Abstract
OBJECTIVES
Cardiac surgery is associated with risk of cerebral injury and mean arterial pressure (MAP) during cardiopulmonary bypass (CPB) is suggested to be associated with cerebral injury. The ‘Perfusion Pressure Cerebral Infarcts’ (PPCI) trial randomized patients undergoing coronary artery bypass grafting (CABG) and/or aortic valve replacement to a MAP of 40–50 or 70–80 mmHg during CPB and found no difference in clinical or imaging outcomes between the groups. We here present PPCI trial predefined secondary end points, consisting of biomarkers of brain injury.
METHODS
Blood was collected from PPCI trial patients at baseline, 24 and 48 h after induction of anaesthesia and at discharge from the surgical ward. Blood was analysed for neuron-specific enolase, tau, neurofilament light and the glial marker glial fibrillary acidic protein. Linear mixed models were used to analyse differences in biomarker value changes from baseline between the 2 MAP allocation groups.
RESULTS
A total of 193 (98%) patients were included. We found no differences in biomarker levels over time from baseline to discharge between the 2 MAP allocation groups (PNSE = 0.14, PTau = 0.46, PNFL = 0.21, PGFAP = 0.13) and the result did not change after adjustment for age, sex and type of surgery.
CONCLUSIONS
We found no significant differences in levels of biomarkers of neurological injury in patients undergoing elective or subacute CABG and/or aortic valve replacement randomized to either a target MAP of 40–50 mmHg or a target MAP of 70–80 mmHg during CBP.
Keywords: Coronary artery bypass grafting, Aortic valve replacement, Neurological injury, Biomarkers
Cardiac surgery, including coronary artery bypass grafting (CABG) and aortic valve replacement (AVR), is associated with a considerable risk of cerebral injury, including silent stroke, overt stroke and postoperative cognitive dysfunction [1–3].
INTRODUCTION
Cardiac surgery, including coronary artery bypass grafting (CABG) and aortic valve replacement (AVR), is associated with a considerable risk of cerebral injury, including silent stroke, overt stroke and postoperative cognitive dysfunction [1–3]. The pathophysiology of cerebral injury remains poorly understood, but cerebral perfusion pressure, which can be approximated as the difference between mean arterial pressure (MAP) and jugular venous pressure, may affect the risk. During on-pump cardiac surgery, cardiopulmonary bypass (CPB) is applied to deliver oxygenated blood to all tissues, and the MAP can be targeted by regulating pump flow or by using vasopressors. The knowledge regarding the optimal MAP during CPB is sparse, as previous randomized trials have yielded conflicting results [4–6], and contemporary guidelines recommend a relative wide MAP interval from 50 to 80 mmHg during CPB [7].
Accordingly, the ‘Perfusion Pressure Cerebral Infarcts’ (PPCI) trial was conducted at our institution, in which patients undergoing CABG and/or AVR were randomized to a target MAP of 40–50 vs 70–80 mmHg during CPB [8]. The study found no difference in the primary end point consisting of total volume of cerebral infarcts at day 3–6 after surgery, detected by diffusion weighted magnetic resonance imaging, nor in a secondary outcome, defined as the occurrence of postoperative cognitive dysfunction at discharge [9]. In addition, the PPCI trial defined a number of biomarkers of cerebral injury as secondary end points with a potential higher sensitivity to detect differences in cerebral damage compared to clinical end points.
We here present PPCI trial predefined secondary end points, consisting of biomarkers of cerebral injury, including the neuronal proteins neuron-specific enolase (NSE), tau, neurofilament light (NFL) and the glial marker glial fibrillary acidic protein (GFAP), measured before and after surgery. We hypothesized that the high target MAP group would have lower release of circulating brain injury biomarkers after cardiac surgery, reflecting minimized neurological injury.
MATERIALS AND METHODS
The present study was approved by the institutional review board (Videnskabsetisk Komité, ref. no. H-3-2013-110). All participants provided verbal and written informed consent prior to enrolment.
