Abstract
OBJECTIVES
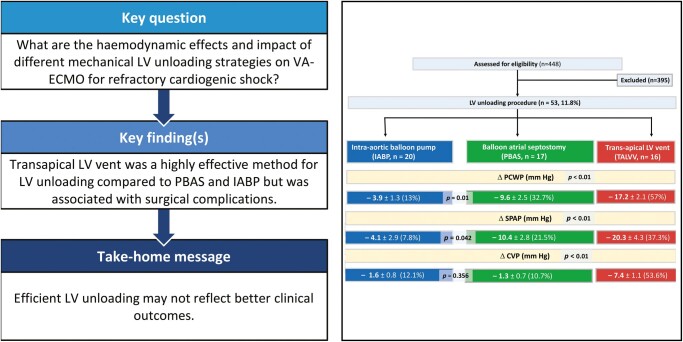
Our goal was to compare the haemodynamic effects of different mechanical left ventricular (LV) unloading strategies and clinical outcomes in patients with refractory cardiogenic shock supported with venoarterial extracorporeal membrane oxygenation (VA-ECMO).
METHODS
A total of 448 patients supported with VA-ECMO for refractory cardiogenic shock between 1 March 2015 and 31 January 2020 were included and analysed in a single-centre, retrospective case–control study. Fifty-three patients (11.8%) on VA-ECMO required LV unloading. Percutaneous balloon atrial septostomy (PBAS), intra-aortic balloon pump (IABP) and transapical LV vent (TALVV) strategies were compared with regards to the composite rate of death, procedure-related complications and neurological complications. The secondary outcomes were reduced pulmonary capillary wedge pressure, pulmonary artery pressure, central venous pressure, left atrial diameter and resolution of pulmonary oedema on a chest X-ray within 48 h.
RESULTS
No death related to the LV unloading procedure was detected. Reduction in pulmonary capillary wedge pressure was highest with the TALVV technique (17.2 ± 2.1 mmHg; P < 0.001) and was higher in the PBAS than in the IABP group; the difference was significant (9.6 ± 2.5 and 3.9 ± 1.3, respectively; P = 0.001). Reduction in central venous pressure with TALVV was highest with the other procedures (7.4 ± 1.1 mmHg; P < 0.001). However, procedure-related complications were significantly higher with TALVV compared to the PBAS and IABP groups (50% vs 17.6% and 10%, respectively; P = 0.015). We observed no significant differences in mortality or neurological complications between the groups.
CONCLUSIONS
Our results suggest that TALVV was the most effective method for LV unloading compared with PBAS and IABP for VA-ECMO support but was associated with complications. Efficient LV unloading may not improve survival.
Keywords: Venoarterial extracorporeal membrane oxygenation, Left ventricular unloading, Percutaneous balloon atrial septostomy, Transapical left ventricular vent
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides immediate bedside cardiopulmonary and circulatory support for refractory cardiogenic shock (RCS) [1–3].
INTRODUCTION
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides immediate bedside cardiopulmonary and circulatory support for refractory cardiogenic shock (RCS) [1–3]. Because clinical indications are expanding, VA-ECMO provides a rescue option for bridging patients to decision and to long-term definitive therapies such as implantable circulatory support, a transplant or, rarely, recovery of native cardiac function [3–5]. However, VA-ECMO support is only temporary; it is limited to days or weeks with potentially adverse effects on the left heart [6, 7].
In patients with poor cardiac reserve, increased left ventricular (LV) afterload, wall stress and myocardial oxygen consumption may lead to LV distension and acute lung injury [6]. Residual flow through the pulmonary circuit, bronchial circulation to the left atrium, coronary circulation and Thebesian drainage may all contribute to LV overload [4]. Furthermore, circulatory stagnation within the left ventricle, left atrium or aortic root may result in intracardiac or aortic root thrombus formation. The incidence of LV distention requiring unloading in VA-ECMO-supported cases has been reported to be 20–30% [8, 9].
LV overload in patients with VA-ECMO support can be diagnosed with worsening pulmonary oedema, elevated pulmonary arterial diastolic pressure greater than 25 mmHg, echocardiographic evidence of LV distention, LV/left atrial (LA) thrombosis and refractory ventricular arrhythmias [8]. Although different techniques have been described for treating LV decompression in patients with VA-ECMO support [7], evidence for ideal solutions in guidelines is missing. The most commonly used method is the intra-aortic balloon pump (IABP), which restores pulsatility and decreases LV afterload during VA-ECMO [10, 11]. Other effective strategies for LV unloading include transapical LV venting (TALVV) [9, 12], transaortic LV venting [13, 14], left atrial decompression by minithoracotomy [15] and percutaneous or surgical pulmonary artery venting [16, 17]. Use of an axial flow catheter, namely the Abiomed Impella® (Abiomed, Danvers, MA, USA), and left atrial to femoral artery bypass, Tandem Heart™ (Tandem Life, Pittsburgh, PA, USA) devices are not available in our institution. Emerging transcatheter techniques are percutaneous transseptal cannula placement [18, 19] and left atrial decompression by percutaneous blade or balloon atrial septostomy (PBAS) [20–23].
In this single-centre study, our goal was to compare the efficiency of LV unloading and outcomes of different LV decompression strategies, namely PBAS, IABP and TALVV for left heart decompression in patients with RCS supported by VA-ECMO.
