Abstract
OBJECTIVES
The aim of this study was to evaluate the clinical implication of tumour spread through air spaces (STAS) as a prognostic factor in pathological stage I lung adenocarcinoma treated with lobectomy and to identify related parameters.
METHODS
Medical records of patients who underwent pulmonary lobectomy for stage I (American Joint Committee on Cancers eighth edition) lung adenocarcinomas between 2012 and February 2018 at our institutions were reviewed retrospectively. Patients with minimally invasive adenocarcinomas and tumours ≥3 cm in size were excluded. Included patients were classified into STAS (+) and STAS (−) groups. Clinical implications of STAS and recurrence in patients were investigated.
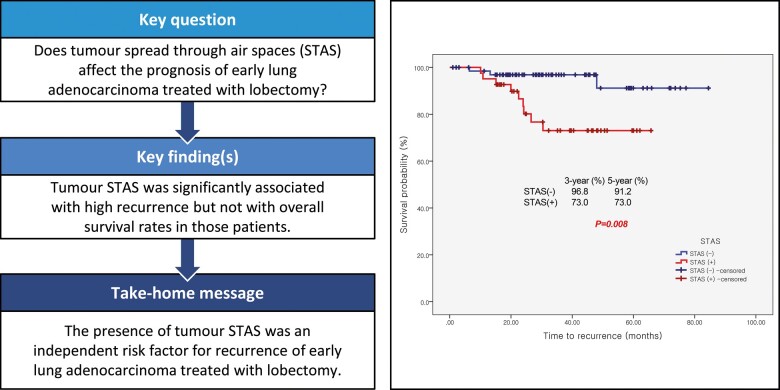
RESULTS
A total of 109 patients was analysed: 41 (37.6%) in the STAS (+) and 68 (62.4%) in the STAS (−) group. STAS was associated with larger consolidation diameter on chest tomography (≥1.5 cm; P = 0.006) or a higher invasive ratio (≥85%; P = 0.012) and presence of a micropapillary pattern in multivariable analysis (P = 0.003) The recurrence-free survival curve showed statistical difference (P = 0.008) with 3-year survival rates of 73.0% (9 patients) and 96.8% (2 patients) in the STAS (+) and STAS (−) group, respectively. However, no statistical significance was observed in the lung cancer-related survival curve (P = 0.648). The presence of STAS was an independent risk factor for recurrence in multivariable analysis (hazard ratio = 5.9, P = 0.031).
CONCLUSIONS
The presence of STAS could be an important risk factor for recurrence in patients with early-stage invasive lung adenocarcinoma treated with pulmonary lobectomy.
Keywords: Invasive adenocarcinoma, Lobectomy, Prognosis
INTRODUCTION
Tumour spread through air spaces (STAS) is a newly adopted pathological pattern of invasion of cancer cell nests spreading into air spaces in the lung parenchyma adjacent to the border of the main tumour [1–3]. Studies have reported that STAS is an independent risk factor for loco-regional recurrence in small early-stage lung adenocarcinoma treated with limited resection [4–6] and a negative survival risk factor for surgically resected stage I squamous cell lung cancers [7]. However, STAS has not been proved to be a risk factor for recurrence in early-stage lung cancer treated with lobectomy.
As imaging technology advances and adoption of screening tests for lung cancer expands, detection of small, peripheral early-stage lung cancers that could be candidates for limited resection is increasing [8]. The negative correlation between STAS and recurrence rate suggests an important indicator for treatment strategies.
The presence of STAS has been reported to be related to invasive pathological characteristics such as larger tumour size, visceral pleural invasion micropapillary and solid predominant subtypes [4, 5]. We found that some patterns of early-stage lung adenocarcinoma such as adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA) are weakly related to the presence of STAS, and most advanced adenocarcinomas are strongly associated with STAS. We were interested in the clinical manifestation of early-stage invasive lung cancers presenting STAS, <3 cm in total size (including lepidic portion) with contained invasive portions larger than 5 mm.
In this study, we investigated the prognostic influence of STAS in early-stage invasive lung adenocarcinoma in patients treated with lobectomy. We investigated the precise clinical implications of STAS in early-stage patients who underwent standard surgical treatments.
MATERIALS AND METHODS
Patient characteristics
A retrospective medical review of 230 patients who underwent surgical treatment for lung adenocarcinoma between January 2012 and February 2018 at Korea University Anam Hospital was performed. Clinical and pathological stages were revised according to the eighth edition of the American Joint Committee on Cancers (AJCC)/Union for International Cancer Control (UICC) lung cancer staging system with 183 patients included in stage I [9, 10]. We excluded patients who (i) had a tumour larger than 3 cm in total tumour size including lepidic portion (33 patients); (ii) were diagnosed as having AIS (10 patients) or MIA (14 patients); or (iii) underwent sublobar resection (15 with wedge resection and 1 with segmentectomy).
Analysed patients were classified into 2 groups by the presence of STAS. Demographic information, preoperative findings, pathological results and follow-up clinical manifestations were reviewed and compared.
Pathology review
All archival slides and pathology reports of patients were reviewed by 2 specialized pulmonary pathologists (J.H.L. and Y.L.). The presence of STAS and relevant pathological features including micropapillary pattern, lymphovascular invasion or inflammatory reactions and invasion of visceral pleura were investigated.
