Abstract
Food and reproduction are the fundamental needs for all animals. However, the neural mechanisms that orchestrate nutrient intake and sexual behaviors are not well understood. Here, we find that sugar feeding immediately suppresses sexual drive of male Drosophila, a regulation mediated by insulin that acts on insulin receptors on the courtship-promoting P1 neurons. The same pathway was co-opted by anaphrodisiac pheromones to suppress sexual hyperactivity to suboptimal mates. Activated by repulsive pheromones, male-specific PPK23 neurons on the leg tarsus release crustacean cardioactive peptide (CCAP) that acts on CCAP receptor on the insulin-producing cells in the brain to trigger insulin release, which then inhibits P1 neurons. Our results reveal how male flies avoid promiscuity by balancing the weight between aphrodisiac and anaphrodisiac inputs from multiple peripheral sensory pathways and nutritional states. Such a regulation enables male animals to make an appropriate mating decision under fluctuating feeding conditions.
Sugar suppresses sexual drive of male Drosophila, and the same pathway is used to suppress courtship to suboptimal mates.
INTRODUCTION
Energy homeostasis and reproduction in animals are orchestrated to maintain the highest reproductive success under fluctuating feeding conditions. It has been proposed that the hormones that respond to availability of oxidizable metabolic fuels regulate the sexual behaviors (1–3). However, the roles of the predominant metabolic hormones, such as insulin, in the control of reproductive behaviors are not well understood. A line of studies have begun to reveal the diverse roles of insulin in regulating sex behaviors in both insects and mammals. For example, Drosophila females with disrupted insulin signaling exhibit elevated receptivity to a male’s chasing (4, 5). A similar role of insulin was observed in mammals (6, 7).
On the other hand, feeding, or the accessibility to food, was found to promote sexual behaviors. The remating rate of a newly mated female fly was substantially enhanced when the courtship ritual occurred on food (8). Males of some insect species even deliver nuptial feeding to attract females (9, 10). In birds and mammals, metabolic challenges, such as food deprivation, inhibit the reproductive behaviors at many aspects, as parenting is among the most energetically costly behaviors (11–13). It thus seems that the availability of nutrients and the metabolic regulating hormones play differential roles in regulating sexual behaviors.
As sexual activities are energy-consuming, male animals must prevent futile courtship, e.g., persistent pursuit of an inappropriate or unreceptive mate, including males, mated females, and virgin females from other species. This is achieved by the integration in the male’s brain of both aphrodisiac and anaphrodisiac signals from the mate (14–17) and the environment (18, 19), as well as the internal state of the male himself (20, 21). However, how the brain integrates all the information and makes the effective decision on mating is largely unknown.
The balance between excitatory or inhibitory inputs onto P1 is the key for the right mating decision for male flies (17, 22, 23). Flies preferentially used chemical cues to identify a mate, as pheromones are highly reliable in distinguishing gender and species identity (24–26). For example, a female Drosophila melanogaster (D.mel) uses a combination of cuticular hydrocarbons (CHCs) as a barcode of her identity and mating states that are distinct from those of a male or a female from another species (27). In addition, pure visual stimuli are sufficient to trigger courtship initiation, albeit with less potency (28). In isolated males, activation of LC10 neurons, a male-specific circuit that is tuned to visual cues from a female, can induce ipsilateral wing extensions. It is proposed that the visual information is integrated with chemical cues in P1 neurons (28–30). P1 neurons can be activated by visual stimuli such as an inanimate moving object of certain size (29), and repetitive visual stimuli from a fly-size moving object cause robust and persistent change in P1 neurons’ state (31). In addition, volatile pheromones from females impose strong activation onto P1 neurons (17, 23). In contrast, contacting pheromones cause rapid adaptation of the tarsal PPK23 (Pickpocket 23) neurons that require minutes to recover (32), indicating that the chemical inhibition may become less potent after several rounds of body contacts. Both the excitatory or inhibitory cues are integrated on P1 neurons, which calculate the value between the two inputs as the appropriateness of the mate. The escalating excitation and diminishing inhibition onto P1 neurons over time raise a question: How is the weight of excitation versus inhibition in P1 neurons adjusted to avoid promiscuity and maintain selectivity toward appropriate mates?
Here, we demonstrate an elegant strategy used by the nervous system to fine-tune central neurons’ activity during courtship behaviors. The peripheral neurons that sense aversive pheromones not only relay the information to P1 neurons via a tandem of neuronal connections but also release a neuropeptide crustacean cardioactive peptide (CCAP) upon repulsive pheromone contacts. CCAP directly activates the insulin-producing cells (IPCs) in the brain to release insulin-like peptides (ILPs) that inhibit P1 neurons via the insulin/insulin-like growth factor signaling. This persistent inhibition on P1 compensates the rapid adaption of peripheral pheromone-sensing neurons and escalating potentiation of excitatory cues so that the males maintain the sensitivity to unappropriated mates. Our finding reveals an interesting heuristic in which neuronal and hormonal pathways orchestrate to enable an optimal tuning of neuronal activities.
RESULTS
Insulin is required for the inhibition of futile courtship
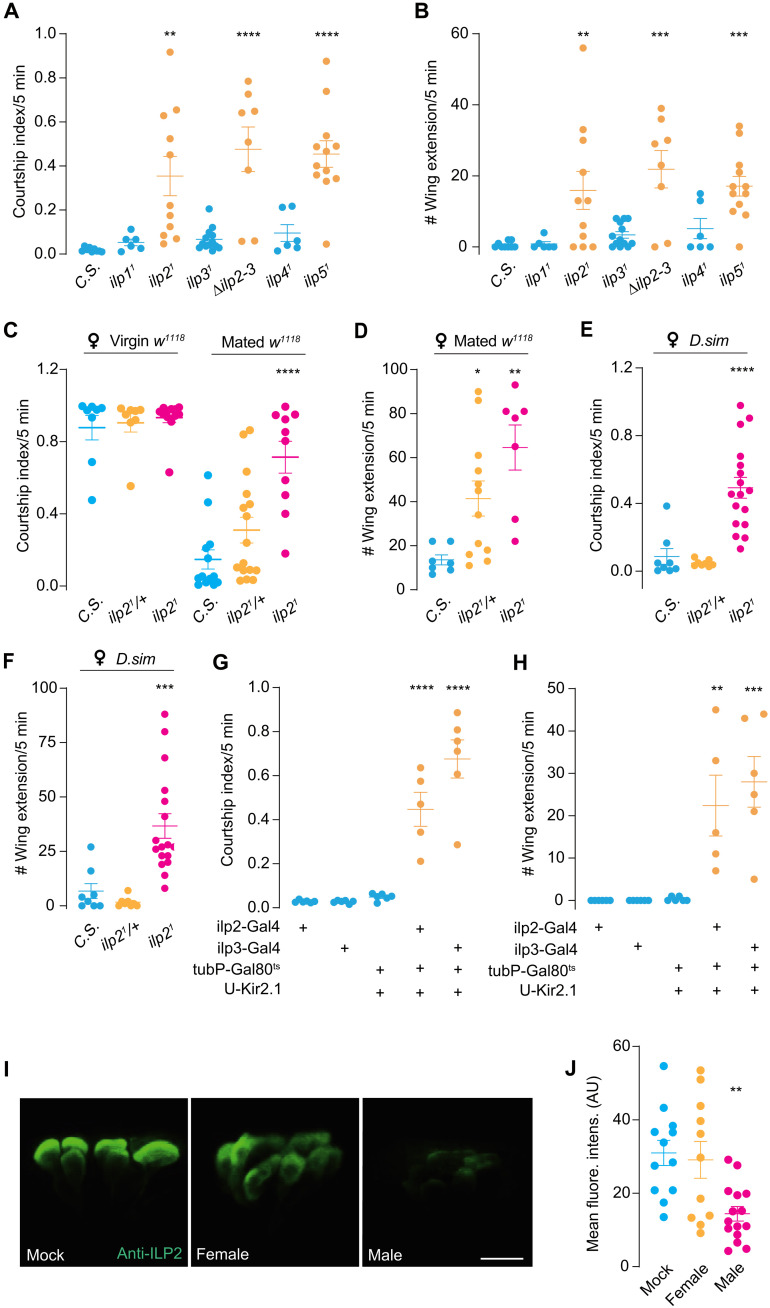
Male flies court rigorously to young virgin females while only intermittently to inappropriate mates, such as a newly mated female, a conspecific male, or a female from other species (24, 25, 33). Here, we found that, unlike control flies, male flies with mutation of the ILP gene ilp2 exhibited an elevated courtship index toward another male (Fig. 1, A and B). In addition, a similar behavioral defect was observed when the ilp2 mutants were grouped with less optimal mates such as newly mated females (Fig. 1, C and D) or virgin females of a closely related Drosophila species [Drosophila simulans (D.sim) and Drosophila mojavensis] (Fig. 1, E and F, and fig. S1, A and B), suggesting that insulin is essential for males to suppress inappropriate courtship.
