Abstract
OBJECTIVES
Longer aortic cross-clamp (ACC) time is associated with decreased early survival after cardiac surgery. Because maximum follow-up in previous studies on this subject is confined to 28 months, it is unknown whether this adverse effect is sustained far beyond this term. We aimed to determine whether longer ACC time was independently associated with decreased late survival after isolated aortic valve replacement in patients with severe aortic stenosis during 25 years of follow-up.
METHODS
In this retrospective cohort study, multivariable analysis was performed to identify possible independent predictors of decreased late survival, including ACC and cardiopulmonary bypass (CPB) time, in a cohort of 456 consecutive patients with severe aortic stenosis, who had undergone isolated aortic valve replacement between 1990 and 1993.
RESULTS
Mean follow-up was 25.3 ± 2.7 years. Median (interquartile range) and mean ACC times were normal: 63.0 (20.0) and 64.2 ± 16.1 min, respectively. Age, operative risk scores and New York Heart Association class were similar in patients with ACC time above, versus those with ACC time below the median. Longer ACC time was independently associated with decreased late survival: hazards ratio (HR) 1.01 per minute increase of ACC time (95% confidence interval [CI] 1.00–1.02; P = 0.012). Longer CPB time was not associated with decreased late survival (HR 1.00 per minute increase of CPB time [95% CI 1.00–1.00; P = 0.30]).
CONCLUSIONS
Longer ACC time, although still within normal limits, was independently associated with decreased late survival after isolated aortic valve replacement in patients with severe aortic stenosis.
Keywords: Aortic valve replacement, Aortic cross-clamping, Cardiopulmonary bypass
Previous studies have shown that longer aortic cross-clamp (ACC) time is independently associated with decreased early survival after cardiac surgery [1].
INTRODUCTION
Previous studies have shown that longer aortic cross-clamp (ACC) time is independently associated with decreased early survival after cardiac surgery [1]. However, maximum follow-up in these studies is confined to 28 months [2]. Therefore, it is unknown whether this adverse effect is sustained far beyond this term. This might be interesting to know because longer cardiopulmonary bypass (CPB) time is, like longer ACC time, supposed to be independently associated with decreased early [3], but not late survival during a maximum follow-up of 14 years [4]. When longer ACC time is independently associated with decreased late survival, then there may be an adverse biologic effect of longer ACC time on the human body and still more effort should be exerted to keep ACC time during cardiac surgery as short as possible. In the current study, we aimed to determine by multivariable analysis whether longer ACC time was independently associated with decreased late survival during 25 years of follow-up after isolated aortic valve replacement (AVR) in patients with severe aortic stenosis.
MATERIALS AND METHODS
Study population
The study population comprised a cohort of 456 consecutive patients with severe aortic stenosis who underwent their first isolated AVR between 1990 and 1993 in a single centre. Exclusion criteria were repeat AVR, multiple valve operations, aortic homograft surgery and AVR combined with coronary artery bypass grafting (CABG), ascending aortic graft replacement surgery, ventricular septum defect, left ventricular (LV) aneurysm resection or lung surgery. The study population was divided into 2 groups, arbitrarily based on the median ACC time of the total study population (63.0 min): 226 patients with ACC times below 63.0 min, and 230 patients with ACC times of 63.0 min or longer. Follow-up data were obtained between 2006 and 2012 and at the end of 2018 from our own or referring cardiology departments, general practitioners and through telephone calls to patients and relatives.
Ethical statement
The study object was agreed upon by the hospital Committee on Ethics and Medical Experiments. Date: 8 June 1998. Protocol number: SQUARES 98.22. Consent of patients was waived.
Operative technique
All surgical procedures were performed using a midsternal approach and applying the same techniques of performing CPB and achieving cardiac arrest. CPB included a membrane oxygenator with moderate haemodilution and hypothermia and single right venous drainage. The LV was decompressed by transmitral drainage through the right superior pulmonary vein. Cardiac arrest was obtained mostly by antegrade perfusion of the aorta or direct coronary perfusion depending on the degree of aortic incompetence. Approximately 1 l of a 4°C oxygenated low sodium and normopotassic Bleese solution was infused by a separate single or double pressure controlled pump circuit [5]. In case of ACC times exceeding 1 h, coronary or graft perfusion was added or repeated. A pericardial well was installed with continuous infusion of Ringer’s Lactate at 4°C and a posterior pericardial gauze pad for insulation.