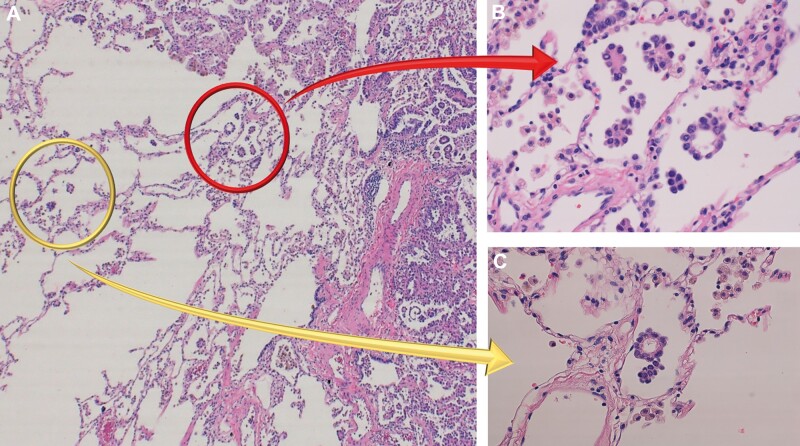
The definition of STAS was based on research from Kadota et al. [4] (i.e. tumour cells within air spaces; Fig. 1), with (i) micropapillary structures of papillary structures without central fibrovascular cores, (ii) solid nests or tumour islands of solid collections of tumour cells filling air spaces and (iii) scattered discohesive single cells. The distance from the edge of a tumour to the farthest STAS was measured using a ruler as well as using numbers of in-between alveolar space.
Figure 1:
Pathological findings of spread through air spaces (STAS). (A) High-power field image (×100) of lung adenocarcinoma. Tumour margin and normal alveolar spaces are observed. (B) STAS adjacent to the edge of tumours (red arrow). STAS are defined as (i) micropapillary structures consisting without central fibrovascular cores, (ii) solid nests or tumour island filing air spaces, and (iii) single cells consisting of scattered discohesive single cells. (C) STAS found within air spaces in the lung parenchyma beyond the edge of the main tumour (yellow arrow).
Categorization of AIS, MIA, differentiation and predominant subtypes of adenocarcinoma followed the new adenocarcinoma classification system of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society [11].
Tumour sizes were measured in 2 ways. Before surgery, the largest diameter of total tumour size and the consolidative part were measured using chest computed tomographic imaging (chest CT). After surgery, total tumour size and invasive tumour size (excluding lepidic portion and invasive component only) were measured from pathological specimens. The total tumour size from the imaging study was defined as the largest diameter of the tumour including ground-glass opacity, and that from the pathological specimen, including the lepidic portion.
The consolidative tumour (CT) ratio was the proportion of consolidative size divided by total size measured on chest CT. The invasive ratio was invasive size divided by total size.
Clinical prognosis
All patients who underwent surgical resection for lung cancer were followed up regularly by the outpatient department. Medical examination including chest CT was scheduled every 4 months after surgery during the first 2 years and then every 6 months for the next 3 years.
Recurrence was confirmed when radiological or pathological evidence was reported. Loco-regional recurrence was defined as evidence of a tumour identified within the diseased hemithorax, ipsilateral lung, ipsilateral mediastinal or hilar lymph nodes. Distant metastasis was any recurrence site outside the ipsilateral haemothorax including contralateral lung and hilum [12, 13]. Simultaneous loco-regional and distant metastases were categorized as loco-regional.
Statistical analysis
χ 2 and Fisher’s exact tests (when the expected value of data was lower than 5) were used for statistical comparison for categorical variables. Student’s t-test for parametric and Mann–Whitney U-test for non-parametric variables were applied to compare continuous variables. Univariable analysis was performed to identify statistically significant variables for recurrence using Cox’s proportional hazard model and STAS presentation using a logistic regression method. Multivariable analysis was performed with variables found to be significantly related to recurrence using Cox’s proportional hazard model and STAS presentation, a logistic regression method.
Cumulative recurrence-free, cancer-related and overall survival rates were calculated using the Kaplan–Meier method, and statistical differences were determined using log-rank tests. All survivals were calculated assuming patients were alive with or without recurrence from the date of surgery. Significant differences were defined when the P-value was <0.05 with 5% significance level. Statistical analyses were performed using SPSS 20.0 (SPSS Inc., Armonk, NY, USA).
RESULTS
Patient characteristics
A total of 109 patients with pathological stage I lung adenocarcinoma with total tumour sizes (including lepidic portion) <3 cm was analysed in this study. The STAS (+) group had 41 patients and the STAS (−) group had 68 patients. All enrolled patients underwent lobectomy with mediastinal node dissection (including at least 3 mediastinal and 2 hilar lymph node stations). The mean follow-up was 37.5 ± 19.68 months. Demographic and preoperative characteristics are in Table 1.