PATIENTS AND METHODS
Setting
The institutional ethics committee of the Ankara University School of Medicine approved this study (2020/I5-319-20). Before the procedures, patients were informed about procedural risks and therapeutic benefits. Written informed consent was obtained from all patients or their relatives. Our research was conducted according to the ethical principles of the World Medical Association Declaration of Helsinki.
Study population and protocol
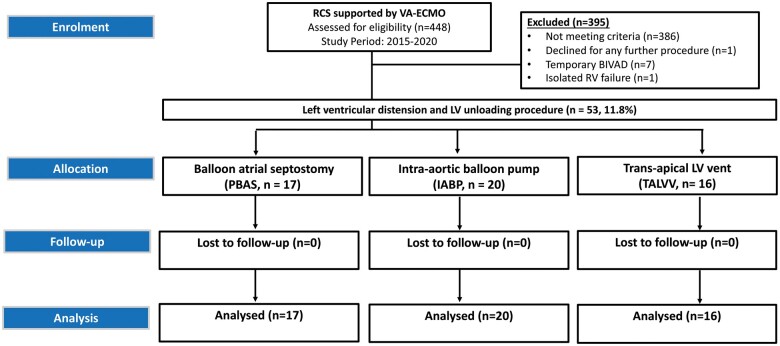
A total of 448 adult patients supported with VA-ECMO for RCS between 01 March 2015 and 31 January 2020 were analysed in this single-centre retrospective case-control study. We identified 53 patients (11.8%) who had an LV unloading procedure during the VA-ECMO run. However, 395 patients did not require an LV unloading procedure during VA-ECMO support. The study flow diagram is demonstrated in Fig. 1. Patients who were treated with VA-ECMO for isolated right ventricular failure and temporary biventricular assist devices were excluded from the analysis.
Figure 1:
Overview of study flow diagram. BIVAD: biventricular assist device; LV: left ventricle; RCS: refractory cardiogenic shock; RV: right ventricle; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
Among the 53 patients who required the LV decompression procedure, 17 patients (32.1%) had the PBAS procedure, 20 patients (37.7%) had IABP support and 16 patients (30.2%) had the TALVV procedure.
The primary end-points were the composite rate of death, procedure-related complications and neurological outcomes with 3 different decompression strategies, namely PBAS, IABP and TALVV. The secondary end-points were delta change in pulmonary capillary wedge pressure (PCWP), systolic pulmonary artery pressure, central venous pressure, left atrial diameter and the resolution of pulmonary oedema on a chest X-ray within 48 h. The resolution of pulmonary oedema on the chest X-ray was followed daily. We compared all the chest X-rays of patients who underwent 3 different LV unloading procedures at 48 h for standardization.
Venoarterial extracorporeal membrane oxygenation
The ECMO circuit used in this study was a continuous flow centrifugal pump (Jostra Rotaflow; Maquet Cardiopulmonary, Rastatt, Germany), an oxygenator (Jostra Quadrox; Maquet Cardiopulmonary) and a heat exchanger. We assessed biventricular filling and function, the interventricular septum position, the aortic valve opening, valvular regurgitation, intra-cardiac or aortic thrombus formation, pericardial effusion and cannula position by daily echocardiographic monitoring.
We used unfractionated heparin 50–100 units/kg bolus and heparin infusion 20–50 units/kg/h for anticoagulation. Activated clotting times were measured every 6 h with a target activated clotting time of 160–200 s (Medtronic ACT Plus™, Minneapolis, MN, USA). Activated partial thromboplastin time was measured twice daily with a goal of 50–60 s. The same protocol was applied for patients who had the LV unloading procedure. We also assessed antifactor Xa, platelet count, prothrombin time/international normalized ratio, fibrinogen and D-dimer when needed.
Timing and selection for left ventricular decompression methods
The multidisciplinary heart transplant team set the timing and type of the LV decompression procedure to initiate LV unloading as early as possible under Swan-Ganz catheterization, echocardiography and chest X-ray findings. LV unloading was considered in patients with evidence of pulmonary oedema on a chest radiogram, increased pulmonary artery diastolic blood pressure (>25 mmHg) or increased PCWP >20 mmHg on VA-ECMO support, inadequate response to increasing doses of inotropes and impaired aortic valve opening for every beat on echocardiography. Before the final decision, we made every effort to reduce LV loading, including reducing ECMO flow rates, increasing inotropic support and initiating vasodilators while maintaining sufficient mean arterial pressure. Our clinical criteria for choosing the type of LV venting were formed according to the clinical status of the patient to minimize the risk of potential complications. IABP was often the first line of LV unloading utilized for patients with post-infarction cardiogenic shock. However, IABP was avoided in patients with peripheral arterial disease. We often preferred PBAS or TALVV techniques in patients with decompensated chronic heart failure. In patients with LV thrombus and in redo cases, PBAS was the first line of the LV unloading method.