Definitions of baseline characteristics
Baseline characteristics were defined as follows: normal, moderate or poor LV function (LV ejection fraction ≥50%, ≥30– <50% or <30%, respectively); no, mild/moderate or severe LV hypertrophy (interventricular septal thickness of <11, 11–15 or >15 mm, respectively); recent or old myocardial infarction (myocardial infarction occurring ≤6 or >6 weeks before operation, respectively); previous ischaemic stroke (focal neurological deficit of sudden onset as diagnosed by a neurologist, lasting 24 h and caused by cerebral ischaemia); peripheral arterial disease (claudication, surgical or percutaneous intervention on the peripheral arteries, excluding carotid disease); paroxysmal or permanent atrial fibrillation; dialysis; hypertension (blood pressure >140/90 mmHg or use of antihypertensive medication); severe pulmonary hypertension (mean invasive pulmonary artery pressure >40 mmHg); and chronic lung disease (chronic obstructive lung disease, asthma or pulmonary fibrosis). Peak gradient across the aortic valve, aortic valve area, LV ejection fraction and interventricular septal thickness were all measured by transthoracic echocardiography.
Candidate independent predictors of decreased late survival
A priori based on previous research [6–8], the following candidate independent predictors of decreased late survival after AVR for multivariable analysis were chosen: age (continuous variable), gender, biologic or mechanical AVR, concomitant annular enlargement procedure, moderate or poor LV function, mild/moderate or severe LV hypertrophy, body mass index <20 or ≥30 kg/m2, previous CABG, recent or old myocardial infarction, previous ischaemic stroke, peripheral arterial disease, paroxysmal or permanent atrial fibrillation, hypertension, severe pulmonary hypertension, insulin- or non-insulin-dependent diabetes, chronic lung disease, serum creatinine, ACC and CPB time (min; continuous variables).
Statistical analysis
Kaplan–Meier survival analysis was performed to determine late survival from date of AVR in the patients with ACC time below versus those above the median. Cox proportional hazards analysis (forward stepwise technique with cut-off P-value of 0.20 for inclusion of candidate independent predictors) was performed to identify independent predictors of decreased late survival, including ACC and CPB time as continuous variables. This analysis was done after excluding the patients who had died within 30 days from AVR. Binary (yes/no) variables were labelled as yes versus no or missing. Missing data on categorical variables were categorized as missing. Continuous outcomes were analysed using Student’s t tests. Data were analysed by IBM SPSS Statistics 25 (IBM City, Armonk, New York, USA).
RESULTS
Baseline characteristics
Median (interquartile range) and mean ACC times of the total study population of 456 patients were 63.0 (20.0) and 64.2 ± 16.1 min, respectively (range 26.0–137.0 min). Median (interquartile range) and mean CPB times of the total study population were 87.0 (25.0) and 92.7 ± 42.5 min, respectively (range 52.0–238.0 min). Baseline characteristics regarding age, operative risk scores and New York Heart Association (NYHA) class of the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min, are depicted in Table 1. Age at time of AVR, operative risk scores and NYHA classes in the patients with ACC time below the median were similar to those with ACC time above the median, although operative risk scores seemed to show an insignificant trend towards being higher in the patients with ACC time above the median. Other baseline characteristics are depicted in Supplementary Material, Table S1. All other baseline characteristics were similar, except frequency of concomitant annular enlargement and incidence of previous old myocardial infarction, which were higher in the patients with ACC time above the median in comparison with those below.
Table 1:
Baseline characteristics of the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min
| Characteristics | ACC time <63.0 min (n = 226) | ACC time ≥63.0 min (n = 230) | P-value |
|---|---|---|---|
| ACC time (min) | 52.1 ± 6.5 | 76.1 ± 13.7 | <0.001 |
| CPB time (min) | 80.4 ± 53.1 | 104.9 ± 22.7 | <0.001 |
| Age (years) | 64.0 ± 11.4 | 65.1 ± 10.8 | 0.327 |
| Logistic EuroSCORE | 4.1 ± 3.4 | 4.6 ± 3.6 | 0.087 |
| EuroSCORE II | 1.5 ± 1.7 | 1.8 ± 1.7 | 0.122 |
| Society of Thoracic Surgeons score | 1.6 ± 1.6 | 1.7 ± 1.3 | 0.394 |
| New York Heart Association class | |||
| I | 22 (9.7) | 32 (13.9) | 0.193 |
| II | 99 (43.8) | 85 (37.0) | 0.152 |
| III | 87 (38.5) | 101 (43.9) | 0.254 |
| IV | 18 (8.0) | 12 (5.2) | 0.261 |
Values are presented as mean ± standard deviation or N (%).
