Abstract
OBJECTIVES
This study analysed the patterns of extraction ranges, characteristics, advantages and disadvantages of median sternotomy (MS) and subxiphoid (SX) approaches for extended thymectomy.
METHODS
This study included patients with anterior mediastinum tumour and myasthenia gravis who underwent extended thymectomy at our institution between 2015 and 2018. There were 5 MS and 6 SX extended thymectomy surgeries with the VINCENT software. On preoperative computed tomography, the thymus area and fat tissue surrounding the thymus, which were planned for extraction, were traced using VINCENT (Ver. 4.0). We then constructed three-dimensional images and calculated the volumes. Evaluation of the extended thymectomy approach based on the residual fat tissue was required to determine the area of extended thymectomy.
RESULTS
No significant differences in operation time (min) [SX: 197.3 ± 34.0, MS: 206.6 ± 91.4, drainage duration (days), SX: 2.2 ± 1.0, MS: 2.2 ± 0.4, hospital stay (days), SX: 11.8 ± 1.2, MS: 13.4 ± 2.1, residual rate (%), SX: 29.9 ± 17.5, MS: 58.7 ± 18.0 (P = 0.0519)] were observed between the 2 groups. Bleeding was significantly lower for SX than for MS. The residual rate was lower for SX than for MS.
CONCLUSIONS
Considering the amount of the residual fat tissue, the SX approach allows an adequate dissection area for extended thymectomy compared with the MS approach.
Keywords: Thoracic surgery, Thymoma, Myasthenia gravis
Median sternotomy (MS) and subxiphoid (SX) approaches are the conventional surgical procedures for extended thymectomy, and endoscopic surgical techniques also have expanded in recent years.
INTRODUCTION
Median sternotomy (MS) and subxiphoid (SX) approaches are the conventional surgical procedures for extended thymectomy, and endoscopic surgical techniques also have expanded in recent years. The SX approach requires only a small incision and is minimally invasive, but in some cases, MS is necessary for a successful thymectomy. When thymectomy is performed for myasthenia gravis (MG), an adequate area needs to be uncovered to access and remove the thymus gland. Therefore, the choice of the surgical method is known to affect the outcomes of extended thymectomy [1].
A recent study demonstrated that thymectomy, compared to other interventions, improved clinical outcomes over a 3-year period in patients with non-thymomatous MG [1], highlighting the importance of surgical therapy for MG. Importantly, the extraction range of the fat tissue around the thymus must be considered when conducting extended thymectomy and a comparison between the SX and MS approaches would be useful for clinical decision-making. However, more evidence is needed to compare these 2 surgical methods. Therefore, this study described the extraction range and characteristics to compare between the MS and SX approaches and the advantages and disadvantages of each technique were also assessed.
PATIENTS AND METHODS
The study design was approved by the Ethics Review Board at Tokushima University (approval number 3384, dated 1 March 2019). This study included consecutive patients with anterior mediastinum tumours and MG who underwent extended thymectomy at our institution between September 2015 and November 2018. Two cases in the MS group were excluded due to outlying data regarding amount of bleeding and hospital stay. Finally, there were 5 MS and 6 SX extended thymectomy surgeries in 11 patients included in the analysis.
VINCENT software analysis
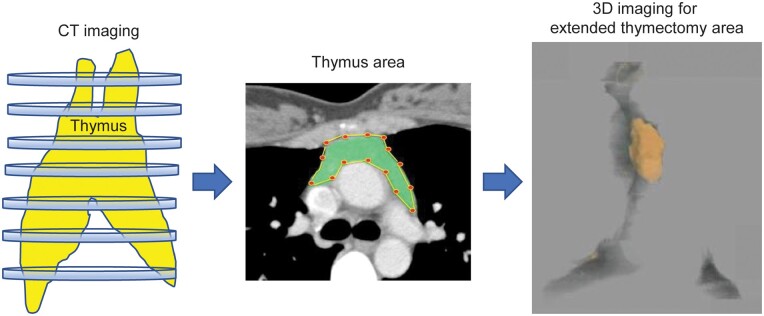
On preoperative computed tomography (CT), the thymus area and the surrounding fat tissue, which were planned for extraction, were traced (∼20 slices) with SYNAPSE VINCENT software v4.0 (Fujifilm Holdings Corp., Tokyo, Japan). The SYNAPSE VINCENT software is an imaging analysis system that provides high-quality three-dimensional (3D) images from CT and magnetic resonance imaging for analysis. VINCENT can remember the constructing process for the organ shape, compute the density of an area and perform histogram analysis. Since the software cannot automatically trace the thymus (Fig. 1), 1 radiologist and 1 thoracic surgeon traced the extended thymectomy area to be resected (Fig. 2). We traced the extended thymectomy area including the thymic tissue around the left brachiocephalic vein and right brachiocephalic artery.
