Abstract
OBJECTIVES
We herein report a single-centre experience with the SAPIEN 3 Ultra balloon-expandable transcatheter aortic valve implantation (TAVI) system.
METHODS
Between March 2019 and January 2020, a total of 79 consecutive patients received transfemoral TAVI using the SAPIEN 3 Ultra device. Data were retrospectively analysed according to updated Valve Academic Research Consortium-2 definitions. Detailed analysis of multislice computed tomography data was conducted to identify potential predictors for permanent pacemaker (PPM) implantation and residual paravalvular leakage (PVL) post TAVI.
Graphical Abstract.
RESULTS
Device success and early safety were 97.5% (77/79) and 94.9% (75/79) with resulting transvalvular peak/mean pressure gradients of 21.1 ± 8.2/10.9 ± 4.4 and PVL >mild in 0/79 patients (0%). Mild PVL was seen in 18.9% (15/79) of cases. Thirty-day mortality was 2.5% (2/79). The Valve Academic Research Consortium-2 adjudicated clinical end points disabling stroke, acute kidney injury and myocardial infarction occurred in 1.3% (1/79), 5.1% (4/79) and 0% (0/79) of patients. Postprocedural PPM implantation was necessary in 7.6% (6/79) of patients. Multislice computed tomography analysis revealed significantly higher calcium amounts of the right coronary cusp in patients in need for postprocedural PPM implantation and a higher eccentricity index in patients with postinterventional mild PVL.
CONCLUSIONS
First experience with this newly designed balloon-expandable-transcatheter heart valve demonstrates adequate 30-day outcomes and haemodynamic results with low mortality, low rates of PPM implantation and no residual PVL >mild. The herein-presented multislice computed tomography values with an elevated risk for PPM implantation and residual mild PVL may help to further improve outcomes with this particular transcatheter heart valve in TAVI procedures.
Keywords: Transcatheter valve therapy, Valve disease, Transcatheter aortic valve implantation Transcatheter aortic valve replacement, Balloon-expandable
Transcatheter aortic valve implantation (TAVI) is an established therapy for severe aortic valve stenosis in patients with an intermediate- or high-risk profile for surgical aortic valve replacement, when a suitable anatomy for the interventional approach is present [1–3].
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is an established therapy for severe aortic valve stenosis in patients with an intermediate- or high-risk profile for surgical aortic valve replacement, when a suitable anatomy for the interventional approach is present [1–3]. Accordingly, TAVI has been incorporated in international guidelines [4, 5]. Recent results of a randomized controlled trial comparing TAVI and surgical aortic valve replacement in low-risk patients suggest superiority of a latest-generation balloon-expandable (BE) transcatheter heart valve (THV) (SAPIEN 3; Edwards Lifesciences Inc., Irvine, CA, USA) compared to surgical aortic valve replacement [6]. While expansion of TAVI indication to low-risk patients remains controversial, mainly due to a higher risk of postprocedural permanent pacemaker (PPM) implantation, residual paravalvular leakage (PVL) and lack of long-term durability data as shown in registry analysis [7–9], evolution of THV and corresponding delivery systems is continuing.
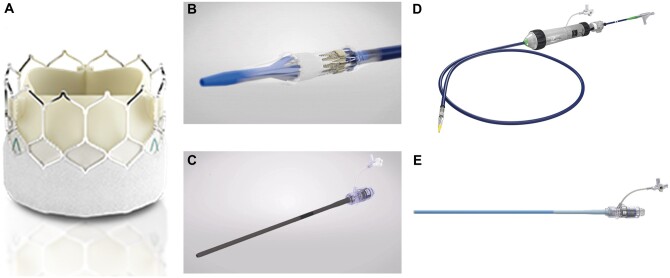
The most recent BE device is the SAPIEN 3 Ultra (S3U) THV System (Edwards Lifesciences Inc.). The S3U THV is the successor of the SAPIEN 3 and consists of a bovine pericardial tissue valve mounted in a low-profile cobalt-chromium frame as known from the SAPIEN 3. Structural modification was added to the outer positron emission tomography skirt, which is now 40% higher compared to the precursor without increase of the inner skirt height. At present, this novel BE THV is available in 3 sizes (20, 23, 26 mm) covering an annulus range of 18.6–26.4 mm according to multislice computed tomography (MSCT) measurements. Further changes were implemented regarding the delivery system which was initially launched with an on-balloon valve crimping technique omitting the need for intraprocedural valve alignment and flex catheter retraction, and the introducer sheath (Axela sheath), which is a seamless expandable 14 Fr sheath for all available valve sizes [10, 11] (see Fig. 1). We herein aimed to evaluate all patients at our centre, who received a S3U THV, since the system received CE mark at the end of 2018.
