Abstract
OBJECTIVES
Primary graft dysfunction after heart transplant is associated with high morbidity and mortality. Extracorporeal membrane oxygenation (ECMO) can be used to wean patients from cardiopulmonary bypass. This study retrospectively reviews a single-centre experience of post-transplant ECMO in regard to outcomes and associated costs.
METHODS
Between May 2006 and May 2019, a total of 267 adult heart transplants were performed. We compared donor and recipient variables, ECMO duration and the incidence of renal failure, bleeding, infection and cost analysis between ECMO and non-ECMO groups.
RESULTS
ECMO support was required postoperatively to manage primary graft dysfunction in 72 (27%) patients. The mean duration of ECMO support was 6 ± 3.2 days. Mean ischaemic times were similar between the groups. There was a significantly higher proportion of ventricular assist device explant to transplant in the ECMO group versus non-ECMO (38.2% vs 14.1%; P < 0.0001). ECMO patients had a longer duration of stay in the intensive care unit (P < 0.0001) and total hospital stay (P < 0.0001). Greater mortality was observed in the ECMO group (P < 0.0001). The median cost of providing ECMO was £18 000 [interquartile range (IQR): £12 750–£24 000] per patient with an additional median £35 225 (IQR: £21 487.25–£51 780.75) for ITU stay whilst on ECMO. The total median cost per patient inclusive of hospital stay, ECMO and dialysis costs was £65 737.50 (IQR: £52 566.50–£95 221.75) in the non-ECMO group compared to £145 415.71 (IQR: £102 523.21–£200 618.96) per patient in the ECMO group (P < 0.0001).
CONCLUSIONS
Patients with primary graft dysfunction following heart transplantation who require ECMO are frequently bridged to a recovery; however, the medium and longer-term survival for these patients is poorer than for patients who do not require ECMO.
Keywords: Extracorporeal membrane oxygenation, Post-heart transplant, Primary graft dysfunction, Cardiopulmonary bypass, Ventricular assist device
INTRODUCTION
Heart transplantation is an established treatment option for patients with end-stage heart failure. In the UK, only 27% of brain stem death hearts are used for transplantation, whilst 43% of patients on the waiting list die or become too sick for surgery [1]. To meet this recipient demand, the organs from older donors or those with comorbidities are increasingly being accepted. However, such organs have been associated with poorer immediate function [2]. Primary graft dysfunction (PGD) following heart transplantation is a well-recognized complication with the incidence ranging from 1.0% to 31% and contributing to 3–75% of mortality depending on severity of dysfunction [3–5]. The International Society for Heart and Lung Transplantation 2014 consensus statement defined PGD as severe dysfunction of the cardiac allograft without obvious anatomic or immunologic cause and is characterized by hypotension, high filling pressures and refractory low-cardiac output. PGD covers right heart dysfunction without evidence of a high afterload, isolated left ventricular dysfunction and biventricular dysfunction [6]. A recent UK national study demonstrated that an incidence of 30-day mortality for PGD patients following heart transplantation was 19% and 4.5% for non-PGD [5].
Extracorporeal membrane oxygenation (ECMO) has been reported to be effective for use in patients following myocardial infarction, cardiac arrest, the development of refractory cardiogenic shock and also for weaning from cardiopulmonary bypass in post-cardiotomy cardiogenic shock [7–9]. ECMO has been reported by other groups to be an excellent tool to salvage patients with cardiorespiratory failure following heart transplantation and is associated with better clinical outcomes than the use of temporary ventricular assist devices (VADs) in such circumstances [10, 11]. We report our single-centre experience of using ECMO as a method of support after heart transplantation for patients with PGD. We compare clinical characteristics and survival outcomes of patients requiring postoperative ECMO with those patients without ECMO requirement following heart transplantation. We have also compared the total cost of post-transplant care between those patients who did and did not require ECMO.
METHODS
Between May 2006 and May 2019, a total of 267 adult Heart transplant (HTX) were performed in our unit. During this period, 72 (27.0%) recipients required ECMO support postoperatively for severe PGD. One patient underwent retransplantation due to severe PGD where the heart never recovered and subsequently also required ECMO support following the retransplant. ECMO is used following heart transplantation in our centre when the patient has failed to satisfactorily wean from cardiopulmonary bypass despite optimization of all parameters and a generous period of reperfusion and the, use of 2 inotropic drugs (milrinone, dopamine, epinephrine and norepinephrine) with intra-aortic balloon pump (IABP) support and inhaled nitric oxide. All direct and indirect costs were calculated using cost–utility analysis. The unit cost of all equipment, disposables, intensive care unit cost, theatre cost, hospital cost, complications and outcomes data were collected from the hospital finance department. All treatment costs were incurred over a period of time, so costs and benefits were discounted. However, we have not used cost-effectiveness or cost–benefit analysis for this study because we do not have any quality of life SF-36 data to analyse Quality Adjusted Life in Years.
