Abstract
OBJECTIVES
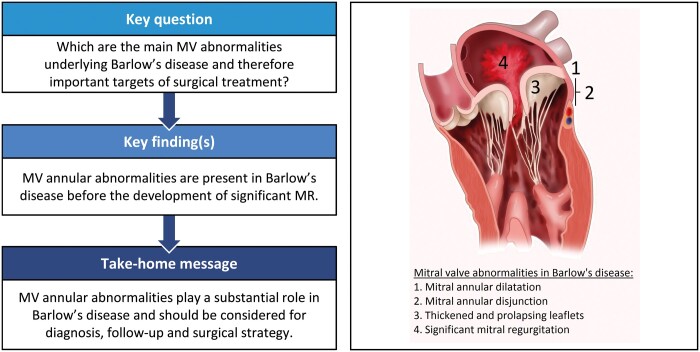
Barlow’s disease (BD) is characterized by thick, redundant mitral valve (MV) leaflets, which can lead to prolapse and significant mitral regurgitation (MR). MV annular abnormalities are also commonly observed and increasingly recognized as possible primary pathology, with leaflet thickening being secondary to increased stress on the MV apparatus. To provide more insights into this hypothesis, the evolution of MV abnormalities over time in patients with BD was assessed.
METHODS
A total of 64 patients (54 ± 12 years, 72% male) with BD who underwent MV surgery and had multiple transthoracic echocardiograms (TTE) before surgery were included. In total, 186 TTE were analysed (median time interval 4.2, interquartile range 2.2–6.5 years) including specific MV characteristics.
RESULTS
At baseline, MV leaflet length, thickness, billowing height and annular diameter were larger in patients with BD compared to 59 healthy subjects. Systolic outward motion (curling) of the annulus was observed in 77% and severe mitral annular disjunction (≥5 mm) in 38% of patients with BD. Forty (63%) patients had MR grade I–II and 24 (37%) MR grade III–IV; at baseline, the 2 groups only differed in left atrial volume and in thickness and billowing height of the posterior leaflet, showing comparable MV annular abnormalities and dilatation despite different grades of MR. Over time, MV annulus diameter, leaflet length and billowing height increased significantly along with MR grade.
CONCLUSIONS
In patients with BD, MV annulus abnormalities are present at an early stage and precede the development of significant MR, suggesting their substantial role in the pathophysiology of this disease and as an important target for surgical treatment.
Keywords: Mitral valve prolapse, Barlow’s disease, Echocardiography, Longitudinal changes, Mitral valve repair
INTRODUCTION
Current classifications of primary mitral regurgitation (MR), and particularly of mitral valve (MV) prolapse, distinguish mainly between Barlow’s disease (BD) and fibro-elastic deficiency (FED). BD is characterized by thick and redundant MV leaflets with elongated chordae and a bi-leaflet prolapse. FED is characterized by single-segment prolapse/flail with thin leaflets or thickening limited to the prolapsing segment [1]. Although the redundant and prolapsing MV leaflets are considered the hallmark of BD, specific MV annular abnormalities, such as annular dilatation, mitral annular disjunction (MAD) and systolic outward motion (curling) of the annulus, are also commonly observed in these patients [2–5]. These annular abnormalities are increasingly recognized as a primary component of the underlying pathology of BD, which might increase the mechanical stress on the MV apparatus, with further thickening and elongation of leaflets and chordae, and possibly also development of ventricular arrhythmias [6, 7]. However, whether these annular abnormalities are concomitant to the leaflet al.terations, secondary to the development of MR or might precede them is not known yet. Longitudinal echocardiographic studies could help understanding the pathophysiology of BD and identifying the main morphological and functional alterations underlying this complex MV pathology, improving timely diagnosis and eventually identifying the main target of surgical repair. Few studies reported on the echocardiographic characteristics (i.e. leaflet alterations) related to the progression of MR in patients with MV prolapse [8–10] but did not assess specific MV annular abnormalities more typically associated with BD. Therefore, the aim of the present study was to evaluate changes in MV morphology and function over time, particularly focusing on annular abnormalities, using echocardiography in a relatively large group of patients with BD who eventually developed a severe MR requiring surgery.
METHODS
Patient population
Patients who underwent MV surgery for severe primary MR due to BD between 2000 and 2017 were identified in 2 different centres: Leiden University Medical Center, Leiden, the Netherlands and Centro Cardiologico Monzino, IRCCS, Milan, Italy. The diagnosis of BD was based on echocardiographic findings and confirmed by intraoperative observations [1, 11]: a bi-leaflet MV prolapse due to excessive tissue and elongated chordae, with annular abnormalities such as annular dilatation with or without calcification and systolic outward motion (curling). Patients with other forms of MV prolapse (such as FED and BD forme fruste), hypertrophic cardiomyopathy, Marfan’s syndrome and/or endocarditis were excluded. Furthermore, to study the echocardiographic changes over time before MV surgery, patients were included in this study when there were ≥2 complete transthoracic echocardiograms (TTE) available before MV surgery with ≥2 years in between. Patient data were collected at the time of the first TTE from the departmental cardiology information systems (EPD-Vision® at Leiden University Medical Center, and W-Hospital at Centro Cardiologico Monzino) and included age, gender, and cardiovascular risk factors. In addition, 59 healthy controls having structural normal hearts were selected from the echocardiography database. These controls were matched based on age, gender and cardiovascular risk factors, by using the ‘case control matching’ function in SPSS. The study complies with the Declaration of Helsinki and was approved by the Institution Review Boards. Due to the retrospective design of this study, the Medical Ethical Committee waived the need for written informed consent.