The study presents the analysis of a predefined secondary end point of the investigator-initiated, assessor-blinded, randomized, controlled PPCI trial. The trial protocol and primary results have previously been published [8, 9]. In brief, the study included adult patients scheduled for elective or subacute CABG and/or AVR. Exclusion criteria included previous stroke, transient ischaemic attack, as well as progressive neurodegenerative disease. Patients were randomized to a low MAP target of 40–50 mmHg or a high MAP target of 70–80 mmHg during CPB. Assigned MAP targets were achieved by boluses of phenylephrine up to a maximum of 2.0 mg, which could be followed by continuous infusion of norepinephrine up to 0.4 μg/kg/min. CPB pump flow was fixed at a flow rate of 2.4 l/min/m2 body surface area plus 10–20% in both groups. Vasodilators were not administered. CPB was performed in accordance with departmental guidelines targeting arterial oxygen saturation above 96% (and PaO2 above 13 kPa), normocapnia (PaCO2 between 4.5 and 6.0 kPa), normothermia (temperature above 36.5°C) and transfusion of packed red blood cells if haematocrit was below 24%; or higher in case of lactic acidosis or low mixed venous saturations [9]. Other perioperative treatment was at the discretion of the treating physician [8].
Biomarker measurements
Blood samples were collected preoperatively prior to initiation of CPB, 24 h after anaesthesia induction, 48 h after anaesthesia induction and at discharge from the surgical department or no later than 7 days after surgery. The first blood sample was drawn from a radial artery catheter, whereas other blood samples were drawn from either a radial artery catheter, a central venous line or a cubital vein. In all cases, a total of 9 ml blood was drawn and divided into ethylenediamine-tetraacetate, citrate-coated and heparin-coated tubes. Samples were centrifuged at 3000 rpm for 10 min at 4°C, after which plasma was transferred to polypropylene test tubes and stored at −80°C until assaying.
Tau, GFAP and NFL concentrations were measured with commercially available Simoa kits (Quanterix, Billerica, MA, USA). For all biomarker assays, calibrators were run in duplicates, and obvious outlier calibrator replicates were masked before curve fitting. Samples were diluted 4-fold and run in singlicate. Two quality control levels were run in duplicates in the beginning and the end of each run.
For tau, a quality control sample with a concentration of 4.9 pg/ml achieved a repeatability of 4.2% with an intermediate precision of 4.4%, while a quality control sample with a concentration of 21.3 pg/ml achieved a repeatability of 5.5% with an intermediate precision of 8.5%. The validated measurement interval for tau was 1.22–317 pg/ml with a lower limit of quantification of 1.22 pg/ml. For GFAP, a quality control sample with a concentration of 82.4 pg/ml achieved a repeatability of 4.5% with an intermediate precision of 5.9%, while a quality control sample with a concentration of 223 pg/ml achieved a repeatability of 8.2% with an intermediate precision of 8.2%. The dynamic range for GFAP was 5.48–4000 pg/ml with a lower limit of quantification of 0.69 pg/ml. For NFL, a quality control sample with a concentration of 6.3 pg/ml achieved a repeatability of 8.3% with an intermediate precision of 9%, while a quality control sample with a concentration of 48.5 pg/ml achieved a repeatability of 8.1% with an intermediate precision of 8.2%. The dynamic range for neurofilament was 1.9–1800 pg/ml with an lower limit of quantification of 1.9 pg/ml. NSE concentrations were measured using standard clinical chemistry assays on a Cobas platform (Roche Diagnostics, Penzberg, Germany).