Figure 1:
A newly designed balloon-expandable transcatheter heart valve with high outer positron emission tomography skirt (A) and corresponding on balloon valve crimping delivery system (B), seamless expandable 14 Fr introducer sheath (C), precursor delivery system ‘Commander’ (D) and introducer sheath ‘e-sheath’ (E).
MATERIALS AND METHODS
Patients
Between March 2019 and January 2020, a consecutive series of 79 patients received transfemoral-TAVI using the S3U system for treatment of severe symptomatic calcified aortic valve stenosis as determined by transthoracic echocardiography (TTE) and transoesophageal echocardiography. In the same period, an overall number of 407 TAVI procedures were conducted at our centre.
Written informed consent was obtained from all patients. Decision to implant this particular THV was left to operators’ discretion and there were no specific anatomical criteria for using the S3U system. However, after ‘eye-balling’ of the preprocedural MSCT, patients with an extensive calcium load of the left ventricular outflow tract (LVOT) were excluded from S3U implantation after consensus of the local heart team due to an increased risk of LVOT rupture caused by the high radial forces of BE THV. Allocation of patients to TAVI followed current international recommendations [4] after consensus of the local dedicated heart team.
Diagnostic work-up and study procedure
As already described in Refs [12, 13], the preprocedural diagnostic work-up followed institutional standards: By routine, all patients received preoperative TTE and transesophageal echocardiography for evaluation of cardiac functional status. Furthermore, diagnostic work-up included contrast-enhanced, electrocardiogram-gated MSCT. Data sets were analysed using the 3mensio Medical Imaging Software (3mensio, Medical Imaging, Bilthoven, Netherlands) for calculation of native aortic annulus dimensions and determination of adequate THV size as well as assessment of aortic root anatomy and morphology (e.g. distribution and severity of valvular calcification, aortic root dimensions or height of coronary ostia take-off), prediction of optimal c-arm angulation and assessment of aorto-iliac and peripheral vascular status.
First-line approach for all procedures was local anaesthesia and/or analgosedation. All procedures were performed in a specially equipped hybrid operating suite by a dedicated team of cardiologists, cardiac surgeons and anaesthesiologists. THV function was assessed by invasive measurements of haemodynamics, aortic root angiography and TTE.
Multislice computed tomography data analysis
Analysis of MSCT was described before [14]. In brief, the aortic annulus was defined as the virtual basal plane containing the basal attachment of the 3 aortic cusps. The cover index was assessed to adjust for oversizing of the THV and calculated as 100 × (THV area − annulus area)/THV area. Eccentricity of the aortic annulus was evaluated with an index calculated as 1 − (minimum diameter/maximum diameter). Asymmetry of calcium distribution was calculated as maximum absolute difference in calcium volume between leaflet sectors for both aortic valve complex and LVOT.
Calcium quantification was performed utilizing an automated volume-scoring tool with an empiric threshold of 500 Hounsfield units. Two regions were evaluated for calcium load: the aortic valve complex including the basal plane to coronary ostia (zone 1) and the LVOT including the subannular region from 10 mm inferior to basal plane (zone 2). Calcium distribution within the aortic valve and the LVOT was sectioned according to left-, right- and non-coronary cusps.
To mitigate interobserver variability, all MSCT were analysed by 1 investigator.
Statistical analysis
Baseline, intraprocedural and acute follow-up data up to 30 days were retrospectively collected and entered into a standardized database and analysed. Clinical end points were adjudicated in accordance with the updated standardized Valve Academic Research Consortium-2 definitions [15]. Data are presented as absolute numbers and percentages for categorical variables and mean values and standard deviation for continuous variables unless stated otherwise. All variables were tested for approximately normal distribution. Continuous variables were compared by unpaired t tests. P-values were reported without correction for multiple testing. A level of significance was set to 2-tailed P-value <0.05. All statistical analyses were performed using Prism© (GraphPad Software, San Diego, CA, USA).