Echocardiographic analysis
Standard TTE was performed with commercially available ultrasound (Vivid 7 and E9, GE-Vingmed, Milwaukee, WI and ie33 and EPIQ system, Philips Medical System, Andover, MA, USA). Images were digitally stored and analysed offline using EchoPAC (version 112, GE Medical Systems) and QLAB (Philips Medical System). All available TTE performed before MV surgery were analysed by 2 observers. Left ventricular end-diastolic diameter (LVEDD) and left ventricular (LV) end-systolic diameter were measured from the parasternal long-axis view and left ventricular ejection fraction (LVEF) and left atrial volume were calculated using Simpson’s biplane method. Left atrial volume was indexed [left atrial volume index (LAVI)] for body surface area [12]. MR severity was graded according to current recommendations using a multi-parametric approach, and MR was classified as mild (grade I), mild–moderate (grade II), moderate–severe (grade III) and severe (grade IV) [13]. Systolic pulmonary artery pressure was estimated by measuring maximal tricuspid regurgitant jet velocity with the simplified Bernoulli equation in combination with an estimation of the right atrial pressure, as recommended [14]. Tricuspid valve annular diameter was measured from a right ventricular focused apical four-chamber view [13].
Mitral valve morphology and function assessment
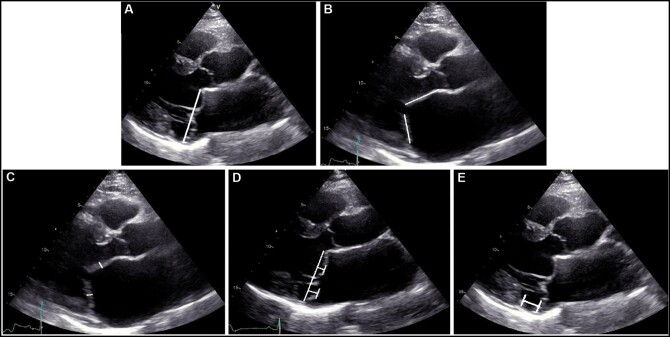
All measurements of MV morphology and function were performed on the parasternal long-axis view according to current recommendations and applying the definition used by previous studies [7, 15, 16]: MV annular diameter was determined in the end-systolic phase, just before opening of the MV leaflets (Fig. 1A). Leaflet length was measured as the distance from the hinge point to the free edge and was measured by slowly reviewing the cardiac cycle to identify the hinge point and the free edge, without including the chordae (Fig. 1B). Leaflet thickness was defined as the maximum distance from atrial to ventricular surface, considering the entire leaflets (Fig. 1C). Leaflet billowing height was defined as the maximum prolapse of the leaflet into the left atrium, measured as the maximum height above the annular level (Fig. 1D) [17]. Furthermore, presence of annular curling, defined as unusual hypermobile outward and downwards systolic motion of the posterior mitral annulus on the adjacent myocardium, was noted [2]. MAD was measured as the separation between the insertion of the posterior leaflet into the left atrial wall and the base of the LV posterior wall (Fig. 1E) and considered relevant when ≥5 mm [18–20].
Figure 1:
Transthoracic echocardiography showing the specific mitral valve measurements: (A) mitral annular diameter; (B) length of mitral valve leaflets; (C) thickness of mitral valve leaflets; (D) billowing height of mitral valve leaflets; and (E) mitral annular disjunction.
Statistical analysis
Statistical analysis was performed with the SPSS software package (version 20, IBM Corp, Armonk, New York, USA) and R version 3.6.0. Continuous variables are reported as mean ± standard deviation, when normally distributed, and as median (interquartile range), when not normally distributed. Categorical variables are presented as absolute numbers and percentages. Differences in clinical and echocardiographic characteristics between control subjects and patients with BD were assessed using Student’s t-test, Mann–Whitney U-test or chi-square, when appropriate. Patients with BD were further divided based on MR grade (patients with MR grade I/II versus MR grade III/IV) at first TTE, to evaluate whether changes in MV morphology were correlated to the severity of MR. For each patient, the average change per year was computed. Within each group (MR grade I/II versus MR grade III/IV), a one-sample signed-rank Wilcoxon test was performed to test whether the location of the average change per year was statistically significantly different from zero. Furthermore, a linear mixed model with a fixed interaction between time and MR grade, and random intercept and time slope per patient, was used to assess echocardiographic changes over time for all patients and for patients with MR grade I/II at baseline compared to patients with MR grade III/IV at baseline. Intra-class correlation coefficients were calculated for interobserver and intra-observer agreement in 10 randomly selected patients, to evaluate reproducibility. A P-value of <0.05 was considered statistically significant.
