Abstract
OBJECTIVES
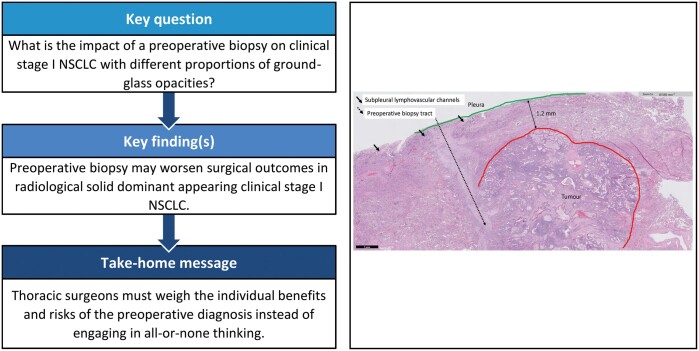
The present study aimed to clarify the association between preoperative biopsy and surgical outcomes in clinical stage I non-small-cell lung cancer (NSCLC) with different proportions of ground-glass opacity (GGO).
METHODS
Data on patients who underwent pulmonary resection for NSCLC from 2006 to 2016 were drawn from a prospective registered database and analysed retrospectively. Patient characteristics collected included tumour size, location and staging, surgical approach, consolidation–tumour ratio, histopathology and the presence or absence of preoperative biopsy to identify the independent prognostic factors of disease-free survival (DFS) and cancer-specific survival. A 1:1 propensity score matching was conducted between the preoperative biopsy and reference groups based on their baseline characteristics measured before the decision for preoperative biopsy.
RESULTS
A total of 1427 patients were collected to achieve an overall 5-year DFS as 84.5% (median follow-up: 67.3 months), stratified to be 99.5% in the GGO-dominant group (n = 430) and 78.2% in the solid-dominant group (n = 997). Only 2 patients (0.5%) in the GGO-dominant group experienced tumour recurrence. For solid-dominant tumours matched with propensity scores (279 in preoperative biopsy vs 279 in reference group), the independent predictors of DFS included preoperative biopsy, sublobar resection, pathological staging and angiolymphatic invasion. Preoperative biopsy was a predictor of cancer-specific survival in univariable analysis but was not in multivariable analysis. Significant differences were also found between matched groups in those with late-delay surgery, but not in patients receiving preoperative biopsy with early-delay surgery (≤21 days).
CONCLUSION
Preoperative biopsy may worsen surgical outcomes in patients with clinical stage I, solid-dominant NSCLC.
Keywords: Non-small-cell lung cancer, Ground-glass opacity, Solid-dominant appearance
INTRODUCTION
A prospective, multi-institutional study conducted by Japan Clinical Oncology Group 0201 has demonstrated the effectiveness of using radiological criteria to distinguish non-invasive from invasive lung adenocarcinoma—a consolidation–tumour ratio (CTR) ≤ 0.5 in cT1a-b on thin-section computed tomography (CT), referred as an excellent prognosis for ‘radiological’ non-invasive adenocarcinomas [1]. A phase III non-randomized confirmatory study of wedge resection for lung cancer ≤2 cm with a CTR ≤ 0.25 has further demonstrated an excellent result of 5-year recurrence-free survival, 99.7%, with no local relapse [2]. Other studies have also reported no cases of recurrence in patients presenting higher proportion of ground-glass opacity (GGO; >50%), regardless of the solid are diameter [3, 4]. In contrast, radiological solid lung cancers without GGO component (CTR = 1) have been found to exhibit a more malignant nature compared with non-solid or part-solid lung cancers based on both pathological and oncological outcomes [5], even with a higher percentage of nodal involvement or staging migration [6]. This accumulated evidence is all directed to indicate that the degree of pathological invasion and growth in lung cancer can be quantified according to CTR, can heavily affect oncological outcomes and can act as a strong prognostic factor of survival [7].
Although preoperative biopsy is essential for the accurate diagnosis and treatment of lung cancer, especially at advanced stage, the possibilities of inducing tumour recurrence and worsening survival therefore caused have already been suggested by several studies on stage I non-small-cell lung cancer (NSCLC) [8–11]. On the other hand, the association between preoperative biopsy and the degree of malignancy at early-stage NSCLC, determined radiologically by the proportion of GGO, has not been well explored. The purpose of the present study was therefore to testify the hypothesis that surgical outcome was associated with preoperative biopsy and could be further stratified based on the proportions of GGO in clinical stage I NSCLC.
MATERIALS AND METHODS
Ethical statement
The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital, and the signed informed consent from patients was waived (Approval No. 2019-01-012BC).