RESULTS
Baseline demographics
Seventy-nine consecutive patients received transfemoral-TAVI using the S3U THV (40.5% female, 79.8 ± 7.1 years, logistic European System for Cardiac Operative Risk Evaluation I 12.1 ± 8.8%). Patients presented with a moderate comorbidity burden, comprising 43/79 (54.4%) patients with concomitant coronary artery disease, 13/79 (16.5%) patients with diabetes mellitus and 6/79 (7.6%) with previous sternotomy. One-third of the herein-investigated patients were highly symptomatic with a New York Heart Association (NYHA) functional class ≥III and baseline left ventricular ejection fraction was 52 ± 14%. Detailed patient demographics are summarized in Table 1.
Table 1:
Baseline demographics
| Study group (n = 79) | |
|---|---|
| Age (years), mean ± SD | 79.8 ± 7.1 |
| Female gender, n (%) | 40.5 (32) |
| BMI (kg/m²), mean ± SD | 27.2 ± 5.5 |
| logEuroSCORE I (%), mean ± SD | 12.1 ± 8.8 |
| Diabetes mellitus, n (%) | 16.5 (13) |
| Arterial hypertension, n (%) | 74.7 (59) |
| Stroke, n (%) | 6.3 (5) |
| Coronary artery disease, n (%) | 54.4 (43) |
| Previous sternotomy, n (%) | 7.6 (6) |
| Extracardiac atheropathy∞, n (%) | 11.4 (9) |
| Arrhythmia, n (%) | 37.9 (30) |
| COPD∞ >Gold II, n (%) | 5.1 (4) |
| Creatinine (mg/dl), mean ± SD | 1.1 ± 0.4 |
| NYHA ≥III, n (%) | 36.7 (29) |
| LVEF (%), mean ± SD | 52 ± 14 |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; logEuroSCORE: logistic European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; ∞extracardiac atheropathy, ∞COPD according to EuroSCORE definitions.
Periprocedural data
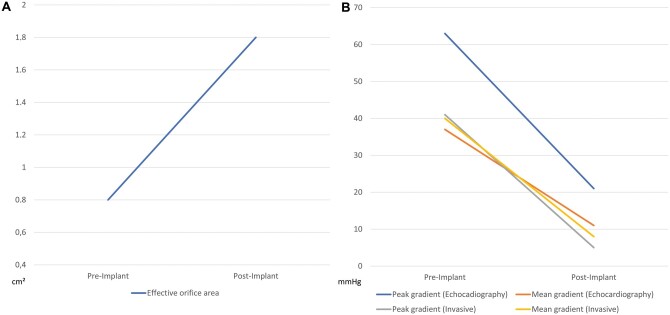
Baseline effective orifice area and peak/mean pressure gradients of the aortic valve were 0.75 ± 0.19 cm2 and 63 ± 23.4/37.5 ± 14.9 mmHg, respectively, as determined by preprocedural transesophageal echocardiography. Procedure time, fluoroscopy time and contrast agent used were 67.4 ± 21.5 min, 18.1 ± 9.1 min and 176.9 ± 53.3 ml, respectively. No S3U valves size 20 mm were used. A cerebral protection system (SENTINEL™ Cerebral Protection System, Boston Scientific Co., Marlborough, MS, USA) was utilized in 9/79 (11.4%) cases. Invasive measurements of pre- and postimplant pressure gradients revealed a decrease in peak gradient from 41.4 ± 20.9 to 4.7 ± 5.8 mmHg (P˂0.001) and decrease in mean gradient from 40.4 ± 17.2 to 8.3 ± 5.6 mmHg (P ˂ 0.001) (see Fig. 2).
Figure 2:
Comparison of pre- and postimplantation effective orifice area (A) and peak and mean pressure gradients of the aortic valve in echocardiography and invasive measurements (B).
Majority of patients were treated under local anaesthesia and/or analgosedation (76/79, 96.2%). One-third of patients received balloon aortic valvuloplasty after THV insertion due to significant residual PVL. Detailed periprocedural data are summarized in Table 2.