Percutaneous balloon atrial septostomy
The PBAS procedure was performed in the cardiac catheterization laboratory. After femoral vein access, the transseptal puncture was performed with a Brockenbrough needle under biplane fluoroscopy and transoesophageal echocardiography (TOE) guidance. Then, the balloon catheter was advanced into the right atrium and positioned in the left atrium. The balloon was quickly inflated with 3–4 ml of dilute radiopaque solution, locked and then sharply withdrawn into the right atrium down to the junction of the inferior caval vein. The catheter was then advanced into the right atrium body, so as not to obstruct inferior caval return, and then deflated rapidly. This manoeuver might be repeated 2–3 times. Finally, the catheter was removed, and left atrial decompression, the interatrial communication dimension and the absence of complications (pericardial haemorrhage, cardiac injury) were verified via TOE. The efficiency of the septostomy was evaluated with post-procedural measurement of PCWP, pulmonary artery diastolic pressure, a postero-anterior chest X-ray and evaluation of the LV dimensions and aortic valve opening on echocardiography.
Intra-aortic balloon pump support
IABP (Maquet Cardiopulmonary) implantation was performed in our hybrid operating room or at the bedside in the intensive care unit (ICU). With the patient under the local anaesthesia, the balloon catheter was placed using the Seldinger technique via a femoral artery. The location of the IABP with its tip lying distal to the left subclavian artery and the proximal portion ending above the origin of renal arteries is considered the ‘safe zone’; the position was confirmed by fluoroscopy.
Transapical left ventricular venting
The TALVV procedure was performed in the operating room via a left anterolateral minithoracotomy. In patients with post-cardiotomy RCS, a pre-existing sternotomy was reopened. The cardiac apex on the chest surface was marked using TOE. A minithoracotomy was usually performed at the 5th or 6th intercostal space. After the pericardium was opened, the apex of the left ventricle was exposed using pericardial slings. We placed 2 purse-string sutures of 3–0 polypropylene, oriented at 90°, at the LV apex cannulation site for haemostasis. Using the Seldinger technique, a high-flow 19- to 21-Fr arterial cannula was placed over the wire into the left ventricle. After confirmation with TOE, the cannula was fixed with pledgeted purse-string sutures and connected via a Y connector to the venous access line of the ECMO circuit. Decompression of the left ventricle and optimal flow regulation were arranged by tightening or loosening the venous occluder device on the TALVV arm, which was placed proximally to the flow sensor. The average flow over the venting cannula was approximately 600–1800 ml. The aortic valve opening was monitored daily by transthoracic echocardiography.
Haemodynamic evaluation
For standardization of the baseline haemodynamic evaluation using a Swan-Ganz catheter, we set the ECMO flows where the aortic valve opening could be detected on the arterial pulse pressure tracing or M-mode echocardiography. Haemodynamic parameters were reassessed 24 h after the decompression procedure. Our ECMO weaning protocol was described in detail previously [24].
Statistical analyses
Statistical analyses were performed using SPSS version 25.0 (IBM SPSS Statistics, Chicago, IL, USA). Continuous variables are expressed as mean with standard deviation unless otherwise specified, and categorical variables are expressed as numbers and percentages. The distribution of continuously measured variables was tested with the Shapiro–Wilk test. We first compared data from baseline characteristics (Table 1) using the traditional χ2analysis. A comparison of means was performed using a one-way analysis of variance with post hoc Tukey honest significance difference or the Games–Howell multiple comparison test to adjust for pairwise mean comparisons between PBAS and IABP and LV vent groups. Dichotomous outcome comparisons between groups were conducted using Pearson’s χ2or Fisher’s exact test when appropriate. Statistical significance was considered to be at the P < 0.05 level.
Table 1:
Venoarterial extracorporeal membrane oxygenation patient characteristics, other clinical variables and laboratory findings, per left ventricular unloading group
| Baseline characteristics | VA-ECMO + IABP (n = 20) | VA-ECMO + PBAS (n = 17) | VA-ECMO + TALVV (n = 16) | P-value |
|---|---|---|---|---|
| Age (years), mean ± SD | 57.3 ± 8.5 | 54.9 ± 16.0 | 56.1 ± 8.0 | 0.751 |
| Male gender, n (%) | 12 (60) | 10 (58.8) | 10 (62.5) | 0.976 |