RESULTS
Patient population
A total of 64 patients with BD (54 ± 12 years, 72% male) were included. Baseline clinical and echocardiographic characteristics (at the time of the first TTE) of these patients were compared with the 59 controls (Table 1). Regarding conventional echocardiographic measurements, patients with BD showed larger LVEDD and slightly higher LVEF. Furthermore, patients with BD showed LA dilatation with a larger median LAVI. All patients with BD showed some degree of MR: 40 (63%) had MR grade I or II and 24 (37%) had MR grade III or IV. In 49 patients (77%), annular curling of the MV was observed. Furthermore, in patients with BD, the mean MAD was 2.9 ± 3 mm and 24 patients (38%) showed an MAD of ≥5 mm. Annular curling and/or MAD were not observed in the controls. In patients with BD, the MV annulus was significantly dilated as compared to controls already at first TTE. Moreover, length, thickness and billowing height of the posterior and anterior MV leaflets were significantly larger in patients with BD compared to controls.
Table 1:
Clinical and echocardiographic characteristics of patients with Barlow’s disease (at the first echocardiographic assessment) as compared to healthy subjects
| Controls (N = 59) | BD (first TTE, N = 64) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years), mean ± SD | 54 ± 11 | 54 ± 12 | 0.82 |
| Male, n (%) | 42 (71) | 46 (72) | 1.00 |
| Hypertension, n (%) | 21 (36) | 24 (38) | 0.60 |
| Hypercholesterolaemia, n (%) | 7 (12) | 14 (22) | 0.16 |
| Diabetes, n (%) | 2 (3) | 1 (2) | 0.51 |
| Echocardiographic characteristics | |||
| LVEDD (mm), mean ± SD | 49 ± 6 | 54 ± 6 | <0.001 |
| LVESD (mm), mean ± SD | 32 ± 7 | 34 ± 7 | 0.071 |
| LVEF (%), mean ± SD | 61 ± 6 | 63 ± 5 | 0.026 |
| LAVI (ml/m2), median (IQR) | 24 (17–28) | 37 (29–48) | <0.001 |
| sPAP (mmHg), median (IQR) | 24 (19–27) | 24 (23–31) | 0.015 |
| TV annulus (mm), mean ± SD | 29 ± 5 | 34 ± 5 | <0.001 |
| MR grade, n (%) | <0.001 | ||
| None | 59 (100) | 0 (0) | |
| I–II | 0 (0) | 40 (63) | |
| III–IV | 0 (0) | 24 (37) | |
| MV annular curling, n (%) | 0 (0) | 49 (77) | <0.001 |
| MAD height (mm), mean ± SD | 0 | 2.9 ± 3 | <0.001 |
| MAD ≥ 5 mm, n (%) | 0 (0) | 24 (38) | <0.001 |
| MV annulus (mm), mean ± SD | 27 ± 3 | 36 ± 5 | <0.001 |
| Length AML (mm), mean ± SD | 17 ± 2 | 23 ± 3 | <0.001 |
| Length PML (mm), mean ± SD | 11 ± 2 | 16 ± 3 | <0.001 |
| Thickness AML (mm), mean ± SD | 2 ± 0.5 | 4 ± 1 | <0.001 |
| Thickness PML (mm), mean ± SD | 2 ± 0.5 | 4 ± 1 | <0.001 |
| Billowing height AML (mm), mean ± SD | 0 | 4 ± 2 | <0.001 |
| Billowing height PML (mm), mean ± SD | 0 | 5 ± 2 | <0.001 |
AML: anterior mitral leaflet; BD: Barlow’s disease; IQR: interquartile range; LAVI: left atrial volume index; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MAD: mitral annular disjunction; MR: mitral regurgitation; MV: mitral valve; PML: posterior mitral leaflet; SD: standard deviation; sPAP: systolic pulmonary artery pressure; TTE: transthoracic echocardiography; TV: tricuspid valve.
Barlow’s disease: changes in mitral valve morphology over time
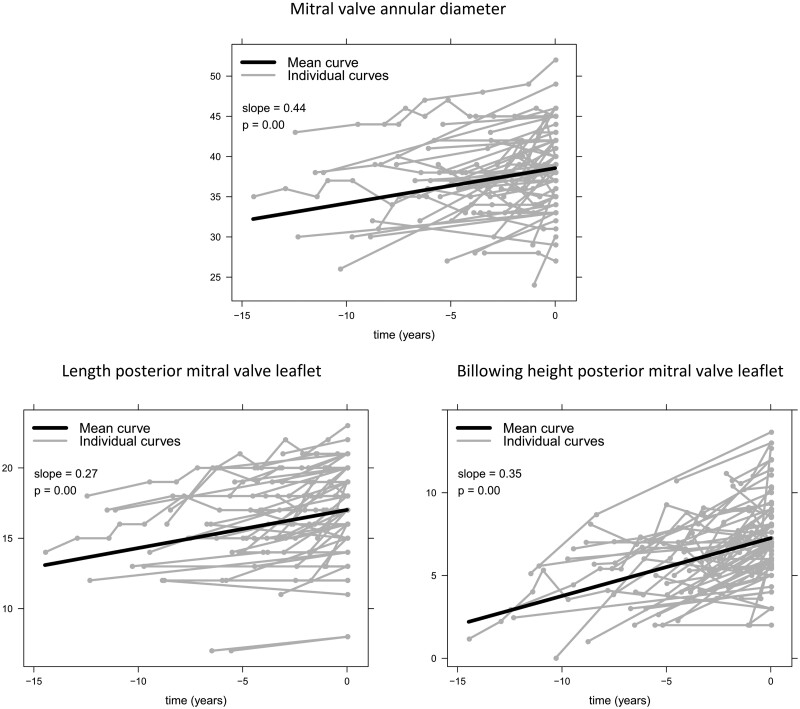
Of the 64 patients with BD, a total of 186 echocardiograms were assessed (range 2–11 echocardiograms per patient). In Table 2, the differences between the first and last echocardiogram {median interval [4.2(2.2–6.5) years]} are presented. At the time of the first TTE, 40 patients (63%) had MR grade I–II, while at the time of the last TTE (before surgery), all patients progressed to MR grade III–IV. Between first and last TTE, left ventricular end-systolic diameter and LVEF remained stable over time, while LVEDD and LAVI showed significant increase. The