Table 2:
Periprocedural data
| Study group (n = 79) | |
|---|---|
| Baseline EOA (cm2), mean ± SD | 0.8 ± 0.2 |
| Baseline peak gradient (mmHg), mean ± SD | 63 ± 23.4 |
| Baseline mean gradient (mmHg), mean ± SD | 37.5 ± 14.9 |
| Invasive pre-implant peak gradient (mmHg), mean ± SD | 41.4 ± 20.9 |
| Invasive pre-implant mean gradient (mmHg), mean ± SD | 40.4 ± 17.2 |
| Procedure time (min), mean ± SD | 67.4 ± 21.5 |
| Fluoroscopy time (min), mean ± SD | 18.1 ± 9.1 |
| Contrast agent (ml), mean ± SD | 176.9 ± 53.3 |
| Valve size (mm), n (%) | |
| 20 | 0 (0) |
| 23 | 31.6 (25) |
| 26 | 68.4 (54) |
| Predilatation, n (%) | 44.3 (35) |
| Postdilatation, n (%) | 32.9 (26) |
| Cerebral protection, n (%) | 11.4 (9) |
| Anaesthesia, n (%) | |
| General anaesthesia | 3.8 (3) |
| Local anaesthesia conscious sedation | 96.2 (76) |
| Invasive postimplant peak gradient (mmHg), mean ± SD | 4.7 ± 5.8 |
| Invasive postimplant mean gradient (mmHg), mean ± SD | 8.3 ± 5.6 |
EOA: effective orifice area; LVEF: left ventricular ejection fraction; SD: standard deviation.
Echocardiographic outcome data
In the study group, peak and mean transvalvular gradients as determined by TTE decreased from 63 ± 23.4 to 21.1 ± 8.2 mmHg and 37.5 ± 14.9 to 10.9 ± 4.4 mmHg (both P < 0.001). Effective orifice area increased from 0.8 ± 0.2 to 1.8 ± 0.3 cm2 (P < 0.001) (see Fig. 2). Postinterventionally, PVL >mild was found in 0% (0/79) of patients. Mild PVL was seen in 18.9% (15/79) of patients. Echocardiographic outcome parameters are documented in Table 3.
Table 3:
Clinical outcome and echocardiographic results at 30 days
| Study group (n = 79) | |
|---|---|
| All-cause mortality (30 days), n (%) | 2.5 (2) |
| Cardiovascular or unknown | 2.5 (2) |
| Stroke (disabling), n (%) | 1.3 (1) |
| Stroke (minor), n (%) | 1.3 (1) |
| Myocardial infarction, n (%) | 0 (0) |
| Bleeding (major/life threatening), n (%) | 6.3 (5) |
| Access site complications (major), n (%) | 6.3 (5) |
| Acute kidney injury (AKINa 2, 3), n (%) | 5.1 (4) |
| PPM implantation, n (%) | 7.6 (6) |
| Device successb, n (%) | 97.5 (77) |
| Early safetyc, n (%) | 94.9 (75) |
| Intensive care unit stay (days), mean ± SD | 1.6 ± 1.2 |
| In-hospital stay (days), mean ± SD | 11.6 ± 5.3 |
| EOA (cm²), mean ± SD | 1.8 ± 0.3 |
| Peak gradient (mmHg), mean ± SD | 21.1 ± 8.2 |
| Mean gradient (mmHg), mean ± SD | 10.9 ± 4.4 |
| Mild PVL, n (%) | 18.9 (15) |
| PVL >mild, n (%) | 0 (0) |
AKIN Acute Kidney Injury Network; VARC-2 definitions.
Device success: absence of procedural mortality, correct positioning of a single prosthetic heart valve into the proper anatomical position, intended performance of the prosthetic heart valve (no prosthesis–patient mismatch and mean aortic valve gradient <20 mmHg or peak velocity <3 m/s and no moderate or severe prosthetic valve regurgitation).
Early safety at 30 days: all-cause mortality (at 30 days), all stroke (disabling and non-disabling), life-threatening bleeding, acute kidney injury stage 2 or 3 (including renal replacement therapy), coronary artery obstruction requiring intervention, major vascular complication, valve-related dysfunction requiring repeat procedure (Balloon aortic valvuloplasty, TAVI or SAVR).
EOA: effective orifice area; PPM: permanent pacemaker; PVL: paravalvular leakage; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; VARC-2: Valve Academic Research Consortium-2.