ACC: aortic cross-clamp; CPB: cardiopulmonary bypass.
Thirty-day mortality
Thirty-day mortality in the patients of the total study population was 2.6% (12 of 456 patients). Thirty-day mortality in the patients with ACC time below versus those above the median was similar: 1.3% (3 of 226 patients) vs 3.9% (9 of 230 patients), respectively (P = 0.141).
In-hospital major adverse cardiac events
Table 2 shows in-hospital major adverse cardiac events (MACE) [new-onset postoperative atrial fibrillation, systolic heart failure, inotropic therapy, adult respiratory distress syndrome, dialysis, sepsis, mediastinitis, cardiac tamponade, ischaemic stroke, non-fatal myocardial infarction, re-thoracotomy, permanent pacemaker implantation and endocarditis] in the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min. Incidence of in-hospital heart failure was higher in the patients with ACC time above the median in comparison with those below. Incidence of the other in-hospital MACE did not differ between both groups. None of the study patients suffered in-hospital haemorrhagic stroke.
Table 2:
In-hospital major adverse cardiac events in the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min
| In-hospital major adverse cardiac event | ACC time <63.0 min (n = 226) | ACC time ≥63.0 min (n = 230) | P-value |
|---|---|---|---|
| New-onset atrial fibrillation | 77 (34.1) | 81 (35.2) | 0.918 |
| Systolic heart failure | 6 (2.7) | 18 (7.8) | 0.019 |
| Inotropic therapy | 15 (6.6) | 17 (7.4) | 0.855 |
| Adult respiratory distress syndrome | 12 (5.3) | 9 (3.9) | 0.510 |
| Dialysis | 4 (1.8) | 6 (2.6) | 0.751 |
| Sepsis | 3 (1.3) | 6 (2.6) | 0.504 |
| Mediastinitis | 0 | 1 (0.4) | 1.000 |
| Cardiac tamponade | 11 (4.9) | 14 (6.1) | 0.682 |
| Ischaemic stroke | 3 (1.3) | 6 (2.6) | 0.504 |
| Non-fatal myocardial infarction | 1 (0.4) | 1 (0.4) | 1.000 |
| Re-thoracotomy | 24 (10.6) | 24 (10.4) | 1.000 |
| Permanent pacemaker implantation | 2 (0.9) | 7 (3.0) | 0.175 |
| Endocarditis | 1 (0.4) | 1 (0.4) | 1.000 |
Values are presented as N (%).
ACC: aortic cross-clamp.
Late follow-up
In the total study population, mean follow-up from date of AVR was 25.3 ± 2.7 years. Follow-up was complete in all, but 9 (2.0%) patients, who were lost to follow-up at 16 (n = 1), 17 (n = 1), 18 (n = 3), 19 (n = 3) and 20 (n = 1) years after AVR.
Late survival
During late follow-up from date of AVR, 371 of 456 (81.4%) patients of the total study population died: 179 of 226 (79.2%) patients with ACC time below, and 192 of 230 (83.5%) patients with ACC above the median. In 46 of 179 (25.7%) deceased patients with ACC time below, versus 69 of 192 (35.9%) deceased patients with ACC time above the median, cause of death was cardiac (P = 0.043). Figure 1 shows Kaplan–Meier survival curves from date of AVR of the 226 study patients with ACC time below, versus the 230 patients with ACC time above the median. Mean late survival from date of AVR in the patients with ACC time above, versus those with ACC time below the median was similar: 13.3 ± 0.6 years (95% confidence interval [CI] 12.1–14.5) vs 14.3 ± 0.6 years (95% CI 13.2–15.5), respectively; log-rank: P = 0.239.