Fig. 1. ILP2 is required to suppress futile courtship.
(A and B) Intermale courtship in ilp21 and ilp51 males. Courtship index and single-wing extension number between two males of various ILP (ilp) mutants. Intermale courtship of Canton-S (C.S.) as the wild-type control. N = 6 to 13 in each group. (C and D) ilp21 males’ courtship toward virgin or newly mated w1118 female. Courtship index and single-wing extension number between tester males and virgin or mated w1118 female. Mated w1118 female indicated female with immediately completed courtship. ilp21/+ was used as the parental control. N = 7 to 16 in each group. (E and F) ilp21 male chased D.sim virgin female intensely. Courtship index and single-wing extension number between tester males and virgin D.sim female. N = 8 to 17 in each group. (G and H) Intermale courtship was induced by silencing IPCs. Courtship index and single-wing extension number between tester males. Ilp2-Gal4 and ilp3-Gal4 were used to drive Kir2.1 expression in IPCs. N = 5 to 6 in each group. (I and J) Male contacts induced the release of ILP2. Immunostainings of ILP2 in male IPCs when males were housed alone (Mock), coupled with female or male. Mean gray values [arbitrary units (AU)] of IPCs were quantified between groups. Scale bar, 10 μm. N = 11 to 15 in each group. In all analyses, one-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons was used, and statistical differences were represented as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data were represented as means ± SEM.
Similarly, the mutants of ilp5 showed an equally strong courtship toward male flies (Fig. 1, A and B), indicating that the neurons that expressed these ilps are critical for the suppression of mating toward suboptimal subjects. In the brain, ilp2 and ilp5 are coexpressed in the IPCs in the par intercerebralis (34). Silencing these neurons with an inward-rectifying potassium channel Kir2.1 (35) caused the male flies to court another male (Fig. 1, G and H).
To ask whether ILP2 release can be triggered upon male contact, we monitored the insulin levels of IPCs in males exposed to different sexes. Exposure to a male for 10 min substantially elicited ILP2 release from IPCs in the male brain, as revealed by anti-ILP2 immunostaining (Fig. 1, I and J, and fig. S3, A to E). In contrast, interacting with virgin female (decapitated to avoid copulation) had no impact on the ILP2 level, although the male courted the female vigorously. These results indicated that male but not female exposure is sufficient to trigger ILP2 release from the IPCs.
Insulin targets insulin receptor to inhibit P1 neurons
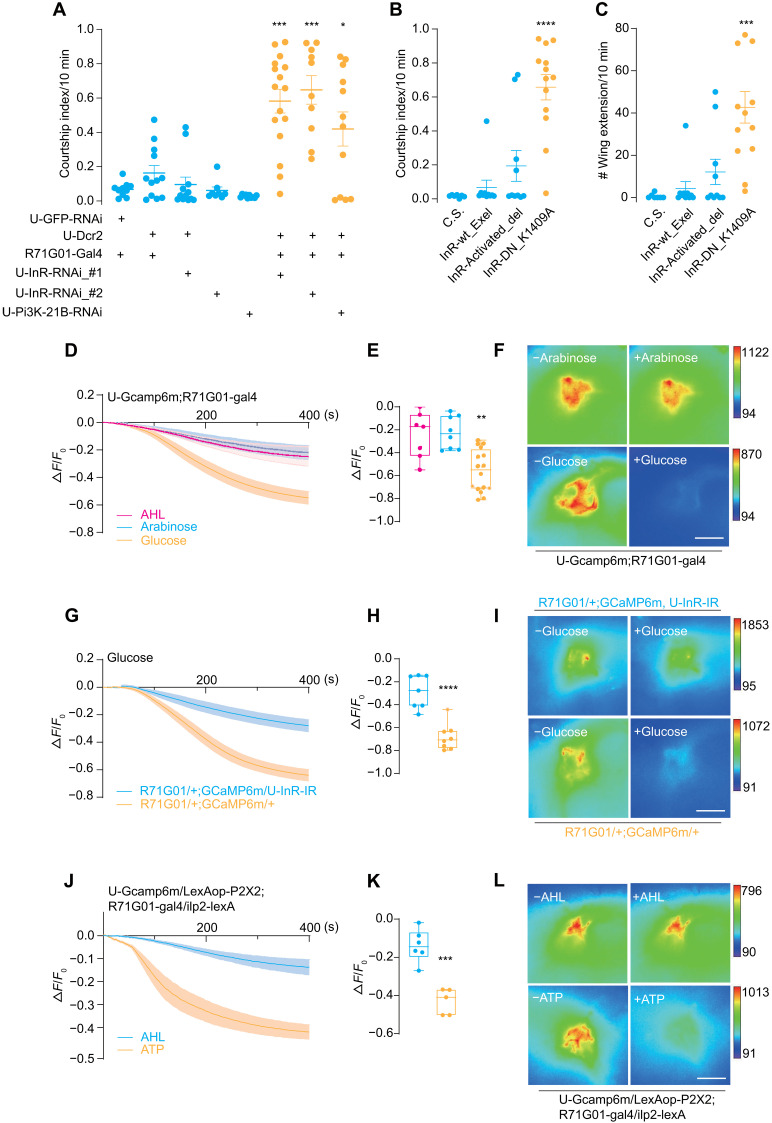
What is the downstream target of ILP2 in the inhibition of futile courtship? As the mutant of ilp2 exhibited hypersexual drive, we speculated that insulin may regulate the activity of P1 neurons that control courtship behaviors. The sole receptor of Drosophila ILP–insulin receptor (InR) is broadly expressed in the brain and the peripheral (36, 37). When InR expression was knocked down with InR-RNAi (RNA interference) driven by R71G01-Gal4 that labeled P1 neurons, the male flies showed strong courtship to other males (Fig. 2A and fig. S2F). Furthermore, disrupting the insulin-InR signaling pathway in P1 neurons by knocking down phosphoinositide 3-kinase (Pi3K)–21B, a key adaptor protein of the functional downstream Pi3K in the InR signaling pathway, resulted in a similar phenotype (Fig. 2A and fig. S2F). In addition, alleles of a dominant-negative form of InR, but neither the activated nor wild-type forms, induced vigorous courtship between males (Fig. 2, B and C).
Fig. 2. Male P1 neurons are inhibited by ILP2 via the insulin-like receptor InR.
(A) Down-regulation of InR signaling in P1 neurons induced intermale courtship. Courtship index between two males with various genotypes. Two separate UAS-InR-RNAi lines and a UAS-Pi3K-21B-RNAi line were expressed in P1 neurons with R71G01-Gal4. N = 10 to 16 in each group. (B and C) P1-specific expression of a dominant-negative form of InR (K1409A) induced intermale courtship. InR-wt_Exel, InR-Activated_del, and InR-DN_K1409A represented wild-type, constitutively active, and dominant-negative forms of InR, respectively. All forms were driven by R71G01-Gal4. N = 7 to 13 in each group. (D to F) Glucose decreased P1 activity. Fluorescence changes (ΔF/F0) of GCaMP6m in P1 neurons after the application of glucose (2.8 mM), AHL buffer, and arabinose (2.8 mM). Sugar solutions were added at 10 s. N = 7 to 10 in each group. (G to I) Glucose-induced inhibition on P1 activity required InR. Fluorescence changes (ΔF/F0) of GCaMP6m intensity in P1 neurons along with InR-RNAi driven by R71G01-Gal4. N = 7 to 8 in each group. (J to L) Activation of IPCs via P2X2 inhibited P1 activity. Fluorescence changes (ΔF/F0) of GCaMP6m in P1 neurons after adding either 1 mM ATP or AHL buffer. N = 5 to 6 in each group. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s test for multiple comparisons (A to C and E) and two-tailed unpaired t test (H and K). In all analyses, statistical differences were represented as follows: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data were represented as means ± SEM except for the box plot: minimum to maximum and median value (E, H, and K). Scale bars, 10 μm (F, I, and L).