Statistical analysis
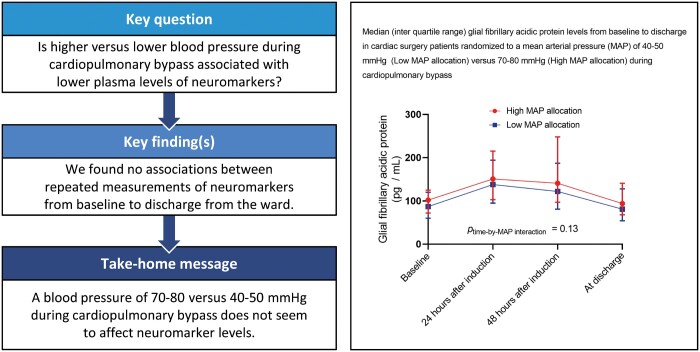
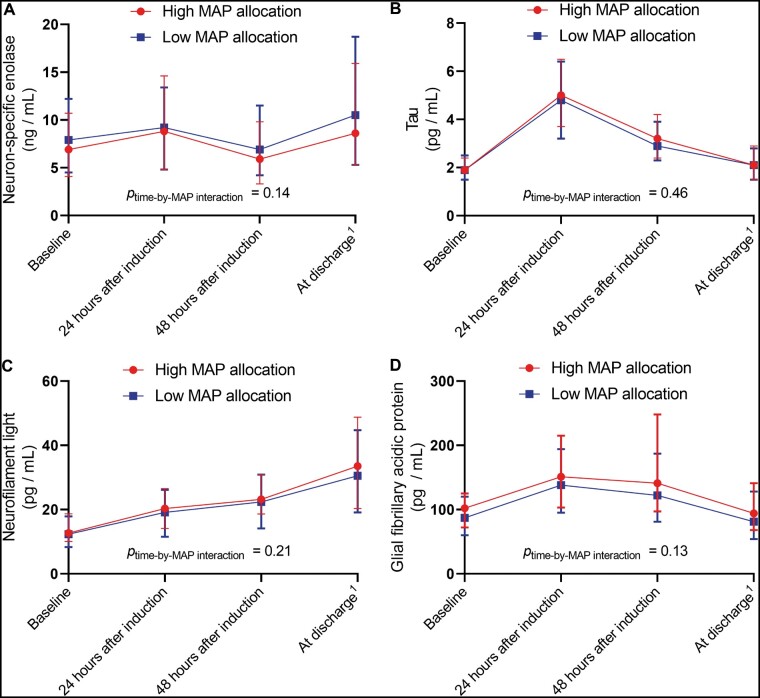
All analyses were conducted on the population of PPCI patients who had biomarker levels measured at baseline. Crude median biomarker values with range from 25th percentile to 75th percentile (interquartile range) were presented at each sampling time point after stratification for MAP allocation (Fig. 1).
Figure 1:
Median (interquartile range) biomarker levels from baseline to discharge, stratified by MAP allocation. Cardiac surgery patients randomized to a mean arterial blood pressure of either 40–50 mmHg (low) or 70–80 mmHg (high). MAP: mean arterial pressure.
For analyses of the development of biomarker levels over time, due to the randomized, longitudinal design, we applied constrained (i.e. baseline corrected) linear mixed models of covariance, including the individual biomarker as the dependent outcome variable and the time of biomarker sampling and MAP allocation group as the 2 independent exposure variables. The primary results were the ‘time × MAP allocation’-interaction, as a marker of different biomarker development over time in each allocation group. We prespecified to apply an unstructured covariance structure for the analyses. In case the distribution of a biomarker was right-skewed, we prespecified to apply logarithmic transformation as appropriate, to approximate normal distribution prior to statistical analysis. Normality was assessed graphically by QQ plots.
As secondary analyses, we repeated the constrained linear mixed models adjusting for suspected confounding factors, including age (included as continuous variable), sex and type of surgery (CABG versus valve replacement). In case of greater than 5% missing biomarker values, we prespecified to conduct the following sensitivity analyses: Repetition of the primary analysis with imputation of missing biomarker values in a ‘best-and-worst’ case scenario: In one analysis, missing biomarker values were replaced with the lowest measured biomarker value for the given MAP allocation group at the given time point (i.e. a best case scenario), and in another analysis, missing biomarker values were replaced with highest measured biomarker value for the given MAP allocation group at the given time point (i.e. a worst case scenario). A significance level of 0.01 was applied throughout in accordance with the trial protocol [8], and SAS software, version 9.4 (SAS institute, Cary, NC, USA) was used for all statistical analysis.
RESULTS
A total of 197 patients were included in the PPCI trial, with 98 patients being randomized to the high MAP allocation group and 99 patients being randomized to the low MAP allocation group [9]. At baseline, 95 (97%) patients in the high MAP allocation group and 98 (99%) patients in the low MAP allocation group had blood analysed for all 4 biomarkers. Patients allocated to the high MAP group were significantly older than patients allocated to the low MAP group (69 ± 8.5 vs 65 ± 11 years, P = 0.002). Otherwise, the groups were well balanced (Table 1), and further analyses were conducted on the presented population of a total of 193 (98%) patients. The achieved mean MAP was 67 ± 4.7 mmHg in the high MAP allocation group compared to 45 ± 4.5 mmHg in the low MAP allocation group. Other periprocedural characteristics can be seen in Table 1.