Extracorporeal membrane oxygenation technique and management
We utilized a central or a peripheral cannulation technique to deliver venoarterial ECMO. In the central ECMO technique, the aortic cannula (Medtronic EOPA®, Minneapolis, MN, USA— Elongated One-Piece Arterial) was tunnelled under the sternum and brought out below the costal margin. The venous cannula was tunnelled under the xiphisternum on the right side using a malleable (Medtronic) size 32-Fr cannula inserted into the right atrium. In the peripheral ECMO technique, a right axillary artery with 8 mm Polytetrafluoroethylene (PTFE) side graft was used for arterial return and a heparin-coated wire-reinforced long-term femoral venous pipe (Maquet®, Gothenburg, Sweden, HLS Cannula with Bioline coating) was used for drainage. The ECMO circuit consisted of a magnetically levitated centrifugal pump (Abbott®, Plymouth, MN, USA, CentriMag™) and polymethyl pentene oxygenators (Paragon™, Cambridge, MA, USA).
The heart was partially rested as soon as the ECMO support was commenced. Most of the inotropic support was weaned and only dopamine at 5 µg/kg/min and IABP support was maintained. The lungs were ventilated normally and the ECMO was run lower than the patients’ full flow to maintain some ventricular ejection. This was also important to maintain flow through the pulmonary vasculature to prevent stasis and reduce the risk of pulmonary venous and left atrial thrombus formation. End tidal CO2 levels and Swan Ganz catheter transduced ejection trace were used as an evidence of reasonable pulmonary blood flow. Heparinization was reversed with protamine and after thorough haemostasis, the chest was closed. In the immediate postoperative period, activated clotting time levels were checked every 4–6 h and heparin was usually not started until the bleeding risk was minimal and the activated clotting time level fell below 180 s. Transoesophageal echo assessment of recovery together with an improved arterial pressure trace and stable blood pressure with a flow of under 2.8 l/min were our indications for a trial of ECMO weaning and potentially decannulation and chest closure. According to English transplant legislation, retrospective studies are a part of transplant outcomes assessment and do not require ethics committee approval. All the cost data such as total hospital stay post-transplant, total days in the ITU, use of blood products and dialysis were obtained from the hospital finance department.
Advantages
Use of ECMO has a number of advantages in this circumstance, as it involves inserting only one additional cannula in the right atrium and the same aortic pipe used for the operation can be tunnelled to provide the ECMO return. More recently, we have started using heparin-coated wire-reinforced, long-term femoral venous pipe (Maquet® HLS Cannula with Bioline coating) for cardiopulmonary bypass for the operation which can be converted to provide ECMO support at the end of operation if needed. To provide better mobilization postoperatively, we now often use right axillary artery with 8 mm side graft for ECMO instead of central aortic cannula.
ECMO supports cardiorespiratory function and helps to completely rest the heart in a way that is not possible if only a left ventricular assist device or right ventricular (RV) assist device is used because ECMO allows inotropic agents that are potentially harmful during the ischaemia–reperfusion period to be weaned. Finally, when an RV assist device is used for a presumed donor RV dysfunction, the underlying ventricular dysfunction can be unmasked, thereby needing left ventricular assist device support. Taghavi et al. [12] have reported that the ECMO use in suspected RV dysfunction is superior to RV assist device in these groups of patients.
The centrifugal pump (CentriMag™, Abbott Laboratories, IL, USA) used in the circuit is a safe device and has an excellent record in the published data regarding its use as for short-term mechanical support [13, 14]. We have not seen any device-related failures in any of these patients even when they were not anticoagulated, and the incidence of thrombo-embolic complications is very low.
Disadvantages
The major disadvantage of ECMO is the maximum duration of 7 days for which a patient can be supported on this device. The oxygenator requires frequent changes while the CentriMag™ pump lasts almost a month. However, most patients need less than a week of ECMO support and our median duration of ECMO post-heart transplantation is 6 days. The cost and ITU resources can also limit the period of support which can be provided at an institution. As ECMO bypasses both ventricles, the incidence of acute lung injury increases with longer duration of support. This has been reported previously in literature by Boulate et al. [15]. As the pulmonary blood flow reduces due to the bypassing effect, the incidence of lung collapse increases, and infiltrates start to appear on chest X-ray. In severe cases, venovenous ECMO may be required to support acute lung injury when the heart is ready to be weaned off venoarterial ECMO. Therefore, if the donor heart function does not improve by the end of 1 week, then we prefer to convert them to Biventricular assist device (BIVAD) to avoid pulmonary complications. We hypothesize that the reduction in pulmonary blood flow might reduce the alveolar surfactant levels that might contribute the collapse and consolidation of the lungs. We closely monitored end tidal CO2 levels from the ventilator and pulmonary artery trace on Swan-Ganz catheter as a measure of pulmonary blood flow. Further studies will be required to assess the relationship of surfactants on patients with ECMO.