Table 1:
Patient characteristics and preoperative findings
| Variables | STAS (+) (N = 41)N (%) |
STAS (−) (N = 68)N (%) |
Total (N = 109)N (%) |
P-value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Age (years) | 66.1 ± 7.92 | 63.6–68.6 | 63.3 ± 9.88 | 70.1–65.7 | 64.4 ± 9.12 | 62.6–66.1 | 0.147 |
| Gender | 0.541 | ||||||
| Men | 28 (68.3) | 26 (38.2) | 39 (35.8) | ||||
| Women | 13 (31.7) | 42 (61.8) | 70 (64.2) | ||||
| Smoking | 0.919 | ||||||
| Never | 31 (73.4) | 49 (72.1) | 80 (73.4) | ||||
| Ex | 8 (19.5) | 15 (22.1) | 23 (21.1) | ||||
| Current | 2 (4.9) | 4 (5.9) | 6 (5.5) | ||||
| PPY | 32.4 ± 19.14 | 19.5–45.3 | 28.9 ± 19.14 | 19.7–38.2 | 30.2 ± 18.53 | 23.1–37.2 | 0.645 |
| Comorbidities | |||||||
| Hypertension | 22 (37.6) | 26 (54.2) | 48 (44.0) | 0.116 | |||
| Diabetes mellitus | 7 (17.1) | 11 (16.2) | 18 (16.5) | 1.000 | |||
| Atrial fibrillation | 2 (4.9) | 3 (4.4) | 5 (4.6) | 1.000 | |||
| Angina | 7 (17.1) | 2 (2.9) | 9 (8.3) | 0.025 | |||
| Heart failure | 3 (7.3) | 2 (2.9) | 5 (4.6) | 0.362 | |||
| Liver cirrhosis | 0 (0.0) | 2 (2.9) | 2 (1.8) | 0.526 | |||
| Renal insufficiency | 2 (4.9) | 4 (5.9) | 6 (5.5) | 1.000 | |||
| Stroke | 0 (0.0) | 3 (4.4) | 3 (2.8) | 0.290 | |||
| COPD | 1 (2.4) | 3 (4.4) | 4 (3.7) | 1.000 | |||
| Interstitial pneumonitis | 5 (12.2) | 0 (0.0) | 5 (4.6) | 0.013 | |||
| Double primary cancer | 0.574 | ||||||
| Lung | 2 (4.9) | 1 (1.5) | 3 (2.8) | ||||
| Other organs | 11 (26.8) | 19 (27.9) | 30 (27.5) | ||||
| CCI | 3.2 ± 1.49 | 2.8–3.7 | 2.9 ± 1.99 | 2.4–3.3 | 3.0 ± 1.82 | 2.7–3.3 | 0.107 |
| Preoperative PFT | |||||||
| FEV1 | 2.3 ± 0.55 | 2.1–2.5 | 2.5 ± 0.51 | 2.3–2.6 | 2.4 ± 0.54 | 2.3–2.5 | 0.113 |
| FEV1 (%) | 90.0 ± 16.49 | 84.8–95.2 | 93.6 ± 14.65 | 90.0–97.1 | 92.2 ± 15.39 | 89.3–95.2 | 0.237 |
| DLCO | 15.4 ± 3.18 | 14.4–16.5 | 16.8 ± 4.02 | 15.8–17.9 | 16.3 ± 3.76 | 15.5–17.1 | 0.109 |
| DLCO (%) | 67.8 ± 30.05 | 57.9–77.7 | 63.5 ± 32.18 | 55.2–71.8 | 65.2 ± 31.09 | 58.9–71.4 | 0.511 |
| CT diameter | |||||||
| Total diameter | 21.0 ± 4.94 | 19.5–22.6 | 19.0 ± 4.90 | 17.8–20.2 | 19.8 ± 4.99 | 18.8–20.7 | 0.051 |
| <2.0 cm | 16 (39.0) | 33 (48.5) | 49 (45.0) | 0.427 | |||
| ≥2.0 cm | 25 (61.0) | 35 (51.5) | 60 (55.0) | ||||
| Solid | 19.5 ± 5.04 | 17.9–21.1 | 14.7 ± 6.27 | 13.1–16.2 | 16.5 ± 6.27 | 15.3–17.7 | <0.001 |
| 1.5 cm | 10 (48.6) | 43 (63.2) | 53 (48.6) | <0.001 | |||
| ≥1.5 cm | 31 (75.6) | 25 (36.8) | 56 (51.4) | ||||
| CT ratio | 0.9 ± 0.12 | 0.89–0.97 | 0.76 ± 0.23 | 0.70–0.81 | 0.82 ± 0.21 | 0.78–0.86 | <0.001 |
| <85% | 8 (19.5) | 40 (58.8) | 48 (44.0) | <0.001 | |||
| ≥85% | 33 (80.5) | 28 (41.2) | 61 (56.0) | ||||
| CT location | 1.000 | ||||||
| Parenchymal | 16 (39.0) | 26 (38.2) | 42 (38.5) | ||||
| Peripheral | 25 (61.0) | 42 (61.8) | 67 (61.5) | ||||
| Clinical stage | |||||||
| T stages | 0.277 | ||||||
| 1a | 2 (4.9) | 4 (5.9) | 6 (5.5) | ||||
| 1b | 9 (22.0) | 23 (33.8) | 32 (29.4) | ||||
| 1c | 3 (7.9) | 3 (4.4) | 6 (5.5) | ||||
| 2a | 25 (61.0) | 38 (55.9) | 63 (57.8) | ||||
| 3 | 2 (4.9) | 0 (0.0) | 2 (1.8) | ||||
| N stages | |||||||
| N0 | 39 (95.1) | 67 (98.5) | 106 (97.2) | ||||
| N1 | 2 (4.9) | 1 (1.5) | 3 (2.8) | ||||
| IA1 | 0 (0.0) | 1 (1.5) | 1 (0.9) | 0.195 | |||
| IA2 | 11 (26.8) | 26 (38.2) | 37 (33.9) | ||||
| IA3 | 2 (4.9) | 3 (4.4) | 5 (4.6) | ||||
| IB | 25 (61.0) | 37 (54.4) | 62 (56.9) | ||||
| IIA | 0 (0.0) | 1 (1.5) | 1 (0.9) | ||||
| IIB | 3 (2.8) | 0 (0.0) | 3 (2.8) | ||||
CCI: Charlson comorbidity index; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CT: consolidative tumour; DLCO: diffuse capacity of lung for carbon monoxide; FEV1: forced expiratory volume in 1 s; PFT: pulmonary function test; PPY: pack per year; SD: standard deviation; STAS: spread through air spaces.