Table 1:
Characteristics of cardiac surgery patients randomized to a mean arterial blood pressure of either 40–50 mmHg (low) or 70–80 mmHg (high)
| Low MAP allocation (n = 98) | High MAP allocation (n = 95) | |
|---|---|---|
| Demographics | ||
| Age (years), mean ± SD | 65 ± 11 | 69 ± 8.5 |
| Male gender, n (%) | 92 (94) | 81 (85) |
| Actively working, n (%) | 61 (62) | 69 (73) |
| Medical history, n (%) | ||
| Recent myocardial infarction | 31 (32) | 23 (24) |
| Hypertension | 82 (84) | 84 (88) |
| Diabetes mellitus | 24 (24) | 24 (25) |
| Current smoker | 18 (18) | 15 (16) |
| Current alcohol abuse | 7 (7.1) | 7 (7.4) |
| Symptoms, n (%) | ||
| CCS score above 1 | 63 (64) | 46 (48) |
| NYHA class, n (%) | ||
| I | 32 (33) | 20 (21) |
| II | 34 (35) | 44 (46) |
| III | 28 (29) | 25 (26) |
| IV | 4 (4.1) | 6 (6.3) |
| Objective findings | ||
| BMI (kg/m2), mean ± SD | 27 ± 3.8 | 27 ± 3.9 |
| Heart rate (bpm), mean ± SD | 67 ± 14 | 69 ± 11 |
| Left ventricular ejection fraction, median (IQR) | 55 (45–60) | 55 (45–60) |
| P-creatinine (µmol/l), mean ± SD | 92 ± 28 | 88 ± 17 |
| EuroSCORE II (%), median (IQR) | 1.6 (0.97–2.9) | 2.0 (1.3–3.1) |
| Surgical procedure | ||
| CABG, n (%) | 67 (68) | 71 (75) |
| No. grafts, median (IQR) | 2 (0 - 3) | 2 (0 - 3) |
| AVR, n (%) | 27 (28) | 29 (31) |
| MVR, n (%) | 4 (4.1) | 6 (6.3) |
| MAP during bypass (mmHg), mean ± SD | 45 ± 4.7 | 67 ± 4.7 |
| Surgery time (min), mean ± SD | 185 ± 49 | 190 ± 57 |
| CPB time (min), mean ± SD | 94 ± 32 | 103 ± 72 |
| Cross-clamp time (min), mean ± SD | 63 ± 27 | 64 ± 29 |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; CCS: Canadian Cardiovascular Society; IQR: interquartile range; MAP: mean arterial pressure; MVR: Mitral valve replacement; NYHA: New York Heart Association; SD: standard deviation.
After 24 and 48 h, between 2% and 5% of patients had missing biomarker measurements, depending on the biomarker and MAP allocation group. At discharge, between 13% and 15% had missing biomarker measurements (Table 2).
Table 2:
Number of analysed blood samples at different time points, stratified by biomarker in cardiac surgery patients randomized to a mean arterial blood pressure of either 40–50 mmHg (low) or 70–80 mmHg (high)
| Sampling time | Neuron-specific enolase, n (%) | Tau, n (%) | Neurofilament light, n (%) | Glial fibrillary acidic protein, n (%) |
|---|---|---|---|---|
| High MAP allocation (n = 98) | ||||
| Baseline | 95 (97) | 96 (98) | 96 (98) | 96 (98) |
| 24 h after induction | 93 (95) | 93 (95) | 93 (95) | 93 (95) |
| 48 h after induction | 93 (95) | 93 (95) | 93 (95) | 93 (95) |
| At dischargea | 83 (85) | 83 (85) | 83 (85) | 83 (85) |
| Low MAP allocation (n = 99) | ||||
| Baseline | 98 (99) | 98 (99) | 98 (99) | 98 (99) |
| 24 h after induction | 97 (98) | 96 (97) | 97 (98) | 97 (98) |
| 48 h after induction | 97 (98) | 95 (96) | 97 (98) | 97 (98) |
| At dischargea | 86 (87) | 86 (87) | 86 (87) |
86 (87) |
At discharge from the surgical department or no later than 7 days postoperatively.
MAP: mean arterial pressure.
We did not find any significant differences in the development of biomarker levels over time from baseline between the 2 MAP allocation groups (Fig. 1).
In models adjusted for age, sex and type of surgery, we did not find any significant differences in the development of biomarker levels over time from baseline between the 2 MAP allocation groups for either NSE (P = 0.14), tau (P = 0.47), NFL (P = 0.22) or GFAP (P = 0.14) (Table 3). Increasing age was found to be significantly associated with higher levels of NFL (P < 0.0001) and GFAP (P < 0.0001), whereas female sex was associated with higher levels of tau (P = 0.0005, Table 3). Excluding 10 (1.3%) NSE samples with a positive haemolysis index (i.e. Roche haemolysis index greater than 20) did not change these results.