tricuspid valve annular diameter showed a trend towards increase; however this was not significantly different. No significant changes in annular curling and presence of MAD ≥5 mm were observed over time, although the MAD height slightly increased. All other MV characteristics changed significantly during follow-up: in particular, MV annulus dilatation progressed over time and the length and the thickness of both anterior mitral leaflet (AML) and posterior mitral leaflet (PML) increased. Furthermore, the prolapse became more evident with an increase in the billowing height of the AML and PML. Figure 2 shows the results of the mixed model analysis of some of the individual MV measurements over time. All available MV measurements of the echocardiograms between the first and last TTE are taken into account in this analysis and show that MV annular dilation (slope = 0.44, P < 0.001), leaflet length (slope for AML = 0.27, P < 0.001; slope for PML = 0.27, P < 0.001) and billowing height (slope for AML = 0.13, P = 0.01; slope for PML = 0.35, P < 0.001) increased over time.
Table 2:
Changes in echocardiographic parameters over time in patients with Barlow’s disease
| Parameter | First TTE | Last TTE | P-value |
|---|---|---|---|
| LVEDD (mm), mean ± SD | 54 ± 6 | 57 ± 7 | 0.008 |
| LVESD (mm), mean ± SD | 34 ± 7 | 35 ± 7 | 0.20 |
| LVEF (%), mean ± SD | 63 ± 5 | 63 ± 7 | 0.44 |
| LAVI (ml/m2), median (IQR) | 37 (29–48) | 51 (38–63) | <0.001 |
| sPAP (mmHg), median (IQR) | 24 (23–31) | 30 (25–37) | <0.001 |
| TV annulus (mm), mean ± SD | 29 ± 5 | 35 ± 5 | 0.10 |
| MR grade, n (%) | <0.001 | ||
| I–II | 40 (63) | 0 (0) | |
| III–IV | 24 (37) | 64 (100) | |
| Annular curling, n (%) | 49 (77) | 52 (81) | 0.25 |
| MAD height (mm), mean ± SD | 2.9 ± 3 | 3.1 ± 3 | 0.004 |
| MAD ≥ 5 mm, n (%) | 24 (38) | 26 (41) | 0.50 |
| MV annulus (mm), mean ± SD | 36 ± 5 | 39 ± 5 | <0.001 |
| Length AML (mm), mean ± SD | 23 ± 3 | 24 ± 4 | <0.001 |
| Length PML (mm), mean ± SD | 16 ± 3 | 17 ± 3 | <0.001 |
| Thickness AML (mm), mean ± SD | 4 ± 1 | 5 ± 1 | <0.001 |
| Thickness PML (mm), mean ± SD | 4 ± 1 | 5 ± 1 | <0.001 |
| Billowing height AML (mm), mean ± SD | 4 ± 2 | 5 ± 3 | 0.010 |
| Billowing height PML (mm), mean ± SD | 5 ± 2 | 7 ± 3 | <0.001 |
AML: anterior mitral leaflet; IQR: interquartile range; LAVI: left atrial volume index; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MAD: mitral annular disjunction; MR: mitral regurgitation; MV: mitral valve; PML: posterior mitral leaflet; SD: standard deviation; sPAP: systolic pulmonary artery pressure; TTE: transthoracic echocardiography; TV: tricuspid valve.
P-values corrected for time in between the echocardiograms [5.1 years (IQR 3.0–8.4) for MR grade I/II and 3.1 years (IQR 1.5–4.9) for MR grade III/IV].
Figure 2:
Changes over time in some of the mitral valve characteristics. The grey lines represent the individual measurements per patient, and the black lines present the mean curve of all patients. Values on y-axis are in millimetres. Patients underwent mitral valve surgery at time point ‘0’.
Differences between MR grade I/II and MR grade III/IV
In Table 3, differences between patients with MR grade I/II and patients with already MR grade III/IV at the first TTE are presented. The echocardiographic measurements at first and last TTE are shown for each group and compared between the 2 groups and within groups over time. At the time of the first TTE, LAVI, thickness of the PML and billowing height of the PML were significantly larger in patients with MR grade III/IV compared to patients with MR grade I/II. All other measurements were not significantly different between the 2 groups at the time of the first TTE.