Relating factors with spread through air spaces
Pathological characteristics and perioperative data of the STAS (+) and STAS (−) groups are shown in Table 2 (and Supplementary Material, D1). Univariable and multivariable analysis searching for factors related to STAS are shown in Table 3. Larger consolidative size on chest CT or higher invasive ratio was significantly related to the presence of STAS, as was the presence of a micropapillary pattern. Pathological stages showed no prognostic power for STAS nor recurrence in multivariable analysis (Tables 3 and 4). The full data of univariable analysis for risk factors of STAS are given in the Supplementary Material, D2.
Table 2:
Major perioperative findings
| Variables | |||||||
|---|---|---|---|---|---|---|---|
| STAS (+) (N = 41)N (%) |
STAS (−) (N = 68)N (%) |
Total (N = 109)N (%) |
P-value | ||||
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Predominant | 0.005 | ||||||
| Lepidic | 0 (0.0) | 19 (27.9) | 19 (17.4) | ||||
| Acinar | 30 (73.2) | 36 (52.9) | 66 (60.6) | ||||
| Papillary | 5 (12.2) | 3 (4.4) | 8 (7.3) | ||||
| Micropapillary | 2 (4.9) | 1 (1.5) | 3 (2.8) | ||||
| Solid | 1 (2.4) | 3 (4.4) | 4 (3.7) | ||||
| Mucinous adenocarcinoma | 3 (7.3) | 3 (8.8) | 9 (8.3) | ||||
| Diameter | |||||||
| Total | 21.0 ± 4.94 | 19.5–22.6 | 19.0 ± 4.90 | 17.8–20.2 | 19.8 ± 4.99 | 18.8–20.7 | 0.098 |
| <2.0 cm | 18 (43.9) | 36 (52.9) | 54 (49.5) | 0.430 | |||
| ≥2.0 cm | 23 (56.1) | 32 (47.1) | 55 (50.5) | ||||
| Invasive | 19.5 ± 5.04 | 17.9–21.1 | 14.7 ± 6.30 | 13.1–16.2 | 16.5 ± 6.27 | 15.3–17.7 | 0.003 |
| <1.5 cm | 10 (24.4) | 33 (48.5) | 43 (39.4) | 0.015 | |||
| ≥1.5 cm | 31 (75.6) | 35 (51.5) | 66 (60.6) | ||||
| Invasive ratio | 0.9 ± 0.12 | 0.89–0.97 | 0.8 ± 0.23 | 0.70–0.81 | 0.8 ± 0.21 | 0.78–0.86 | 0.003 |
| <85% | 3 (7.3) | 29 (42.6) | 32 (29.4) | <0.001 | |||
| ≥85% | 38 (92.7) | 39 (57.4) | 77 (70.6) | ||||
| Pathological features | |||||||
| Visceral pleural invasion | 0.058 | ||||||
| P0 | 15 (36.6) | 29 (42.6) | 44 (40.4) | ||||
| P1 | 21 (51.2) | 38 (55.9) | 59 (54.1) | ||||
| P2 | 5 (12.2) | 1 (1.5) | 6 (5.5) | ||||
| Vascular invasion | 3 (7.3) | 2 (2.9) | 5 (4.6) | 0.362 | |||
| Lymphatic invasion | 3 (7.3) | 3 (4.4) | 6 (5.5) | 0.670 | |||
| Necrosis | 6 (14.6) | 7 (10.3) | 13 (11.9) | 0.550 | |||
| Micropapillary pattern | |||||||
| Positive | 29 (70.7) | 12 (17.6) | 41 (37.6) | <0.001 | |||
| STAS | |||||||
| Distance (mm) | 0.6 ± 1.02 | 0.4–0.8 | |||||
| Alveolar space | 2.4 ± 3.82 | 1.69–3.14 | |||||
| Pathological stage | 0.109 | ||||||
| IA2 (T1bN0) | 7 (17.1) | 23 (33.8) | 30 (27.5) | ||||
| IA3 (T1cN0) | 8 (19.5) | 7 (10.3) | 15 (13.8) | ||||
| IB (T2aN0) | 26 (63.4) | 38 (55.9) | 64 (58.7) | ||||
| EGFR | 1.000 | ||||||
| Positive | 17.0 | 35.0 | 52.0 | ||||
| Exon18 | 0.0 | 1.0 | 1.0 | 0.907 | |||
| Exon19 | 7.0 | 17.0 | 24.0 | ||||
| Exon21 | 9.0 | 16.0 | 25.0 | ||||
| Multiple variant | 1.0 | 1.0 | 2.0 | ||||
| ALK | 0.801 | ||||||
| Positive | 5.0 | 6.0 | 11.0 | ||||
| Negative | 19.0 | 35.0 | 54.0 | ||||
| ALK (%) | 8.5 ± 11.24 | 4.9–12.0 | 9.2 ± 13.83 | 3.4–15.1 | 8.7 ± 12.16 | 5.7–11.8 | 0.823 |
ALK: anaplastic lymphoma receptor tyrosine kinase; CI: confidence interval; EGFR: epidermal growth factor receptor; SD: standard deviation; STAS: spread through air spaces.