Patient selection
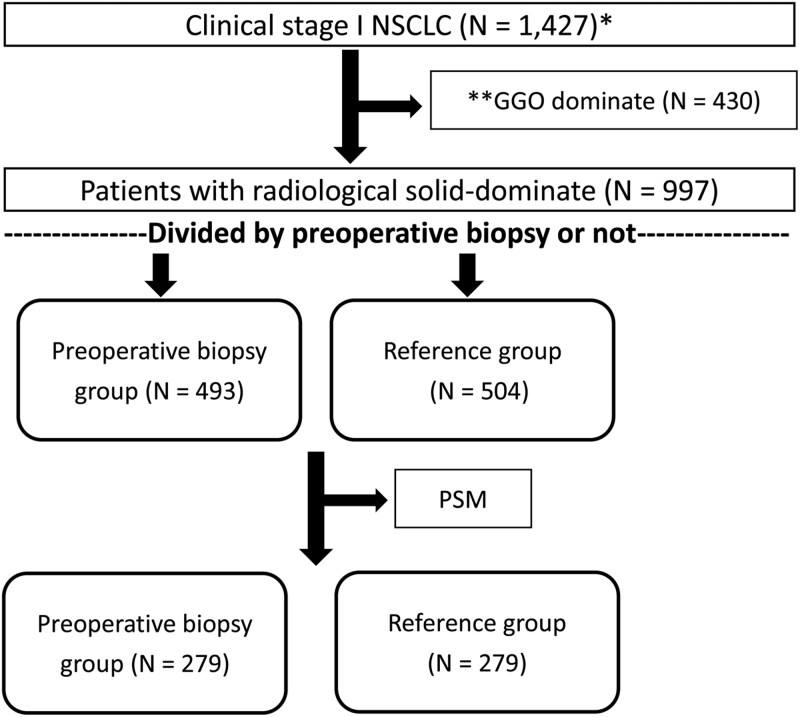
Data of patients who underwent pulmonary resection for lung cancer from January 2006 to December 2016 at Taipei Veterans General Hospital were extracted from the hospital’s prospective registered database and analysed retrospectively. During the study period, surgical resection was performed in 425 patients diagnosed as having a benign pulmonary lesion (23.8%) without preoperative definitive diagnosis and 1427 patients diagnosed as having clinical stage I NSCLC (Fig. 1).
Figure 1:
Flow diagram for patient selection. NSCLC: non-small-cell lung cancer; PSM: propensity score matching.
Surgery
Mediastinal evaluation included mediastinoscopy, intraoperative lymphadenectomy or preoperative positron emission tomography (PET)/CT scan. Six surgeons were involved in operating on these patients. Patients underwent either radical mediastinal lymphadenectomy (the majority) or mediastinal node sampling, according to the surgeon’s preference. The pathological stage was diagnosed using the 8th tumor, node, and metastasis (TNM) system for lung cancer [12]. Visceral pleural invasion, angiolymphatic invasion and comprehensive histological subtyping were defined as previously described [8, 13]. Sublobar resection included anatomic segmentectomy and wedge resection.
Preoperative radiological evaluation
The extent of GGO of all tumours was estimated radiologically using the same thin-section CT scan with a 2-mm collimation (GE Healthcare, Chicago, IL, USA) as previously described [13]. Briefly, CTR was defined as the ratio of the maximum size of consolidation to the maximum tumour size on thin-section CT scan. Based on CTR, a part-solid tumour was defined as a tumour with both focal nodular opacity and GGO (0 ≤ CTR ≤1.0), classified into 2 groups: GGO dominant (0 ≤ CTR ≤ 0.5) and solid dominant (0.5 < CTR ≤1.0) in appearance [7].
Preoperative biopsy versus reference
The majority of patients in the preoperative biopsy group received CT-guided percutaneous core needle biopsy (n = 524, 82.8%), including 18 patients who failed transbronchial biopsy and 2 patients who repeated CT-guided biopsy after the first failure. The other patients had transbronchial biopsy (n = 109, 17.2%). The patients without preoperative biopsy, failing the biopsy or undergoing to surgical intervention directly (n = 27), were classified into reference group, and the majority of them received intraoperative wedge resection for frozen section to verify malignancy before lobectomy or sublobar resection. Eight patients undergoing direct lobectomy with only sputum cytology confirmation were stratified into the reference group (n = 8). The choices of preoperative biopsy or intraoperative wedge resection were made after thoracic and oncological judgement on the probability of malignancy and discussion with the patients the pros and cons of different approaches. In general, preoperative biopsy was performed trans-bronchially for the centrally located tumours by medical pulmonologists, and was CT-guided for the peripherally located lesions by radiologists. ‘Time-lag’ was defined as the interval from malignant tissue verification to surgical intervention and was defined as zero in the reference group.
Follow-up
Postoperative follow-up was scheduled every 3 months for the first 2 years, every 6 months for the third to fifth year, and then annually thereafter. Chest CT scan was performed every 6 months for 2 years and then annually. Recurrences were confirmed by tissue biopsy or clinically determined by the multidisciplinary lung cancer committee. Patients with synchronous un-resected GGOs and metachronous tumours were excluded to distinguish between ipsilateral and contralateral recurrence at the beginning of the study [14]. DFS was defined as the interval between the date of surgical resection and the date of first recurrence or death. Patients without an event were censored at the time of last date of follow-up. Cancer-specific survival (CSS) was defined as the interval between the date of surgical resection and the date of death from lung cancer. Patients still alive were censored at the time of last date of follow-up. Observations were censored at the last follow-up session at which the patients were still alive with recurrence-free status, or in the patients who died afterward without recurrence. As of 30 November 2019, all patients had been followed with the exception of 97 patients lost to follow-up (follow-up rate 93.2%).