Outcome measures
The primary outcome measure was to determine the incidence of ECMO for PGD in our unit and assess recent trends in use. Secondary outcomes would be the 30-day and 1-year survival, complication rates and length of ITU and hospital stay in ECMO- and non-ECMO-supported patients.
Statistical analysis
Data distribution was determined by the Shapiro–Wilk test. Demographic data are expressed as descriptive values using mean ± standard deviation or median (min–max) as appropriate depending upon data distribution. Demographic differences between the groups were assessed by Fisher’s exact test or unpaired T test. The outcome measures of incidence of renal failure, bleeding, re-exploration, infection and 30-day/1-year survival were analysed using Fisher’s exact test. The difference in duration of stay in ITU or total hospital stay between the groups was determined by the Mann–Whitney test. Survival curve comparison between groups was performed using the Mantel–Cox test. ECMO-associated costs as well as costs for in-hospital stay were recorded and comparisons between groups were performed by Mann–Whitney test. All statistical analyses were performed using Prism version 7 software.
RESULTS
During the study period, we performed 267 heart transplants, with 72 patients requiring the use of ECMO. A total of 4 patients were excluded, as ECMO was instituted as a salvage procedure due to (i) major air embolism, (ii) superior vena cava obstruction leading to severe intra-cranial oedema, (iii) delayed rescue ECMO for sudden cardiac arrest 28 days after heart transplant and (iv) VV ECMO only for respiratory support rather than as a result of PGD. The incidence of PGD requiring mechanical support varied from 0% to 46% during the 13 years. In the last few years, there has been an increase in the incidence of PGD requiring ECMO support and this could be attributed to the significantly increased number of transplants performed following VAD explant in recent years (P = 0.0005, analysed as proportion of transplants performed following VAD explant vs transplant alone). Between 2016 and 2019, 29–46% of patients received ECMO support post-heart transplantation at our centre (Fig. 1). Most demographics variables were found to be similar except true-recipient blood group and VAD pretransplant were significantly different between groups (Table 1). No differences were discovered between ischaemic times between those who required ECMO and those who did not (P = 0.40). Patient requiring ECMO had neither older donors nor were older themselves at the time of surgery (P = 0.77 and P = 0.99, respectively).
Figure 1:
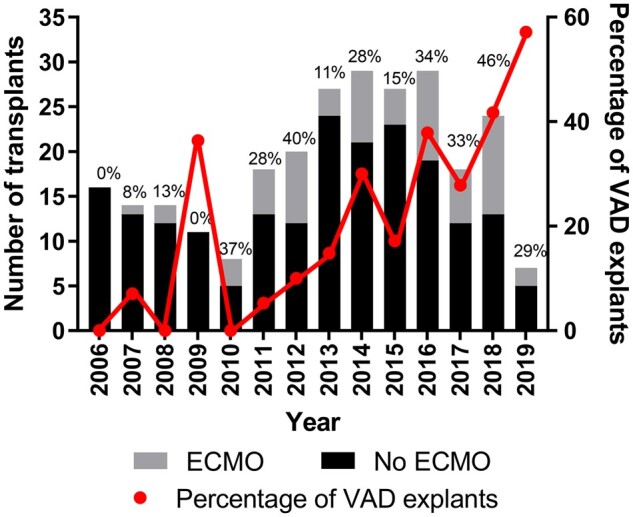
The number of patients received ECMO and VAD explants. ECMO: extracorporeal membrane oxygenation; VAD: ventricular assist device.