Figure 1:
Preoperative computed tomography (CT). The thymus area and fat tissue surrounding the thymus, which was planned for extraction, were traced using VINCENT (Ver. 4.0) (∼30 slices). Preoperative computed tomography was conducted manually with 1 radiologist and 2 thoracic surgeons; three-dimensional (3D) imaging was performed, and the volume was calculated.
Figure 2:
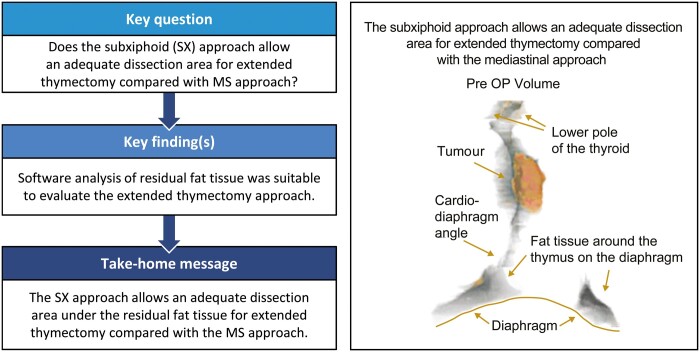
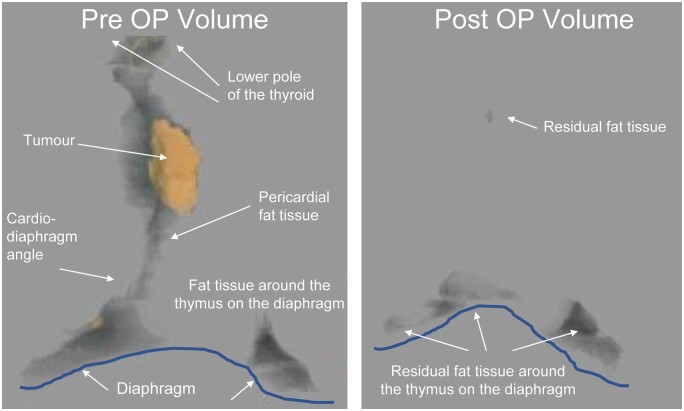
Representative three-dimensional images—pre- and postoperation. We constructed three-dimensional imaging using VINCENT (Ver. 4.0) software. Two thoracic surgeons and 1 radiologist constructed the extended thymectomy area as precisely as possible. The area was discussed thoroughly until all parties agreed. The grey area is the anterior mediastinal fat tissue, including the thymus, and the orange area is the tumour. VINCENT (Ver. 4.0) was used to calculate the volume of the grey area and the orange area (ml). There was the tendency that we had the residual fat tissue on the diaphragm side.
The pericardium fat tissue and the cardio-diaphragm angle fat tissue were very difficult to trace automatically because the thymus area has complex low- and high-density areas and the shape needed for extended thymectomy is different for each patient. Therefore, the decision to trace was discussed between the radiologist and thoracic surgeon. The VINCENT software was used to construct the slices (∼20 slices from the head side to leg side) to make a 3D image for use in the extended thymectomy. After surgery, we traced the residual fat tissue using the same method and the 3D imaging was used to calculate the volume. The CT imaging was conducted 3–9 months postoperatively for the SX group and 7–8 months postoperatively for the MS group. Because the area of the fat tissue on the diaphragm was difficult to determine preoperatively, we evaluated the volumes, which included the fat tissue volume on the diaphragm. We defined the residual rate (%) as follows: [postoperative volume/(preoperative volume − tumour volume)] × 100 (%) (Fig. 3).