Clinical outcome data
All-cause 30-day mortality was 2.5% (2/79) (overall mortality of all TAVI patients during the same time period: 2.9%) due to 1 pulseless electric activity immediate prior to PPM implantation on postoperative day 4 and 1 unclear fulminant cerebral embolism on postoperative day 3. Device success and early safety were 97.5% (77/79) and 94.9% (75/79), respectively. Rates of the Valve Academic Research Consortium-2-adjudicated clinical end points disabling stroke, myocardial infarction and acute kidney injury were 1.3% (1/79), 0% (0/0) and 5.1% (4/79). Postprocedural PPM implantation due to atrioventricular block was indicated in 7.6% of patients (6/79). All patients who received postprocedural PPM implantation presented with atrial fibrillation, left/right bundle branch block or both prior to TAVI.
Intensive care unit and hospital stay were 1.6 ± 1.2 and 11.6 ± 5.3, respectively. Postinterventional NYHA functional class assessment presented 1 patient in NYHA functional class III (1.3%) and 78/79 patients with NYHA functional class I or II.
Detailed clinical outcome data are summarized in Table 3. Key outcomes are depicted in Fig. 3.
Figure 3:
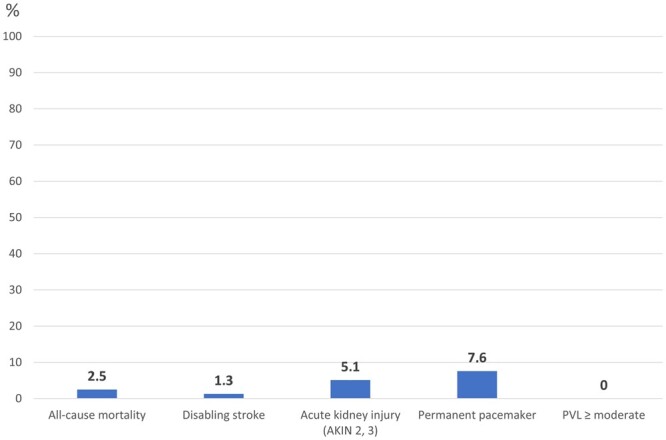
Key 30-day outcomes of patients provided with a latest-generation balloon-expanding transcatheter heart valve. AKIN: Acute Kidney Injury Network; PVL: paravalvular leakage
Multislice computed tomography and electrocardiogram data
The study group exhibited the following calcium loads of both predefined zones of the aortic annulus: zone 1 (aortic annulus to coronary ostia) showed a mean total calcium load of 942.2 ± 631.8 mm³ and zone 2 (subannular from AA 10 mm in LVOT) a mean total calcium load of 36.1 ± 87 mm³. Patients provided with postprocedural PPM implantation presented with significant higher calcium loads of the right coronary cusp in zone 1 (269.4 ± 197.8 vs 467.6 ± 131.6 mm³, P = 0.02).
Cover index, eccentricity index and annulus diameter were 3.7 ± 3.7, 0.23 ± 0.06 and 24.1 ± 1.6 mm with a significant higher eccentricity index for patients with residual mild PVL (0.21 ± 0.06 vs 0.25 ± 0.07, P = 0.02).
Detailed MSCT data are summarized in Table 4.
Table 4:
Preprocedural MSCT imaginga
| Study group (n = 79) | PPM (n = 6) | P-valueb | Mild PVL (n = 15) | P-valueb | |
|---|---|---|---|---|---|
| Calcium load zone 1c (mm³), mean ± SD | |||||
| Right-coronary cusp | 273.7 ± 197.3 | 467.6 ± 131.6 | 0.02 | 244.8 ± 196.3 | 0.52 |
| Left-coronary cusp | 246 ± 189.5 | 235.3 ± 169.7 | 0.89 | 187.6 ± 125.7 | 0.18 |
| Non-coronary cusp | 422.5 ± 340.3 | 389.7 ± 295.6 | 0.80 | 392.5 ± 246.1 | 0.71 |
| Total calcium load zone 1 | 942.2 ± 631.8 | 1090.1 ± 514.4 | 0.57 | 824.9 ± 515.1 | 0.49 |
| Calcium load zone 2d (mm³), mean ± SD | |||||
| Right-coronary cuspLVOT | 7.9 ± 24.3 | 18.1 ± 42.7 | 0.29 | 7.4 ± 27 | 0.92 |
| Left-coronary cuspLVOT | 12.3 ± 38.6 | 3.8 ± 5.9 | 0.58 | 6.2 ± 9.8 | 0.50 |
| Non-coronary cuspLVOT | 15.9 ± 35.7 | 21.3 ± 41.3 | 0.70 | 11.1 ± 26.8 | 0.57 |
| Total calcium load zone 2 | 36.1 ± 87.2 | 41.8 ± 85.4 | 0.89 | 24.1 ± 54.9 | 0.67 |