Statistical analysis
All continuous data are expressed as means and standard deviations. Continuous variables were analysed by the 2-sample t-test. Categorical variables (counts and % frequencies) were analysed by using Pearson’s chi-square test where appropriate (expected frequency >5), otherwise by Fisher’s exact test. Survival curves were calculated by the Kaplan–Meier method. Since non-random assignment can lead to selection bias and invalid estimates of survival, a 1:1 propensity score matching (PSM) was conducted based on the following baseline characteristics measured prior to the determination of preoperative biopsy: age, gender, smoking history, Carlson comorbidity score, tumour solid size, received sublobar resection, and received preoperative PET/CT scan. A multivariable logistic regression model was used to calculate the propensity score for each patient receiving preoperative biopsy. Among the independent variables first analysed with univariable analysis, those showing significant associated with survival were entered into a Cox proportional hazards regression model for multivariable analysis. Statistically significant level was defined as P ≤ 0.05. Results are presented as hazard ratios and 95% confidence intervals (CI). Statistical analysis was performed using the Statistical Package for the Social Sciences, version 17.0 (SPSS, Chicago, IL, USA) and SAS statistical software (version 9.4, SAS Inc., NC, USA).
RESULTS
Clinicopathological demographics
As shown in Table 1, totally 630 males (44.1%) and 797 females (55.9%) were included. The mean patient age was 62.1 (standard deviation = 11.3) years. The majority of patients were non-smokers (70.4%), had preoperative whole-body PET/CT scan to rule out mediastinal and distant metastasis (53.8%), underwent lobectomy (or above) as surgical intervention (68.3%) and underwent pulmonary resection by video-assisted thoracic surgery approach (79.5%).
Table 1:
Demographic, clinical and pathological characteristics of 1427 patients
| Characteristic | All patients, n (%); mean ± SD | GGO dominant (n = 430), n (%); mean ± SD | Solid dominant (n = 997), n (%); mean ± SD |
|---|---|---|---|
| Demographic | |||
| Age (y/o) | 62.1 ± 11.3 | 58.9 ± 10.8 | 63.5 ± 11.3 |
| Gender (male) | 630 (44.1) | 147 (34.2) | 483 (48.4) |
| Smoking history (smoker) | 426 (29.6) | 76 (17.7) | 350 (35.1) |
| Clinical | |||
| Charlson comorbidity score | 2.49 ± 1.80 | 2.0 ± 1.6 | 2.7 ± 1.8 |
| Preoperative serum CEA level (abnormal) | 145 (10.2) | 23 (5.3) | 122 (12.2) |
| Maximum tumour solid size (cm) | 1.28 ± 0.87 | 0.28 ± 0.33 | 1.72 ± 0.64 |
| Tumour location (subpleural lesion) | 725 (50.8) | 177 (41.2) | 548 (55.0) |
| Tumour location (centrally located) | 164 (11.5) | 15 (3.5) | 149 (14.9) |
| Surgical approaches (lobectomy or above) | 975 (68.3) | 192 (44.7) | 783 (78.5) |
| Consolidation/tumour ratio | 0.67 ± 0.36 | 0.18 ± 0.17 | 0.89 ± 0.13 |
| Preoperative PET scan (yes) | 768 (53.8) | 164 (38.1) | 604 (60.6) |
| Surgical method (VATS) | 1135 (79.5) | 405 (94.2) | 730 (73.2) |
| Preoperative biopsy | 633 (44.4) | 140 (32.6) | 493 (49.4) |
| Time-laga (day; biopsy group only) | 24.0 ± 18.2 | 23.5 ± 15.1 | 24.2 ± 19.0 |
| Pathological | |||
| Adenocarcinoma | 1305 (91.5) | 428 (99.5) | 877 (88.0) |
| Squamous cell carcinoma | 66 (4.6) | 0 | 66 (6.6) |
| Carcinoid tumour | 21 (1.5) | 0 | 21 (2.1) |
| Large cell carcinoma | 16 (1.1) | 0 | 16 (1.6) |
| Adenosquamous cell carcinoma | 10 (0.7) | 0 | 10 (1.0) |
| Mucoepidermoid carcinoma | 5 (0.4) | 0 | 5 (0.5) |
| Lymphoepithelioma-like carcinoma | 4 (0.3) | 0 | 4 (0.4) |
| Pathological stages (8th edition) | |||
| Tis | 138 (9.7) | 138 (30.1) | 0 |
| IA1 | 321 (22.5) | 197 (45.8) | 124 (12.4) |
| IA2 | 185 (13.0) | 6 (1.4) | 179 (18.0) |
| IA3 | 96 (6.7) | 0 | 96 (9.6) |
| IB | 580 (40.6) | 86 (20) | 494 (45.5) |
| IIB | 55 (3.9) | 2 (0.4) | 53 (5.3) |
| IIIA | 32 (2.2) | 0 | 32 (3.2) |
| IIIB | 1 (0.1) | 0 | 1 (0.1) |
| IV | 19 (1.3) | 1 (0.2) | 18 (18.0) |
| Stage migration | 107 (7.5) | 3 (0.7) | 104 (10.4) |
| Adjuvant chemotherapy (yes) | 327 (22.9) | 29 (6.7) | 298 (29.9) |
Tissue diagnosis to surgery.
GGO: ground-glass opacity; PET: positron emission tomography; SD: standard deviation; VATS: video-assisted thoracic surgery.
The majority of patients underwent sublobar resection via wedge resection (n = 380) and only 72 patients underwent anatomic segmentectomy (5% in overall cohort). A total of 107 patients (7.5%) had stage migration determined pathologically (55 upstaged to stage IIB, 32 to stage IIIA, 1 to stage IIIB and 19 to stage IVA). A total of 430 patients (30.1%) and 997 patients (69.9%) were classified into GGO-dominant and solid-dominant groups, respectively. Adjuvant chemotherapy was prescribed for 327 patients (22.9%). The average ‘time-lag’ in the preoperative biopsy group was 24.0 days (2–267; median 20).