As the activity of P1 neurons is positively correlated with a male fly’s sexual drive, we hypothesized that insulin signaling inhibited P1 neurons to suppress mating drive to another male. To test this, we performed Ca2+ imaging on P1 neurons and applied 2.8 mM glucose to the brain that was shown to substantially activate IPCs and evoked insulin release (38). As a result, Ca2+ level in P1 neurons was largely reduced upon glucose application (Fig. 2, D to F). In contrast, application of artificial hemolymph (AHL) only or a non-nutritious sugar arabinose of the same concentration did not significantly suppress P1 neurons. The glucose-induced inhibition was eliminated when InR was specifically knocked down in P1 neurons (Fig. 2, G to I). In addition, activating IPCs expressing the adenosine triphosphate (ATP) receptor P2X2 by adding ATP (39) induced a comparable level of inhibition on P1 neurons (Fig. 2, J to L). Such an inhibition may be critical to tone down the activity of P1 during fly interaction to balance the excitatory and inhibitory inputs onto P1 neurons.
Sugar feeding suppresses mating drive
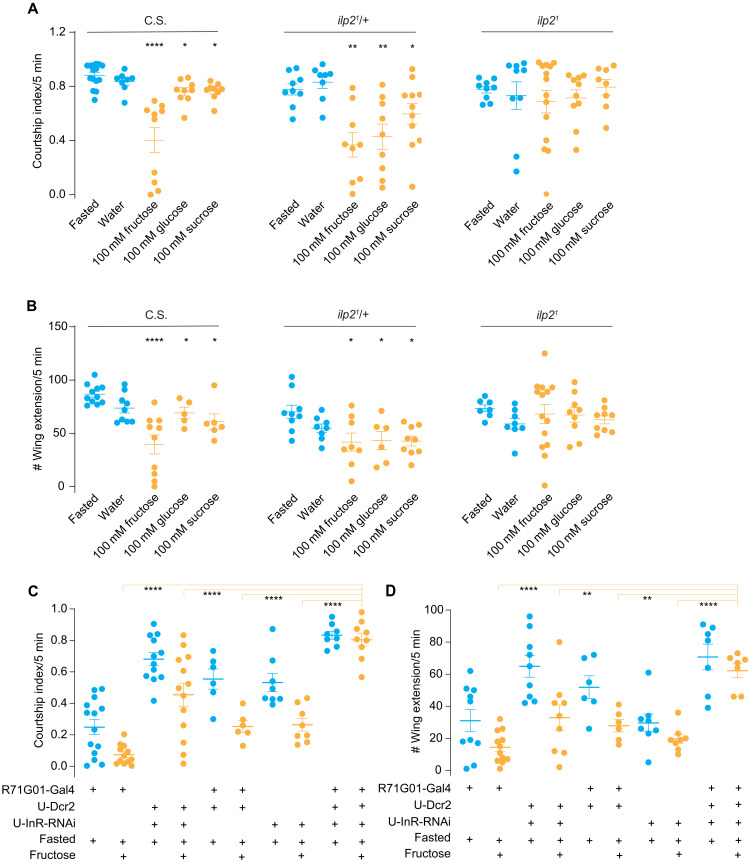
Insulin is the major hormonal peptide in blood sugar regulation (40). We then asked whether insulin release triggered by sugar ingestion would affect a male fly’s mating drive. Males were fasted for 7 to 9 hours and then fed with 100 mM sugar for 3 min before being grouped with a virgin female. The courtship intensity of Canton-S (CS) males fed with different sugars toward a virgin female was substantially decreased (Fig. 3, A and B). The postprandial inhibition of courtship is mediated by insulin, as the ilp2 mutant flies exhibited equally strong courtship after sugar feeding (Fig. 3, A and B). In addition, fasted males with the supplement of water showed a decreased courtship intensity, indicating that water was dispensable for the courtship inhibition (fig. S7, A and B). Intracellular ILP2 level in IPCs was significantly reduced after sugar feeding (fig. S7, C to G), demonstrating that feeding sugars promoted the release of ILP2 within a similar time window. Furthermore, the fructose intake-induced courtship suppression required the intact InR in the courtship-promoting center P1 neurons (Fig. 3, C and D). Together, mating drive is regulated by the InR on P1 neurons as a downstream of metabolic state changes.
Fig. 3. Sugar feeding attenuates male’s courtship intensity toward females via ILP2 signaling.
(A and B) Ilp21 mutant males maintained high courtship intensity after sugar feeding. Courtship index and single-wing extension number of indicated D.mel males (C.S., ilp21/+ control and Ilp21 mutant) toward w1118 virgin females were quantified. N = 9 to 15 in each group of (A) and n = 5 to 14 in each group of (B). (C and D) Courtship intensity after sugar feeding in males with InR knockdown in P1 neurons. Courtship index and single-wing extension number of indicated D.mel males toward w1118 virgin females were quantified. InR was specifically suppressed in P1 neurons using R71G01-Gal4. Males were fed with 100 mM fructose. N = 8 to 14 in each group of (C) and n = 7 to 12 in each group of (D). One-way ANOVA followed by Dunnett’s test for multiple comparisons (A and B) and two-way ANOVA followed by Dunnett’s multiple comparisons test (C and D) were used for statistical significance assessment. Statistical differences were represented as follows: *P < 0.05, **P < 0.01, and ****P < 0.0001. Data were represented as means ± SEM.
CCAP receptor in IPCs is required for suppressing male-male courtship
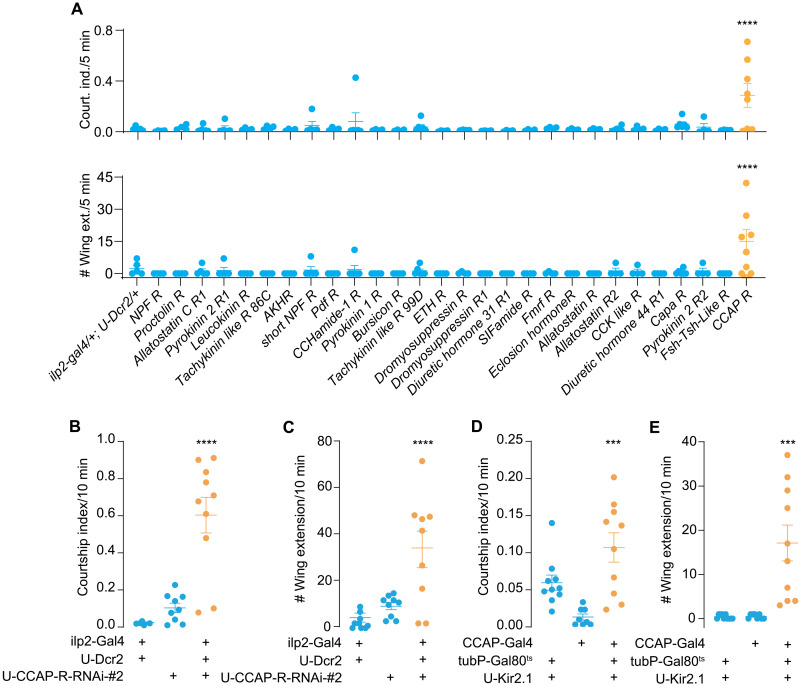
IPCs express a cohort of neurotransmitter and neuropeptide receptors (41) and are subjected to regulation by multiple external and internal cues (40). To search for other factors that may mediate ILP2 release during male-male interaction, we performed RNAi-based screening for 28 neuropeptide receptor genes with the ilp2-Gal4 and also looked at the involvement of a series of neurotransmitter receptor genes in male-male courtship. Among those, knockdown of the CCAP receptor (CCAP-R) in IPCs resulted in vigorous male-male courtship (Fig. 4A), indicating that CCAP may promote ILP release via CCAP-R expressed in IPCs. Expression of another independent RNAi line against CCAP-R led to comparable intermale courtship intensity (Fig. 4, B and C). This function of CCAP was further validated by the fact that silencing CCAP neurons resulted in an elevated male-male courtship (Fig. 4, D and E). We also carried out a mutant screening for neuromodulator and neurotransmitter receptor genes on IPCs and found that none of them except for TrhKO (tryptophan hydroxylase encoding gene, the first and rate-limiting step in the synthesis of serotonin) appeared indispensable in male-male courtship suppression (fig. S1, C and D).
Fig. 4. CCAP-R on IPCs is required for suppression of intermale courtship.
(A) Screening for neuropeptide receptor genes using IPC-specific RNAi knockdown. Courtship index (top) and single-wing extension number (bottom) was counted between two males of the same genotype. N = 4 to 8 in each group. (B and C) Knockdown of CCAP-R in IPCs induced intermale courtship. Courtship index and single-wing extension number were counted between males. U-CCAP-R-RNAi #2 is another independent RNAi line from the one used in the screening (B). N = 9 to 10 in each group. (D and E) Silencing CCAP cells induced intermale courtship. Courtship index and single-wing extension number were counted between males. N = 8 to 10 in each group. In all analyses, one-way ANOVA followed by Dunnett’s test for multiple comparisons was used, and statistical differences were represented as follows: ***P < 0.001 and ****P < 0.0001. Data were represented as means ± SEM.