Table 3:
Univariable and multivariable analysis for identifying independent risk factors for the presence of STAS
| Variables | Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | ||
| CT diameter | |||||||
| Total diameter | 1.1 | 1.00–1.18 | 0.044 | ||||
| Consolidation | ≥1.5 cm | 6.8 | 2.38–19.39 | <0.001 | 1.44 | 1.108–1.862 | 0.006 |
| CT ratio (%) | ≥85% | 5.9 | 2.37–14.65 | <0.001 | |||
| Diameter | |||||||
| Invasive | ≥1.5 cm | 3.2 | 1.31–7.62 | 0.010 | |||
| Invasive ratio | ≥85% | 6.9 | 2.20–21.47 | 0.001 | 1.11 | 1.024–1.212 | 0.012 |
| Pathological features | |||||||
| Visceral pleural invasion | P2 | 9.7 | 1.03–90.41 | 0.047 | |||
| Micropapillary pattern | Positive | 11.3 | 4.51–28.22 | 0.000 | 6.09 | 1.826–20.284 | 0.003 |
| Pathological stage | IA3 | 3.8 | 1.00–14.07 | 0.050 | |||
CI: confidence interval; CT: consolidative tumour; STAS: spread through air spaces.
Table 4:
Univariable and multivariable analysis for identifying independent risk factors for recurrence in any sites during follow-up periods
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Variables | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
| Age | ||||||
| >65 | 5.0 | 1.11–23.16 | 0.036 | 5.4 | 0.87–33.35 | 0.070 |
| Gender | ||||||
| Men | 5.8 | 1.63–20.49 | 0.007 | 3.9 | 0.86–17.24 | 0.078 |
| Smoking | ||||||
| Current/ex | 3.6 | 1.12–11.4 | 0.032 | |||
| PPY | 1.0 | 1.00–1.06 | 0.043 | |||
| CT diameter | ||||||
| Total diameter | 1.2 | 1.05–1.35 | 0.009 | |||
| ≥2.0 cm | 4.8 | 1.05–22.03 | 0.043 | |||
| Solid | 1.2 | 1.09–1.41 | 0.001 | |||
| ≥1.5 cm | 2.0 | 0.57–7.23 | 0.269 | |||
| CT ratio (%) | 1.1 | 1.00–1.13 | 0.045 | 6.9 | 0.72–65.63 | 0.095 |
| ≥85% | 9.6 | 1.23–74.42 | 0.031 | |||
| Diameter | ||||||
| Total | 1.2 | 1.08–1.44 | 0.003 | |||
| ≥2.0 cm | 5.4 | 1.19–24.77 | 0.029 | |||
| Invasive | 1.2 | 1.08–1.38 | 0.002 | |||
| ≥1.5 cm | 7.5 | 0.96–57.90 | 0.054 | |||
| Pathological features | ||||||
| Lymphatic invasion | 10.4 | 1.83–59.52 | 0.008 | |||
| Micropapillary pattern | ||||||
| Positive | 10.6 | 2.20–51.53 | 0.003 | |||
| STAS | 4.9 | 1.33–18.18 | 0.017 | 5.9 | 1.18–30.09 | 0.031 |
| Distance | 1.6 | 1.02–2.63 | 0.040 | |||
| Changes in stages | ||||||
| Up | 4.7 | 1.25–17.54 | 0.022 | |||
CI: confidence interval; CT: consolidative tumour; PPY: pack per year; STAS: spread through air spaces.