Table 3:
Echocardiographic changes over time between patients with Barlow’s disease with mitral regurgitation grade I/II versus mitral regurgitation grade III/IV (at the time of the first echocardiography)
|
Parameter |
MR grade I/II (N = 40) | MR grade III/IV (N = 24) | P-value between groups | P-value group-time interaction |
|---|---|---|---|---|
| LVEDD (mm), mean ± SD | 0.94 | |||
| First TTE | 54 ± 7 | 56 ± 6 | 0.26 | |
| Last TTE | 57 ± 7* | 56 ± 6 | 0.64 | |
| LVESD (mm), mean ± SD | 0.70 | |||
| First TTE | 33 ± 7 | 36 ± 7 | 0.16 | |
| Last TTE | 35 ± 8* | 36 ± 6 | 0.63 | |
| LVEF (%), mean ± SD | 0.082 | |||
| First TTE | 64 ± 5 | 63 ± 6 | 0.63 | |
| Last TTE | 62 ± 8 | 64 ± 6 | 0.19 | |
| LAVI (ml/m2), median (IQR) | 0.50 | |||
| First TTE | 33 (17-43) | 46 (35-54) | 0.005 | |
| Last TTE | 50 (37-60)* | 53 (42-66) | 0.23 | |
| sPAP (mmHg), median (IQR) | 0.58 | |||
| First TTE | 25 (22-31) | 25 (23-31) | 0.66 | |
| Last TTE | 29 (25-37)* | 31 (27-33)* | 0.76 | |
| MV annulus (mm), mean ± SD | 0.65 | |||
| First TTE | 36 ± 5 | 37 ± 4 | 0.36 | |
| Last TTE | 38 ± 5* | 39 ± 4* | 0.66 | |
| Length AML (mm), mean ± SD | 0.014 | |||
| First TTE | 23 ± 3 | 24 ± 4 | 0.25 | |
| Last TTE | 24 ± 3* | 25 ± 4* | 0.12 | |
| Length PML (mm), mean ± SD | 0.49 | |||
| First TTE | 15 ± 3 | 16 ± 3 | 0.15 | |
| Last TTE | 17 ± 3* | 18 ± 1* | 0.35 | |
| Thickness AML (mm), mean ± SD | 0.88 | |||
| First TTE | 4 ± 1 | 4 ± 1 | 0.16 | |
| Last TTE | 5 ± 1* | 5 ± 1* | 0.37 | |
| Thickness PML (mm), mean ± SD | 0.19 | |||
| First TTE | 4 ± 1 | 5 ± 1 | 0.003 | |
| Last TTE | 5 ± 1* | 6 ± 2* | 0.063 | |
| Billowing height AML (mm), mean ± SD | 0.99 | |||
| First TTE | 4 ± 2 | 5 ± 2 | 0.076 | |
| Last TTE | 5 ± 3* | 5 ± 3 | 0.64 | |
| Billowing height PML (mm), mean ± SD | 0.46 | |||
| First TTE | 5 ± 2 | 6 ± 3 | 0.016 | |
| Last TTE | 7 ± 2* | 8 ± 3* | 0.16 |
AML: anterior mitral leaflet; IQR: interquartile range; LAVI: left atrial volume index; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; MR: mitral regurgitation; MV: mitral valve; PML: posterior mitral leaflet; SD: standard deviation; sPAP: systolic pulmonary artery pressure; TTE: transthoracic echocardiography.
P-value <0.05 within group: first versus last measurement, corrected for time between echocardiograms.
In the group of patients with MR grade I/II at baseline, all echocardiographic measurements showed an increase over time, except LVEF. In the group of patients with MR grade III/IV at baseline, LV dimensions, LVEF and LAVI remained stable, while annular diameter, leaflet length, thickness and billowing height increased over time, except for billowing height of the AML. In addition, the group-time interaction showed whether the echocardiographic variables changed differently over time between the 2 groups: most parameters showed similar changes over time in the 2 groups except for the length of the AML, which showed a slightly higher increase (24 ± 4–25 ± 4mm) for patients with MR grade III/IV as compared to patients with MR grade I/II (23 ± 3–24 ± 3mm, P = 0.010).
Inter- and intra-observer variabilities
The intra-class correlation coefficients for repeated measurements by the same observer (intra-observer agreement) was excellent for all parameters; the intra-class correlation coefficients for measurements between 2 different observers (interobserver agreement) were generally good or excellent (Supplementary Material, File S1).
DISCUSSION
The present study provides novel insights into the pathophysiology of specific MV abnormalities in patients with BD who eventually developed severe MR. In particular, MV annular abnormalities, such as dilatation, systolic curling and disjunction, represent a common feature of these patients and are present already before the development of significant MR, which is in turn associated to increasing leaflet thickness and billowing height over time.