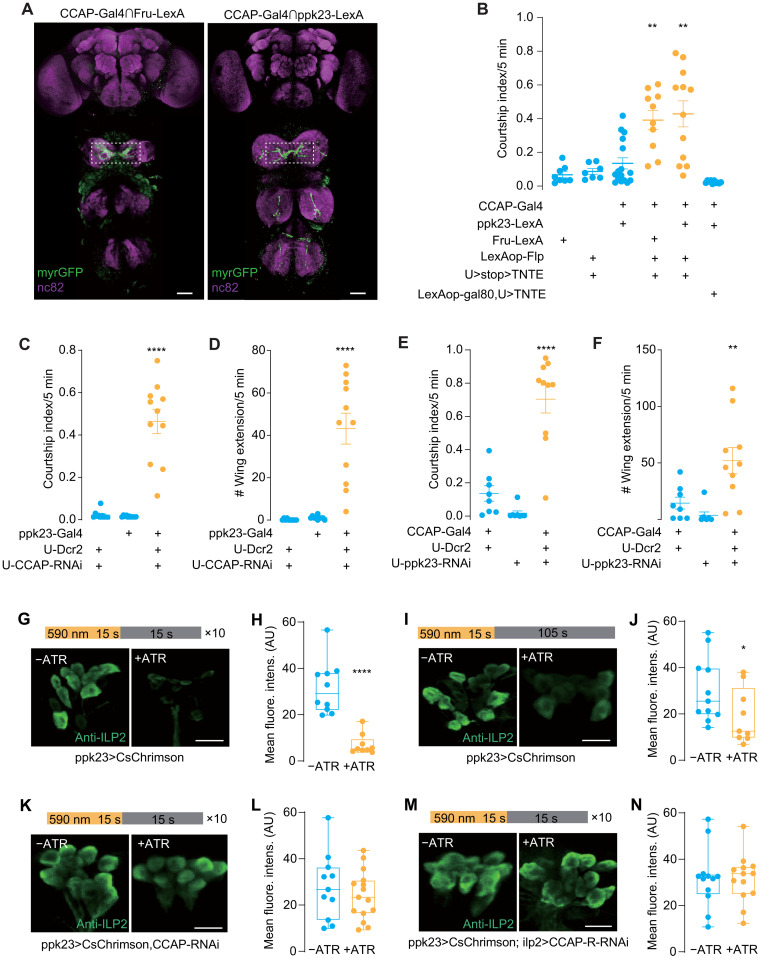
CCAP is expressed in pheromone-sensing PPK23 neurons
What is the source of CCAP during male-male contact? Among the female CHCs, 7,11-heptacosadiene plays a predominant role in attracting males. It activates PPK23+ F cells (female pheromone specific) on a male’ foreleg tarsus to elicit vigorous courtship (15, 22, 32). In contrast, a male predominant CHC 7-tricosene is detected by PPK23+ M cells (male pheromone specific) (22) to deter courtship (15). Male cues are relayed to P1 neurons via inhibitory mAL neurons (15, 22). Since both PPK23 neurons and mAL neurons are Fru-positive, we thus intersected a CCAP-Gal4 with the Fru-lexA, and this intersection labeled a group of tarsal neurons whose axons projected to ventral nerve cord (VNC) with a middle-line crossing pattern, resembling some of the PPK23-expressing M cells and/or F cells (Fig. 5A). Silencing the Fru+/CCAP+ neurons induced vigorous intermale courtship, confirming that these neurons were M cells, which are necessary to suppress futile courtship (Fig. 5B and fig. S2G). To test whether these neurons were the PPK23 pheromone-sensing neurons, we intersected the CCAP-Gal4 with the PPK23-LexA and observed a similar axonal projection pattern in the VNC (Fig. 5A). Furthermore, inactivation of these neurons caused male-male courtship (Fig. 5B and fig. S2G). These results are consistent with previous finding that silencing PPK23-expressing neurons resulted in male-male courtship (15).
Fig. 5. CCAP functions in leg tarsal PPK23 neurons to inhibit intermale courtship.
(A) Cross-midline projections into VNC from intersections of CCAP/Fruitless (left) or CCAP/PPK23 (right). Dotted box indicated anti-GFP (green fluorescent protein) signals. Scale bars, 20 μm. (B) Silencing CCAP-Gal4/Fru-LexA and CCAP-Gal4/PPK23-LexA colabeled neurons induced intermale courtship. Courtship index was quantified between males of indicated genotypes. N = 7 to 12. (C and D) Knockdown of PPK23 in CCAP neurons induced intermale courtship. Courtship index (C) and single-wing extension number (D) were counted. N = 8 to 10. (E and F) Knockdown of CCAP in PPK23 neurons induced intermale courtship. Courtship index (E) and single-wing extension number (F) were counted. N = 10 to 12. (G and H) Repeated activation of PPK23 neurons induced ILP2 release from IPCs. CsChrimson was driven by ppk23-Gal4. Light stimulation program was listed. All-trans retinal (ATR) was supplied (+ATR) or not (−ATR). N = 7 to 8. (I and J) Single activation of PPK23 neurons. Similar to (G and H) except for light stimulation of 15 s. ATR was supplied (+ATR) or not (−ATR). N = 10 to 11. (K and L) Knockdown of CCAP in PPK23 neurons blocked ILP2 release upon stimulating PPK23 neuron. CsChrimson and CCAP-RNAi were expressed with ppk23-Gal4. N = 14 to 15. (M and N) Knockdown of CCAP-R in IPCs blocked ILP2 release upon PPK23 neuron stimulation. CsChrimson and CCAP-R-RNAi were driven by ppk23-LexA and ilp2-Gal4, respectively. N = 8 to 12. Mean fluorescence intensity (AU) of IPCs was compared between groups (G to N). One-way ANOVA followed by Dunnett’s test for multiple comparisons (B to F) and two-tailed unpaired t test (H, J, L, and N) were used. Statistical differences were represented as follows: *P < 0.05, **P < 0.01, and ****P < 0.0001. Data were represented as means ± SEM, except for the box plot: minimum to maximum and median value (H, J, L, and N). Scale bars, 10 μm (G, I, K, and M).
To further validate that CCAP functions in PPK23 neurons upon pheromone contact, we used the PPK23-Gal4 to knock down CCAP with RNAi. The flies exhibited strong courtship toward another male (Fig. 5, C and D), indicating that the activation of M cells during male contact are the major source for CCAP release. In addition, knocking down PPK23 with the CCAP-Gal4 resulted in a similar defect (Fig. 5, E and F). Courtship activity of both groups to virgin females was not changed (fig. S2, A to C), suggesting that the elevated courtship activity derived from the down-regulation of CCAP was specific to male targets, as the same males showed normal courtship to virgin females. Furthermore, CCAP transcript level was significantly reduced in male legs when CCAP was knocked down using M cell–Gal4 (fig. S8C), indicating that CCAP was released from leg tarsal PPK23 “M” cells. This conclusion was further strengthened by the fact that CCAP+ neurons in the brain was dispensable for the suppression of intermale courtship (Fig. 5B and fig. S2G).
PPK23 neurons release CCAP upon contacting aversive pheromones
To test whether CCAP release from PPK23 neurons directly activates CCAP-R on the IPCs, we carried out two sets of experiments. We first optogenetically activated PPK23 neurons via CsChrimson and found that ILP2 were released from IPCs (Fig. 5, G and H, and fig. S4A). Notably, optogenetic activation of PPK23 neurons for 15 s was sufficient to trigger ILP2 release from IPCs after 2 min of resting, suggesting that a transient pheromone contact can induce persistent state change of the brain neurons (Fig. 5, I and J, and fig. S4B). In addition, red fluorescent protein (RFP) was introduced into IPCs as an internal reference to better quantify the release of ILP2 during stimulation. Normalization of ILP2 immunostainings to RFP intensity revealed a reduction of fluorescence activity when males were provided with all-trans retinal (fig. S5, A to D). This light-induced ILP2 release was greatly reduced when CCAP was knocked down in PPK23 neurons (Fig. 5, K and L, and fig. S4C) or when CCAP-R expression was knocked down in IPCs (Fig. 5, M and N, and fig. S4D). Last, we determined that M cell axonal projections on VNC were the releasing sites of CCAP, by using preproANF-Emerald that was reported to label dense core vesicles (fig. S6, A to D) (42). This supports the notion that CCAP released from M cell axons travels a long distance to reach IPCs, possibly via circulation.