Figure 1:
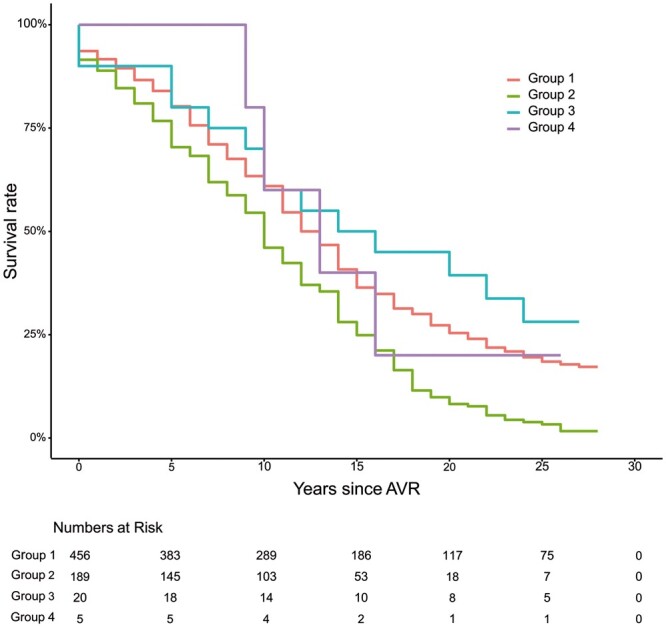
Kaplan–Meier survival curves of the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min. ACC: aortic cross-clamp; AVR: aortic valve replacement.
Independent predictors of decreased late survival
Table 3 shows the independent predictors of decreased late survival after excluding the 12 patients who had died within 30-days from AVR. In order of decreasing significance, these were: poor LV function, chronic lung disease, non-insulin dependent diabetes, moderate LV function, male gender, age and ACC time. Per minute increase of ACC time, the hazards ratio (HR) for decreased late survival was 1.01 (95% CI 1.00–1.02; P = 0.012). Longer CPB time was not an independent predictor of decreased late survival (HR 1.00 per minute increase of CPB time [95% CI 1.00–1.00; P = 0.30]).
Table 3:
Independent predictors of decreased late survival after excluding the 12 patients who had died within 30-days from aortic valve replacement
| Predictor | Hazards ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Poor left ventricular function | 2.20 | 1.31–3.68 | 0.003 |
| Chronic lung disease | 1.55 | 1.13–2.12 | 0.007 |
| Non-insulin dependent diabetes | 1.55 | 1.05–2.30 | 0.029 |
| Moderate left ventricular function | 1.46 | 1.06–2.02 | 0.022 |
| Male gender | 1.31 | 1.06–1.63 | 0.015 |
| Age | 1.09 | 1.08–1.11 | <0.001 |
| Aortic cross-clamp time | 1.01a | 1.00–1.02 | 0.012 |
Per minute.
Late major adverse cardiac events
Table 4 shows cumulative incidence of late MACE (atrial fibrillation [new-onset or new, paroxysmal or permanent], systolic heart failure, ischaemic stroke, haemorrhagic stroke, non-fatal myocardial infarction, permanent pacemaker implantation and endocarditis) in the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min. Cumulative incidence of late MACE during late follow-up did not differ between both groups.
Table 4:
Cumulative incidence of late major adverse cardiac events in the 226 patients with ACC time below, versus the 230 patients with ACC time above the median ACC time of 63.0 min
| Late major adverse cardiac event | ACC time <63.0 min (n = 226) | ACC time ≥63.0 min (n = 230) | P-value |
|---|---|---|---|
| Atrial fibrillationa | 53 (23.5) | 73 (31.7) | 0.059 |
| Systolic heart failure | 55 (24.3) | 65 (28.3) | 0.395 |
| Ischaemic stroke | 24 (10.6) | 28 (12.2) | 0.660 |
| Haemorrhagic stroke | 18 (8.0) | 12 (5.2) | 0.261 |
| Non-fatal myocardial infarction | 15 (6.6) | 8 (3.5) | 0.138 |
| Permanent pacemaker implantation | 19 (8.4) | 18 (7.8) | 0.865 |
| Endocarditis | 6 (2.7) | 13 (5.7) | 0.158 |
Values are presented as N (%).
New-onset or known, paroxysmal or permanent.
ACC: aortic cross-clamp.