Mitral valve morphology in patients with BD
The MV apparatus is a complex structure, whose proper function is guaranteed by normal leaflet morphology and attachment to papillary muscles and to the MV annulus. Particularly, MV annulus contributes to valve competence not only being the anchoring point of the leaflets but also by active systolic contraction. Normally, the MV annulus has a saddle shape and is attached posteriorly to the LV myocardium; therefore, when systolic LV contraction occurs, MV annulus also contracts along its antero-posterior diameter enhancing the coaptation of the AML and PML and preventing MR [21]. Major abnormalities of MV annulus morphology and function have been observed in BD, including hypermobility of the posterior annulus (systolic curling), attachment of the PML above the LV myocardium (MAD) and annular dilatation [2–5]. From histological samples, Hutchins et al. [18] were the first recognizing MV annular abnormalities in patients with MV prolapse and also introduced the term MAD, defined as a separation between the LV myocardium and the attachment of the PML in the atrial wall. The authors also suggested for the first time that a defect in the annulus fibrosus could be the primary abnormality in BD and that the other morphologic and functional alterations of the leaflets might be secondary. Since then, several studies have described MV annular abnormalities in patients with MV prolapse using echocardiography or magnetic resonance imaging, but interestingly with some variation in the definition and in the reported prevalence [7, 15, 19–24]. Lee et al. [20] defined MAD when ≥5-mm displacement was observed and reported a prevalence of 42% in patients who underwent MV surgery because of degenerative MR. Eriksson et al. [19] evaluated 109 patients who underwent MV surgery, with transoesophageal echocardiography, and reported a prevalence of MAD, defined as any displacement, of 98% in patients with advanced MV degeneration and 9% in patients with mild MV degeneration. In contrast, Konda et al. [23] reported MAD (defined as ≥2-mm displacement) also in healthy subjects and therefore questioned the pathological significance of this finding. Of note, most articles did not describe the presence of systolic curling of the MV annulus, although these 2 abnormalities are considered to reflect similar annular alterations [6]. Furthermore, the discrepancies in the prevalence of annular abnormalities between studies might be explained by the difference in patient populations included (i.e. any type of MV prolapse or organic MR in general). Studies evaluating differences in annular dynamics between patients with FED and BD have demonstrated that the MV annulus in patients with FED has relatively normal function and is mildly dilated compared to BD [3, 5]. These observations also contribute to the hypothesis that the diffuse thickening and elongation of MV leaflets, not observed in FED, are probably partially a primary alteration in BD but partially also compensatory to altered stress on the MV apparatus due to the abnormal annular dynamics. However, only studies including serial echocardiographic examinations focusing on MV annular abnormalities could help supporting this hypothesis. Therefore, the present study selected only patients with a clear phenotype of BD and showed that MV annular abnormalities are detectable with TTE at an early stage, before significant MR develops.
Evolution of mitral valve abnormalities in BD
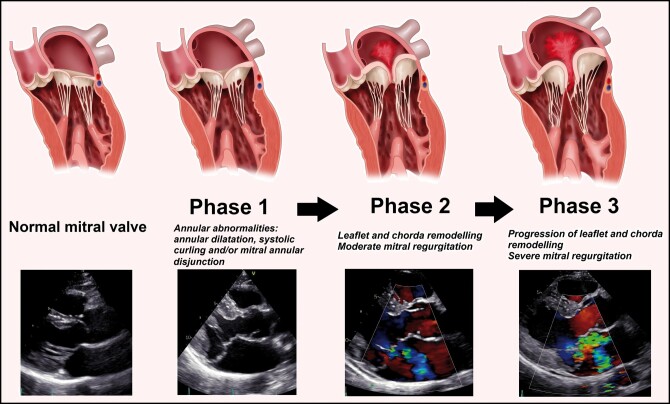
Few studies assessed changes in MV morphology and function over time in patients with MV prolapse. Avierinos et al. [10, 25] evaluated the natural history of asymptomatic MV prolapse and showed that age and MR grade at baseline were independent predictors of MR progression. The rate and determinants of MR progression were evaluated by Enriquez-Sarano et al. [9] in 74 patients with organic MR and showed that MR progressed over time, which was correlated with an increase in MV annular diameter. However, the progression of MR was poorly predictable using baseline characteristics and it was unclear which MV alterations preceded development of MR. Furthermore, more specific MV abnormalities, like leaflet characteristics, MAD and systolic curling of the annulus, were not evaluated. In another study, by Delling et al. [8], longitudinal changes in MV characteristics were evaluated in patients from the Framingham Offspring, including 63 patients with MV prolapse, 60 patients with ‘non-diagnostic MV prolapse morphologies’ and 138 healthy subjects. They showed that a large proportion of patients from both groups, MV prolapse and ‘non-diagnostic MV prolapse morphologies’, showed changes over time with increasing leaflet thickness, displacement and progression of MR, demonstrating that also patients with mild MV abnormalities can progress to a more severe phenotype of MV prolapse. MV prolapse and LV diameter were identified as predictors of progression of MR. However, the MV alterations that preceded the development of MR and specific annular abnormalities were not evaluated. The present study is the first to describe the longitudinal changes in specific MV characteristics in a population of patients with a clear BD phenotype. At the time of the first TTE, MV annular dilatation, (any) disjunction and curling were observed in the majority of patients, even in the absence of significant MR and signs of LV or LA remodelling, whereas elongation and thickening of the MV leaflets were also observed, but to less extent. Over time, leaflet elongation, thickening and prolapsing height progressed, leading to impaired leaflet coaptation and severe MR and to LV and LA dilatation. In Fig. 3, these phases are presented schematically. These results support the hypothesis that MV annular abnormalities might play a primary role in the pathology of BD leading to secondary alterations in the whole MV apparatus. Correction of these abnormalities should be therefore a crucial target of the surgical treatment.