PPK23-CCAP-ILP2 pathway suppresses futile courtship
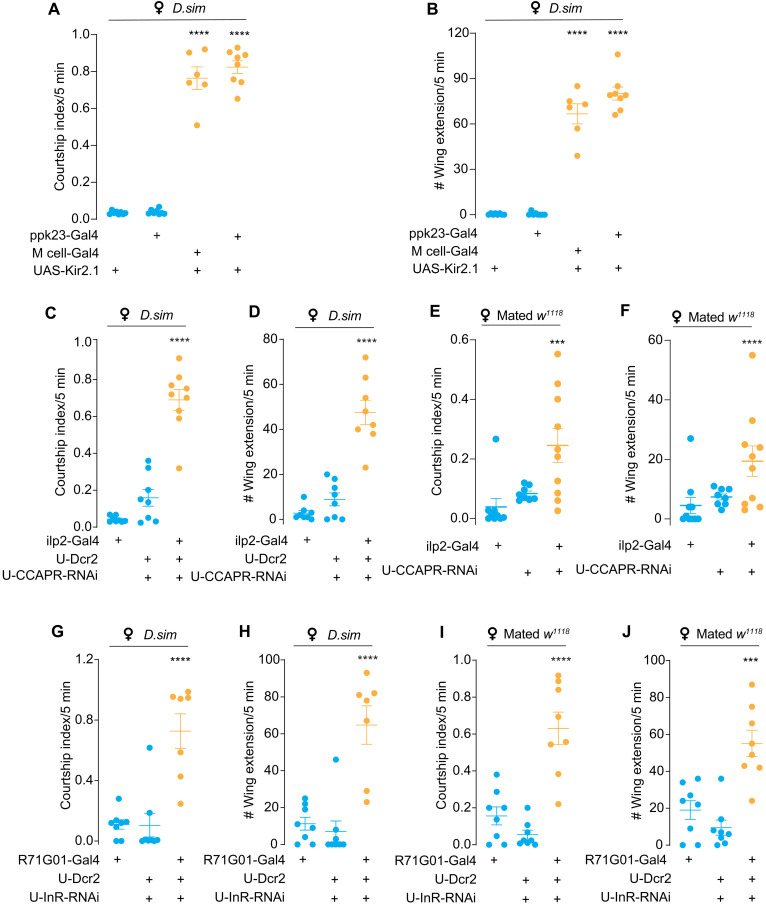
We next asked whether the PPK23-CCAP-ILP2 pathway is involved in suppressing courtship toward other futile females. Silencing PPK23 neurons or the M cells resulted in vigorous courtship toward D.sim virgin females (Fig. 6, A and B). Moreover, a similar phenotype was observed when CCAP-R was knocked down in IPCs (Fig. 6, C and D) and CCAP-R was found to colocalize with IPCs (fig. S6E). Besides, knocking down CCAP-R in IPCs resulted in male’s courtship toward newly mated w1118 females (Fig. 6, E and F, and fig. S2, D and E), suggesting that CCAP activates IPCs to suppress futile courtship. Besides, specific suppression of CCAP-R in neuropeptide F (NPF)-Gal4, which was shown to modulate intermale courtship (43), resulted in elevated intermale courtship (fig. S2, D and E), suggesting that CCAP may have multiple targets in the brain. In addition, specifically knocking down InR expression on P1 neurons caused the males to court D.sim virgin females (Fig. 6, G and H) and newly mated w1118 females (Fig. 6, I and J). Dual labeling of P1 neurons and IPCs demonstrated that their arborizations are in close proximity (fig. S8B). GFP Reconstitution Across Synaptic Partners (GRASP) signals were also detected between P1 cells and IPCs, suggesting that they may form direct synaptic connections (fig. S8A). These results suggest that ILPs from IPCs may target P1 neurons via paracrine mechanisms.
Fig. 6. PPK23-CCAP-ILP2 axis suppresses futile courtship.
(A and B) Silencing leg tarsal PPK23 neurons induced courtship toward D.sim virgin females. Courtship index and single-wing extension number toward D.sim females of D.mel males with PPK23 neurons silenced. Tarsal pheromone-sensitive neurons were labeled with ppk23-Gal4 or M cell–Gal4 (ppk23-Gal4; vGlut-Gal80). N = 6 to 8 in each group. (C and D) Knockdown of CCAP-R expression in IPCs induced futile courtship toward D.sim virgin females. Courtship index and single-wing extension number of D.mel males with UAS-CCAP-R-RNAi using ilp2-Gal4. N = 8 to 9 in each group. (E and F) Knockdown of CCAP-R expression in IPCs induced futile courtship toward mated w1118 females. Courtship index and single-wing extension number of D.mel males with UAS-CCAP-R-RNAi using ilp2-Gal4. N = 8 to 9 in each group. (G and H) Knockdown of InR expression in P1 neurons induced futile courtship toward D.sim virgin females. Courtship index and single-wing extension number of D.mel males with UAS-InR-RNAi using R71G01-Gal4. N = 7 to 8 in each group. (I and J) Knockdown of InR expression in P1 neurons induced futile courtship toward mated w1118 females. Courtship index and single-wing extension number of D.mel males with UAS-InR-RNAi using R71G01-Gal4. N = 8 in all group. In all analyses, statistical significance was assessed by one-way ANOVA followed by Dunnett’s test for multiple comparisons, and statistical differences were represented as follows: ***P < 0.001 and ****P < 0.0001. Data were represented as means ± SEM.
CCAP-ILP2-P1 signaling mediates delayed inhibition of male-male courtship
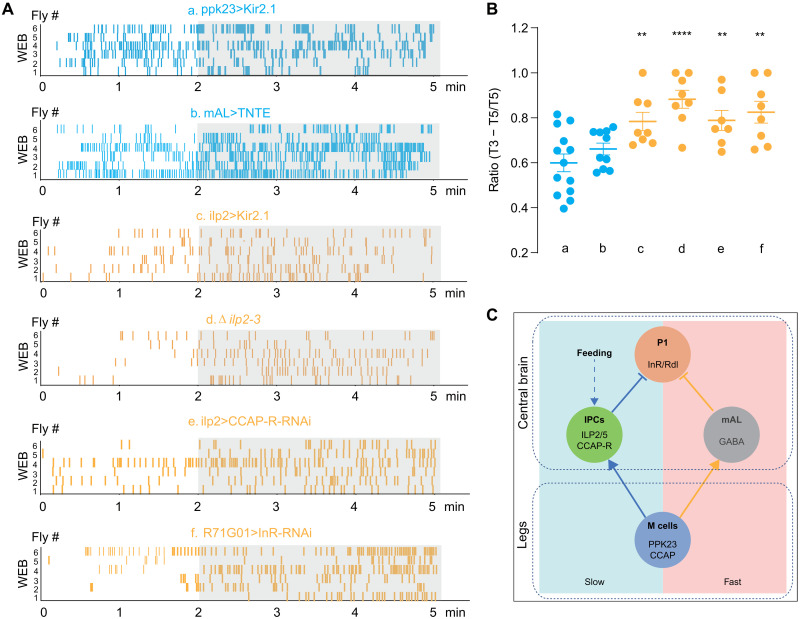
Previous studies have revealed the circuits that relay male specific chemical cues to the central P1 neurons to ultimately suppress courtship behaviors (17, 22). However, studies indicated that these pheromone-sensing neurons quickly adapted to multiple stimulations. Thus, there might be a chronic or delayed inhibition mechanism to counteract the escalating mating drive fueled by the olfactory and visual signals. Although ilp2 mutant males courted other males vigorously, the single-wing extension bouts exhibited a delayed onset of around 2 min after the males were grouped together, unlike the immediate initiation of courtship between males with their silenced PPK23+ neurons (Fig. 7, A and B). Besides, the similar courtship delay was also observed in males when any component of CCAP-ILP2-P1 signaling was disrupted (Fig. 7, A and B). We also proved that mAL neurons were directly involved in fast suppression. Blocking mAL neurons using tetanus toxin evoked immediate intermale courtship bouts, with similar temporal dynamics as seen in blocking ppk23 neurons but distinct from that observed in flies with defective CCAP-ILP2 pathway (Fig. 7, A and B). Thus, both PPK23+ neurons and their downstream mAL pathways are necessary to suppress intermale courtship in the initial stage. Together, we speculated that the activation of IPCs was required for sustained suppression of selecting inappropriate mating target, and this neuropeptidergic pathway relays the combination of metabolism state and pheromone cues to maintain long-lasting courtship inhibitory effects (Fig. 7C).
Fig. 7. CCAP-ILP2-P1 axis induces a delayed inhibition of male-male courtship.