The median follow-up period was 67.3 months: death within 30 postoperative days occurred in 4 (0.3%) patients, death from lung cancer occurred in 136 (9.5%) and tumour recurrence occurred in 227 (15.9%). Loco-regional recurrences, distant recurrences and distant plus local recurrences occurred in 96 (6.7%), 56 (3.9%) and 75 (5.3%) patients, respectively.
Disease-free survival (preoperative biopsy versus reference group)
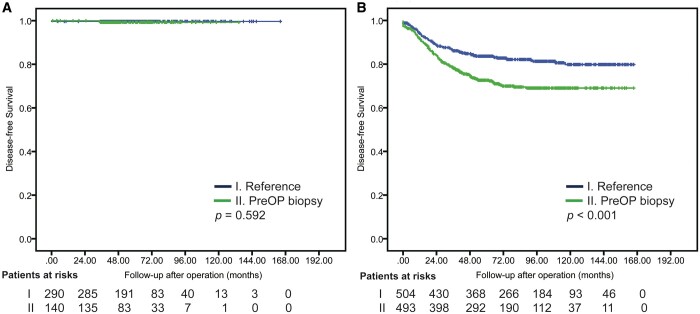
The overall 5-year DFS was 84.5%. Five-year DFS and CSS for preoperative biopsy versus reference groups in the overall cohort was 78.3% vs 89.4% and 88.5% vs 95.6%, respectively (P < 0.001; Supplementary Material, Fig. S1). Among patients with GGO-dominant tumours, 5-year DFS for preoperative biopsy and reference groups was 99.7% and 99.3%, respectively (P = 0.592); among patients with solid-dominant tumours, 5-year DFS for preoperative biopsy and reference groups was 72.6% and 83.6%, respectively (P < 0.001, Fig. 2). The differences in DFS between preoperative biopsy and reference groups were significant for solid-dominant tumours but not for GGO-dominant tumours.
Figure 2:
Disease-free survival in ground-glass opacity-dominant group (A) and solid-dominant group (B) of patients; preoperative biopsy versus reference group.
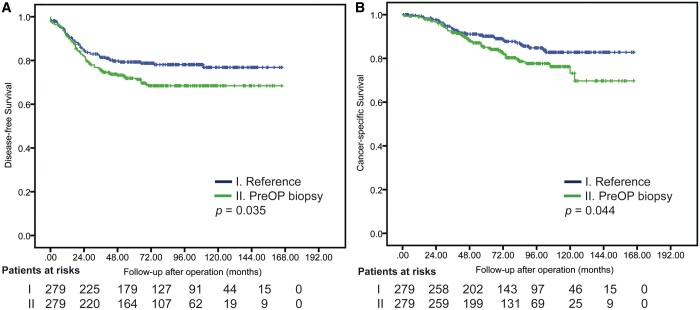
Preoperative biopsy, abnormal preoperative serum carcinoembryonic antigen (CEA) level, tumour solid size, tumour located at subpleural area, tumour located at central area and sublobar resection were identified by multivariable Cox analyses as the independent prognostic factors of DFS after surgical resection in clinical stage I solid-dominant NSCLC (Table 2). Table 3 shows that 279 patients in each group (preoperative biopsy versus reference) were identified by PSM for further comparison in solid-dominant appearance. No statistically significant differences were found in any variable between groups except for CTR results (P = 0.009; higher CTR in reference group). Cox regression modelling (Table 4) identified preoperative biopsy (hazard ratio [HR], 1.42; 95% CI, 1.01–2.00), sublobar resection (HR, 2.57; 95% CI, 1.65–3.99), pathological stages (HR, 1.60; 95% CI, 1.41–1.83) and angiolymphatic invasion (HR 2.50; 95% CI, 1.65–3.77) as independent prognostic factors for DFS. Figure 3 depicts DFS and CSS after PSM.
Table 2:
Risk analysis of disease-free survival in patients with 997 radiologically solid-dominant, clinical stage I non-small-cell lung cancer
| Variables | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | aHR | 95% CI | P-value | |
| Preoperative biopsy | 1.74 | 1.33–2.27 | <0.001 | 1.36 | 1.02–1.80 | 0.034 |
| Demographic characteristics | ||||||
| Age (y/o) | 1.02 | 1.01–1.03 | 0.002 | 0.99 | 0.99–1.04 | 0.800 |
| Gender (male) | 1.51 | 1.16–1.96 | 0.002 | 1.16 | 0.81–1.65 | 0.421 |
| Smoking history (smoker) | 1.63 | 1.26–2.13 | <0.001 | 1.04 | 0.72–1.49 | 0.834 |
| Clinical characteristics | ||||||
| Charlson comorbidity score | 1.13 | 1.06–1.20 | <0.001 | 1.04 | 0.96–1.13 | 0.299 |
| Preoperative serum CEA level (abnormal) | 2.99 | 2.20–4.07 | <0.001 | 2.24 | 1.64–3.06 | <0.001 |
| Maximum tumour dimension (cm; solid) | 2.21 | 1.79–2.74 | <0.001 | 1.86 | 1.43–2.40 | <0.001 |
| Tumour location (subpleural lesion) | 1.94 | 1.46–2.57 | <0.001 | 1.67 | 1.25–2.22 | 0.001 |
| Tumour location (centrally located) | 2.01 | 1.48–2.74 | <0.001 | 1.87 | 1.34–2.60 | <0.001 |
| Consolidation/tumour ratio | 7.18 | 2.32–22.24 | 0.001 | 1.55 | 0.41–5.91 | 0.518 |
| Sublobar resection | 1.62 | 1.21–2.18 | 0.001 | 2.13 | 1.51–3.00 | <0.001 |
| Preoperative PET/CT (nil) | 0.91 | 0.69–1.19 | 0.486 | – | – | – |
| Surgical method (VATS) | 1.25 | 0.93–1.70 | 0.145 | – | – | – |
Calculated by Cox regression method; only variables with P ≤ 0.05 after univariable analyses were entered into the multivariable model.