Figure 3:
Evolution of mitral valve abnormalities in patients with Barlow’s disease over time. Left panel: normal mitral valve; ‘Phase 1’: annular abnormalities, including annular dilatation, mitral annular disjunction and systolic curling, without mitral regurgitation; ‘Phase 2’: remodelling of leaflets and chordae, including elongation, thickening and prolapse of the mitral valve leaflets with moderate mitral regurgitation; and ‘Phase 3’: progression of leaflet and chorda remodelling and development of severe mitral regurgitation and left atrial dilatation. See also the video (Supplementary Material, Video) showing the echocardiogram (parasternal long-axis view with and without colour Doppler) of a Barlow’s disease mitral valve with bi-leaflet prolapse, thickened leaflets, mitral annular disjunction and mitral regurgitation.
Clinical implications
The observations of the present study may be relevant in the choice of surgical strategy for these patients. Annular remodelling and stabilization should represent the cornerstone of surgical treatment in BD, to correct the dilatation and abnormal annulus movement, which in some cases is the main cause of anterior leaflet prolapse [26]. Furthermore, proper annuloplasty reduces the stress applied to native valvular leaflet, preventing further leaflet degeneration and possible recurrence of MR. The results of the current study also underline the importance of recognizing MV annular abnormalities in patients with BD. Since the present study suggested annular abnormalities to be the early sign of BD, assessment of MV abnormalities could be important when screening family members in the setting of familial BD [27]. Patients without significant MR but important annular dysfunction might be considered for regular follow-up, also considering the recently suggested association between MAD and ventricular arrhythmias [7, 22]. More studies with larger population are needed to confirm these results and demonstrate the prognostic value of these MV annular abnormalities and how and when to intervene.
Limitations
The following limitations should be mentioned: Due to the retrospective design of this study, longitudinal follow-up was not standardized and, therefore, the amount and time in between echocardiograms varied among patients. Therefore, the mixed model analysis was corrected for time and included all available echocardiograms, instead of only ‘first’ and ‘last’. To evaluate the changes over time leading to severe MR, only patients who had multiple echocardiographic examinations and ultimately underwent MV surgery were included. Although of clinical interest, patients with BD who did not develop severe MR over time could not be assessed since no echocardiographic data were systematic available. Although all patients had severe MR at the last echocardiogram, at the time of the first echocardiogram, the stage of the disease differed among patients. To minimize bias regarding the heterogeneity in disease stage at baseline, we compared patients with MR grade I/II with patients with MR grade III/IV and the changes in echocardiographic characteristics over time. In this study, only two-dimensional TTE was used. Although other imaging techniques such as cardiac magnetic resonance and three-dimensional and/or transoesophageal echocardiography could have provided more accurate and reproducible measures, they were not systematically available in these patients and future prospective studies need to confirm our results using three-dimensional imaging techniques; however, TTE is widely and readily available and likely to be used in clinical practice and also in previous studies showed to be able to accurately detect MV abnormalities [15, 22, 23].
CONCLUSION
In patients with BD, abnormalities of the MV annulus are present at an early stage and precede the development of significant MR, suggesting a substantial role in the pathophysiology of this disease and an important target for surgical treatment.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Funding
V.D. received consulting fees from Abbott Vascular. N.A.M. received speaker fees from Abbott Vascular and GE Healthcare. The Department of Cardiology of Leiden University Medical Centre received research grants from Biotronik, Medtronic, Boston Scientific and Edwards Lifesciences.
Conflict of interest: none declared.
Author contributions
Yasmine L. Hiemstra: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft. Anton Tomsic: Conceptualization; Data curation; Methodology; Writing—review & editing. Paola Gripari: Conceptualization; Data curation; Formal analysis; Methodology; Writing—review & editing. Aniek L. van Wijngaarden: Conceptualization; Data curation; Formal analysis; Writing—review & editing. Stephanie L. van der Pas: Data curation; Formal analysis; Methodology; Writing—review & editing. Meindert Palmen: Conceptualization; Data curation; Methodology; Writing—review & editing. Robert J.M. Klautz: Conceptualization; Data curation; Methodology; Writing—review & editing. Mauro Pepi: Conceptualization; Data curation; Methodology; Supervision; Writing—review & editing. Jeroen J. Bax: Conceptualization; Supervision; Writing—original draft; Writing—review & editing. Victoria Delgado: Conceptualization; Methodology; Supervision; Writing—original draft; Writing—review & editing. Nina Ajmone Marsan: Conceptualization; Formal analysis; Methodology; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Bruno J. Messmer, Tomasz Plonek and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- AML
Anterior mitral leaflet
- BD
Barlow’s disease
- FED
Fibro-elastic deficiency
- LAVI
Left atrial volume index
- LV
Left ventricular
- LVEDD
Left ventricular end-diastolic diameter
- LVEF