(A) Raster plot of wing-extension bouts (WEB) for males of indicated phenotypes. From top to bottom: a, males with silenced PPK23 cells (ppk23-Gal4>UAS-Kir2.1); b, males with silenced mAL cells (R43D01-Gal4>UAS-TNTE); c, silenced IPCs (ilp2-Gal4>UAS-Kir2.1); d, ilp2 mutant males; e, males with CCAP-R knocking down in IPCs using ilp2-Gal4; and f, InR knocking down in P1 neurons using R71G01-Gal4. Raster plots of WEB in 5 min were presented. (B) Quantification of male WEB in listed males. Percentage of bouts in the last 3 min of total 5 min (T3 − T5/T5) was calculated. N = 7 to 13 in each group. The corresponding genotypes were correlated with a to f in (A). (C) Schematic of the neural circuits in males that suppress futile courtship. Left (light blue): The neural circuit that controlled the delayed suppression to inappropriate mates. Right (light red): The pathway that rapidly suppresses male’s sexual drive toward futile mates. In all analyses, statistical significance was assessed by one-way ANOVA followed by Dunnett’s test for multiple comparisons, and statistical differences were represented as follows: **P < 0.01 and ****P < 0.0001. Data were represented as means ± SEM. GABA, γ-aminobutyric acid.
DISCUSSION
This work identified a neuropeptidergic neural circuit underlying mating decision, and this study reveals a direct link between the “metabolic center” and the “sex center” in the Drosophila brain. First, sugar feeding largely suppressed male’s sexual drive toward a virgin female, and this metabolic state–dependent neural control relied on ILP2 and ILP5. A suppression on P1 neurons was induced in vitro with the activation of IPCs, which was triggered by loading sugar (Fig. 2, D to L). This conclusion was further validated by the fact that the decrease of P1 neurons’ activity was weaker when InR was knock down specifically on P1 neurons. We also demonstrated that leg tarsal PPK23 M cells release CCAP once contacting aversive pheromone and this inhibitory signal activates IPCs in the brain via CCAP-R to release ILP2/5. IPC-derived ILP2/5 furthermore suppresses the activity of courtship-promoting P1 neurons to ultimately shut down male’s sexual drive toward inappropriate mating targets (Fig. 7C). Together with inhibition on P1 by the tandem connections of PPK23 M cells and mAL neurons (22), male flies maintain high selectivity against inappropriate mates.
Integration of external and internal information on P1 during courtship
As the central hub in control of male courtship, P1 neurons in the brain integrate both the external and internal information. The former includes sensory inputs from a potential mating target. While visual (28, 31) and olfactory cues (16, 18) are reported to trigger a male’s propensity to court, contact pheromones can be either attractive or repulsive and gate the perception for other sensory pathways (17). The internal state, on the other hand, represents the male’s readiness to mate, e.g., his mating history and nutritional state (21, 44). P1 neurons constantly monitor the internal state of a male to evaluate the readiness to court or mate (20, 21). For example, dopaminergic neurons control the mating drive of a male (21), and neuropeptide Drosulfakinin (DSK) and NPF impose an inhibitory tone on P1 neurons by encoding either mating experiences or nutritional state (43, 45). In addition, sleep regulates mating via the functional interaction between circadian neurons and P1 (46, 47). Insulin signaling reportedly plays a well-established role in homeostatic regulation. Here, we revealed an unprecedented role of insulin in modulating male sexual behavior. On the basis of the fact that insulin level changes under numerous physiological conditions, such as feeding (38), temperature change (48), and sleep (49), our findings raised the question whether, under such conditions, insulin regulates sexual activity via InR on P1. Our present data support the existence of feeding/mating interaction via the insulin signaling.
The link between metabolism and sexual behaviors
Further investigation is needed to reveal the biological significance of courtship inhibition immediately after a sugar meal. In the studies of human and mouse, sugar intake reduces the level of testosterone, an effect likely mediated with insulin (50, 51). This adversity of sugar intake on libido may be important for the animals’ fitness. There is an immediate boost of the blood sugar level after a sugar meal. Insulin is then secreted to help move the sugar from the blood into the cells. During this process, the sexual activity may be momentarily inhibited to foster sugar uptake. It still needs further investigation how decreased sexual behaviors contribute to energy storage. Another interesting question is whether other nutrition-related hormones such as adipokinetic hormone (Akh) and unpaired 2 (upd2) also regulate sexual activity in flies. It is already reported that DSK and NPF are both required to tune a male’s sexual drive (43, 45). Recently, it was reported that protein intake caused postprandial sleepiness that may be critical for protein metabolism (52), suggesting that animals’ nutritional homeostasis is critical in maintaining the balance of their feeding, reproduction, and other behaviors.
Another intriguing question that warrants further investigation is whether insulin released upon contacting aversive pheromones would cause certain metabolic consequences to the males. It was reported that exposing male flies to female pheromone but preventing them from mating reduced males’ life span (53). As deterring courtship is relatively fast, it would be interesting to look at the long-term effects after a male is exposed to aversive pheromones.
Sexual dimorphic roles of insulin in mating behaviors
Insulin was implicated to regulate female sexual receptivity (5). The female flies with mutations in their ilp genes exhibit a higher sexual activity (54), a similar defect as seen in male flies in current study. However, the neural circuit controlling mating in male and female shows profound sexual dimorphism. It thus raises an interesting question: How does insulin regulate the sexual drive in females? A virgin female’s receptivity is controlled by double-sex–positive neurons in the brain. Among them, clusters of neurons of pCd and pC1 play a determinant role in female’s sex behaviors (55). It is still an open question whether these neurons express InR and, if so, under what circumstance are they inhibited by insulin release. Insulin is essential for vitellogenesis in female flies (56), suggesting that insulin signaling may play differential roles at different reproductive stages.
MATERIALS AND METHODS
Fly stocks
All fly strains were maintained with a 12-hour light/12-hour dark cycle at 25°C (unless otherwise noted) and 60% humidity (Percival incubator). For experiments using tubulin-gal80ts, flies were raised in 22°C for egg laying and larval/pupal development, and adults were immediately transferred into 30°C after eclosion until behavior assay. w1118 and CS were used as wild-type control. Other mutants and transgenic lines from Bloomington Drosophila Stock Center included the following: ilp11 (30880), ilp21 (30881), ilp31 (30882), ilp41 (30883), ilp51 (30884), Δilp2-3 (30888), Ilp2-Gal4 (37516), Ilp3-Gal4 (52660), R71G01-Gal4 (39599), R43D01-Gal4 (mAL-Gal4, 64345), R64B05-Gal4 (CCAP-R-Gal4, 39292), UAS-Dicer2 (24650 and 24651), UAS-InR-RNAiJF01482 #2 (31037), UAS-Pi3K-21B-RNAiHMS01907 (38991), UAS-InR.K1409A (8252), UAS-InR.Exel (8262), UAS-InR.del (8248), UAS-GCaMP6m (42748), LexAop2-CsChrimson (55138), UAS-CsChrimson (55135), UAS-Kir2.1 (6596), CCAP-Gal4 (25685), FruP1-LexA (66698), UAS-ppk23-RNAiJF02986 (28350), UAS-CCAP-RNAiHMJ23953 (62470), vGlut-Gal80 (58448), UAS-TNTE (28837), UAS-preproANF-Emerald (7001), and UAS-mCD8::GFP (32186). UAS-InR-RNAi #1 (v992), UAS-5-HT7-RNAi (v104804), and UAS-CCAP-R-RNAi #2 (v14767) are from Vienna Drosophila Resource Center. D. mojavensis (BCF#96) was from Core Facility of Drosophila Resource and Technology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences. A screening list of RNAi lines and null mutants of various neuropeptides and neurotransmitters is supplemented (table S1). We thank L. Wang at Zhejiang University for sharing RNAi lines of neuropeptide receptors. PPK23-LexA was a gift from B. Dickson at Janelia Farm Research Campus. PPK23-Gal4 was provided by Y. Rao at Peking University. We thank Y. Pan at Southeast University, China for providing LexAop2-p2x2, dilp2-LexA, 8xLexAop2-Flp, UAS>Stop>myrGFP, UAS>Stop>TNT; 8xLexAop2-Flp, UAS>Stop>dTrpA1; 8xLexAop2-Flp, and tubp-GAL80ts; UAS-Kir2.1.