Table 1:
Basic demographics
| No ECMO (n = 199) | ECMO (n = 68) | P-value | |
|---|---|---|---|
| Donor sexa, n (%) |
Male: 131 (65.8) Female: 68 (34.2) |
Male: 44 (64.7) Female: 23 (33.8) |
>0.99 |
| Donor age (years)a | 37.5 (12.0), 15–59 | 37.0 (12.6), 16–61 | 0.77 |
| Donor BMI (kg/m2)a | 25.6 (4.1), 16.7–40.5 | 25.7 (4.9), 16.8–45.2 | 0.87 |
| Donor cause of death | |||
| Hypoxia | 115 | 41 | Not Available |
| Tumour | 5 | 4 | |
| Vascular problems | 4 | 2 | |
| Hypoxia | 22 | 10 | |
| Trauma | 40 | 7 | |
| Cardiac arrest (out of hospital) | 3 (2) | 3 (2) | |
| Infection | 10 | 1 | |
| Donor blood groupa, n (%) | |||
| O (−/+) | 22 (11.2)/80 (40.8) | 5 (7.4)/32 (47.1) | 0.66 |
| A (−/+) | 13 (6.6)/63 (32.1) | 3 (4.4)/19 (27.9) | |
| B (−/+) | 0 (0.0)/15 (7.7) | 2 (2.9)/6 (8.8) | |
| AB (−/+) | 1 (0.5)/2 (1.0) | 1 (1.5)/0 (0.0) | |
| Donor-recipient weight mismatch | 33.5% | 23.4% | 0.13 |
| Donor-recipient height mismatch | 1.2% | 0.0% | >0.99 |
| Ischaemic time (min) | 197 (49.7), 57–332 | 191 (53.6), 50–313 | 0.40 |
| Recipient sexa, n (%) |
Male: 151 (75.9) Female: 48 (24.1) |
Male: 54 (79.4) Female: 13 (19.1) |
0.50 |
| Recipient age (years)a | 46.3 (13.6), 15–66 | 46.8 (11.6), 18–66 | 0.79 |
| Recipient BMI (kg/m2)a | 25.7 (3.8), 16.7–34.4 | 26.4 (3.6), 19.2–32.3 | 0.19 |
| Recipient blood groupa, n (%) | |||
| O (−/+) | 9 (4.5)/64 (32.2) | 5 (7.5)/27 (40.9) | 0.03 |
| A (−/+) | 20 (10.1)/74 (37.2) | 6 (9.1)/14 (21.2) | |
| B (−/+) | 3 (1.5)/21 (10.6) | 2 (3.0)/5 (7.6) | |
| AB (−/+) | 1 (0.5)/7 (3.5) | 0 (0.0)/7 (10.6) | |
| Recipient primary diagnosis | |||
| Dilated cardiomyopathy | 38 | 118 | NA |
| Hypertrophic cardiomyopathy | 3 | 8 | |
| Viral cardiomyopathy | 1 | 2 | |
| Chemotherapy induced cardiomyopathy | 3 | 4 | |
| Restrictive cardiomyopathy | 0 | 8 | |
| Arrhythmogenic cardiomyopathy | 8 | 5 | |
| Cardiogenic shock | 0 | 1 | |
| Danon disease | 0 | 1 | |
| Fabry’s disease | 0 | 1 | |
| Ventricular failure | 8 | 22 | |
| Retransplant | 1 | 1 | |
| Viral myocarditis | 2 | 2 | |
| Ischaemic heart disease | 3 | 25 | |
| Takayasu disease | 1 | 0 | |
| Wolff–Parkinson white syndrome | 0 | 1 | |
| Mean PA pressure (mmHg) | 29.0 (9.6), 7–59 | 29.4 (8.0), 8–41 | 0.76 |
| VAD pretransplant (%) | 14.1% | 38.2% | <0.0001 |
| Time on waiting list (days) | 161 (288), 0–1798 | 244 (564), 0–3522 | 0.12 |
| Duration of ECMO (days) | NA | 6 (3.2), 1–22b | NA |
| Duration of VAD (days) | NA | 34 (22.0), 11–69 | NA |
Results presented as mean (standard deviation), minimum–maximum.
Data available for 221/223 transplants—data missing for 1 donor/recipient pair in each group.
Duration across 2 separate ECMO periods.
ECMO: extracorporeal membrane oxygenation; VAD: ventricular assist device.
Extracorporeal membrane oxygenation support
The mean duration of ECMO support was 6 ± 3.2 days. There was a significantly higher proportion of VAD explants in the ECMO group compared to non-ECMO group (P < 0.0001). The ECMO patients had significantly longer ITU stay with a median duration of 26 (min–max: 1–201) days as compared to the non-ECMO group who had median duration of stay of 8 (min–max: 2–85 days; P < 0.0001). The total duration of hospital stay was also significantly longer for ECMO recipients with a median in-hospital stay of 44 (min–max: 2–233) days compared to 27 (min–max: 16–189) days in the non-ECMO group (P < 0.0001) (Table 2).