| Body surface area (kg/m2), mean ± SD | 1.85 ± 0.1 | 1.86 ± 0.1 | 1.86 ± 0.1 | 0.909 |
| Body mass index (kg/m2), mean ± SD | 25.1 ± 2.3 | 25.3 ± 2.9 | 25.6 ± 2.4 | 0.862 |
| Hypertension, n (%) | 9 (45) | 8 (47) | 8 (50) | 0.956 |
| Diabetes, n (%) | 6 (30) | 5 (29.4) | 5 (31.2) | 0.993 |
| Hyperlipidaemia, n (%) | 8 (40) | 7 (41.1) | 6 (37.5) | 0.976 |
| History of peripheral vascular disease, n (%) | 2 (10) | 2 (11.7) | 2 (12.5) | 0.970 |
| History of cardiac arrest, n (%) | 1 (5) | 1 (5.8) | 1 (6.2) | 0.986 |
| Previous hospital admission for heart failure, n (%) | 5 (25) | 4 (25.1) | 4 (25) | 0.993 |
| Previous implantable defibrillator, n (%) | 4 (20) | 3 (17.6) | 3 (18.7) | 0.983 |
| Cardiac resynchronization therapy, n (%) | 4 (20) | 4 (25.1) | 4 (25) | 0.933 |
| Previous stroke, n (%) | 1 (5) | 1 (5.8) | 1 (6.2) | 0.986 |
| Current tobacco use, n (%) | 7 (35) | 6 (35.2) | 5 (31.2) | 0.963 |
| COPD, n (%) | 5 (25) | 4 (25.1) | 4 (25) | 0.993 |
| Hgb (g/dl), mean ± SD | 11.1 ± 0.8 | 10.9 ± 0.7 | 11 ± 0.7 | 0.754 |
| Platelet count (×109/l), mean ± SD | 111.7 ± 15.9 | 109.5 ± 13.3 | 105.1 ± 14.6 | 0.413 |
| Serum creatinine level (mg/dl), mean ± SD | 1.7 ± 1.2 | 1.7 ± 1.3 | 1.7 ± 1.4 | 0.790 |
| Renal failure, n (%) | 2 (10) | 2 (11.7) | 2 (12.5) | 0.970 |
| LVEF (%), median (IQR) | 20 (20–25) | 20 (20–25) | 20 (20–30) | 0.836 |
| Previous IABP support, n (%) | 5 (25) | 4 (23.5) | 4 (25) | 0.993 |
| Multiorgan failure, n (%) | 6 (30) | 5 (29.4) | 4 (25) | 0.940 |
| pH at VA-ECMO before LV unloading, U, mean ± SD | 7.38 ± 0.11 | 7.37 ± 0.09 | 7.36 ± 0.10 | 0.557 |
| Lactate at VA-ECMO before LV unloading, mg/dl, mean ± SD | 6.1 ± 2.6 | 5.9 ± 2.5 | 6.3 ± 2.56 | 0.905 |
| Mean arterial pressure at VA-ECMO (mmHg), mean ± SD | 57.9 ± 8.2 | 57.3 ± 8.1 | 57.1 ± 8.7 | 0.956 |
| Arterial access for VA-ECMO | 0.868 | |||
| Femoral artery, n (%) | 16 (80) | 14 (82.4) | 12 (75) | |
| Axillary artery, n (%) | 4 (20) | 3 (17.6) | 4 (25) | |
| Venous access for VA-ECMO | ||||
| Femoral vein, n (%) | 20 (100) | 17 (100) | 16 (100) |
Data are presented as n (%) or mean ± SD and median with interquartile range.
COPD: chronic obstructive pulmonary disease; Hgb: haemoglobin; IABP: intra-aortic balloon pump; IQR: interquartile range; LV: left ventricular; LVEF: left ventricular ejection fraction; PBAS: percutaneous balloon atrial septostomy; SD: standard deviation; TALVV: transapical left ventricular vent; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
RESULTS
In this single-centre cohort of patients with RCS on VA-ECMO (n = 448) support, we identified 53 patients (11.8%) who had developed left ventricular distension and required an LV unloading procedure. Overall, the mean age of patients with VA-ECMO support was 55.8 ± 12.4; 276 (61.6%) were men. The mean pre-VA-ECMO left ventricular ejection fraction was 25.1 ± 4.8%. The median ECMO duration was 16 (interquartile range, 9–21) days. There were no significant differences in baseline characteristics between patients who underwent 3 different LV decompression strategies (Table 1). There was no conversion between the LV decompression methods and no unloading procedure-related deaths in this study cohort.
Decompensated chronic heart failure (n = 148, 33.1%) was the leading cause of RCS. As reported in Table 2, there were no significant differences between the 3 LV decompression groups in terms of RCS aetiology.
Table 2:
Indications for venoarterial extracorporeal membrane oxygenation support for patients with refractory cardiogenic shock, per left ventricular unloading group
| Indication | Only VA-ECMO (n = 448) | VA-ECMO + IABP (n = 20) | VA-ECMO + PBAS (n = 17) | VA-ECMO + TALVV (n = 16) | P-value |
|---|---|---|---|---|---|
| Acute myocardial infarction, n (%) | 63 (14.1) | 3 (15) | 2 (11.7) | 2 (12.5) | 0.954 |
| Decompensated chronic heart failure*, n (%) | 148 (33.1) | 7 (35) | 6 (35.2) | 6 (37.5) | 0.986 |
| Post-cardiotomy heart failure, n (%) | 126 (28.1) | 5 (25) | 5 (29.4) | 4 (25) | 0.944 |
| Tx-PGF, n (%) | 6 (1.3) | 1 (5) | 0 (0) | 0 (0) | 0.431 |
| Fulminant myocarditis, n (%) | 9 (2.0) | 1 (5) | 1 (5.8) | 1 (6.2) | 0.986 |
| Post LVAD right heart failure, n (%) | 9 (2.0) | 1 (5) | 1 (5.8) | (0) | 0.632 |
| Sepsis-associated cardiomyopathy, n (%) | 28 (6.2) | 1 (5) | 1 (5.8) | 1 (6.2) | 0.986 |
| Pulmonary hypertension with right heart failure, n (%) | 15 (3.3) | 0 (0) | 0 (0) | 0 (0) | |
| Pulmonary embolism, n (%) | 26 (5.8) | 0 (0) | 0 (0) | 0 (0) | |
| Intractable arrhythmias, n (%) | 6 (1.3) | 1 (5) | 1 (5.8) | 2 (12.5) | 0.665 |
| ECPR, n (%) | 12 (2.7) | 0 (0) | 0 (0) | 0 (0) |
Data are presented as n (%) or mean ± SD.