Courtship assay
All behavioral experiments were done under 25°C and 45 to 50% humidity. Flies were aspirated into a courtship chamber with height of 3 mm and diameter of 10 mm. Damped filter paper was put on the bottom of the courtship chamber to keep the moisture (unless otherwise noted). Virgin females were kept in groups (15 flies per vial) for 4 to 6 days, while males were raised individually for 8 to 12 days. All male flies were collected immediately after eclosion to make sure that they were naïve to social experience. Courtship behaviors were video-recorded using a stereoscopic microscope mounted with a charge-coupled device camera (Basler ORBIS OY-A622f-DC) and the Virtual VCR software. The total duration from each step of courtship ritual was manually analyzed, and the courtship index was defined as the percentage of time that a male exhibited any step of the ritual in 5 or 10 min. For screening, two strategies were used: chemoconnectome (CCT) mutants of various neurotransmitter genes were tested in behavior, and 28 neuropeptide receptors and three 5-hydroxytryptamine (serotonin) receptor (5-HT) receptors were knocked down in IPCs driven with ilp2-Gal4. A pair of male flies of same genotype (10 to 12 days old) were aspirated into the courtship chamber, and the number of wing extension and courtship index in 5 min were analyzed.
For sugar-feeding assay, male flies were deprived of food for 16 hours or of both food and water for 7 to 9 hours, fed with water only or sugar solutions with indicated concentrations for 3 min, and then introduced into a courtship chamber with the bottom covered with dry filter paper. A virgin female was introduced immediately into the same chamber. For courtship test with mated females, all female flies were 5 to 6 days old and newly mated with CS males.
Quantification of dILP2 release in IPCs
A single newly eclosed male fly was fed with sugar-retinal solution (500 μM all-trans retinal; Sigma-Aldrich, R2500; diluted in 100 mM sucrose solution) in darkness and used for optogenetic manipulation after 4 to 5 days. Control files were fed with 100 mM sucrose solution. For RNAi experiment, flies were tested after 8 to 10 days. Two stimulation patterns were used. The first contained 15 s of light and 105 s of darkness, and brain was dissected and immunostained with anti-ILP2 immediately; the second consisted of 10 cycles of 15 s of light and 15 s of darkness, and subsequent immunostaining was performed. For sugar-feeding assay, male flies were deprived of food or water for 7 to 9 hours and fed with sugar solutions with indicated concentrations for 3 min, and then, subsequent immunostaining was performed.
Rabbit anti-DILP2 (1:1000), mouse anti-RFP (1:500; Rockland, 200-301-379), Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG), and Alexa Fluor 647 goat anti-mouse IgG were used for staining. Images were captured using 60× water immersion objective (Olympus). All optogenetic manipulations and sugar feeding were carried out in the courtship chamber. All images were captured using an Olympus FV1000 confocal microscope with 60× water immersion lens, using identical laser power and scan settings for each experiment. The mean fluorescence intensity (arbitrary units) was measured from the total Z stack using ImageJ software. Regions of interest (ROIs) were selected on the basis of the position of IPC soma, and the minimum range or area that surrounds all visible soma was selected as ROI. The experimenter that defined ROIs was blinded to the experimental conditions. Plotting and statistical analyses were conducted with GraphPad Prism software.
Quantification of wing extension bouts
Courtship intensity was partially represented by the number of single-wing extension bouts tempospatially. The number and distribution of wing extension occurrence in 5 min were manually annotated with BORIS software. The ratio of extension bouts in the last 3 min (T3 − T5) over the total 5 min (T5) represented the temporal dynamics of courtship intensity. Plotting was performed with Adobe Illustrator software.
Immunohistochemistry
Brains or VNC were dissected in dissection buffer [0.015% Triton X-100 in 1× phosphate-buffered saline (PBS)] and fixed in 4% paraformaldehyde (PFA) at room temperature on a nutator for 20 min. The tissues were then washed three times for 10 min in wash buffer (30% Triton X-100 in 1× PBS). Subsequently, the samples were blocked in block buffer (1× heat-inactivated normal goat serum with 30% Triton X-100 in 1× PBS) for 30 min at room temperature and incubated with primary antibody overnight at 4°C. The primary antibodies included rabbit anti–green fluorescent protein (1:500; Invitrogen), mouse anti-nc82 [1:500; Developmental Studies Hybridoma Bank (DSHB)], mouse anti-RFP (1:500; Invitrogen), and rabbit anti-DILP2 (1:1000; gift from Y. Li, Institute of Biophysics, CAS). On the second day, tissues were washed three times for 10 min in wash buffer and then incubated in secondary antibodies. The secondary antibodies included the following: Alexa Fluor 555 goat anti-rabbit IgG (1:500; Invitrogen), Alexa Fluor 488 goat anti-rabbit IgG (1:500; Invitrogen), and Alexa Fluor 647 goat anti-mouse IgG (1:500; Invitrogen). Last, brains or VNC were mounted for imaging with an Olympus FV1000 confocal microscope with 20× air lens or 60× water immersion lens.
Calcium imaging
The male flies were raised single-housed for 7 to 11 days, and the brains were dissected in a recording chamber filled with the AHL [108 mM NaCl, 5 mM KCl, 4 mM NaHCO3, 1 mM NaH2PO4, 15 mM ribose, and mM Hepes (pH 7.5); 300 mosM]. CaCl2 (2 mM) and MgCl2 (8.2 mM) were added to the AHL before use. Calcium imaging was performed using an Olympus BX51WI microscope with a 60× water immersion objective, an Andor Zyla camera, and a Uniblitz shutter. GCaMP6 medium (GCaMP6m) signals were collected at 1 Hz. Two microliters of either 2.8 M sugar solution (glucose or arabinose) or AHL was added into the recording chamber to reach the final concentration of 2.8 mM. For P2X2 stimulation, 10 μl of 0.2 M ATP solution or AHL was loaded into the recording chamber to reach the final concentration of 1 mM. ROIs were manually selected from the P1 commissural fibers area with ImageJ. Fluorescent change was calculated as (Fpeak − F0)/F0, where F0 was calculated from the average intensity of 10 frames of background-subtracted baseline fluorescence before glucose or arabinose or AHL application, and Fpeak corresponds to the lowest fluorescence after application.
Quantitative reverse transcription PCR
Legs of 8- to 10-day-old males were collected and used for total RNA extraction with TRIzol reagent (Invitrogen, 15596026). Control males (UAS-CCAP-RNAi/+ heterozygous) and CCAP knockdown males (expression of UAS-CCAP-RNAi driven by ppk23-Gal4; vGlut-Gal80) were used. First-strand cDNA synthesis (TransGen Biotech, AT311-02) was performed from control and treatment groups and were then used for quantitative polymerase chain reaction (qPCR) using ABI QuantStudio 6 Flex (Thermo Fisher Scientific) with SuperReal SYBR Green PreMix Plus (TianGen Biotech, FP205). qPCR was performed with the following program: predegeneration, 95°C for 15 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Primer pairs for CCAP (CG4910) and internal reference Elongation Factor 1 alpha100 (EF1, CG1873) were listed as follows: CCAP-q, AGTGGCGTTATACAATGGA (forward) and TCGTTGCGTTTGAATAGC (reverse), 116 base pairs (bp), and EF1-q, GCGTGGGTTTGTGATCAGTT (forward) and GATCTTCTCCTTGCCCATCC (reverse), 125 bp. Three replicates were performed with controls without reverse transcriptase or template control added to the reaction. Melting curve analysis and primer efficiency tests were performed for all primer sets. Plotting and statistical analyses were conducted with GraphPad Prism software.
Statistical analysis
Experimental and control groups were tested at the same condition. Statistical analysis was performed with GraphPad Prism Software. Error bars in all data represented means ± SEM. The statistical significance between two datasets was determined by two-tailed unpaired t test and Mann-Whitney nonparametric test or between groups using one-way or two-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons.
Acknowledgments
We thank the members of the Zhang laboratory for discussions. We thank K. Scott for suggestion on the manuscript.
Funding: This work was supported by grants 31871059 and 32022029 from the National Natural Science Foundation of China, grant Z181100001518001 from the Beijing Municipal Science and Technology Commission, and a “Brain+X” Seeds grant from the IDG/McGovern Institute for Brain Research at Tsinghua to W.Z. This work is supported by Collaborative Research Fund of Chinese Institute for Brain Research, Beijing. W.Z. is an awardee of the Young Thousand Talents Program of China.