Table 2:
Postoperative outcomes
| No ECMO | ECMO | Odds ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Renal failure, n (%) | 83 (42.3) | 58 (87.9) | 9.9 (4.5–20.8) | <0.0001 |
| Bleeding, n (%) | 33 (16.8) | 52 (80.0) | 19.8 (9.7–38.5) | <0.0001 |
| Re-exploration, n (%) | 34 (17.3) | 51 (78.5) | 19.4 (8.1–42.6) | <0.0001 |
| Infection, n (%) | 68 (34.7) | 46 (75.4) | 6.5 (3.0–14.6) | <0.0001 |
| Median Critical care unit (CCU) stay [days (min–max)] | 8 (2–85) | 26 (1–201) | <0.0001 | |
| Median hospital stay [days (min–max)] | 27 (16–189) | 44 (2–233) | <0.0001 | |
| 30-day survival, n (%) | 193 (98.5) | 53 (84.1) | 0.1 (0.02–0.3) | <0.0001 |
| 1-year survival, n (%) | 189 (95.9) | 47 (74.6) | 0.1 (0.1–0.3) | <0.0001 |
| Recipient cause of death (3-year post-transplant) | 35/199 | 25/68 | NA | |
| Pulmonary embolism | 2 | 2 | ||
| Myocardial infarction | 6 | 2 | ||
| Cardiac failure | 6 | 2 | ||
| Acute respiratory distress syndrome | 3 | 1 | ||
| Renal failure | 0 | 1 | ||
| Multiorgan failure | 2 | 9 | ||
| Cancer | 4 | 1 | ||
| Sepsis | 2 | 2 | ||
| Donor heart failure | 3 | 1 | ||
| Acute rejection | 2 | 3 | ||
| Unknown | 5 | 1 | ||
CI: confidence interval; ECMO: extracorporeal membrane oxygenation.
Extracorporeal membrane oxygenation complications
In addition to a longer ITU and total hospital stay, the ECMO patients also developed more complications. ECMO patients more frequently developed renal failure (87.9% vs 42.3%, P < 0.0001), experienced more postoperative bleeding (80.0% vs 16.8%, P < 0.0001), had a higher re-exploration rate (78.5% vs 17.3%, P < 0.0001) and a higher rate of infection (75.4% vs 34.7%, P < 0.0001) when compared with the non-ECMO group (Table 2).
Survival
The 30-day survival was 84.1% and 98.5% in ECMO and non-ECMO groups, respectively (P < 0.0001). The 1-year survival for the ECMO group is 74.6% and non-ECMO group is 95.9% (P < 0.0001) (Table 2). Survival for each group to the time of analysis was determined (41.7% vs 63.0% for ECMO vs non-ECMO, respectively, log-rank test P < 0.0001, Fig. 2). Recipients’ cause of death is listed in Table 2.
Figure 2:
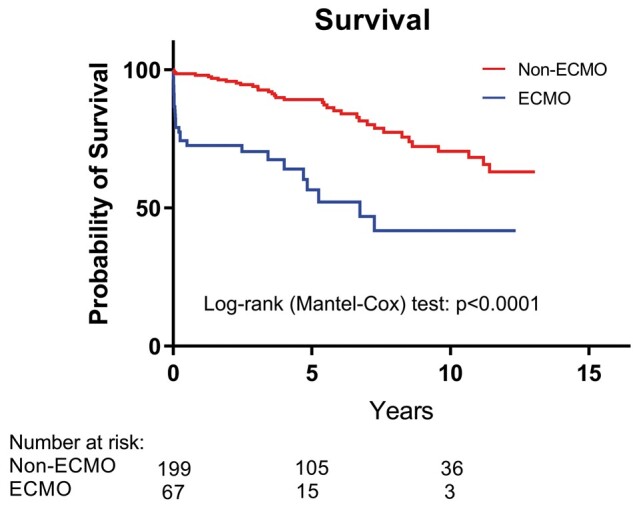
The survival analysis between patients who received ECMO and non-ECMO interventions. ECMO: extracorporeal membrane oxygenation.
