scored below 20, again indicating that a subset has relevant impairments. For the NIHSS, dichotomization at 1 has been proposed [19]. More than half of our patients scored ≤1. While the mRS is a functional outcome scale describing handicap relevant to daily activities, the NIHSS describes neurological deficits in detail. Full re-integration into social life as prior to ECLS was achieved in 58.3%. Of those younger than 65 years, 39.4% had returned to work life, while another 39.4% were retired.
Previous, less-extensive studies support our findings [9, 10, 13, 20]. Orbo et al. [13] analysed 74 adults and children with venovenous or venoarterial ECLS from 1988 to 2015 for cardiac or respiratory failure, and CPR. Twenty patients completed follow-up questionnaires (follow-up 6.5 ± 7 years). No clinical examination was performed. Global outcome was positive, with impairments in a subset: 10% were living at home with help from others and 40% were on disability pension. HRQL was comparable, whereas general health, role emotional and role physical were below normal. No relevant depression but anxiety was reported in 15% [13]. Combes et al. analysed 28 survivors with varying cannulation strategies and indications, including postcardiotomy patients. The median follow-up was only 11 months. They reported satisfactory mental health and vitality but persistent problems with work or other daily activities because of physical health, which interfered with social life [9]. In a double-centre study on acute myocardial infarction patients, 138 underwent follow-up with the analysis of HRQL, anxiety, depression and post-traumatic stress disorder [10]. After 32 (18–54) months, 41 survivors were assessed, with persisting physical and emotional difficulties in one-third [10].
In summary, our results demonstrate a positive outcome for the majority, with high rates of independence in daily life and self-care. However, a subset of ∼10–20% suffers from relevant impairments. These interfere with other functions, social and work life. Tailored rehabilitation to address physical, neurocognitive and psycho-social handicap resulting from the index or subsequent adverse events is essential, with early rehabilitation starting in the ICU. In addition, intensified follow-up care by the ECLS centre should gain relevance and will be able to further increase survival and improve functional status. The focus of care in ECLS has to be expanded from acute care to a coverage beyond 30 days.
Limitations
This study provides a comprehensive follow-up, and several assessments have never been reported until now. However, follow-up was performed at only 1 occasion in a cross-sectional manner, resulting in varying follow-up times. All regression analyses considered follow-up time to account for this to the most possible extent. Six patients were lost to follow-up, and 9 survivors declined participation in the study. This impairs survival analyses mildly. However, the clinical status of these patients remains unclear, and we cannot exclude an incomplete representation of the clinical condition due to selection bias. In addition, the functional status prior to ECLS is unknown, and most assessments were non-disease specific. These factors limit the generalizability of our results. Lastly, studies covering greater implantation periods and patient numbers are warranted to confirm our findings.
CONCLUSION
While short-term survival is the initial landmark in ECLS, long-term survival free from functional impairment remains the ultimate therapy goal. In this comprehensive follow-up analysis, medium-term outcome of patients treated with ECLS for cardiocirculatory failure was positive regarding both survival and functional status, although a high rate of early functional deficits had been observed. For 10–20%, impairments will sustain. Peri-implantation parameters lacked predictive power in this study; however, downstream morbidity and functional status at discharge or 30 days can help identify patients at risk for poor recovery. Effective prevention and management of common adverse events with related morbidity is the key to success, and establishment of competence centres to create an ideal infrastructure needs to be discussed. Individually tailored rehabilitation and close follow-up care may address the observed impairments and improve outcome further.
Conflict of interest: none declared.
Author contributions
Sabina P.W. Guenther: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Writing—original draft; Writing—review & editing. Roman Hornung: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft; Writing—review & editing. Dominik Joskowiak: Conceptualization; Data curation; Formal analysis; Methodology; Writing—review & editing. Polyxeni Vlachea: Conceptualization; Investigation; Writing—review & editing. Katharina Feil: Conceptualization; Investigation; Methodology; Writing—review & editing. Martin Orban: Conceptualization; Methodology; Writing—review & editing. Sven Peterss: Conceptualization; Methodology; Writing—review & editing. Frank Born: Conceptualization; Methodology; Writing—review & editing. Jörg Hausleiter: Conceptualization; Methodology; Writing—review & editing. Steffen Massberg: Conceptualization; Methodology; Writing—review & editing. Christian Hagl: Conceptualization; Methodology; Supervision; Writing—review & editing).