Table 3:
Type III tests of fixed effects from constrained linear mixed models for each biomarker measured in cardiac surgery patients randomized to a mean arterial blood pressure of either 40–50 mmHg (low) or 70–80 mmHg (high)
| Covariate | Neuron-specific enolase | Tau | Neurofilament light | Glial fibrillary acidic protein |
|---|---|---|---|---|
| P-value | P-value | P-value | P-value | |
| MAP allocation group | 0.86 | 0.02 | 0.68 | 0.20 |
| Age (years) | 0.44 | 0.37 | <0.0001 | <0.0001 |
| Sex | 0.63 | 0.0005 | 0.01 | 0.04 |
| Type of surgery (CABG versus AVR) | 0.27 | 0.27 | 0.11 | 0.26 |
| MAP allocation group-by-time interaction | 0.14 | 0.47 | 0.22 | 0.14 |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; MAP: mean arterial pressure.
In a ‘best case’ sensitivity analysis, where missing biomarker levels were replaced with the lowest recorded value in the MAP allocation group at the given time point, we did not find any significant differences in the development of biomarker levels over time from baseline between the 2 MAP allocation groups for NSE (P = 0.29), tau (P = 0.59), NFL (P = 0.33) or GFAP (P = 0.13).
In a ‘worst case’ sensitivity analysis, where missing biomarker levels were replaced with the highest recorded value in the MAP allocation group at the given time point, we did not find any significant differences in the development of biomarker levels over time from baseline between the 2 MAP allocation groups for NSE (P = 0.15), tau (P = 0.02) and NFL (P = 0.13). We found a significantly different development of GFAP levels over time between MAP allocation groups in the ‘worst case’ scenario (P = 0.006) with GFAP levels being higher in the high MAP allocation group at 48 and 72 h after induction. This difference was driven by a single patient in the high MAP allocation group with a GFAP level of 2673 pg/ml 48 h after induction and a GFAP level of 4765 pg/ml 72 h after induction. This patient had a complicated postoperative course with cognitive impairment postoperatively. The second highest levels of GFAP in the cohort were 982 pg/ml 48 h after induction and 786 pg/ml 72 h after induction. If the outlier (patient no. 115) was excluded, we found no differences in GFAP levels over time between the 2 MAP allocation groups (P = 0.18) in the sensitivity analysis.
DISCUSSION
In this secondary end point analysis of the PPCI trial, we discovered no significant differences in biomarkers of neurological injury in cardiac surgery patients being randomized to a low MAP target of 40–50 mmHg versus a high MAP target of 70–80 mmHg during CPB. This corresponds well with previously published primary and secondary end point analyses, in which no differences were found in volume and number of new cerebral infarcts [9], domain-specific patterns of postoperative cognitive dysfunction [10] or long-term mortality or occurrence of postoperative cognitive dysfunction [11] between the 2 MAP allocation groups.
While the PPCI trial found no clinical differences in clinical end points between high versus low target MAP during CBP, the Cornell Coronary Artery Bypass Outcomes Trial Group Gold found a significantly higher rate of major cardiac and neurological complications in patients randomized to MAP of 50–60 vs 80–100 mmHg; however, in this trial, the high MAP allocation group reached a lower target of 69 ± 7 mmHg [4]. In another RCT, patients with a MAP of 60–70 mmHg had more cognitive dysfunction and delirium compared to patients with a MAP of 80–90 mmHg [6]. Accordingly, the contemporary 2019 EACTS/EACTA/EBCP guidelines on CPB in adult cardiac surgery suggest that MAP should be targeted between 50 and 80 mmHg, and the optimal MAP during CPB remains an area of equipoise [7]. While the PPCI trial found no difference in clinical end points, the trial was powered to detect a 50% reduction in the primary end point (total volume of diffusion weighted imaging verified cerebral infarcts), and it cannot be ruled out that the lack of clinical difference between groups could be a type II error. Therefore, the PPCI trial protocol prespecified secondary analyses of biomarkers of neurological injury, with the purpose of including end points with a higher sensitivity to detect subclinical cerebral injury between the MAP allocation groups [8].