aHR: adjusted hazard ratio; CI: confidence interval; CT: computed tomography; HR: hazard ratio; PET: positron emission tomography; VATS: video-assisted thoracic surgery.
Table 3:
Descriptive statistics of patients with radiologically solid-dominant, clinical stage I non-small-cell lung cancer, stratified by the presence of preoperative biopsy after matching
| With PSM matching | Preoperative biopsy | Reference | P-value |
|---|---|---|---|
| (n = 279) | (n = 279) | ||
| n (%); mean ± SD | n (%); mean ± SD | ||
| Pre-exposure variables | |||
| Age (y/o) | 63.7 ± 12.0 | 63.6 ± 10.7 | 0.914 |
| Gender (male) | 138 (49.5) | 134 (48.0) | 0.735 |
| Smoking history (smoker) | 107 (38.4) | 103 (36.9) | 0.727 |
| Clinical characteristics | |||
| Charlson comorbidity score | 2.71 ± 1.90 | 2.67 ± 1.78 | 0.800 |
| Preoperative CEA level (abnormal) | 34 (12.2) | 39 (14.0) | 0.530 |
| Maximum tumour dimension (solid size) | 1.74 ± 0.58 | 1.73 ± 0.61 | 0.775 |
| Tumour location (subpleural lesion) | 165 (59.1) | 159 (57.0) | 0.607 |
| Tumour location (central lesion) | 44 (15.8) | 43 (15.4) | 0.907 |
| Surgical approaches (sublobar) | 50 (17.9) | 58 (20.8) | 0.391 |
| Consolidation/tumour ratio | 0.87 ± 0.14 | 0.90 ± 0.12 | 0.009 |
| Preoperative PET scan (yes) | 195 (69.9) | 199 (71.3) | 0.710 |
| Surgical method (VATS) | 213 (76.3) | 196 (70.3) | 0.104 |
| Histopathology (invasive adenocarcinoma) | 248 (88.9) | 244 (87.5) | 0.600 |
| Stage migration | 36 (12.9) | 33 (11.8) | 0.700 |
| Pathological stages | 0.299 | ||
| IA1 | 17 (6.1) | 24 (8.6) | |
| IA2 | 55 (19.7) | 54 (19.4) | |
| IA3 | 21 (7.5) | 34 (12.8) | |
| IB | 150 (53.8) | 134 (48.0) | |
| IIB | 17 (6.1) | 21 (7.5) | |
| IIIA | 11 (3.9) | 9 (3.2) | |
| IIB | 1 (0.4) | 0 | |
| IV | 7 (2.5) | 3 (1.1) | |
| Pleural invasion (PL1, PL2) | 174 (62.4) | 157 (56.2) | 0.143 |
| Angiolymphatic invasion (yes) | 63 (22.6) | 61 (21.9) | 0.839 |
| Histology grade (high-grade predominant) | 47 (16.8) | 52 (19.6) | 0.580 |
| Adequate lymph node sampling (≥15) | 161 (57.7) | 169 (60.6) | 0.491 |
| Adjuvant chemotherapy (yes) | 91 (32.6) | 96 (34.4) | 0.654 |
| Patterns of recurrence | 0.870 | ||
| Local only | 36 (12.9) | 26 (9.3) | |
| Distal only | 21 (7.5) | 13 (4.7) | |
| Local + distal | 24 (8.6) | 19 (6.8) | |
PET: positron emission tomography; PSM: propensity score matching; VATS: video-assisted thoracic surgery.