Figure 3:
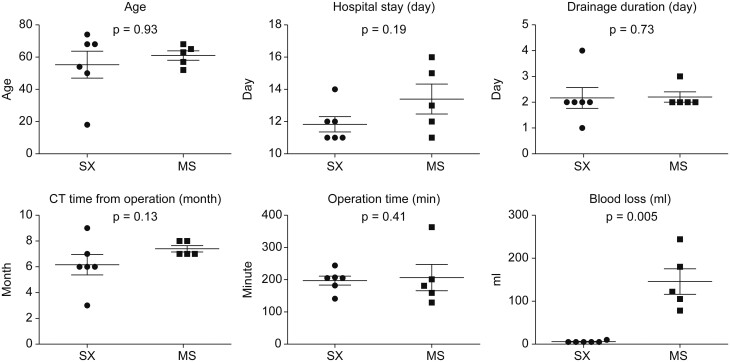
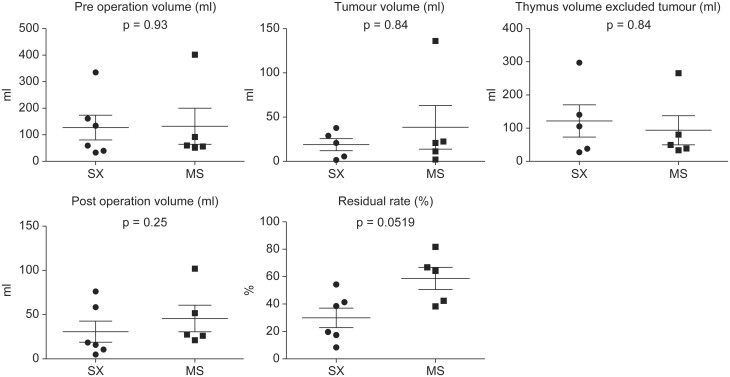
Comparison between the median sternotomy and subxiphoid approaches for extended thymectomy. No significant differences in age, operation time, drainage duration, hospital stay and computed tomography time from operation. Subxiphoid led to significantly lower bleeding than median sternotomy (p = 0.005).
Blood loss
At the study site, blood loss between 0 and 10 ml is recorded as a ‘small amount’ on the intraoperative patient records. Therefore, we defined a small amount of blood loss as the mean amount (5 ml) in this study.
Operative procedures
Extended thymectomy was performed using Masaoka’s procedure [2] via the MS and SX approaches. Briefly, an en bloc resection of the anterior mediastinal fat tissue, including the thymus, was conducted, and dissection was performed bluntly from the pericardium and pleura. The adipose tissues around the lower poles of the thymus, around both brachiocephalic veins, and on the pericardium were resected meticulously; the adipose tissue was resected up to the diaphragm caudally, thyroid gland cranially and phrenic nerves laterally.
The SX approach is a comparatively new technique as described by Suda et al. [3] and adopted at our institution for the treatment of MG with and without thymoma. When the procedure was first introduced at our institution, we adopted a dual-port SX approach. On occasion, an additional 5-mm port is needed (three-port method). In the procedure, after the bilateral visceral pleura is opened, the dissection starts from the left lower pole of the thymus. The thymus is dissected from the pericardium to avoid damaging the left phrenic nerve and the thymus is exposed at the inflow area of the left brachiocephalic vein. The right side of the lower pole of the thymus is then dissected from the pericardium and the bilateral upper pole of the thymus from the thyroid to expose the caudal end of the left brachiocephalic vein. Subsequently, the thymus vein is dissected using a vessel sealing device (LigaSure V; Covidien, Mansfield, MA, USA). The equivalent accuracy for extended thymectomy with MS can be achieved using the three-port method.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Groups were compared by unpaired t-tests using Prism v.8 software (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
No significant differences in age, operation time, drainage duration, hospital stay, timing of postoperative CT, preoperative thymus area and the surrounding fat tissue volume, tumour volume, postoperative thymus area and the surrounding fat tissue volume or postoperative volume with tumour excluded (postop volume − tumour volume) were observed between patients who underwent MS and SX approaches for extended thymectomy.
The SX cases were associated with significantly lower amounts of bleeding than the MS cases (P = 0.005) (Table 1 and Fig. 3). The residual rates were 58.7% ± 18.0% and 29.9% ± 17.5% for the MS and SX approaches, respectively, with no significant difference between groups (P = 0.0519) (Table 1 and Fig. 3).