ECPR: extracorporeal cardiopulmonary resuscitation; IABP: intra-aortic balloon pump; LVAD: left ventricular assist device; PBAS: percutaneous balloon atrial septostomy; SD: standard deviation; TALVV: transapical left ventricular vent; Tx-PGF: post-transplant primary graft failure; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
However, after LV unloading procedures, we noted a clinical and statistically significant difference between the PBAS, IABP and TALVV groups (Table 3). Reduction in PCWP was highest with the TALVV technique (17.2 ± 2.1 mmHg; P < 0.001) and was higher in the PBAS than in the IABP group; this difference was significant (9.6 ± 2.5 and 3.9 ± 1.3, respectively; P = 0.001) (Fig. 2). The TALVV technique was more effective than the PBAS and IABP strategies (20.3 ± 4.3 mmHg; P < 0.001) in reducing pulmonary arterial pressure. Similarly, reduction in the LA diameter and central venous pressure was highest with the TALVV technique (14.8 ± 3.2 mm and 7.4 ± 1.1 mmHg, respectively; P < 0.001).
Table 3:
Haemodynamics during venoarterial extracorporeal membrane oxygenation support for refractory cardiogenic shock and after 3 different left decompression methods per left ventricular unloading group
| Variables | VA-ECMO + IABP (n = 20) | VA-ECMO + PBAS (n = 17) | VA-ECMO + TALVV (n = 16) | P-value | |
|---|---|---|---|---|---|
| VA-ECMO run (days), median (IQR) | 16 (14–20) | 16 (11–23.5) | 16 (14–22.5) | 0.962 | |
| Mean duration from VA-ECMO to LV unloading (h), mean ± SD | 13.9 ± 3.6 | 46.1 ± 3.6 | 52.1 ± 4.9 | <0.001 | |
| VA-ECMO output expressed as CI (l/min/m2) | 2.1 ± 0.2 | 2.2 ± 0.3 | 2.1 ± 0.3 | 0.485 | |
| Measurements | |||||
| Preintervention | PCWP (mmHg) | 29.1 ± 2.5 | 29.3 ± 2.5 | 30.2 ± 2.8 | 0.431 |
| SPAP (mmHg) | 52.3 ± 9.1 | 48.4 ± 9.4 | 54.4 ± 6.8 | 0.137 | |
| LA diameter (mm) | 56.6 ± 4.5 | 56.0 ± 7.2 | 56.9 ± 4.3 | 0.879 | |
| CVP (mmHg) | 13.2 ± 2.3 | 12.1 ± 2.1 | 13.8 ± 2.5 | 0.116 | |
| (Δ) change | PCWP (mmHg) | −3.9 ± 1.3 | −9.6 ± 2.5b | −17.2 ± 2.1a | <0.001 |
| SPAP (mmHg) | −4.1 ± 2.9 | −10.4 ± 2.8b | −20.3 ± 4.3a | <0.001 | |
| LA diameter (mm) | −2.9 ± 1.1 | −5.1 ± 1.1b | −14.8 ± 3.2a | <0.001 | |
| CVP (mmHg) | −1.6 ± 0.8 | −1.3 ± 0.7b | −7.4 ± 1.1a | <0.001 | |
Data are presented as I (%) or mean ± SD and median with interquartile range.
Reduction in PCWP, SPAP, LA diameter and CVP was significantly higher in the TALVV group compared to the PBAS and IABP groups.
The PBAS technique was more effective than the IABP strategies in terms of reduction in PCWP (P = 0.001), SPAP (P = 0.042) and LA diameter (P = 0.003). There was no significant difference between PBAS and IABP in reducing CVP (P = 0.356).
: change; CI: cardiac index; CVP: central venous pressure; IABP: intra-aortic balloon pump; IQR: interquartile range; LA: left atrial; LV: left ventricular; PBAS: percutaneous balloon atrial septostomy; PCWP: pulmonary capillary wedge pressure; SD: standard deviation; SPAP: systolic pulmonary artery pressure; TALVV: transapical left ventricular vent.
Figure 2:
Haemodynamic changes after 3 different left ventricular unloading strategies. CVP: central venous pressure; LV: left ventricle; PCWP: pulmonary capillary wedge pressure; SPAP: systolic pulmonary artery pressure.
A chest X-ray evaluation 48 h after the LV unloading procedure revealed significant resolution of pulmonary oedema with all techniques (Fig. 3). However, TALVV was the most effective method for LV unloading compared to PBAS and IABP. The TALVV group was also associated with significant complications, including bleeding at the access site (n = 3, 18.8%), thrombosis of the cannula (n = 1, 6.3%), ventricular arrhythmias (n = 2, 12.5%) and malposition of the cannula (n = 2, 12.5%) (Table 4). There was no significant difference among the groups regarding neurological complications. In addition, patient care and mobilization in the ICU were challenging in the TALVV group. Overall, there were no significant differences in the duration of VA-ECMO support, ICU stay and hospital stay among the groups. Nine (52.9%) patients in the PBAS group were weaned from VA-ECMO support, whereas 7 (43.7%) patients in the TALVV group and 11 (55%) patients in the IABP group (P = 0.783) were weaned.
Figure 3:
Chest X-ray illustrations of the resolution of pulmonary oedema before and 48 h after LV unloading procedures. IABP: intra-aortic balloon pump; LV: left ventricle; PBAS: percutaneous balloon atrial septostomy; TALVV: transapical left ventricular vent.