The past decade, several novel biomarkers of cerebral injury have emerged. Neuron-specific enolase (NSE) is a dimeric glycoprotein present in cells of neuroectodermic origin. In out-of-hospital cardiac arrest, NSE is a specific marker of neurological outcome [12–14], and it has been shown as a marker of brain injury after CPB [15]. Tau protein originates from the axonal cytoskeleton, and serum levels of tau have been associated with poor clinical outcome after cardiac arrest [16] and with neurocognitive deficits after CPB [15]. GFAP originating from the astroglia cell cytoskeleton has been associated with diseases of acute brain injury, including hypoxic brain injury, stroke, traumatic brain injury and subarachnoid haemorrhage [17–19], and it has been suggested that GFAP may be a marker of acute brain injury after cardiac surgery [20]. Neurofilaments are a group of proteins integrated in the neuronal and axonal cytoskeleton [21]. Blood (plasma or serum) concentration of NFL is a sensitive, but disease-unspecific, biomarker for both neurodegeneration and acute neuronal injury [21, 22]. Blood NFL has been suggested to serve as a potential biomarker for subtle neuronal injury after circulatory arrest [23], as higher levels after cardiac arrest accurately predict poor clinical outcome [24]. Importantly, a study specifically including patients operated with or without CPB during cardiac surgery found more pronounced increases in blood tau and NFL levels in patients with CPB than in those without [25]. Consistent with previous studies, levels of tau and GFAP reached peak concentrations rapidly following CABG, whereas NFL levels were steadily increasing in the days after CABG [20, 25]. Accordingly, it is possible that NFL levels could separate at a later time point than at discharge.
While our primary and secondary analyses were neutral between the 2 MAP allocation groups, 1 sensitivity analysis, in which missing biomarker values were replaced with the highest recorded value in the same MAP allocation group at the same time point, suggested a significantly higher level of GFAP in the high MAP allocation group. One weakness of this pre-specified sensitivity analysis is that a single outlier can inflate the results to a great degree. In accordance, replacing the missing GFAP levels with the second highest recorded GFAP level resulted in no significant differences between groups, and our results suggest that the difference in the sensitivity analysis was caused by this single outlier.
The intervention in the current study was target MAP; however, it is the likely that the driver behind cerebral injury after cardiac surgery is cerebral oxygenation during CPB, rather than systemic MAP. Accordingly, the PPCI trial used near-infrared spectroscopy in an attempt to assess the regional cerebral oxygen saturation. In contrast to the predefined hypothesis, allocation to the high-target MAP group was associated with lower mean regional oxygen saturation and more pronounced cerebral desaturation load during CPB. It is unknown whether this finding was caused by actual lower cerebral perfusion in the high MAP group or whether the near-infrared spectroscopy signal could have been contaminated by extracranial perfusion [26].
Limitations
The presented results should be interpreted in light of some limitations. Patients randomized to the high MAP allocation group were older compared to patients in the low MAP allocation group, which could confound the associations between MAP allocation and biomarker levels. We did, however, use baseline-corrected linear mixed models that correct for any differences in biomarker levels at baseline, i.e. prior to initiation of the intervention. Furthermore, our results remained robust to adjustment for age, and we consider the presented results valid. In the PPCI trial protocol, in addition to the included biomarkers, we defined the matrix metallopeptidase 9 and ubiquitin C-terminal hydrolase 1 as secondary end points [8]; however, we have not been able to complete those analyses at present. We also chose to analyse tau, although this was not prespecified in the original trial protocol. The reason for this was the promising results of tau as a marker of neurological injury [27], as well as feasibility, as tau was analysed using the same assay as GFAP and NFL. We saw a relatively high frequency of missing blood samples at discharge (13–15%), but a low frequency of missing blood samples at earlier sampling time points (1–5%). The higher frequency of missingness at discharge was primarily caused by logistic difficulties in obtaining blood samples prior to patient discharge. As NSE originates from cells of neuroectodermic origin, it is present in erythrocytes and platelets. Accordingly, haemolysis occurring during CPB can result in falsely elevated NSE levels; however, removing patients with a positive haemolysis index did not alter the results. For all biomarkers of cerebral injury, pre-existing cerebral disease may result in elevated levels; however, we found no differences in biomarker levels at baseline, and our results should be unaffected by this. While any neutral results could be caused by a lack of power, we saw no tendencies to higher biomarker values in one of the 2 randomized MAP allocation groups and it is difficult to clearly define what a clinically important difference would be.