Table 1:
Characteristics for extended thymectomy case
| MS | SX | |
|---|---|---|
| Operation time (min) | 230.3 ± 80.0 | 197.3 ± 31.1 |
| Drainage (days) | 2.4 ± 0.7 | 2.2 ± 0.9 |
| Hospital stay (days) | 15.3 ± 5.4 | 11.8 ± 1.1 |
| Bleeding (ml) | 360.7 ± 356.4 | 1.7 ± 3.7 |
| Preoperative volume (ml) | 128.8 ± 122.6 | 138.9 ± 102.6 |
| Tumour volume (ml) | 41.6 ± 45.7 | 18.9 ± 12.5 |
| Postop volume (ml) | 40.5 ± 25.5 | 38.3 ± 31.4 |
| Postop volume—tumour excluded (ml) | 87.1 ± 81.7 | 123.2 ± 90.5 |
| Residual rate (%) | 54.1 ± 9.4 | 34.5 ± 20.7 |
Bleeding was significantly lower with SX than with MS. There was no significant difference in other characteristics evaluated between SX and MS.
MS: median sternotomy; SX: subxyhoid.
MG patients
The study had 3 MG cases who were reviewed by a neurologist to investigate the clinical course of MG symptoms. Case I in the SX approach group was a patient with non-thymomatous MG. The patient’s presurgery activities of daily living (ADL) score was 10 and the MG Foundation of America (MGFA) classification was 3A. Her prednisolone (PSL) dose was 35 mg/day. The residual fat tissue rate was 8.4%. Two years post-surgery, the patient’s ADL score and MGFA classification were 0 and she did not need PSL.
Case II in the MS group had thymoma with MG. The patient’s presurgery ADL score was 12 and MGFA classification was 3B. Her PSL dose was 15 mg/day. The residual fat tissue rate was 51.7%. About 4 years later, the patient’s post-surgery ADL score was 5 and MGFA classification was 2B. Her PSL dose was 5 mg/day.
Case III in the MS group also had thymoma with MG. The patient continued to experience MG symptoms after surgery. After presenting with the first MG-related symptom, the patient’s ADL score was 4 and MGFA classification was 2B. About 4 years later, her ADL score was 2 and MGFA classification was 2B. The residual fat tissue rate was 21.1%. The patient started PSL after surgery with a dose of 60 mg/day, but the dosage fluctuated, making the PSL analysis difficult.
DISCUSSION
This study aimed to compare the SX and MS approaches regarding the extraction range of the fat tissue around the thymus during an extended thymectomy. The results show no significant differences between SX and MS approaches except for blood loss, which was significant lower in SX cases than in MS cases and the residual rate, which tended to be lower in SX than in MS cases [however, a significant difference was shown when one of the consecutive patient’s data was re-included (P = 0.0519)].
The bilateral phrenic nerve could be clearly identified using the SX approach, and it was easier to dissect the thymus on the diaphragm using the SX approach compared to the MS approach. With the MS approach, it was easier to dissect around the brachiocephalic vein and the cephalic aspect of the thymic tissue. Notably, the SX approach had an overwhelming advantage in reduced bleeding and drainage duration (Fig. 3).
We evaluated volumes because the area of the fat tissue on the diaphragm was difficult to analyse using CT preoperatively (Fig. 2). It is possible that the endoscopic procedures using the SX approach, rather than the macroscopic procedures, magnified the tissue resulting in a more accurate dissection. It is generally considered that the dissected area tends to be more accurate using the SX approach compared to the MS approach. In addition, the present study shows that a modified SX approach tends to have lower residual rates than the MS approach (Fig. 4).
Figure 4:
Comparison between the median sternotomy and subxiphoid approaches for extended thymectomy. Preoperative volume, tumour volume, postoperative volume or postoperative volume with tumour excluded (postop volume − tumour volume) were observed between the median sternotomy and subxiphoid approaches for extended thymectomy. The residual rates (%) were 58.7 ±18.0 (%) and 29.9 ± 17.5 (%) for the median sternotomy and subxiphoid approaches, respectively. There was no significant difference.
In addition, we adopted Masaoka’s procedure for extended thymectomy [2], although the lower boundary for the procedure has not been defined to date. In this study, we defined the area for extended thymectomy as the pericardial fat tissue around the thymus at the lower edge of the cardiac diaphragm angle. The 3D imaging results showed that it was difficult to dissect the fat tissue at the bilateral pericardiophrenic angle and, therefore, the fat tissues might remain on the diaphragm and the surrounding areas based on pre- and postoperative CT imaging. Therefore, an additional finding indicates that the 3D imaging analysis has high reproducibility for assessment of the appropriate area for extended thymectomy.