Economic analysis
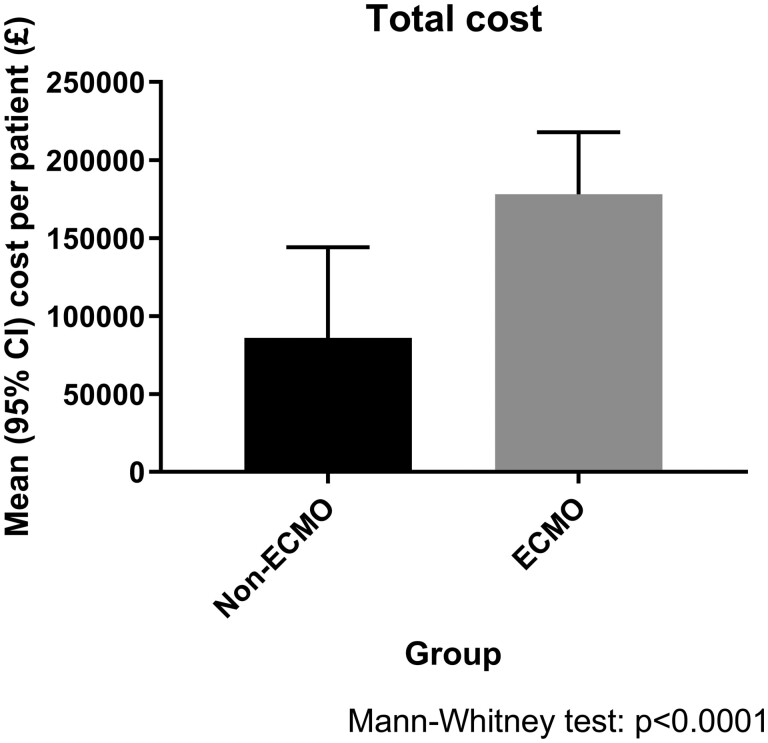
The mean cost of providing ECMO was £18 250 ± 7396 per patient with an additional £49 637.90 for ITU stay whilst on ECMO. The mean cost for renal dialysis was calculated at £201.10 per patient in the non-ECMO group and £427.10 per patient in the ECMO group (P < 0.0001), reflecting the greater requirement for dialysis. The mean cost for total hospital stay post-transplant was determined as £66 784 per patient in the non-ECMO group compared to £104 240 per patient in the ECMO group (P < 0.0001). Blood products, including red blood cells, platelets, fresh frozen plasma and cryoprecipitate, were a mean (±standard deviation) additional cost in the ECMO group of £2695.66 ± £2360.42 per patient. The total mean cost per patient inclusive of hospital stay, ECMO and dialysis costs was £85 944 ± 58 180 in the non-ECMO group compared to £178 056 ± 137 026 per patient in the ECMO group (P < 0.0001, Fig. 3).
Figure 3:
The total cost between ECMO and non-ECMO patients. CI: confidence interval; ECMO: extracorporeal membrane oxygenation
DISCUSSION
PGD after heart transplantation is a serious life-threatening and a devastating complication requiring significant intervention. This retrospective study shows that there is an increased incidence of ECMO using CentriMag™ short-term pump in salvaging patients who develop PGD after HTX and is now over 40% in our centre. The role of ECMO for such patients has been previously reported in literature [10, 11, 16–20].
In the UK, there has been an increase in the incidence of PGD after heart transplantation similar to what we have seen in our institution [5]. There are a number of possible reasons for this increase which includes an increase in mean donor age and greater incorporation of marginal donors into the donor pool. The UK national annual report [21] on mechanical circulatory support 2016–17, reported that during 2016/2017, there were 33 implantations of ECMO/VAD for PGD, which was 3 times higher than in 2007/2008. The combination of marginal donor hearts with complex recipients with various comorbidities and urgent in patients increases the risk of PGD [21, 22]. We have similarly found increased incidence of PGD in our population in recent years.
Our local practice for PGD management is to avoid stressing the heart with increasing levels of inotropic support. Instead, we have a reduced threshold for initiation of ECMO support. We only use low-dose epinephrine (up to 0.06 µg/kg/min) in our unit and if the heart fails to wean from bypass on inodilator (phosphodiesterase inhibitors), dopamine (up to 5 µg/kg/min) and IABP support despite resting and reperfusion on cardiopulmonary bypass for a period, then we initiate ECMO. This may explain a higher ECMO rate in our unit compared to other centres in the UK.
Ventricular assist device versus extracorporeal membrane oxygenation for primary graft dysfunction
Takeda et al. [11] compared venoarterial ECMO and temporary VAD support for severe forms of PGD requiring mechanical circulatory support. Out of 597 patients who received a heart transplant, severe PGD developed in 44 patients (7.4%), and they received a continuous-flow external VAD (n = 17) or ECMO (n = 27) support within 24 h after transplant. They reported that implantation of the temporary VAD required longer cardiopulmonary bypass time compared with ECMO (323 ± 86 min vs 216 ± 65 min, P < 0.0001). Patients who received a VAD were more likely to have longer support time (14 ± 17 days vs 5.2 ± 3.9 days, P = 0.011), a higher incidence of major bleeding requiring chest re-exploration (77% vs 30%, P = 0.0047), and a higher incidence of renal failure requiring renal replacement therapy (53% vs 11%, P = 0.0045) after surgery. Overall hospital mortality was 27%. In-hospital mortality for VAD and ECMO patients was 41% and 19%, respectively (P = 0.16). They reported that 10 patients (59%) were weaned from VAD support, and 24 (89%) were weaned from ECMO support after adequate graft function recovery (P = 0.03). The 3-year post-transplant survival was 41% in the VAD group and 66% in the ECMO group (P = 0.13). They concluded that for severe PGD, support with ECMO appeared to result in better clinical outcomes than VAD support [10].