| Asymmetry calcium loade(mm³), mean ± SD | |||||
| Zone 1 asymmetry | 268.2 ± 245.4 | 175 ± 103.9 | 0.34 | 238 ± 146.8 | 0.60 |
| Zone 2 asymmetry | 18.3 ± 34.8 | 21.3 ± 38.8 | 0.79 | 13.7 ± 25.4 | 0.57 |
| Sinotubular junction length (mm), mean ± SD | 24.4 ± 2.5 | 23.7 ± 2.8 | 0.52 | 23.9 ± 2.2 | 0.39 |
| Distance to RCA (mm), mean ± SD | 18.2 ± 2.9 | 18.6 ± 2.3 | 0.74 | 18.9 ± 3.3 | 0.34 |
| Distance to LCA (mm), mean ± SD | 15.1 ± 2.8 | 17.1 ± 2.5 | 0.09 | 15.5 ± 3.1 | 0.71 |
| Area of AA (mm²), mean ± SD | 448.6 ± 58.9 | 473.1 ± 58.8 | 0.23 | 449.5 ± 56.3 | 0.95 |
| Perimeter of AA (mm), mean ± SD | 75.9 ± 5.5 | 78.2 ± 4.9 | 0.31 | 76.3 ± 4.8 | 0.42 |
| Annulus diameter (mm), mean ± SD | 24.1 ± 1.6 | 24.5 ± 1.3 | 0.89 | 24 ± 1.4 | 0.32 |
| Eccentricity indexf | 0.23 ± 0.06 | 0.19 ± 0.07 | 0.02 | 0.25 ± 0.07 | 0.02 |
| Cover indexg | 3.7 ± 3.7 | 3.7 ± 1.3 | 1 | 4.7 ± 3.2 | 0.33 |
Threshold 500–600 HU.
P-values for comparison of subgroup vs study group-subgroup (values of study group-subgroup are not quoted).
From AA to coronary ostia.
Subannular, from AA 10 mm in LVOT.
Calculated by maximum absolute difference in calcium volume between leaflet sectors for AVC/LVOT.
Calculated by 1 – (minimum diameter/maximum diameter), & ([nominal THV diameter-measured diameter]/nominal THV diameter)*100.
([nominal THV diameter-measured diameter]/nominal THV diameter)*100.
AA: aortic annulus; AVC: aortic valve complex; ECG: electrocardiogram; LCA: left coronary artery; LVOT: left ventricular outflow tract; MSCT: multi-slice computed tomography; PPM: permanent pacemaker; RCA: right coronary artery; THV: transcatheter heart valve.
DISCUSSION
Main findings
Main findings of the herein-conducted study are: (i) use of the investigated BE-THV resulted in adequate 30-day outcomes regarding mortality and Valve Academic Research Consortium-2-adjudicated clinical end points, (ii) echocardiography demonstrated adequate postprocedural effective orifice area and transvalvular pressure gradients with no cases of PVL >mild, (iii) patients presenting with mild PVL after 30 days showed a significantly higher eccentricity index in preprocedural MSCT, (iv) rate of postinterventional PPM implantation was low with 7.6% and (v) patients who received PPM implantation after TAVI presented with preoperative conduction disorders and significantly higher calcium loads of the right coronary cusp at the height of the aortic annulus.
Real-world data for S3U system were recently reported by Saia and colleagues. In this multicentre registry, comparable clinical outcomes are reported with a mortality of 0%, a rate of PVL ≥moderate of 1.4% and a rate of PPM implantation of 4.4% in 139 patients [16]. Registry data of the precursor THV also demonstrated similar or at least comparable outcomes with a mortality of 2.2%, PPM rate of 12% and a rate of residual postoperative PVL ≥moderate of 3.1% in the SOURCE (SAPIEN Aortic Bioprosthesis European Outcome)-3 registry [7]. Also, stroke rates were low with an incidence between 0% and 1.4% in the mentioned studies.