Survival analysis
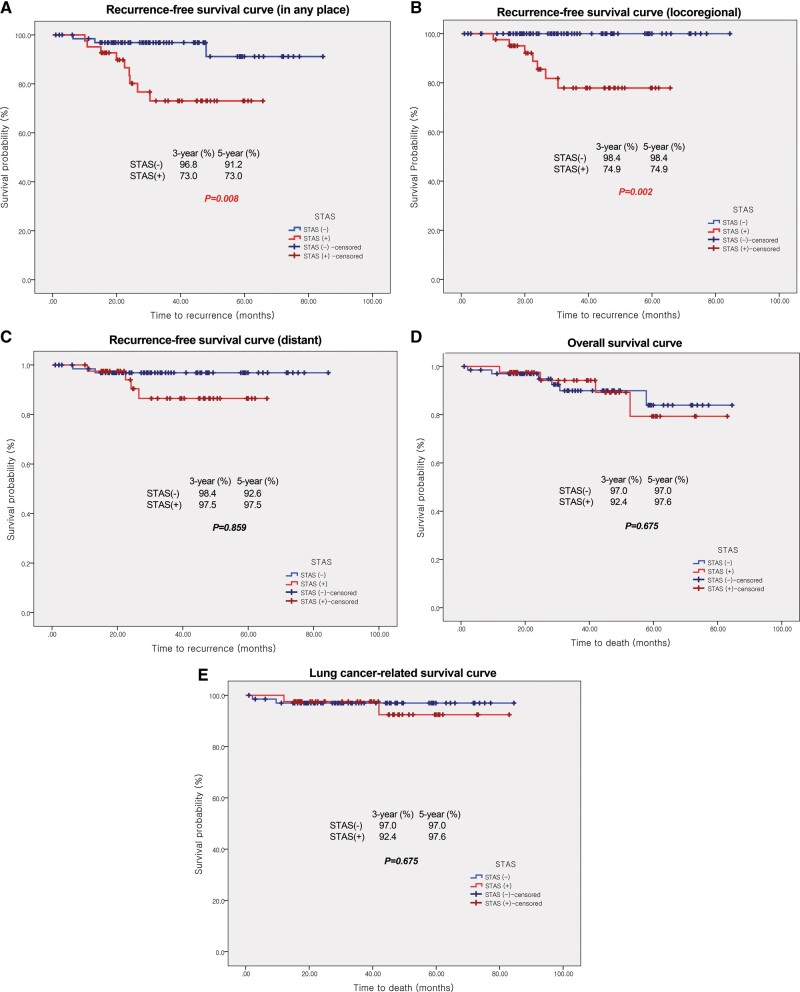
Total recurrence rates were 11.0% (12 patients, Supplementary Material, D3). Univariable and multivariable analyses of recurrence-free survival were performed (Supplementary Material, D4 and Table 4). The presence of STAS and more than 10% micropapillary pattern were significant risk factors for recurrence. The recurrence-free survival curve showed statistical difference with a P-value of 0.008 (log-rank test), 3-year survival rates of 73.0% (9 patients) in STAS (+) and 96.8% (2 patients) in STAS (−) group. However, no statistical significance was observed in the lung cancer-related survival curve (P = 0.648 in log-rank test) with 97.6% (1 patient) in the STAS (+), and 97.0% (2 patients) in the STAS (−) group. The results recurrence-free and overall survival analyses are in shown in the Supplementary Material, D5 and Fig. 2. From the logistic regression test, the recurrence rate at any site in the STAS (+) group was significantly higher than in the STAS (−) group (9% and 22% vs 3% and 4.4%, P = 0.009) but only for loco-regional recurrence (8% and 19.5% vs 1% and 1.5%, P = 0.002) and not for distant metastasis (P = 1.000, Supplementary Material, D3).
Figure 2:
Recurrence-free and overall survival curves of STAS (+) and STAS (−) groups. (A) Recurrence-free survival curve in any place. (B) Recurrence-free survival curve related with loco-regional recurrence. (C) Recurrence-free survival curve related with distant metastasis. (D) Overall survival curve. (E) Lung cancer-related overall survival. Number of patients with recurrence and mortality was described in Supplementary Material, D3. Results of Kaplan–Meier survival analysis with log-rank tests were described in Supplementary Material, D5. STAS: spread through air spaces.
DISCUSSION
We investigated the prognostic ability of STAS in early-stage lung adenocarcinoma for invasive tumours larger than 5 mm but not exceeding 3 cm in total tumour size for patients treated with standard major pulmonary resection. Our study showed a correlation between higher recurrence rates and the presence of STAS in those patients. The 5-year recurrence-free survival values for any recurrences and loco-regional recurrence were significantly lower in the STAS (+) than the STAS (−) group (Fig. 2).
Results from our study seemed to be contrary to the conclusions of previous studies [4]. This difference could be due to the fact that we excluded AIS and MIA and instead included larger tumours (<3 cm). When we excluded larger tumours and included AIS or MIA, similar to other studies, we found no statistical differences in recurrence between the STAS (+) and (−) groups (STAS positive 18 and STAS negative 46, P = 0.447).
Therefore, a certain amount of invasive component (tumour with an invasive component larger than 5 mm) was thought to be related to the presence of STAS. Although the visual appearance of STAS was of ‘tumour islands’ floating in alveolar spaces, the islands were known to be interconnected to each other and attached to the main tumour [14, 15]. Therefore, early lung adenocarcinoma, which consists of mostly non-invasive components, was not expected to be related to STAS. We found neither STAS nor recurrence in any excluded cases of AIS or MIA in our study.
Our hypothesis was supported by the results of the multivariable analysis for risk factors of STAS. Two pathological components of invasive ratio, meaning the proportion of the invasive part of the total tumour size, and micropapillary pattern showed a correlation with STAS. A higher invasive ratio seemed to be correlated with a higher probability of STAS positivity. Only 4 patients (9.85%) in the STAS (+) group had an invasive ratio <0.85, while 29 in the STAS (−) group (42.9%) did. All STAS (+) patients showed a higher than 0.5 invasive ratio (mean ratio 0.96 ± 0.08, range 0.59–1.0), while negative patient ratios were from 0.18 to 1.0 (mean ratio 0.81 ± 0.23). This finding could imply that STAS was important in the early phase of the invasion.