Cost–benefit
ECMO is a useful option for salvaging patients who develop PGD. If used at the appropriate time, the majority of patients can be successfully weaned off the support with reasonable 30-day and 1-year survival [10, 15]. However, it is an invasive device and does carry high morbidity as shown in our study. It significantly increases bleeding requiring re-exploration, increases the need for blood transfusion, haemodialysis for renal failure, increases infections and increases both ITU and total hospital stay. It is an expensive modality of treatment; however, it can save lives. As ECMO patients stay longer in the ITU, this leads to cancellation of standard adult cardiac surgery activity due to lack of ITU bed capacity. This additional has an indirect impact on hospital finances, as this is a lost activity for the hospital. We have not included lost activity cost in our financial calculations, but it should be noted that the cost would be higher if this is included.
Most of our ECMO-salvaged survivors are currently in New York Heart Association class 1 and are living a good quality of life (from clinic review). At present, post-transplant ECMO support is not commissioned, and this is an expense borne by the hospitals; therefore, future commissioning for heart transplantation should incorporate the need for ECMO insertion and its associated costs must be reimbursed.
Limitations
This is a retrospective study and we only included patients who have suffered severe PGD requiring ECMO and compared with non-ECMO group. Unfortunately, we do not have data on patients who have suffered lesser degree of PGD requiring IABP and/or prolonged inotropic support. The financial analysis was based on reviewing ITU charts and the number of indirect effects of prolonged ITU stay has not been calculated. Therefore, the actual costs could be much higher in the ECMO group. We have only added cost analysis for ECMO patients who had blood products. We have not included non-ECMO patients’ blood product usage because we routinely do not collect these in our department.
CONCLUSION
In conclusion, ECMO support, although expensive, is an excellent tool to salvage patients who develop severe PGD post-heart transplantation. Without this, the actual mortality would be even higher in this group of patients. Our results with post-transplant ECMO are consistent with other studies in the literature [11, 23]. It is associated with increased morbidity and complications as well as poorer early and 1-year survival rates. Therefore, efforts must be taken to reduce the incidence of severe PGD. This includes adapting newer technology related to organ preservation, ex vivo perfusion and transportation.
Conflict of interest: none declared.
Author contributions
Bhuvaneswari Krishnamoorthy: Conceptualization; Methodology; Writing—original draft; Writing—review & editing. Vipin Mehta: Writing—review & editing. William Critchley: Formal analysis; Writing—review & editing. Paul Callan: Writing—review & editing. Steve Shaw: Data curation; Writing—review & editing. Rajamiyer Venkateswaran: Conceptualization; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Christoph Knosalla and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- ECMO
Extracorporeal membrane oxygenation
- IABP
Intra-aortic balloon pump
- IQR
Interquartile range
- PGD
Primary graft dysfunction
- RV
Right ventricular
- VAD
Ventricular assist device
REFERENCES
- 1. Page A, Messer S, Large SR.. Heart transplantation from donation after circulatory determined death. Ann Cardiothorac Surg 2018;7:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine 1999. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, (DC): The National Academies Press. 10.17226/9628. [DOI] [PubMed]
- 3. Nicoara A, Ruffin D, Cooter M, Patel CB, Thompson A, Schroder JN. et al. Primary graft dysfunction after heart transplantation: incidence, trends, and associated risk factors. Am J Transplant 2018;18:1461–70. [DOI] [PubMed] [Google Scholar]
- 4. DePasquale EC, Ardehali A.. Primary graft dysfunction in heart transplantation. Curr Opin Organ Transplant 2018;23:286–94. [DOI] [PubMed] [Google Scholar]
- 5. Avtaar Singh SS, Banner NR, Rushton S, Simon AR, Berry C, Al-Attar N.. ISHLT primary graft dysfunction incidence, risk factors, and outcome: a UK national study. Transplantation 2019;103:336–43. [DOI] [PubMed] [Google Scholar]