The presence of ectopic thymic tissue in patients with MG following thymectomy has attracted increasing attention and should be considered when determining the efficacy of thymectomy in patients with MG. However, the specific role of the ectopic thymic tissue is still under debate. In a systematic review, Li et al. [4] evaluated the rate of the ectopic thymic tissue in patients with MG with or without thymoma. According to the study, the most common locations for ectopic thymic tissue were the anterior mediastinal fat, pericardiophrenic angles, aortopulmonary window, cervical region (pretracheal fat) and lateral to the phrenic nerves. Of the 9 studies that analysed the effect of the tissue on clinical outcomes in MG patients, 6 studies found that the presence of the ectopic thymic tissue in MG patients was a significant predictor of poor outcomes after thymectomy; however, 3 studies found no association. Because the ectopic thymic tissue was likely present in more than a half of the patients undergoing thymectomy for MG, MG patients with the ectopic thymic tissue after thymectomy might not have as good outcomes as those without the ectopic thymic tissue [5–12]. These results are important for the future of extended thymectomy techniques for patients with MG.
In the present study, MG symptoms improved in the patients with non-thymomatous MG and the residual fat tissue rate was 8.4% in this group, indicating a good course of treatment. In the 2 patients with thymoma and MG, 1 patient experienced improvement in MG symptoms and their residual fat tissue rate was 51.7%. One patient had recurring MG symptoms after surgery. This patient’s residual fat tissue rate was 21.1%. From this study, we cannot establish the precise mechanism between the residual fat tissue, ectopic thymic tissue, germinal centre and MG symptoms, but further investigation is warranted to establish the relationship between these factors.
The extent of tissues that should be removed for extended thymectomy has not been established. Mineo et al. found that the perithymic standardized uptake value significantly correlated with the discovery of ectopic active thymic tissue. In the study, ectopic thymic tissue was detected in 37 patients (54%), most of whom (23/37, 62%) exhibited ectopic germinal centres. In addition, perithymic standardized uptake value significantly correlated with the discovery of ectopic active thymic tissue [13]. Thus, the physiology between MG and germinal centres may be complicated and we propose to evaluate ectopic thymic tissues in a future study.
Although the single-port procedure is commonly used in the field of abdominal surgery, reduced port surgery is regaining popularity owing to improved manoeuvrability of the endoscopic devices [14, 15]. The SX procedure is advantageous because it does not require cutting of the sternum and causes minimal pain; in fact, it does not involve an intercostal incision and thus does not cause intercostal nerve damage. Suda et al. reported that although ‘complete’ extended thymectomy is feasible, the application of single-port thymectomy (SPT) should be reviewed carefully in cases where [1] the handling of forceps is difficult owing to a sternal anomaly, such as funnel chest; [2] the visualization of the pericardial fat tissue on the left side is difficult owing to cardiomegaly or other disorders; or [3] combined resection is required owing to invasion of the surrounding organs [3]. Notably, identification of the cervical region and the bilateral phrenic nerves is easier with a dual-port procedure than with a video-assisted thoracoscopic surgery (VATS) approach from the lateral chest. Because we presumed that the VATS approach does not allow enough dissected area on the contralateral side for extended thymectomy, we did not adopt the VATS procedure for extended thymectomy. Instead, we used the MS approach to conduct extended thymectomy. Suda et al. [3] also supported the use of dual-port thymectomy; if surgeons are learning to perform SPT and are not yet skilled enough to complete it, they can complete the operation using dual-port thymectomy. Therefore, the dual-port procedure can be used by surgeons who have not been sufficiently trained for SPT as an alternative procedure in the event of technical difficulty during SPT or as a novel approach for thymectomy [3].
However, no study has evaluated the residual fat tissue in the extended thymectomy area.
Our study found that, if the residual tissue was on the left side, it tended to remain at the pericardiophrenic angles of fat tissues, probably due to the left ventricle overhang to the left side thoracic cavity. Therefore, the fat tissues on the left pericardiophrenic angles might have inadequate excision. The present findings may contribute to the development of the organ surgery technique.
Limitations
This was a retrospective study, and several different surgeons conducted the MS and SX thymectomy surgeries. We do not have the ectopic thymic tissue data. We checked the fat tissue of the diaphragm site for some patients. However, we could not find germinal centres in these tissues (data not shown). However, at our institution, surgeons followed the same protocol for extended thymectomy surgical procedures.