CONCLUSION
In this secondary end point analysis of the PPCI trial, we found no significant differences in daily recorded levels of biomarkers of neurological injury in patients undergoing elective or subacute CABG and/or AVR being randomized to a target MAP of 40–50 mmHg versus a target MAP of 70–80 mmHg during CBP.
Abbreviations
- AVR
Aortic valve replacement
- CABG
Coronary artery bypass grafting
- CPB
Cardiopulmonary bypass
- GFAP
Glial fibrillary acidic protein
- MAP
Mean arterial pressure
- NFL
Neurofilament light
- NSE
Neuron-specific enolase
- PPCI
Perfusion Pressure Cerebral Infarcts
Funding
This work was supported by the Danish Heart Foundation [24-R97-A5179-22868, 15-R99-A6034-22905]; the Research Foundations at Rigshospitalet [E-22329-01]; University of Copenhagen, Denmark, the Lundbeck Foundation [R186-2015-2132]; the Swedish Research Council [#2017-00915, #2018-02532]; the Alzheimer Drug Discovery Foundation (ADDF), USA [#201809-2016615, #201809-2016862]; the European Research Council [#681712]; the Swedish Alzheimer Foundation [#AF-742881]; Hjärnfonden, Sweden [#FO2017-0243]; the Swedish state under the agreement between the Swedish government and the County Councils; the ALF-agreement [#ALFGBG-715986, #ALFGBG-720931]; and NovoNordisk Foundation [NNF17OC0028706 to J.K.].
Conflict of interest: Henrik Zetterberg is a Wallenberg Scholar. All other authors declare no conflict of interest.
Author contributions
Sebastian Wiberg: Formal analysis; Methodology; Writing—original draft. Frederik Holmgaard: Investigation; Methodology; Project administration; Writing—review & editing. Kaj Blennow: Methodology; Resources; Supervision; Writing—review & editing. Jens C. Nilsson: Conceptualization; Funding acquisition; Methodology; Project administration; Supervision; Writing—review & editing. Jesper Kjaergaard: Methodology; Supervision; Writing—review & editing. Michael Wanscher: Methodology; Writing—review & editing. Annika R. Langkilde: Methodology; Writing—review & editing. Christian Hassager: Methodology; Supervision; Writing—review & editing. Lars S. Rasmussen: Conceptualization; Funding acquisition; Methodology; Supervision; Writing—review & editing. Henrik Zetterberg: Methodology; Resources; Supervision; Writing—review & editing. Anne Grønborg Vedel: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Hanna Dorota Golab, Alexander Horke, Jarle Vaage and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Filsoufi F, Rahmanian PB, Castillo JG, Bronster D, Adams DH. Incidence, imaging analysis, and early and late outcomes of stroke after cardiac valve operation. Am J Cardiol 2008;101:1472–8. [DOI] [PubMed] [Google Scholar]
- 2. Tarakji KG, Sabik JF, Bhudia SK, Batizy LH, Blackstone EH. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA 2011;305:381–90. [DOI] [PubMed] [Google Scholar]
- 3. Sun X, Lindsay J, Monsein LH, Hill PC, Corso PJ. Silent brain injury after cardiac surgery: a review: cognitive dysfunction and magnetic resonance imaging diffusion-weighted imaging findings. J Am Coll Cardiol 2012;60:791–7. [DOI] [PubMed] [Google Scholar]
- 4. Gold JP, Charlson ME, Williams-Russo P, Szatrowski TP, Peterson JC, Pirraglia PA et al. Improvement of outcomes after coronary artery bypass. J Thorac Cardiovasc Surg 1995;110:1302–14. [DOI] [PubMed] [Google Scholar]
- 5. Charlson ME, Peterson JC, Krieger KH, Hartman GS, Hollenberg JP, Briggs WM et al. Improvement of outcomes after coronary artery bypass II: a randomized trial comparing intraoperative high versus customized mean arterial pressure. J Card Surg 2007;22:465–72. [DOI] [PubMed] [Google Scholar]
- 6. Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg 2011;40:200–7. [DOI] [PubMed] [Google Scholar]
- 7. Wahba A, Milojevic M, Boer C, De Somer FMJJ, Gudbjartsson T, van den Goor J et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg 2020;57:210–51. [DOI] [PubMed] [Google Scholar]