DISCUSSION
In the current study, the effect of ACC time on late survival after isolated AVR was determined in a cohort of 456 consecutive patients with severe aortic stenosis during a mean follow-up of 25.3 ± 2.7 years. In multivariable analysis, longer ACC time was independently associated with decreased late survival (HR 1.01 per minute increase of ACC time [95% CI 1.00–1.02]; P = 0.012). Longer CPB time, added into the multivariable model together with ACC time, was not an independent predictor of decreased late survival (HR 1.00 per minute increase of CPB time [95% CI 1.00–1.00; P = 0.30]). From previous studies, it was already known that longer ACC time was independently associated with decreased early survival after cardiac surgery [1]. However, maximum follow-up in these studies was confined to 28 months [2]. Therefore, according to the findings of the current study, the adverse effect of longer ACC time on early survival after cardiac surgery seems to be sustained far beyond this term. This apparently sustained adverse effect of longer ACC time on late survival cannot be easily explained. In previous studies, the adverse effect of longer ACC time on early survival has been related to myocardial damage due to ischaemia, resulting in a higher rate of postoperative complications including prolonged ventilation, low cardiac output, higher requirements for blood transfusion and renal impairment [9, 10]. However, there are no data on the possible adverse effects of longer ACC time on late survival. Although the incidence in the current study of most in-hospital MACE was similar in patients with ACC time above versus those with ACC time below the median of 63.0 min, the incidence of in-hospital systolic heart failure was higher in the patients with ACC time above the median. However, because the need for in-hospital inotropic therapy was similar in the patients with ACC time above versus those with ACC time below the median, the in-hospital systolic heart failure cases had a relatively mild course. Also, because cumulative incidence of systolic heart failure during late follow-up was similar between patients with ACC time above versus those below the median, persistent heart failure was probably not the cause of the decreased late survival in patients with longer ACC time in the current study. Although cumulative incidence of the other MACE during late follow-up did not differ between the patients with ACC time above versus those with ACC time below the median, cause of death during late follow-up in the patients with ACC time above the median was more often cardiac in comparison with those with ACC time below the median (35.9% vs 25.7%, respectively; P = 0.043). For the interpretation of the findings of the current study, it is important to realize that (i) based on the rather low operative risk scores, most study patients were low-risk patients and (ii) both ACC and CPB times in the study patients had been rather low (mean 64.2 ± 16.1 and 92.7 ± 42.5 min, respectively) in comparison with the literature. In a large previous study of 3280 patients who had undergone AVR and/or mitral valve replacement with or without concomitant CABG or other procedures, normal mean ACC and CPB times after isolated AVR (n = 236) were 83 ± 26 and 106 ± 34 min, respectively [11]. In this study, it was also found that ACC and CPB times below 150 and 240 min, respectively, were associated with a rather low risk of 30-day morbidity and mortality, independently of the patient’s operative risk and the complexity of the procedure. In the current study, there were no patients with ACC or CPB times above 150 or 240 min (maximum ACC and CPB times in the study patients were 137 and 238 min, respectively). So, in the current study, longer ACC time was independently associated with decreased late survival, even with ACC times still within normal limits and even in low-risk patients. However, association does not imply causality. The patients in the current study with longer ACC time were probably sicker than the patients with shorter ACC time because operative risk scores showed a non-significant trend towards being higher in the patients with ACC time above the median, in comparison with those with ACC time below the median. Also, possibly other, yet unknown, independent predictors of decreased late survival after isolated AVR, not adjusted for by multivariable analysis, may have contributed to, or might have been the cause of, the decreased late survival of the patients with longer ACC time in the current study. Last, but not least, the interaction between ACC and CPB time regarding possible adverse effects of longer ACC and/or CPB time on early and late survival is complicated. CPB provokes a complex systemic inflammatory response, mainly triggered by contact activation of blood by artificial surfaces of the extracorporeal circuit [12]. This inflammatory response includes complement activation, endotoxin release, leucocyte activation, expression of adhesion molecules and release of many inflammatory mediators including oxygen-free radicals, arachidonic acid metabolites, cytokines, platelet-activating factor, nitric oxide and endothelins, which may contribute to the development of perioperative organ dysfunction and decreased early survival [13, 14]. However, akin to previous research on the effect of CPB time on late survival after isolated AVR during 14 years of follow-up [4], longer CPB time in the current study was not independently associated with decreased late survival. Because the adverse effect in the current study of longer ACC time on late survival was relatively weak (HR 1.01 per minute increase of ACC time), and therefore uncertainties regarding the real effect of longer ACC time on late survival will remain, it is probably wise to keep ACC time during cardiac surgery as short as possible.
Limitations
The most important limitation of the current study is its retrospective and therefore non-randomized design, implying inherent drawbacks on inclusion of study patients. Another important limitation is the rather low number of study patients, limiting the robustness of our findings. Finally, the findings of the current study are done only in low-risk patients with ACC and CPB time within normal limits.