- 6. Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F, Luu M. et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014;33:327–40. [DOI] [PubMed] [Google Scholar]
- 7. Pabst D, Foy AJ, Peterson B, Soleimani B, Brehm CE.. Predicting survival in patients treated with extracorporeal membrane oxygenation after myocardial infarction. Crit Care Med 2018;46:e359–e63. [DOI] [PubMed] [Google Scholar]
- 8. Chonde M, Sappington P, Kormos R, Althouse A, Boujoukos A.. The use of ECMO for the treatment of refractory cardiac arrest or postarrest cardiogenic shock following in-hospital cardiac arrest: a 10-year experience. J Intensive Care Med 2019;34:615–21. [DOI] [PubMed] [Google Scholar]
- 9. Khorsandi M, Dougherty S, Bouamra O, Pai V, Curry P, Tsui S. et al. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg 2017;12:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bermudez CA, McMullan DM.. Extracorporeal life support in preoperative and postoperative heart transplant management. Ann Transl Med 2017;5:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda K, Li B, Garan AR, Topkara VK, Han J, Colombo PC. et al. Improved outcomes from extracorporeal membrane oxygenation versus ventricular assist device temporary support of primary graft dysfunction in heart transplant. J Heart Lung Transplant 2017;36:650–56. [DOI] [PubMed] [Google Scholar]
- 12. Taghavi S, Zuckermann A, Ankersmit J, Wieselthaler G, Rajek A, Laufer G. et al. Extracorporeal membrane oxygenation is superior to right ventricular assist device for acute right ventricular failure after heart transplantation. Ann Thorac Surg 2004;78:1644–9. [DOI] [PubMed] [Google Scholar]
- 13. Takeda K, Garan AR, Ando M, Han J, Topkara VK, Kurlansky P. et al. Minimally invasive CentriMag ventricular assist device support integrated with extracorporeal membrane oxygenation in cardiogenic shock patients: a comparison with conventional CentriMag biventricular support configuration. Eur J Cardiothorac Surg 2017;52:1055–61. [DOI] [PubMed] [Google Scholar]
- 14. Borisenko O, Wylie G, Payne J, Bjessmo S, Smith J, Yonan N. et al. Thoratec CentriMag for temporary treatment of refractory cardiogenic shock or severe cardiopulmonary insufficiency: a systematic literature review and meta-analysis of observational studies. ASAIO J 2014;60:487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boulate D, Luyt CE, Pozzi M, Niculescu M, Combes A, Leprince P. et al. Acute lung injury after mechanical circulatory support implantation in patients on extracorporeal life support: an unrecognized problem. Eur J Cardiothorac Surg 2013;44:544–9. [DOI] [PubMed] [Google Scholar]
- 16. Lima EB, Cunha CR, Barzilai VS, Ulhoa MB, Barros MR, Moraes CS. et al. Experience of ECMO in primary graft dysfunction after orthotopic heart transplantation. Arq Bras Cardiol 2015;105:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su JA, Kelly RB, Grogan T, Elashoff D, Alejos JC.. Extracorporeal membrane oxygenation support after pediatric orthotopic heart transplantation. Pediatr Transplant 2015;19:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim JH, Hwang HY, Yeom SY, Cho HJ, Lee HY, Kim KB.. Percutaneous extracorporeal membrane oxygenation for graft dysfunction after heart transplantation. Korean J Thorac Cardiovasc Surg 2014;47:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kittleson MM, Patel JK, Moriguchi JD, Kawano M, Davis S, Hage A. et al. Heart transplant recipients supported with extracorporeal membrane oxygenation: outcomes from a single-center experience. J Heart Lung Transplant 2011;30:1250–6. [DOI] [PubMed] [Google Scholar]
- 20. Listijono DR, Watson A, Pye R, Keogh AM, Kotlyar E, Spratt P. et al. Usefulness of extracorporeal membrane oxygenation for early cardiac allograft dysfunction. J Heart Lung Transplant 2011;30:783–9. [DOI] [PubMed] [Google Scholar]
- 21.Report NBaTA. Annual Report on Mechanical Circulatory Support Related to Heart Transplantation. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/6543/nhsbt-annual-report-on-mcs-201617.pdf (20 May 2020, date last accessed).
- 22. See SB, Clerkin KJ, Kennel PJ, Zhang F, Weber MP, Rogers KJ. et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant 2017;36:862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Alessandro C, Aubert S, Golmard JL, Praschker BL, Luyt CE, Pavie A. et al. Extra-corporeal membrane oxygenation temporary support for early graft failure after cardiac transplantation. Eur J Cardiothorac Surg 2010;37:343–9. [DOI] [PubMed] [Google Scholar]