The relevance of STAS and micropapillary patterns has been reported [4, 16]. Micropapillary patterns were referred to as independent risk factors of recurrence before STAS was identified [17–19]. STAS is occasionally suspected as an early form or misinterpretation of micropapillary patterns [4, 17]. The presence of STAS could precede an invasion mechanism, with other invasive features appearing subsequently [20]. Micropapillary patterns and invasive tumour size could be a feature of mature invasion in lung adenocarcinoma. Currently, few question the existence of STAS, and the micropapillary pattern is considered a pathological factor that is intimately related to STAS [21].
We expect certain genetic mutations will be related. Although we found no correlation with any epidermal growth factor receptor mutations in our study as the study of Toyokawa et al. [22], studies report lower rates of epidermal growth factor receptor mutations [23] and higher BRAF [21] and k-ras mutations [2, 23].
Limitations
Our research has several limitations. First, this was a retrospective study at a single institute with a small study population with its inherent selection bias. As a result, pathological stages (IA2, IA3 or IB) which were proved to be obvious prognostic factors showed no statistical significance in our study. Similarly, invasive tumour size showed no prognostic significance for recurrence in multivariable analysis. Also, only 109 patients were not sufficient to prove prognostic power [24] of other histological features including the influence of predominant subtype or differentiation. Moreover, quantitative analysis of STAS could not be performed as we had not measured the amount. The number of STAS sites spreading over the tumour edge has a leading function in recurrence [22], and measuring the quantitative effects of STAS is important.
Our study involved controversies related to ex vivo artefacts. The presence of free-floating tumour cell clusters was possibly due to surgical collapse, smooth muscle contraction of lung specimens or spread through knife surface [25, 26]. However, no definite differentiation standards with artefacts exist, and reporting of proven relationships between free-floating cells in alveolar space and negative long-term outcomes cannot be explained by artefacts alone [21].
Finally, our results were possibly due to inadequate regional lymph node dissection, since the prevalence of local recurrence was significantly associated with nodal status [13]. Although our data showed qualified lymph node dissection (average number of hilar and mediastinal stations was 5, the average number of dissected nodes was 15.4), and only 2 cases of regional lymph node recurrence were found (Supplementary Material, D6), the completeness based on its definition [27] was questionable.
Several issues related to STAS need to be addressed. Factors that contribute to the genesis of STAS from the invasive component of adenocarcinoma need to be identified. Several lung cancer-related molecules and biomarkers including anaplastic lymphoma kinase and ROS1 rearrangement were associated with STAS [3]. The lead-lag relationship between STAS and micropapillary patterns should be examined more precisely. Preoperative or intraoperative identification of STAS will suggest an important clinical turning point.
The presence of STAS was not reported during frozen section analysis at our institute. The reason is largely because our pathologists worried about the presence of spread through the knife surface instead of STAS in frozen procedures; however, this limitation could restrict clinical importance. The identification of STAS during frozen procedures could change surgical methods intraoperatively.
Preoperative identification of STAS might shorten the clinical decision-making process. Preoperative identification would be helpful for determining the extent of surgical resection or a follow-up strategy. Our study found that a consolidative size larger than 1.5 cm on chest CT was highly related to the presence of STAS, which as correlated with the study of Toyokawa et al. [28]. An imaging study using multiplex immunofluorescence reconstructed 3-dimensional images of STAS attached to the alveolar wall adjacent to the main tumour [12]. Rapid advances in microscopic imaging techniques will contribute to preoperative or intraoperative detection of STAS.
CONCLUSION
The presence of STAS was an independent risk factor for recurrence in patients with pathological stage I lung adenocarcinoma (3 cm≤ total size, excluding MIA). Patients with STAS tended to have higher rates of loco-regional recurrence (P = 0.001). Relevant factors for STAS were larger consolidation on chest CT, larger invasive tumour size, higher invasive ratio and the presence of micropapillary patterns.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENT
This study has been approved by the Institutional Review Board of Korea University Anam Hospital (IRB Number; 2019AN0146).
Funding
This research was technically supported by the Core Laboratory for Convergent Medical Research in College of Medicine, Korea University [O1700401]. This research was supported by a grant of Korea University Anam Hospital, Seoul, Republic of Korea [K1924691].
Conflict of interest: none declared.
ABBREVIATIONS
- AIS
Adenocarcinoma in situ
- Chest CT
Chest computed tomography
- MIA
Minimally invasive adenocarcinoma
- STAS
Spread through air spaces
Author contributions
Eunjue Yi: Conceptualization; Funding acquisition; Writing—original draft; Writing—review & editing. Jeong Hyeon Lee: Conceptualization; Formal analysis; Resources; Writing—original draft. Younggi Jung: Data curation; Investigation. Jae Ho Chung: Investigation; Supervision. Youngseok Lee: Data curation; Investigation; Supervision. Sungho Lee: Conceptualization; Formal analysis; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Frank C. Detterbeck, Mohammad Behgam Shadmehr and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Accepted for presentation at the European Society of Thoracic Surgeons Annual Meeting 2020.