Author contributions: L.Z. and X.G. performed the experiments and analyzed data. W.Z. supervised the project and wrote the manuscript. All authors discussed, wrote, and commented on the final version of the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Table S1
REFERENCES AND NOTES
- 1.Fernandez-Fernandez R., Martini A. C., Navarro V. M., Castellano J. M., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M., Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 254-255, 127–132 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Hill J. W., Elmquist J. K., Elias C. F., Hypothalamic pathways linking energy balance and reproduction. Am. J. Physiol. Endocrinol. Metab. 294, E827–E832 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider J. E., Energy balance and reproduction. Physiol. Behav. 81, 289–317 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Sakai T., Watanabe K., Ohashi H., Sato S., Inami S., Shimada N., Kitamoto T., Insulin-producing cells regulate the sexual receptivity through the painless TRP channel in Drosophila virgin females. PLOS ONE 9, e88175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wigby S., Slack C., Grönke S., Martinez P., Calboli F. C. F., Chapman T., Partridge L., Insulin signalling regulates remating in female Drosophila. Proc. Biol. Sci. 278, 424–431 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neirijnck Y., Papaioannou M. D., Nef S., The insulin/IGF system in mammalian sexual development and reproduction. Int. J. Mol. Sci. 20, 4440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs P., Parlow A. F., Karkanias G. B., Effect of centrally administered insulin on gonadotropin-releasing hormone neuron activity and luteinizing hormone surge in the diabetic female rat. Neuroendocrinology 76, 357–365 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Harshman L. G., Hoffmann A. A., Prout T., Environmental effects on remating in Drosophila melanogaster. Evolution 42, 312–321 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Arnqvist G., Jones T. M., Elgar M. A., Insect behaviour: Reversal of sex roles in nuptial feeding. Nature 424, 387 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Dorkova M., Nado L., Jarcuska B., Kanuch P., Size-dependent mating pattern in a nuptial gift-giving insect. Ecol. Evol. 9, 454–462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asikainen J., Mustonen A. M., Nieminen P., Pasanen S., Araja-Matilainen H., Hyvarinen H., Reproduction of the raccoon dog (Nyctereutes procyonoides) after feeding or food deprivation in winter. J. Anim. Physiol. Anim. Nutr. 86, 367–375 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Bronson F. H., Marsteller F. A., Effect of short-term food deprivation on reproduction in female mice. Biol. Reprod. 33, 660–667 (1985). [DOI] [PubMed] [Google Scholar]
- 13.Kim E., Lim U. T., Effect of food deprivation period on the development and reproduction of riptortus pedestris (Hemiptera: Alydidae), and its egg parasitism. J. Econ. Entomol. 107, 1785–1791 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Kurtovic A., Widmer A., Dickson B. J., A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K., Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dweck H. K., Ebrahim S. A. M., Thoma M., Mohamed A. A. M., Keesey I. W., Trona F., Lavista-Llanos S., Svatos A., Sachse S., Knaden M., Hansson B. S., Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 112, E2829–E2835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clowney E. J., Iguchi S., Bussell J. J., Scheer E., Ruta V., Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87, 1036–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosjean Y., Rytz R., Farine J. P., Abuin L., Cortot J., Jefferis G. S. X. E., Benton R., An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236–240 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Stensmyr M. C., Dweck H. K. M., Farhan A., Ibba I., Strutz A., Mukunda L., Linz J., Grabe V., Steck K., Lavista-Llanos S., Wicher D., Sachse S., Knaden M., Becher P. G., Seki Y., Hansson B. S., A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Hoopfer E. D., Jung Y., Inagaki H. K., Rubin G. M., Anderson D. J., P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S. X., Rogulja D., Crickmore M. A., Dopaminergic circuitry underlying mating drive. Neuron 91, 168–181 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Kallman B. R., Kim H., Scott K., Excitation and inhibition onto central courtship neurons biases Drosophila mate choice. eLife 4, e11188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohatsu S., Koganezawa M., Yamamoto D., Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498–508 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Billeter J. C., Atallah J., Krupp J. J., Millar J. G., Levine J. D., Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 461, 987–991 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Fan P., Manoli D. S., Ahmed O. M., Chen Y., Agarwal N., Kwong S., Cai A. G., Neitz J., Renslo A., Baker B. S., Shah N. M., Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell 154, 89–102 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeholzer L. F., Seppo M., Stern D. L., Ruta V., Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564–569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferveur J. F., Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 35, 279–295 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro I. M. A., Drews M., Bahl A., Machacek C., Borst A., Dickson B. J., Visual projection neurons mediating directed courtship in Drosophila. Cell 174, 607–621.e18 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Kohatsu S., Yamamoto D., Visually induced initiation of Drosophila innate courtship-like following pursuit is mediated by central excitatory state. Nat. Commun. 6, 6457 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Pan Y., Meissner G. W., Baker B. S., Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc. Natl. Acad. Sci. U.S.A. 109, 10065–10070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hindmarsh Sten T., Li R., Otopalik A., Ruta V., Sexual arousal gates visual processing during Drosophila courtship. Nature 595, 549–553 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toda H., Zhao X., Dickson B. J., The Drosophila female aphrodisiac pheromone activates ppk23+ sensory neurons to elicit male courtship behavior. Cell Rep. 1, 599–607 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Han X., Mehren J., Hiroi M., Billeter J. C., Miyamoto T., Amrein H., Levine J. D., Anderson D. J., Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat. Neurosci. 14, 757–762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broughton S. J., Piper M. D. W., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., Partridge L., Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baines R. A., Uhler J. P., Thompson A., Sweeney S. T., Bate M., Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez R., Tabarini D., Azpiazu N., Frasch M., Schlessinger J., The Drosophila insulin receptor homolog: A gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 14, 3373–3384 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garofalo R. S., Rosen O. M., Tissue localization of Drosophila melanogaster insulin receptor transcripts during development. Mol. Cell. Biol. 8, 1638–1647 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S., Alfa R. W., Topper S. M., Kim G. E. S., Kockel L., Kim S. K., A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLOS Genet. 10, e1004555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima S. Q., Miesenbock G., Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Nassel D. R., Liu Y., Luo J., Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 221, 255–266 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Nässel D. R., Vanden Broeck J., Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 73, 271–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao S., Lang C., Levitan E. S., Deitcher D. L., Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49, 159–172 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Liu W., Ganguly A., Huang J., Wang Y., Ni J. D., Gurav A. S., Aguilar M. A., Montell C., Neuropeptide F regulates courtship in Drosophila through a male-specific neuronal circuit. eLife 8, e49574 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S. X., Miner L. E., Boutros C. L., Rogulja D., Crickmore M. A., Motivation, perception, and chance converge to make a binary decision. Neuron 99, 376–388.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu S., Guo C., Zhao H., Sun M., Chen J., Han C., Peng Q., Qiao H., Peng P., Liu Y., Luo S. D., Pan Y., Drosulfakinin signaling in fruitless circuitry antagonizes P1 neurons to regulate sexual arousal in Drosophila. Nat. Commun. 10, 4770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D., Sitaraman D., Chen N., Jin X., Han C., Chen J., Sun M., Baker B. S., Nitabach M. N., Pan Y., Genetic and neuronal mechanisms governing the sex-specific interaction between sleep and sexual behaviors in Drosophila. Nat. Commun. 8, 154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beckwith E. J., Geissmann Q., French A. S., Gilestro G. F., Regulation of sleep homeostasis by sexual arousal. eLife 6, e27445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Q., Gong Z., Cold-sensing regulates Drosophila growth through insulin-producing cells. Nat. Commun. 6, 10083 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown E. B., Shah K. D., Faville R., Kottler B., Keene A. C., Drosophila insulin-like peptide 2 mediates dietary regulation of sleep intensity. PLOS Genet. 16, e1008270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caronia L. M., Dwyer A. A., Hayden D., Amati F., Pitteloud N., Hayes F. J., Abrupt decrease in serum testosterone levels after an oral glucose load in men: Implications for screening for hypogonadism. Clin. Endocrinol. 78, 291–296 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Xie Y. M., Pei J. H., Kuang J., Chen H. M., Chen Z., Li Z. W., Fu X. Y., Wang L., Lai S. Q., Zhang S. T., Chen Z. J., Lin J. X., Sugar-sweetened beverage intake and serum testosterone levels in adult males 20-39 years old in the United States. Reprod. Biol. Endocrinol. 16, 61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murphy K. R., Deshpande S. A., Yurgel M. E., Quinn J. P., Weissbach J. L., Keene A. C., Dawson-Scully K., Huber R., Tomchik S. M., Ja W. W., Postprandial sleep mechanics in Drosophila. eLife 5, e19334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gendron C. M., Kuo T. H., Harvanek Z. M., Chung B. Y., Yew J. Y., Dierick H. A., Pletcher S. D., Drosophila life span and physiology are modulated by sexual perception and reward. Science 343, 544–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe K., Sakai T., Knockout mutations of insulin-like peptide genes enhance sexual receptivity in Drosophila virgin females. Genes Genet. Syst. 90, 237–241 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Zhou C., Pan Y., Robinett C. C., Meissner G. W., Baker B. S., Central brain neurons expressing doublesex regulate female receptivity in Drosophila. Neuron 83, 149–163 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Richard D. S., Rybczynski R., Wilson T. G., Wang Y., Wayne M. L., Zhou Y., Partridge L., Harshman L. G., Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: Female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 51, 455–464 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Table S1