Table 4:
Risk analysis of disease-free survival in 558 matched patients
| Variables | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Preoperative biopsy | 1.44 | 1.02–2.01 | 0.036 | 1.42 | 1.01–2.00 | 0.049 |
| Demographic characteristics | ||||||
| Age (y/o) | 1.03 | 1.01–1.05 | <0.001 | 1.02 | 0.99–1.04 | 0.129 |
| Gender (male) | 1.27 | 0.91–1.78 | 0.154 | – | – | – |
| Smoking history (smoker) | 1.28 | 0.92–1.80 | 0.148 | – | – | – |
| Clinical characteristics | ||||||
| Charlson comorbidity score | 1.17 | 1.08–1.27 | <0.001 | 1.02 | 0.90–1.16 | 0.733 |
| Preoperative serum CEA level (abnormal) | 3.04 | 2.07–4.47 | <0.001 | 1.22 | 0.80–1.86 | 0.354 |
| Maximum tumour dimension (cm; solid) | 1.91 | 1.43–2.54 | <0.001 | 1.15 | 0.83–1.60 | 0.404 |
| Tumour location (subpleural lesion) | 1.77 | 1.23–2.53 | 0.002 | 1.03 | 0.67–1.52 | 0.885 |
| Tumour location (centrally located) | 1.59 | 1.06–2.40 | 0.026 | 1.34 | 0.87–2.06 | 0.185 |
| Consolidation/tumour ratio | 3.77 | 0.97–3.77 | 0.056 | – | – | – |
| Sublobar resection | 2.05 | 1.41–2.98 | <0.001 | 2.57 | 1.65–3.99 | <0.001 |
| Preoperative PET/CT (nil) | 1.65 | 1.17–2.32 | 0.004 | 1.02 | 0.67–1.48 | 0.933 |
| Surgical method (VATS) | 1.31 | 0.89–1.93 | 0.182 | – | – | – |
| Pathological characteristics | ||||||
| Pleural invasion (PL1, PL2) | 1.69 | 1.18–2.43 | 0.004 | 1.02 | 0.67–1.50 | 0.914 |
| Pathological stage | 1.89 | 1.69–2.11 | <0.001 | 1.60 | 1.41–1.83 | <0.001 |
| Angiolymphatic invasion | 4.52 | 3.69–7.20 | <0.001 | 2.50 | 1.65–3.77 | <0.001 |
| Predominate pattern group (high grade) | 2.44 | 1.69–3.51 | <0.001 | 1.42 | 0.96–2.11 | 0.083 |
| Adequate lymph node sampling (nil) | 1.14 | 0.82–1.60 | 0.440 | – | – | – |
Calculated by Cox regression method; only variables with P ≤ 0.05 after univariable analyses were entered into the multivariable model.
CI: confidence interval; CT: computed tomography; HR: hazard ratio; PET: positron emission tomography; VATS: video-assisted thoracic surgery.
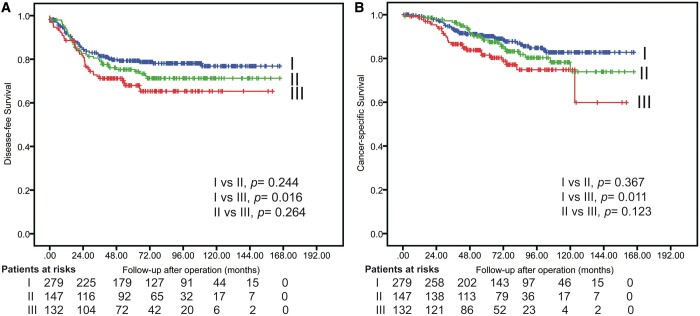
Figure 3:
Disease-free survival (A) and cancer-specific survival (B) after propensity score matching.
Cancer-specific survival (preoperative biopsy versus reference group)
In CSS analysis, although significant differences were observed in the overall cohort and after PSM in solid-dominant patients between preoperative biopsy and reference groups (Supplementary Material, Fig. S1 and Fig. 3), the Cox regression modelling did not identify preoperative biopsy as an independent predictor for CSS before and after PSM in patients with radiologically solid-dominant, clinical stage I NSCLC (Supplementary Material, Table S1 and Table 2).
Considering the potential mechanisms underlying the influence of preoperative biopsy to worsen DFS and CSS, we further divided the patients who underwent preoperative biopsy into early- and late-delayed surgery subgroups (early-delay versus late-delay surgeries) to compare with the reference group using a median delay of 21 days as a cut-off (Fig. 4). Significantly different outcomes were found between reference and preoperative biopsy groups in patients with late delay surgery (DFS, P = 0.016; CSS, P = 0.011), but not in those with early-delay surgery (<21 days).
Figure 4:
Disease-free survival (A) and cancer-specific survival (B) after propensity score matching, subgrouped by time-lag.
DISCUSSION
The present study was performed to evaluate the correlations between preoperative biopsy and proportions of GGO in lung cancer tumours based on preoperative thin-section CT scans. Overall, in patients undergoing surgical resection for lung cancer, the prognostic adverse impact of preoperative biopsy was significant in clinical stage I NSCLC presenting in radiologically solid-dominant tumours, but not in those with GGO-dominant tumours. Only 2 patients (0.5%) in the present study with GGO-dominant tumours experienced tumour recurrence, 1 in the preoperative biopsy group and the other 1 in the reference group.
Other studies on GGO-dominant and solid-dominant lung cancers have yielded similar results. As revealed in Matsuguma et al.’s [3] report, the proportion of GGO was a significant prognostic factor for DFS and, regardless of the solid area diameter, no patient with a higher proportion of GGO (>50%) experienced recurrence. Conversely, all the recurrent tumours occurred in patients with solid-dominant tumours [3]. Similarly, Kashiwabara et al. [15] have also reported no pleural recurrence in part-solid nodules. Briefly, although 140 (32.6%) patients in the present study with GGO-dominant tumours underwent preoperative biopsy, the manipulation of preoperative biopsy itself rarely affected the clinical outcomes. The less-invasive characteristics and natural course in this group of patients with NSCLC might possibly count for favourable outcomes.
The incidence of needle-tract seeding and dissemination of tumour cells into the pleural cavity following percutaneous biopsy of NSCLC has been reported as being extremely low [16]. However, as the pleural cavity is a lymphatic space, tumour recurrence can occur not only when the tumour is exposed onto the pleural surface but also when subpleural lymphatics are invaded by the tumour [17]. Not surprisingly, the presentation of visceral pleural invasion has been reported as a significant prognostic factor associated with pleural seeding after complete resection in pathological stage I lung adenocarcinoma [18].