REFERENCES
- 1. Lee JS, Kim EK, Kim M, Shim HS. Genetic and clinicopathologic characteristics of lung adenocarcinoma with tumor spread through air spaces. Lung Cancer 2018;123:121–6. [DOI] [PubMed] [Google Scholar]
- 2. Onozato ML, Kovach AE, Yeap BY, Morales-Oyarvide V, Klepeis VE, Tammireddy S et al. Tumor islands in resected early stage lung adenocarcinomas are associated with unique clinicopathological and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao D, Sha J, Cui R, Han S. Advances in research of spreading through air spaces and the effects on the prognosis of lung cancer. Cancer Manag Res 2019;11:9725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Masai K, Sakurai H, Sukeda A, Suzuki S, Asakura K, Nakagawa K et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol 2017;12:1788–97. [DOI] [PubMed] [Google Scholar]
- 6. Eguchi T, Kameda K, Lu S, Bott MJ, Tan KS, Montecalvo J et al. Lobectomy is associated with better outcomes than sublobar resection in spread through air spaces (STAS)-positive T1 lung adenocarcinoma: a propensity score-matched analysis. J Thorac Oncol 2019;14:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yanagawa N, Shiono S, Endo M, Ogata SY. Tumor spread through air spaces is a useful predictor of recurrence and prognosis in stage I lung squamous cell carcinoma, but not in stage II and III. Lung Cancer 2018;120:14–21. [DOI] [PubMed] [Google Scholar]
- 8. Aokage K, Yoshida J, Hishida T, Tsuboi M, Saji H, Okada M et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7–11. [DOI] [PubMed] [Google Scholar]
- 9. Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204–23. [DOI] [PubMed] [Google Scholar]
- 10. Rami-Porta R, Asamura H, Travis WD, Rusch VW. Chapter 3—Lung. In: Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK et al. (eds). AJCC Cancer Staging Manual, 8th edn. Springer, 2017. [Google Scholar]
- 11. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor MD, Nagji AS, Bhamidipati CM, Theodosakis N, Kozower BD, Lau CL et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg 2012;93:1813–20; discussion 1820–1. [DOI] [PubMed] [Google Scholar]
- 13. Isaka M, Kojima H, Takahashi S, Omae K, Ohde Y. Risk factors for local recurrence after lobectomy and lymph node dissection in patients with non-small cell lung cancer: implications for adjuvant therapy. Lung Cancer 2018;115: 28–33. [DOI] [PubMed] [Google Scholar]
- 14. Yagi Y, Aly RG, Tabata K, Barlas A, Rekhtman N, Eguchi T et al. Three-dimensional histologic, immunohistochemical, and multiplex immunofluorescence analyses of dynamic vessel co-option of spread through air spaces in lung adenocarcinoma. J Thorac Oncol 2020;15:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morales-Oyarvide V, Mino-Kenudson M. Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol Int 2016;66:1–7. [DOI] [PubMed] [Google Scholar]
- 16. Yi E, Bae MK, Cho S, Chung JH, Jheon S, Kim K. Pathological prognostic factors of recurrence in early stage lung adenocarcinoma. ANZ J Surg 2018;88:327–31. [DOI] [PubMed] [Google Scholar]
- 17. Miyoshi T, Satoh Y, Okumura S, Nakagawa K, Shirakusa T, Tsuchiya E et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101–9. [DOI] [PubMed] [Google Scholar]
- 18. Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2 cm or smaller. J Natl Cancer Inst 2013;105:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makimoto Y, Nabeshima K, Iwasaki H, Miyoshi T, Enatsu S, Shiraishi T et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (≤20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours). Histopathology 2005;46:677–84. [DOI] [PubMed] [Google Scholar]
- 20. Dai C, Xie H, Su H, She Y, Zhu E, Fan Z et al. Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052–60. [DOI] [PubMed] [Google Scholar]
- 21. Shih AR, Mino-Kenudson M. Updates on spread through air spaces (STAS) in lung cancer. Histopathology 2020;77:173–80. [DOI] [PubMed] [Google Scholar]
- 22. Toyokawa G, Yamada Y, Tagawa T, Kozuma Y, Matsubara T, Haratake N et al. Significance of spread through air spaces in resected pathological stage I lung adenocarcinoma. Ann Thorac Surg 2018;105:1655–63. [DOI] [PubMed] [Google Scholar]
- 23. Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol 2015;39:793–801. [DOI] [PubMed] [Google Scholar]
- 24. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:990–1003. [DOI] [PubMed] [Google Scholar]
- 25. Thunnissen E, Blaauwgeers HJLG, de Cuba EMV, Yick CY, Flieder DB. Flieder DBEx vivo artifacts and histopathologic pitfalls in the lung. Arch Pathol Lab Med 2016;140:212–20. [DOI] [PubMed] [Google Scholar]
- 26. Blaauwgeers H, Flieder D, Warth A, Harms A, Monkhorst K, Witte B et al. A prospective study of loose tissue fragments in non-small cell lung cancer resection specimens: an alternative view to “spread through air spaces”. Am J Surg Pathol 2017;41:1226–30. [DOI] [PubMed] [Google Scholar]
- 27. Rami-Porta R, Wittekind C, Goldstraw P. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25–33. [DOI] [PubMed] [Google Scholar]
- 28. Toyokawa G, Yamada Y, Tagawa T, Kamitani T, Yamasaki Y, Shimokawa M et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg 2018;156:1670–6.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.