- 8. Vedel AG, Holmgaard F, Rasmussen LS, Paulson OB, Thomsen C, Danielsen ER et al. Perfusion Pressure Cerebral Infarct (PPCI) trial—the importance of mean arterial pressure during cardiopulmonary bypass to prevent cerebral complications after cardiac surgery: study protocol for a randomised controlled trial. Trials 2016;17:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vedel AG, Holmgaard F, Rasmussen LS, Langkilde A, Paulson OB, Lange T et al. High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial. Circulation 2018;137:1770–80. [DOI] [PubMed] [Google Scholar]
- 10. Vedel AG, Holmgaard F, Siersma V, Langkilde A, Paulson OB, Ravn HB et al. Domain-specific cognitive dysfunction after cardiac surgery. A secondary analysis of a randomized trial. Acta Anaesthesiol Scand 2019;63:730–8. [DOI] [PubMed] [Google Scholar]
- 11. Larsen MH, Draegert C, Vedel AG, Holmgaard F, Siersma V, Nilsson JC et al. Long-term survival and cognitive function according to blood pressure management during cardiac surgery. A follow-up. Acta Anaesthesiol Scand 2020;64:936–44. [DOI] [PubMed] [Google Scholar]
- 12. Tiainen M, Roine RO, Pettilä V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke 2003;34:2881–6. [DOI] [PubMed] [Google Scholar]
- 13. Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 2009;80:784–9. [DOI] [PubMed] [Google Scholar]
- 14. Stammet P, Collignon O, Hassager C, Wise MP, Hovdenes J, Åneman A et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33°C and 36°C. J Am Coll Cardiol 2015;65:2104–14. [DOI] [PubMed] [Google Scholar]
- 15. Ramlawi B, Rudolph JL, Mieno S, Khabbaz K, Sodha NR, Boodhwani M et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg 2006;244:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randall J, Mörtberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation 2013;84:351–6. [DOI] [PubMed] [Google Scholar]
- 17. Schiff L, Hadker N, Weiser S, Rausch C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol Diagn Ther 2012;16:79–92. [DOI] [PubMed] [Google Scholar]
- 18. Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol 2016;12:563–74. [DOI] [PubMed] [Google Scholar]
- 19. Larsson IM, Wallin E, Kristofferzon ML, Niessner M, Zetterberg H, Rubertsson S. Post-cardiac arrest serum levels of glial fibrillary acidic protein for predicting neurological outcome. Resuscitation 2014;85:1654–61. [DOI] [PubMed] [Google Scholar]
- 20. Brunetti MA, Jennings JM, Easley RB, Bembea M, Brown A, Heitmiller E et al. Glial fibrillary acidic protein in children with congenital heart disease undergoing cardiopulmonary bypass. Cardiol Young 2014;24:623–31. [DOI] [PubMed] [Google Scholar]
- 21. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018;14:577–89. [DOI] [PubMed] [Google Scholar]
- 22. Blennow K, Brody DL, Kochanek PM, Levin H, McKee A, Ribbers GM et al. Traumatic brain injuries. Nat Rev Dis Primers 2016;2:16084. [DOI] [PubMed] [Google Scholar]
- 23. Hernández-García C, Rodríguez-Rodríguez A, Egea-Guerrero JJ. Brain injury biomarkers in the setting of cardiac surgery: still a world to explore. Brain Inj 2016;30:10–17. [DOI] [PubMed] [Google Scholar]
- 24. Moseby-Knappe M, Mattsson N, Nielsen N, Zetterberg H, Blennow K, Dankiewicz J et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol 2019;76:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alifier M, Olsson B, Andresson U, Cullen N, Czyżewska J, Jakubów P et al. Cardiac surgery is associated with biomarker evidence of neuronal damage. J Alzheimer Dis 2020;75:1211–20. [DOI] [PubMed] [Google Scholar]
- 26. Holmgaard F, Vedel AG, Lange T, Nilsson JC, Ravn HB. Impact of 2 distinct levels of mean arterial pressure on near-infrared spectroscopy during cardiac surgery: secondary outcome from a randomized clinical trial. Anesth Analg 2019;128:1081–8. [DOI] [PubMed] [Google Scholar]
- 27. Mattsson N, Zetterberg H, Nielsen N, Blennow K, Dankiewicz J, Friberg H et al. Serum tau and neurological outcome in cardiac arrest. Ann Neurol 2017;82:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]