Left ventricular ejection fraction
- MAD
Mitral annular disjunction
- MR
Mitral regurgitation
- MV
Mitral valve
- PML
Posterior mitral leaflet
- TTE
Transthoracic echocardiography
REFERENCES
- 1. Anyanwu AC, Adams DH.. Etiologic classification of degenerative mitral valve disease: Barlow's disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90–6. [DOI] [PubMed] [Google Scholar]
- 2. Grewal J, Suri R, Mankad S, Tanaka A, Mahoney DW, Schaff HV. et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation 2010;121:1423–31. [DOI] [PubMed] [Google Scholar]
- 3. van Wijngaarden SE, Kamperidis V, Regeer MV, Palmen M, Schalij MJ, Klautz RJ. et al. Three-dimensional assessment of mitral valve annulus dynamics and impact on quantification of mitral regurgitation. Eur Heart J Cardiovasc Imaging 2018;19:176–84. [DOI] [PubMed] [Google Scholar]
- 4. Levack MM, Jassar AS, Shang EK, Vergnat M, Woo YJ, Acker MA. et al. Three-dimensional echocardiographic analysis of mitral annular dynamics: implication for annuloplasty selection. Circulation 2012;126:S183–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clavel MA, Mantovani F, Malouf J, Michelena HI, Vatury O, Jain MS. et al. Dynamic phenotypes of degenerative myxomatous mitral valve disease: quantitative 3-dimensional echocardiographic study. Circ Cardiovasc Imaging 2015;8:e002989. [DOI] [PubMed] [Google Scholar]
- 6. Basso C, Perazzolo Marra M.. Mitral annulus disjunction: emerging role of myocardial mechanical stretch in arrhythmogenesis. J Am Coll Cardiol 2018;72:1610–2. [DOI] [PubMed] [Google Scholar]
- 7. Perazzolo Marra M, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi B. et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delling FN, Rong J, Larson MG, Lehman B, Fuller D, Osypiuk E. et al. Evolution of mitral valve prolapse: insights from the Framingham heart study. Circulation 2016;133:1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Enriquez-Sarano M, Basmadjian AJ, Rossi A, Bailey KR, Seward JB, Tajik AJ.. Progression of mitral regurgitation: a prospective Doppler echocardiographic study. J Am Coll Cardiol 1999;34:1137–44. [DOI] [PubMed] [Google Scholar]
- 10. Avierinos JF, Detaint D, Messika-Zeitoun D, Mohty D, Enriquez-Sarano M.. Risk, determinants, and outcome implications of progression of mitral regurgitation after diagnosis of mitral valve prolapse in a single community. Am J Cardiol 2008;101:662–7. [DOI] [PubMed] [Google Scholar]
- 11. Adams DH, Rosenhek R, Falk V.. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J 2010;31:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 13. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA. et al. ; On behalf of the Scientific Document Committee of the European Association of Cardiovascular Imaging: Thor Edvardsen, Oliver Bruder, Bernard Cosyns, Erwan Donal, Raluca Dulgheru, Maurizio Galderisi, Patrizio Lancellotti, Denisa Muraru, Koen Nieman, Rosa S. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611–44. [DOI] [PubMed] [Google Scholar]
- 14. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K. et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713. quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 15. Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA.. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghulam Ali S, Fusini L, Tamborini G, Muratori M, Gripari P, Mapelli M. et al. Detailed transthoracic and transesophageal echocardiographic analysis of mitral leaflets in patients undergoing mitral valve repair. Am J Cardiol 2016;118:113–20. [DOI] [PubMed] [Google Scholar]
- 17. Lee AP, Hsiung MC, Salgo IS, Fang F, Xie JM, Zhang YC. et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation 2013;127:832–41. [DOI] [PubMed] [Google Scholar]
- 18. Hutchins GM, Moore GW, Skoog DK.. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–40. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson MJ, Bitkover CY, Omran AS, David TE, Ivanov J, Ali MJ. et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr 2005;18:1014–22. [DOI] [PubMed] [Google Scholar]
- 20. Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S.. Functional implication of mitral annular disjunction in mitral valve prolapse: a quantitative dynamic 3D echocardiographic study. J Am Coll Cardiol Cardiovasc Imaging 2017;10:1424–33. [DOI] [PubMed] [Google Scholar]
- 21. Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE. et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989;80:589–98. [DOI] [PubMed] [Google Scholar]
- 22. Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom F. et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–9. [DOI] [PubMed] [Google Scholar]
- 23. Konda T, Tani T, Suganuma N, Nakamura H, Sumida T, Fuji Y. et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J Echocardiogr 2017;15:176–85. [DOI] [PubMed] [Google Scholar]
- 24. Newcomb AE, David TE, Lad VS, Bobiarski J, Armstrong S, Maganti M.. Mitral valve repair for advanced myxomatous degeneration with posterior displacement of the mitral annulus. J Thorac Cardiovasc Surg 2008;136:1503–9. [DOI] [PubMed] [Google Scholar]
- 25. Avierinos JF, Gersh BJ, Melton LJ, Bailey KR, Shub C, Nishimura RA. et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002;106:1355–61. [DOI] [PubMed] [Google Scholar]
- 26. Klautz RJ, Tomsic A, Palmen M, van Brakel TJ, Perier P.. Optimal surgical mitral valve repair in Barlow's disease: the concept of functional prolapse. Multimed Man Cardiothorac Surg 2016; Dec 6; 2017; [DOI] [PubMed] [Google Scholar]
- 27. Delling FN, Li X, Li S, Yang Q, Xanthakis V, Martinsson A. et al. Heritability of mitral regurgitation: observations from the Framingham heart study and Swedish population. Circ Cardiovasc Genet 2017;10:e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.