In this, so far, largest single-centre experience utilizing the S3U, first detailed MSCT measurements were conducted for identification of possible calcification patterns leading to residual mild PVL and PPM implantation. Here, a higher calcium load of the right coronary cusp in zone 1 was seen in patients with postinterventional PPM implantation. Although it has to be emphasized that the herein-compared patient groups are rather small, this is a different finding compared to self-expandable THV in which higher calcium loads of the left coronary cusp were shown to be predictive for postinterventional PPM implantation [13]. Also, all patients receiving postinterventional PPM implantation suffered from preoperative atrial fibrillation or a left or right bundle branch block. These factors are also known for promoting post-TAVI conduction disorders [17]. In our patient cohort, no PVL >mild was observed. Whether this is an effect of the higher outer skirt of the S3U is speculative in a small patient cohort, but the higher skirt may be the main advantage of the S3U compared to the SAPIEN 3. However, in 18.9% of patients, mild PVL was found during 30-day follow-up. In those patients, MSCT presented a higher eccentricity of the aortic annulus, which is already known as a risk factor for postinterventional mild PVL for the SAPIEN 3 THV [18]. As PPM implantation and mild PVL are known to increase 1-year mortality in TAVI patients [19, 20], it should be of paramount interest for the heart team to further improve these particular outcomes, especially when expansion of TAVI indications to younger low-risk patients should be taken into consideration. The herein-documented first findings for this latest generation THV may be helpful regarding patient selection and periprocedural aspects (e.g. utilization of pre- and postdilatation, oversizing strategy).
With the new introducer sheath and delivery system of the S3U, omitting some major procedural steps of the SAPIEN 3 implantation, shorter procedure and fluoroscopy times as well as amount of used contrast agent were expected. However, these values were rather mildly increased in this patient cohort compared to earlier reports of the SAPIEN 3 [21], most likely due to a more cautious implantation procedure with novel or altered THV devices. It has to be mentioned that after half of the herein-performed cases, the delivery system and the Axela sheath were recalled from the market due to increased rates of adverse clinical events such as rupture of delivery balloons [22]. Since then, implantations were performed using the eSheath and the Commander delivery system known from the SAPIEN 3 with the same procedural steps and operability known from the SAPIEN 3 system (see Fig. 1).
Limitations
Limitations are inherent in the retrospective, single-centre study design with limited patient numbers: patients were not randomized to a specific treatment or valve; therefore, patient preselection with hidden confounders may apply. Also, MSCT measurements were not implemented in a multivariate statistical model comprising e.g. valve implantation depth or length of the membranous septum, and therefore also here hidden confounders may interfere. Furthermore, a patient preselection regarding high LVOT calcium loads was conducted, which may influence results of this study.
CONCLUSIONS
First experience with this latest-generation BE-THV resulted in adequate 30-day outcomes and haemodynamic results with low mortality, low rates of PPM implantation and no residual PVL>mild. The herein-presented MSCT values with an elevated risk for PPM implantation and residual mild PVL may help to further improve outcomes with this particular THV in TAVI procedures.
Conflict of interest: Moritz Seiffert received travel compensation from Edwards Lifesciences Inc.; Lenard Conradi is a proctor and consultant for Edwards Lifesciences Inc. All other authors declared no conflict of interest.
Author contributions
Andreas Schaefer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Fabienne Plassmeier: Formal analysis; Methodology; Writing—review & editing. Niklas Schofer: Formal analysis; Methodology; Writing—review & editing. Lukas Vogel: Data curation; Formal analysis; Methodology; Writing—review & editing. Sebastian Ludwig: Formal analysis; Methodology; Writing—review & editing. Yvonne Schneeberger: Data curation; Formal analysis; Methodology; Writing—review & editing. Matthias Linder: Formal analysis; Methodology; Writing—review & editing. Till Demal: Data curation; Formal analysis; Writing—review & editing. Moritz Seiffert: Data curation; Formal analysis; Methodology; Writing—review & editing. Stefan Blankenberg: Methodology; Supervision; Writing—review & editing. Hermann Reichenspurner: Investigation; Supervision; Writing—review & editing. Dirk Westermann: Formal analysis; Investigation; Methodology; Supervision; Writing—review & editing. Lenard Conradi: Conceptualization; Formal analysis; Investigation; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks David Schibilsky, Wolfgang Schiller, Tomoki Shimokawa and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