Table 4:
Clinical outcomes, per left ventricular unloading group
| Outcomes | VA-ECMO + IABP (n = 20) | VA-ECMO + PBAS (n = 17) | VA-ECMO + TALVV (n = 16) | P-value |
|---|---|---|---|---|
| Successful weaning from VA-ECMO, n (%) | 11 (55) | 9 (52.9) | 7 (43.7) | 0.783 |
| Post-ECMO mechanical ventilation (days), mean ± SD | 2.0 ± 0.4 | 1.9 ± 0.3 | 2.1 ± 0.3 | 0.381 |
| Days in ICU, mean ± SD | 16.2 ± 3.9 | 15.8 ± 4.3 | 17.4 ± 5.9 | 0.428 |
| Days in hospital, mean ± SD | 28.7 ± 4.6 | 28.2 ± 5.2 | 30.5 ± 4.4 | 0.360 |
| Bridged to LVAD or heart transplantation | 8 (40) | 6 (35.3) | 4 (25) | 0.633 |
| Complications related to LV unloading procedure, n (%) | 2 (10) | 3 (17.6) | 8 (50) | |
| Access site bleeding, n (%) | 0 | 1 (5.9) | 3 (18.8) | |
| Thrombosis of cannulae/balloon, n (%) | 0 | 0 | 1 (6.3) | |
| Cannulae/balloon malposition, n (%) | 1 (5) | 0 | 2 (12.5) | |
| Ventricular arrhythmias, n (%) | 0 | 1 (5.9) | 2 (12.5) | |
| Vascular complications, n (%) | 1 (5) | 0 | 0 | |
| Aortic dissection, n (%) | 0 | 0 | 0 | |
| Pericardial tamponade, n (%) | 0 | 1 | 0 | |
| Neurological complications | 0 | 1 | 2 | |
| Intracranial haemorrhage, n (%) | 0 | 0 | 1 (6.3) | |
| Acute ischaemic stroke, n (%) | 0 | 1 (5.9) | 1 (6.3) | |
| Seizure, n (%) | 0 | 0 | 0 | |
| Survival | ||||
| ICU deaths, n (%) | 8 (40) | 7 (41.2) | 7 (43.8) | 0.974 |
| In-hospital deaths, n (%) | 9 (45) | 8 (47.1) | 9 (56.3) | 0.783 |
| Cause of death | ||||
| Sepsis, n (%) | 1 (5) | 1 (5.9) | 1 (6.3) | 0.985 |
| Multiorgan failure, n (%) | 7 (35) | 6 (35.3) | 6 (37.5) | 0.986 |
| Cerebrovascular event, n (%) | 1 (5) | 1 (5.9) | 2 (12.5) | 0.664 |
Data are presented as n (%) or mean ± SD and median with interquartile range.
IABP: intra-aortic balloon pump; ICU: intensive care unit; LV: left ventricular; LVAD: left ventricular assist device; PBAS: percutaneous balloon atrial septostomy; SD: standard deviation; TALVV: transapical left ventricular vent; VA-ECMO: venoarterial extracorporeal membrane oxygenation.
DISCUSSION
This case–control study shows that in patients with VA-ECMO for RCS, about 12% of the patients develop LV distension and pulmonary oedema. However, no randomized controlled clinical trials have reported the optimal management strategy for LV unloading in patients on VA-ECMO support. Furthermore, a recent systematic review and meta-analysis on LV unloading with VA-ECMO by Kowalewski et al. [25] pointed out certain methodological flaws in previously published data. VA-ECMO induces reverse aortic flow and leads to an increase in LV afterload. Myocardial oxygen demand and supply mismatch may worsen after VA-ECMO support. These phenomena can be associated with pulmonary oedema, poor outcomes and high mortality.
Initial management strategies for LV distension and pulmonary oedema in patients on VA-ECMO include reducing ECMO flow and medical therapies, including inotropic support, diuresis or ultrafiltration and fluid restriction. However, unless the left heart is vented or unloaded, these deleterious effects of VA-ECMO may compromise myocardial recovery and may prolong lung injury [4]. Effective left heart decompression can minimize ventricular distension, decrease end-diastolic pressures, increase subendocardial perfusion, resolve pulmonary oedema and allow myocardial recovery. The advantages of LV/LA decompression theoretically depend on 2 mechanisms. First, the reduction of LA pressure promotes early lung recovery. Second, ameliorated LV wall stress improves subendocardial perfusion, decreases myocardial oxygen consumption and, as a result, provides LV functional recovery [19]. So far, there is no consensus concerning the type and timing of left heart unloading on VA-ECMO. However, studies generally conclude that earlier LA decompression may result from a rapid weaning process and reduced complication risks related to prolonged VA-ECMO support. LV decompression can be successfully provided via various percutaneous methods, such as placement of IABP [10], transaortic LV venting [13, 14], percutaneous transseptal cannula placement [18, 19] and LA decompression by PBAS [20–23].
IABP typically increases coronary perfusion by increasing aortic diastolic pressures and reduces the LV afterload [4, 10]. Volume shifting of approximately 40 ml per beat by the IABP increases LV stroke volume and cardiac output by up to 1 l/min [7]. However, it reduces systolic blood pressure and provides an unreliable degree of LV unloading associated with slightly reduced LV preload [4]. As noted in our study, IABP causes a mild reduction in PCWP by an average of ≈3 to 4 mmHg [10].