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- ACS
Acute coronary syndromes
- BDI-II
Beck Depression Inventory-II
- BP
Bodily pain
- CS
Cardiogenic shock
- CPR
Cardiopulmonary resuscitation
- ECLS
Extracorporeal life support
- GH
General health
- HRQL
Health-related quality of life
- HTx
Heart transplantation
- HR
Hazard ratio
- ICU
Intensive care units
- 6MWT
6-Minute walk test
- MCS
Mental component summary
- MH
Mental health
- mRS
Modified Rankin Scale
- MoCA
Montreal Cognitive Assessment test
- NIHSS
National Institutes of Health Stroke Scale
- PCS
Physical component summary
- PF
Physical functioning
- RRT
Renal replacement therapy
- RE
Role emotional
- RP
Role physical
- SF
Social functioning
- SF-36
36-Item Short Form Survey
- VAD
Ventricular assist device
- VT
Vitality
REFERENCES
- 1. Guenther SP, Brunner S, Born F, Fischer M, Schramm R, Pichlmaier M. et al. When all else fails: extracorporeal life support in therapy-refractory cardiogenic shock. Eur J Cardiothorac Surg 2016;49:802–9. [DOI] [PubMed] [Google Scholar]
- 2. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD. et al. ; ELSO member centers. Extracorporeal life support organization registry international report 2016. ASAIO J 2017;63:60–7. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT. et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246–56. [DOI] [PubMed] [Google Scholar]
- 4. Vdovin N, Gunther SPW, de Waha S, Seizer P, Brunner S, Schlensak C. et al. Early risk stratification in patients with cardiogenic shock complicating acute myocardial infarction treated with extracorporeal life support and primary percutaneous coronary intervention. JACC Cardiovasc Interv 2017;10:2469–71. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 6. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK. et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232–e68. [DOI] [PubMed] [Google Scholar]
- 7. Hardin JW, Hilbe JM.. Generalized Linear Models and Extensions, 2nd edn. Texas, USA: Stata Press, 2007. [Google Scholar]
- 8. Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T. et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302–11, 311.e1. [DOI] [PubMed] [Google Scholar]
- 9. Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P. et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008;36:1404–11. [DOI] [PubMed] [Google Scholar]
- 10. Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N. et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 2016;42:370–8. [DOI] [PubMed] [Google Scholar]
- 11. de Waha S, Graf T, Desch S, Fuernau G, Eitel I, Poss J. et al. Outcome of elderly undergoing extracorporeal life support in refractory cardiogenic shock. Clin Res Cardiol 2017;106:379–85. [DOI] [PubMed] [Google Scholar]
- 12. Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM. et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med 2015;191:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Orbo MC, Karlsen SF, Pedersen EP, Hermansen SE, Ronning PB, Nergaard KA. et al. Health-related quality of life after extracorporeal membrane oxygenation: a single centre's experience. ESC Heart Fail 2019;6:701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schowalter M, Gelbrich G, Stork S, Langguth JP, Morbach C, Ertl G. et al. Generic and disease-specific health-related quality of life in patients with chronic systolic heart failure: impact of depression. Clin Res Cardiol 2013;102:269–78. [DOI] [PubMed] [Google Scholar]
- 15. Spangenberg T, Schewel J, Dreher A, Meincke F, Bahlmann E, van der Schalk H. et al. Health related quality of life after extracorporeal cardiopulmonary resuscitation in refractory cardiac arrest. Resuscitation 2018;127:73–8. [DOI] [PubMed] [Google Scholar]
- 16. Hsieh FT, Huang GS, Ko WJ, Lou MF.. Health status and quality of life of survivors of extra corporeal membrane oxygenation: a cross-sectional study. J Adv Nurs 2016;72:1626–37. [DOI] [PubMed] [Google Scholar]
- 17. Rossetti HC, Lacritz LH, Cullum CM, Weiner MF.. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 2011;77:1272–5. [DOI] [PubMed] [Google Scholar]
- 18. Thomann AE, Goettel N, Monsch RJ, Berres M, Jahn T, Steiner LA. et al. The Montreal Cognitive Assessment: normative data from a German-speaking cohort and comparison with international normative samples. JAD 2018;64:643–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young FB, Weir CJ, Lees KR.. Comparison of the National Institutes of Health Stroke Scale with disability outcome measures in acute stroke trials. Stroke 2005;36:2187–92. [DOI] [PubMed] [Google Scholar]
- 20. Delmas C, Conil JM, Sztajnic S, Georges B, Biendel C, Dambrin C. et al. Early prediction of 3-month survival of patients in refractory cardiogenic shock and cardiac arrest on extracorporeal life support. Indian J Crit Care Med 2017;21:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]