Abbreviations
- BE
Balloon-expandable
- LVOT
Left ventricular outflow tract
- NYHA
New York Heart Association
- PPM
Permanent pacemaker
- PVL
Paravalvular leakage
- S3U
SAPIEN 3 Ultra
- TAVI
Transcatheter aortic valve implantation
- THV
Transcatheter heart valve
- TTE
Transthoracic echocardiography
REFERENCES
- 1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 2. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK. et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 6. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M. et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. [DOI] [PubMed] [Google Scholar]
- 7. Wendler O, Schymik G, Treede H, Baumgartner H, Dumonteil N, Ihlberg L. et al. SOURCE 3 registry: design and 30-day results of the European Postapproval Registry of the latest generation of the SAPIEN 3 transcatheter heart valve. Circulation 2017;135:1123–32. [DOI] [PubMed] [Google Scholar]
- 8. Grube E, Van Mieghem NM, Bleiziffer S, Modine T, Bosmans J, Manoharan G. et al. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: the international FORWARD study. J Am Coll Cardiol 2017;70:845–53. [DOI] [PubMed] [Google Scholar]
- 9. Schaefer A, Schofer N, Goßling A, Seiffert M, Schirmer J, Deuschl F. et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in low-risk patients: a propensity score-matched analysis. Eur J Cardiothorac Surg 2019;56:1131–9. [DOI] [PubMed] [Google Scholar]
- 10. https://www.edwards.com/gb/devices/heart-valves/transcatheter-SAPIEN-3-Ultra (13 February 2020, date last accessed).
- 11. https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140031c.pdf (13 February 2020, date last accessed).
- 12. Schaefer A, Linder M, Treede H, Deuschl F, Schofer N, Seiffert M. et al. Applicability of next generation balloon-expandable transcatheter heart valves in aortic annuli exceeding formally approved dimensions. Clin Res Cardiol 2016;105:585–91. [DOI] [PubMed] [Google Scholar]
- 13. Schaefer A, Neumann N, Linder M, Schofer N, Schneeberger Y, Deuschl F. et al. Outcomes with a latest generation self-expandable, intra-annular, re-sheathable transcatheter heart valve system: analysis of patients with impaired left ventricular function and determinants for pacemaker implantation. Clin Res Cardiol 2018;107:914–23. [DOI] [PubMed] [Google Scholar]
- 14. Seiffert M, Fujita B, Avanesov M, Lunau C, Schön G, Conradi L. et al. Device landing zone calcification and its impact on residual regurgitation after transcatheter aortic valve implantation with different devices. Eur Heart J Cardiovasc Imaging 2016;17:576–84. [DOI] [PubMed] [Google Scholar]
- 15. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH. et al. ; Valve Academic Research Consortium-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013;145:6–23. [DOI] [PubMed] [Google Scholar]
- 16. Saia F, Gandolfo C, Palmerini T, Berti S, Doshi SN, Laine M. et al. In-hospital and thirty-day outcomes of the SAPIEN 3 Ultra balloon-expandable transcatheter aortic valve: the S3U registry. EuroIntervention 2020;15:1240–7. [DOI] [PubMed] [Google Scholar]
- 17. Pollari F, Großmann I, Vogt F, Kalisnik JM, Cuomo M, Schwab J. et al. Risk factors for atrioventricular block after transcatheter aortic valve implantation: a single-centre analysis including assessment of aortic calcifications and follow-up. Europace 2019;21:787–95. [DOI] [PubMed] [Google Scholar]
- 18. Tang GHL, Zaid S, Schnittman SR, Ahmad H, Kaple R, Undemir C. et al. Novel predictors of mild paravalvular aortic regurgitation in SAPIEN 3 transcatheter aortic valve implantation. EuroIntervention 2018;14:58–68. [DOI] [PubMed] [Google Scholar]
- 19. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L. et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: a systematic review and meta-analysis. Eur Heart J 2020;41:2771–81. [DOI] [PubMed] [Google Scholar]
- 20. Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V. et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards SAPIEN valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015;36:449–56. [DOI] [PubMed] [Google Scholar]
- 21. Pellegrini C, Rheude T, Trenkwalder T, Mayr NP, Joner M, Kastrati A. et al. One year VARC-2-defined clinical outcomes after transcatheter aortic valve implantation with the SAPIEN 3. Clin Res Cardiol 2019;108:1258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. https://www.fda.gov/medical-devices/medical-device-recalls/edwards-lifesciences-llc-recalls-sapien-3-ultra-delivery-system-due-burst-balloons-during-surgery (21 March 2020, date last accessed).