CONCLUSION
Longer ACC time, although still within normal limits, was independently associated with decreased late survival after isolated AVR in patients with severe aortic stenosis. Although association does not imply causality, ACC times should be kept as short as possible.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Yvonne van Hees for her effort to develop the databases and enter data; Diane Vermeulen, for her effort to enter data; Geert J.M.G. van der Heijden and Henry A. van Swieten for their efforts to develop the databases; and Edgar J. Daeter for providing the video recording of an AVR procedure (date: 7 September 2016) at the same hospital where the study patients had been operated upon, from which the snapshot for the Central Image was taken.
Funding
This work was supported by: Stichting Hartenzorg St. Antonius, Nieuwegein; Stichting Nuts Ohra; and the former Jacques de Jong Stichting; all from the Netherlands.
Conflict of interest: none declared.
Author contributions
Ben M. Swinkels: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing—original draft; Writing—review & editing. Jurriën M. ten Berg: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing. Johannes C. Kelder: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Supervision; Validation; Writing—original draft; Writing—review & editing. Freddy E. Vermeulen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Wim Jan van Boven: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Bas A. de Mol: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Jarle Vaage and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
Abbreviations
- ACC
Aortic cross-clamp
- AVR
Aortic valve replacement
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CPB
Cardiopulmonary bypass
- HR
Hazards ratio
- LV
Left ventricle/ventricular
- MACE
Major adverse cardiac event
- NYHA
New York Heart Association
REFERENCES
- 1. Iino K, Miyata H, Motomura N, Watanabe G, Tomita S, Takemura H et al. Prolonged cross-clamping during aortic valve replacement is an independent predictor of postoperative morbidity and mortality: analysis of the Japan Cardiovascular Surgery Database. Ann Thorac Surg 2017;103:602–9. [DOI] [PubMed] [Google Scholar]
- 2. Lehrke S, Steen H, Sievers HH, Peters H, Opitz A, MüLler-Bardorff M et al. Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem 2004;50:1560–7. [DOI] [PubMed] [Google Scholar]
- 3. Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814–22. [DOI] [PubMed] [Google Scholar]
- 4. Chalmers J, Pullan M, Mediratta N, Poullis M. A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact CardioVasc Thorac Surg 2014;19:21–6. [DOI] [PubMed] [Google Scholar]
- 5. Bleese N, Döring V, Kalmar P, Pokar H, Polonius MJ, Steiner D et al. Intraoperative myocardial protection by cardioplegia in hypothermia. J Thorac Cardiovasc Surg 1978;75:405–13. [PubMed] [Google Scholar]
- 6. Tjang YS, van Hees Y, Körfer R, Grobbee DE, van der Heijden GJ. Predictors of mortality after aortic valve replacement. Eur J Cardiothorac Surg 2007;32:469–74. [DOI] [PubMed] [Google Scholar]
- 7. de Waard GA, Jansen EK, de Mulder M, Vonk AB, Umans VA. Long-term outcomes of isolated aortic valve replacement and concomitant AVR and coronary artery bypass grafting. Neth Heart J 2012;20:110–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verheul HA, van den Brink RB, Bouma BJ, Hoedemaker G, Moulijn AC, Dekker E et al. Analysis of risk factors for excess mortality after aortic valve replacement. J Am Coll Cardiol 1995;26:1280–6. [DOI] [PubMed] [Google Scholar]
- 9. Al-Sarraf N, Thalib L, Hughes A, Houlihan M, Tolan M, Young V et al. Cross-clamp time is an independent predictor of mortality and morbidity in low- and high-risk cardiac patients. Int J Surg 2011;9:104–9. [DOI] [PubMed] [Google Scholar]
- 10. Salsano A, Giacobbe DR, Sportelli E, Olivieri GM, Natali R, Prevosto M et al. Aortic cross-clamp time and cardiopulmonary bypass time: prognostic implications in patients operated on for infective endocarditis. Interact CardioVasc Thorac Surg 2018;27:328–35. [DOI] [PubMed] [Google Scholar]
- 11. Nissinen J, Biancari F, Wistbacka JO, Peltola T, Loponen P, Tarkiainen P et al. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion 2009;24:297–305. [DOI] [PubMed] [Google Scholar]
- 12. Day JR, Taylor KM. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005;3:129–40. [DOI] [PubMed] [Google Scholar]
- 13. Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 1997;112:676–92. [DOI] [PubMed] [Google Scholar]
- 14. Squiccimarro E, Labriola C, Malvindi PG, Margari V, Guida P, Visicchio G et al. Prevalence and clinical impact of systemic inflammatory reaction after cardiac surgery. J Cardiothorac Vasc Anesth 2019;33:1682–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.