Theoretically, transthoracic biopsy must puncture through the visceral pleura before targeting the tumour, and lymphatic vessels enrich pleura with an intercommunicating network arranged over the lung surface that penetrates into the lung parenchyma, joining the bronchial lymph vessels with drainage to various hilar lymph nodes. In other words, tumour recurrence does not present with rare needle-tract seeding or pleural seeding through the exfoliation of cancer cells into the pleural cavity during biopsy, which may possibly increase the risk of loco-regional recurrence and systemic metastases through lymphatic or haematogenous dissemination in damaged pleura [19]. Similarly, the neoplastic tissue is torn bluntly or brushed away from the main tumour via trans-bronchoscopic biopsy, just as in incisional biopsy, to cause tumour cells to seed and/or disseminate vascular and lymphatic structures of the bronchi, alveoli and even pleura [11].
Another interesting hypothesis of recurrence advocated that tumour cells could dislodge from the tumour into the pulmonary vein even during intraoperative manipulation in lobectomy [20], and consequently affected survival [21]. The general concept of ‘no touch isolation’ or minimal manipulation technique therefore becomes a golden rule in surgical oncological fields. However, applying this principle to preoperative diagnosis of NSCLC is still controversial due to conflicting results.
In the present study, time-lag was significantly different between patients with preoperative biopsy and those without, consistent with the findings that timeline of lung cancer treatment is an important factor in quality of care and may possibly affect the clinical outcomes [13, 22]. From the initial suspicion of malignancy, patients have to spend lots of time waiting for sequential of tissue proof, pathology report, referral to the right specialists and the results of tumour staging determined by whole-body PET, brain magnetic resonance, etc. Our study indicated different outcomes between patients in the reference group and those undergoing preoperative biopsy with late-delay surgery (DFS, P = 0.016; CSS, P = 0.011), but not in those undergoing preoperative biopsy with early-delay surgery (<21 days). These results hint that longer delay in surgical intervention after preoperative biopsy might in part associated with poorer clinical outcomes for patients with clinical stage I NSCLC. In other words, intense follow-up, instead of preoperative biopsy for histological diagnosis, may shorten the delays for patients hesitant about surgical intervention.
Obtaining the most representative portion of stromal invasion from preoperative biopsy is important for nonsurgical candidates or patients with high comorbidities who will receive chemotherapy or other treatment modalities [23]. It almost goes without saying that the potential benefits of preoperative biopsy are to reduce unnecessary thoracotomy/video-assisted thoracic surgery or over diagnosis and to avoid non-randomization before entering a clinical trial [24]. Furthermore, preoperative biopsy should be considered as a way to rule out metastatic disease when patients have an underlying malignancy. We believe, along with other authors, that preoperative biopsy has a crucial role in lung cancer treatment and is an appropriate diagnostic procedure with high accuracy and safety [25]. In clinical practice, certain factors lead clinicians to request a biopsy for 1 patient but not another, but it is difficult to know all the factors that contribute to these decisions. As a result, thoracic surgeons must properly weigh the benefits and risks for individual patients about the preoperative diagnosis and should not engage in all or none thinking.
This study had a few limitations associated with interpretation of results. First, it used retrospective design from a single institution. The performance of preoperative biopsy or not for patients with lung cancer with different tumour characteristics would potentially affect the likelihood of recurrence patterns. Second, DFS was affected by confirmation of true recurrence, but this remains difficult in clinical practice. Although stronger evidence was provided for identifying the factors of CSS, the possible bias could limit the interpretation of results. Further prospective randomized, controlled study is needed to confirm the effects of preoperative biopsy on surgical outcomes in patients with NSCLC.
CONCLUSION
Preoperative biopsy has the potential to worsen the survival in radiologically solid-dominant, clinical stage I NSCLC. Further prospective, in depth study is needed before clinicians can consider adopting new practice patterns into this issue. We suggest that future studies on the association between preoperative biopsy and recurrence patterns in clinical stage I NSCLC should exclude GGO-dominant tumours and focus exclusively on solid-dominant ones.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflicts of interest: none declared.