PBAS is also a straightforward, feasible method for LV unloading compared to surgical LV decompression strategies. Published data describing the use of PBAS for left heart unloading are still limited. In our study, PBAS was the preferred method in high-risk patients due to its ease of application and the low risk of complications. Some case series have demonstrated its influence on LA decompression and LV functional recovery or survival. Aiyagari et al. [19] reported the results of 7 patients with reasonable LV decompression at a median time of 11 h after VA-ECMO implantation. In this study, 57% of patients were decannulated and survived. Kotani et al. [26] described a cohort, including 23 paediatric patients with VA-ECMO, who required LV decompression; 70% of these patients were successfully weaned from ECMO support. They showed that the early timing of the LV decompression method facilitates the VA-ECMO weaning process. On the contrary, Hacking et al. [12] were not able to prove the beneficial effects on survival of elective LV decompression at the initiation of VA-ECMO.
Surgical strategies for LV unloading include TALVV through a left anterior minithoracotomy [12], left atrial decompression by minithoracotomy [15] and surgical pulmonary artery venting [16, 17]. TALVV was the most efficient LV unloading method in our study compared to IABP and PBAS. However, as detected in this study, surgical techniques for LV unloading may have life-threatening complications, including bleeding, haemolysis and increased risk of infection. We also noted cannula malposition, thrombosis and ventricular arrhythmias. Some technical considerations may minimize the early complications of TALVV. In our experience, both transthoracic and TOE guidance, use of the Seldinger technique, identification of an LV apical ‘dimple’ for future potential left ventricular assist device implantation and appropriately positioned purse-string sutures are critical for a safe TALVV technique.
Limitations
This study has some limitations. First, defining the necessity of LV unloading is not standardized globally. Second, our study has a small sample size, and the research is a retrospective, case-control study. Selection bias is unavoidable if the selection of different treatment modalities is decided by the heart transplant team and may impact the clinical outcomes. However, our study was not designed to investigate the superiority of any decompression procedure. Rather, the goal was to evaluate the haemodynamic effects of different decompression strategies in a VA-ECMO setting and share procedure-related complications. Furthermore, the TandemHeart™ and Impella are other effective percutaneous options for decompression of the left chambers, but these options are unavailable in our country. We propose adopting a universal definition for the indications for LV unloading in patients with VA-ECMO, and then we may compare unloading strategies in a randomized controlled trial.
CONCLUSIONS
In this patient cohort, 12% of patients with VA-ECMO for RCS required an LV decompression procedure. In terms of the haemodynamic effects of LV unloading, TALVV was the most effective method when compared to PBAS and IABP. However, TALVV was associated with significant surgical complications. Future technical advances may minimize procedure-related complications. Currently, efficient transapical LV unloading did not result in better clinical outcomes. These results need to be confirmed in a large prospective randomized trial.
ACKNOWLEDGEMENTS
We sincerely thank Atilla Elhan, Ankara University School of Medicine, for statistical review of the manuscript.
FUNDING
Data were extracted from the hospital database developed at the Ankara University Hospitals and from a specified cardiac surgery research database.
Conflict of interest: none declared.
Authors contributions
Ali İhsan Hasde: Conceptualization; Data curation; Methodology; Formal analysis; Investigation; Writing—original draft. Mehmet Cahit Sarıcaoğlu: Investigation; Methodology; Validation; Formal analysis. Nur Dikmen Yaman: Data curation; Methodology; Formal analysis; Writing—original draft. Çağdaş Baran: Conceptualization; Validation; Evren Özçınar: Validation; Resources; Supervision. Mehmet Çakıcı: Project administration; Validation; Visualization; Writing—original draft. Mustafa Bahadır İnan: Conceptualization; Methodology; Project administration; Formal analysis. Ahmet Ruchan Akar: Conceptualization; Project administration; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Jarle Vaage and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
ABBREVIATIONS
- IABP
Intra-aortic balloon pump
- ICU
Intensive care unit
- LA
Left atrial
- LV
Left ventricle
- PCWP
Pulmonary capillary wedge pressure
- PBAS
Percutaneous balloon atrial septostomy
- RCS
Refractory cardiogenic shock
- TOE
Transoesophageal echocardiography
- VA-ECMO
Venoarterial extracorporeal membrane oxygenation
- TALVV
Transapical left ventricular vent
REFERENCES
- 1. Guglin M, Zucker MJ, Bazan VM, Bozkurt B, El Banayosy A, Estep JD. et al. Venoarterial ECMO for adults: JACC scientific expert panel. J Am Coll Cardiol 2019;73:698–716. [DOI] [PubMed] [Google Scholar]
- 2. Abrams D, Combes A, Brodie D.. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769–78. [DOI] [PubMed] [Google Scholar]