CONCLUSION
The SX approach allowed a consistent and adequate dissection area for extended thymectomy, and the amount of the residual fat tissue could be evaluated. Our findings suggest that the SX approach should be promoted in clinics.
ABBREVIATIONS
- ADL
Activities of daily living
- CT
Computed tomography
- 3D
Three-dimensional
- MS
Median sternotomy
- MG
Myasthenia gravis
- MGFA
MG Foundation of America
- PSL
Prednisolone
- SPT
Single-port thymectomy
- SUV
Standardized uptake value
- SX
Subxiphoid
- VATS
Video-assisted thoracoscopic surgery
Presented at the 36th Annual Meeting of the Japanese Association for Chest Surgery, Osaka, Japan, 16–17 May 2019.
Conflict of interest: none declared.
Author contributions
Mitsuteru Yoshida: Conceptualization; Formal analysis; Investigation; Project administration; Software; Supervision; Writing—original draft. Masao Yuasa: Formal analysis; Software. Kazuya Kondo: Conceptualization; Project administration. Mitsuhiro Tsuboi: Investigation. Naoya Kawakita: Investigation. Akira Tangoku: Project administration.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Apostolos Nakas, Meinoshin Okumura, Enrico Ruffini and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med 2016;375:511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masaoka A, Yamakawa Y, Niwa H, Fukai I, Kondo S, Kobayashi M et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853–9. [DOI] [PubMed] [Google Scholar]
- 3. Suda T, Ashikari S, Tochii D, Tochii S, Takagi Y. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570–2. [DOI] [PubMed] [Google Scholar]
- 4. Li F, Tao Y, Bauer G, Elsner A, Li Z, Swierzy M et al. Unraveling the role of ectopic thymic tissue in patients undergoing thymectomy for myasthenia gravis. J Thorac Dis 2019;11:4039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632–5. [DOI] [PubMed] [Google Scholar]
- 6. Mineo TC, Pompeo E, Lerut TE, Bernardi G, Coosemans W, Nofroni I. Thoracoscopic thymectomy in autoimmune myasthenia: results of left-sided approach. Ann Thorac Surg 2000;69:1537–41. [DOI] [PubMed] [Google Scholar]
- 7. Ozdemir N, Kara M, Dikmen E, Nadir A, Akal M, Yucemen N et al. Predictors of clinical outcome following extended thymectomy in myasthenia gravis. Eur J Cardiothorac Surg 2003;23:233–7. [DOI] [PubMed] [Google Scholar]
- 8. Essa M, El-Medany Y, Hajjar W, Hariri Z, Al-Mulhim F, Salih M et al. Maximal thymectomy in children with myasthenia gravis. Eur J Cardiothorac Surg 2003;24:187–9, discussion 190–1. [DOI] [PubMed] [Google Scholar]
- 9. El-Medany Y, Hajjar W, Essa M, Al-Kattan K, Hariri Z, Ashour M. Predictors of outcome for myasthenia gravis after thymectomy. Asian Cardiovasc Thorac Ann 2003;11:323–7. [DOI] [PubMed] [Google Scholar]
- 10. Zieliński M, Kużdżał J, Szlubowski A, Soja J. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann Thorac Surg 2004;78:253–8. [DOI] [PubMed] [Google Scholar]
- 11. Ponseti JM, Gamez J, Vilallonga R, Ruiz C, Azem J, López-Cano M et al. Influence of ectopic thymic tissue on clinical outcome following extended thymectomy in generalized seropositive nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2008;34:1062–7. [DOI] [PubMed] [Google Scholar]
- 12. Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601–6. [DOI] [PubMed] [Google Scholar]
- 13. Mineo TC, Ambrogi V, Schillaci O. May positron emission tomography reveal ectopic or active thymus in preoperative evaluation of non-thymomatous myasthenia gravis? J Cardiothorac Surg 2014;9:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bucher P, Pugin F, Ostermann S, Ris F, Chilcott M, Morel P. Population perception of surgical safety and body image trauma: a plea for scarless surgery? Surg Endosc 2011;25:408–15. [DOI] [PubMed] [Google Scholar]
- 15. Gagner M. Needlescopic splenectomy: a safer alternative to single incision laparoscopic splenectomy (SILS). J Gastrointest Surg 2010;14:1473. [DOI] [PubMed] [Google Scholar]