Author contributions
Chien-Sheng Huang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation. Hung-Che Chien: Data curation. Chun-Ku Chen: Data curation; Investigation. Yi-Chen Yeh: Data curation; Investigation. Po-Kuei Hsu: Conceptualization; Formal analysis. Hui-Shan Chen: Data curation. Chih-Cheng Hsieh: Resources; Supervision. Han-Shui Hsu: Resources; Supervision. Biing-Shiun Huang: Conceptualization; Resources; Supervision. Chun-Che Shih: Conceptualization; Supervision.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Larry R. Kaiser and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CI
Confidence interval
- CSS
Cancer-specific survival
- CT
Computed tomography
- CTR
Consolidation–tumour ratio
- DFS
Disease-free survival
- GGO
Ground-glass opacity
- HR
Hazard ratio
- NSCLC
Non-small-cell lung cancer
- PET
Positron emission tomography
- PSM
Propensity score matching
REFERENCES
- 1. Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K. et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751–6. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki K, Watanabe S, Wakabayashi M, Moriya Y, Yoshino I, Tsuboi M. et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J Clin Oncol 2017;35:8561. [Google Scholar]
- 3. Matsuguma H, Oki I, Nakahara R, Suzuki H, Kasai T, Kamiyama Y. et al. Comparison of three measurements on computed tomography for the prediction of less invasiveness in patients with clinical stage I non-small cell lung cancer. Ann Thorac Surg 2013;95:1878–84. [DOI] [PubMed] [Google Scholar]
- 4. Ito H, Suzuki K, Mizutani T, Aokage K, Wakabayashi M, Fukuda H. et al. Long-term survival outcome after lobectomy in patients with clinical T1 N0 lung cancer. J Thorac Cardiovasc Surg 2020; [DOI] [PubMed] [Google Scholar]
- 5. Hattori A, Hirayama S, Matsunaga T, Hayashi T, Takamochi K, Oh S. et al. Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adaenocarcinoma. J Thorac Oncol 2019;14:265–75. [DOI] [PubMed] [Google Scholar]
- 6. Omori T, Aokage K, Nakamura H, Katsumata S, Miyoshi T, Sugano M. et al. Growth patterns of small peripheral squamous cell carcinoma of the lung and their impacts on pathological and biological characteristics of tumor cells. J Cancer Res Clin Oncol 2019;145:1773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K.. Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg 2016;102:407–15. [DOI] [PubMed] [Google Scholar]
- 8. Huang CS, Hsu PK, Chen CK, Yeh YC, Chen HS, Wu MH. et al. Preoperative biopsy and tumor recurrence of stage I adenocarcinoma of the lung. Surg Today 2020;50:673–84. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Honda O, Tomiyama N, Minami M, Sawabata N, Kadota Y. et al. Risk of pleural recurrence after computed tomographic-guided percutaneous needle biopsy in stage I lung cancer patients. Ann Thorac Surg 2011;91:1066–71. [DOI] [PubMed] [Google Scholar]
- 10. Matsuguma H, Nakahara R, Kondo T, Kamiyama Y, Mori K, Yokoi K.. Risk of pleural recurrence after needle biopsy in patients with resected early stage lung cancer. Ann Thorac Surg 2005;80:2026–31. [DOI] [PubMed] [Google Scholar]
- 11. Nakajima J, Sato H, Takamoto S.. Does preoperative transbronchial biopsy worsen the postsurgical prognosis of lung cancer? A propensity score-adjusted analysis. Chest 2005;128:3512–8. [DOI] [PubMed] [Google Scholar]
- 12. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G. et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990–1003. [DOI] [PubMed] [Google Scholar]
- 13. Huang C-S, Hsu P-K, Chen C-K, Yeh Y-C, Shih C-C, Huang B-S.. Delayed surgery after histologic or radiologic-diagnosed clinical stage I lung adenocarcinoma. J Thorac Dis 2020;12:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu YC, Hsu PK, Yeh YC, Huang CS, Hsieh CC, Chou TY. et al. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg 2013;96:1966–74. [DOI] [PubMed] [Google Scholar]
- 15. Kashiwabara K, Semba H, Fujii S, Tsumura S.. Preoperative percutaneous transthoracic needle biopsy increased the risk of pleural recurrence in pathological stage I lung cancer patients with sub-pleural pure solid nodules. Cancer Invest 2016;34:373–7. [DOI] [PubMed] [Google Scholar]
- 16. Robertson EG, Baxter G.. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol 2011;66:1007–14. [DOI] [PubMed] [Google Scholar]
- 17. Kondo H, Asamura H, Suemasu K, Goya T, Tsuchiya R, Naruke T. et al. Prognostic significance of pleural lavage cytology immediately after thoracotomy in patients with lung cancer. J Thorac Cardiovasc Surg 1993;106:1092–7. [PubMed] [Google Scholar]
- 18. Jiwangga D, Cho S, Kim K, Jheon S.. Recurrence pattern of pathologic stage I lung adenocarcinoma with visceral pleural invasion. Ann Thorac Surg 2017;103:1126–31. [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez-Panadero F, Borderas Naranjo F, Lopez Mejias J.. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989;2:366–9. [PubMed] [Google Scholar]
- 20. Yao X, Williamson C, Adalsteinsson VA, D'Agostino RS, Fitton T, Smaroff GG. et al. Tumor cells are dislodged into the pulmonary vein during lobectomy. J Thorac Cardiovasc Surg 2014;148:3224–31 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei S, Guo C, He J, Tan Q, Mei J, Yang Z. et al. Effect of vein-first vs artery-first surgical technique on circulating tumor cells and survival in patients with non-small cell lung cancer: a randomized clinical trial and registry-based propensity score matching analysis. JAMA Surg 2019;154:e190972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang CJ, Wang H, Kumar A, Wang X, Hartwig MG, D'Amico TA. et al. Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma. Chest 2017;152:1239–50. [DOI] [PubMed] [Google Scholar]
- 23. Lu CH, Hsiao CH, Chang YC, Lee JM, Shih JY, Wu LA. et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol 2012;7:143–50. [DOI] [PubMed] [Google Scholar]
- 24. Kohman LJ, Gu L, Altorki N, Scalzetti E, Veit LJ, Wallen JM. et al. Biopsy first: lessons learned from Cancer and Leukemia Group B (CALGB) 140503. J Thorac Cardiovasc Surg 2017;153:1592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen CK, Chang HT, Chen YC, Chiang SC, Chou HP, Chen TJ.. Utilization and safety of percutaneous lung biopsy: a 10-year nationwide population-based study. Int J Environ Res Public Health 2019;16:1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.