- 3. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD. et al. ; ELSO member centers. Extracorporeal life support organization registry international report 2016. ASAIO J 2017;63:60–7. [DOI] [PubMed] [Google Scholar]
- 4. Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD.. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail 2018;11:e004905. [DOI] [PubMed] [Google Scholar]
- 5. Lorusso R, Gelsomino S, Parise O, Mendiratta P, Prodhan P, Rycus P. et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock in elderly patients: trends in application and outcome from the Extracorporeal Life Support Organization (ELSO) Registry. Ann Thorac Surg 2017;104:62–9. [DOI] [PubMed] [Google Scholar]
- 6. Rupprecht L, Florchinger B, Schopka S, Schmid C, Philipp A, Lunz D. et al. Cardiac decompression on extracorporeal life support: a review and discussion of the literature. ASAIO J 2013;59:547–53. [DOI] [PubMed] [Google Scholar]
- 7. Werdan K, Gielen S, Ebelt H, Hochman JS.. Mechanical circulatory support in cardiogenic shock. Eur Heart J 2014;35:156–67. [DOI] [PubMed] [Google Scholar]
- 8. Xie A, Forrest P, Loforte A.. Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann Cardiothorac Surg 2019;8:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centofanti P, Attisani M, La Torre M, Ricci D, Boffini M, Baronetto A. et al. Left ventricular unloading during peripheral extracorporeal membrane oxygenator support: a bridge to life in profound cardiogenic shock. J Extra Corpor Technol 2017;49:201–5. [PMC free article] [PubMed] [Google Scholar]
- 10. Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S. et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Crit Care Med 2014;42:2075–82. [DOI] [PubMed] [Google Scholar]
- 11. Aso S, Matsui H, Fushimi K, Yasunaga H.. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med 2016;44:1974–9. [DOI] [PubMed] [Google Scholar]
- 12. Hacking DF, Best D, d'Udekem Y, Brizard CP, Konstantinov IE, Millar J. et al. Elective decompression of the left ventricle in pediatric patients may reduce the duration of venoarterial extracorporeal membrane oxygenation. Artif Organs 2015;39:319–26. [DOI] [PubMed] [Google Scholar]
- 13. Fumagalli R, Bombino M, Borelli M, Rossi F, Colombo V, Osculati G. et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs 2004;27:410–3. [DOI] [PubMed] [Google Scholar]
- 14. Hong TH, Byun JH, Lee HM, Kim YH, Kang GH, Oh JH. et al. Initial experience of transaortic catheter venting in patients with venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J 2016;62:117–22. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto M, Oshima Y, Matsuhisa H, Higuma T, Iwaki R, Matsushima S. et al. Left atrial decompression by minithoracotomy during extracorporeal life support. Ann Thorac Surg 2019;107:e227–28. [DOI] [PubMed] [Google Scholar]
- 16. Kimura M, Kinoshita O, Fujimoto Y, Murakami A, Shindo T, Kashiwa K. et al. Central extracorporeal membrane oxygenation requiring pulmonary arterial venting after near-drowning. Am J Emerg Med 2014;32:197.e1. [DOI] [PubMed] [Google Scholar]
- 17. Avalli L, Maggioni E, Sangalli F, Favini G, Formica F, Fumagalli R.. Percutaneous left-heart decompression during extracorporeal membrane oxygenation: an alternative to surgical and transeptal venting in adult patients. ASAIO J 2011;57:38–40. [DOI] [PubMed] [Google Scholar]
- 18. Hlavacek AM, Atz AM, Bradley SM, Bandisode VM.. Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2005;130:595–6. [DOI] [PubMed] [Google Scholar]
- 19. Aiyagari RM, Rocchini AP, Remenapp RT, Graziano JN.. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603–6. [DOI] [PubMed] [Google Scholar]
- 20. Alkhouli M, Narins CR, Lehoux J, Knight PA, Waits B, Ling FS.. Percutaneous decompression of the left ventricle in cardiogenic shock patients on venoarterial extracorporeal membrane oxygenation. J Card Surg 2016;31:177–82. [DOI] [PubMed] [Google Scholar]
- 21. Alhussein M, Osten M, Horlick E, Ross H, Fan E, Rao V. et al. Percutaneous left atrial decompression in adults with refractory cardiogenic shock supported with veno-arterial extracorporeal membrane oxygenation. J Card Surg 2017;32:396–401. [DOI] [PubMed] [Google Scholar]
- 22. Lin YN, Chen YH, Wang HJ, Hung JS, Chang KC, Lo PH.. Atrial septostomy for left atrial decompression during extracorporeal membrane oxygenation by inoue balloon catheter. Circ J 2017;81:1419–23. [DOI] [PubMed] [Google Scholar]
- 23. Baruteau AE, Barnetche T, Morin L, Jalal Z, Boscamp NS, Le Bret E. et al. Percutaneous balloon atrial septostomy on top of venoarterial extracorporeal membrane oxygenation results in safe and effective left heart decompression. Eur Heart J Acute Cardiovasc Care 2018;7:70–9. [DOI] [PubMed] [Google Scholar]
- 24. Cakici M, Gumus F, Ozcinar E, Baran C, Bermede O, Inan MB. et al. Controlled flow diversion in hybrid venoarterial-venous extracorporeal membrane oxygenation. Interact CardioVasc Thorac Surg 2018;26:112–8. [DOI] [PubMed] [Google Scholar]
- 25. Kowalewski M, Malvindi PG, Zielinski K, Martucci G, Slomka A, Suwalski P. et al. Left ventricle unloading with veno-arterial extracorporeal membrane oxygenation for cardiogenic shock. systematic review and meta-analysis. J Clin Med 2020;9:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotani Y, Chetan D, Rodrigues W, Sivarajan VB, Gruenwald C, Guerguerian AM. et al. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: current strategy and clinical outcomes. Artif Organs 2013;37:29–36. [DOI] [PubMed] [Google Scholar]