Abstract
Epigenetic modifications are chemical changes that can modify gene expression without changing the sequence of the gene. These modifications are potentially identifiable and reversible, making the epigenome an important area of research for discovering biomarkers to identify those who may be at risk and providing therapeutic interventions to prevent adverse health outcomes. African Americans bear a disproportionate risk of adverse health outcomes (e.g., hypertension, cancer). Indeed, African American women experience preterm birth (PTB; <37 completed weeks gestation) at more than twice the rate of non-Hispanic White women. Research suggests that environmental influences may play a significant role in PTB outcomes for this population. However, the biological pathways by which these influences contribute to PTB are poorly understood. This paper describes research methods and ethical considerations for the collection and analysis of biological samples based on our study examining the epigenetic regulation of stress pathways in PTB in pregnant African American women.
Keywords: Pregnancy, Premature birth, DNA methylation, Gene expression, Ethics
The epigenome consists of chemical “tags” or “marks” that attach to DNA. These marks occur naturally in the developmental/tissue differentiation process but may also change in response to environmental factors or lifestyle (e.g., stress, smoking, diet) (National Institutes of Health, 2020). Some epigenetic marks are reset with each generation; however, some marks may persist from one generation to the next (National Institutes of Health, 2020). Epigenetic modifications are related to the regulation of gene expression and changes in chromatin structure (Feinberg, 2018). DNA methylation (DNAm) is the most studied epigenetic mechanism and occurs primarily at cytosine bases located at cytosine-guanine dinucleotides known as CpG sites (Bird, 2002). DNA methylation is stable, yet potentially reversible, making it an important area of study in relation to biomarkers—marks or altered epigenetic mechanisms that can be measured in body fluids or tissues—for understanding complex health problems, identifying those who may be at risk, and providing therapeutic interventions to prevent adverse health outcomes (García-Giménez et al., 2017). Nursing’s holistic, interdisciplinary approach to health and well-being allows for approaches inclusive of environmental, psychological, and physiological changes that modify health outcomes. Data are limited on collection and analyses of samples for epigenetic and gene expression analysis, particularly among African Americans with whom there are additional ethical challenges. This paper describes the research methods and ethical considerations for the collection and analysis of biologic samples based on our study examining DNAm and gene expression in glucocorticoid candidate genes and PTB in African American women.
Preterm Birth: A Complex Syndrome
Preterm birth (PTB; birth <37 completed weeks gestation) is a serious health problem worldwide. In the United States, after several years of decline, PTB rates have now increased for five consecutive years (2015–2019) (Martin et al., 2020). Surviving infants are at higher risk for respiratory distress syndrome, necrotizing enterocolitis, sepsis, cerebral palsy, developmental and language delays, visual and auditory problems, and long-term respiratory and cardiovascular disease (Beligere et al., 2015; Crump, 2020; Isayama et al., 2017; Lowe et al., 2019; McGowan et al., 2019). African American women experience a significant disparity in PTB rates of more than 1.5 times that of non-Hispanic White women (Hamilton et al., 2020). Previous research has examined environmental factors in African American and Non-Hispanic White women including maternal behavioral determinants (e.g., smoking, nutritional status, short pregnancy intervals), the physical environment (e.g., neighborhood, toxins), and psychosocial factors (e.g., socioeconomic status, anxiety, depression) (Daskalakis et al., 2019; Nowak & Giurgescu, 2017; Sealy-Jefferson et al., 2015; Sealy-Jefferson et al., 2016). While individual environmental and behavioral factors have been associated with PTB, they do not fully explain the high rates of PTB in the United States or the increasing disparity for African American women suggesting that other factors may be involved. Research is quickly evolving around the biological underpinnings of environmental and behavioral influences. However, the pathways by which these changes occur are not yet well understood.
Despite decades of research, there are currently no biomarkers or reliable methods of early prediction for PTB (Polettini et al., 2017). Shortened cervical length or early ripening of the cervix is a predictive mechanism of PTB. However, clinical interventions such as cervical cerclage and progesterone—thought to promote uterine quiescence and decrease inflammation—have had mixed results and have not been shown to significantly reduce rates of PTB (Femandez-Macias et al., 2019; Norman, 2020; Norman et al., 2018; Singh et al., 2020). Two-thirds of PTBs are spontaneous—due to preterm labor and/or preterm premature rupture of membranes (PPROM) rather than a specific medical indication (e.g., shortened cervix, preeclampsia, intrauterine growth restriction) (Goldenberg et al., 2008; Romero et al., 2014). History of a previous PTB continues to be the most important risk factor for subsequent PTB with a 3–6-fold increased risk, suggesting a genetic component (Laughon et al., 2014). However, a study of over one million parent-offspring pairs estimated the heritability of gestational age (GA) at birth at only 13.3–24.5% and found a greater percentage (60.3%) of the variance of GA attributable to individual environmental factors (Wu et al., 2015). These findings suggest other potential pathways by which environmental influences may affect biological changes. The aggregate of these exogenous influences is tightly interwoven with genetic predisposition and biological changes that are associated with PTB.
Epigenetics and Precision Health in Preterm Birth
Epigenomics is one of many “omics.” Omics are defined as technologies that allow for the assessment of biological assays simultaneously in a comprehensive, global way (Conesa & Beck, 2019) and include: genomics (gene interaction and physiological processes), epigenomics (gene modifications not involving the gene sequence), transcriptomics (RNA transcripts produced by the genome), proteomics (protein production and function), metabolomics (metabolic substrates and products), and microbiomics (human microbial colonies) (Institute of Medicine, 2012) (Table 1). Omics studies are important aspects of the expansion of precision health—the ability to direct therapies based on an individual’s genetic, environmental, and behavioral influences (National Institutes of Health, 2019). Identifying women at risk for adverse birth outcomes will allow for opportunities to intervene with personalized therapies that alter or prevent negative birth outcomes such as PTB. This paper describes the research methods and ethical considerations for the collection and analysis of genomic samples that we encountered conducting our study examining the epigenetic regulation of stress pathways in PTB in African American women. The purpose of this study is to contribute to nursing knowledge and understanding of epigenomic research with consideration for minority and vulnerable populations.
Table 1.
Types of Omics Study.
| Type of Omics | Definition | Molecule |
|---|---|---|
| Genomics | Complete DNA sequence of a cell or organism | DNA 
|
| Epigenomics | Reversible chemical modifications to the DNA sequence or histones | DNA 
|
| Proteomics | Complete set of proteins expressed by an organism | Protein 
|
| Transcriptomics | Complete set of RNA transcripts from a cell, tissue, organ, or organism | RNA 
|
| Metabolomics | Complete set of small molecule metabolites and substrates (e.g., amino acids, fatty acids, carbohydrates) | Metabolites 
|
| Microbiomics | Aggregate of microorganisms (microbiota) in the body—bacteria, viruses, fungi | Microorganisms 
|
Epigenetic Regulation of Gene Transcription
The epigenome is the molecular biological family of chemical modifications that affect gene expression without altering the DNA gene sequence (National Human Genome Research Institute, 2016). As opposed to single-nucleotide polymorphisms and mutations within the gene sequence itself, epigenetic modifications or marks occur “above” the nucleotides, regulating the expression of genes without affecting the underlying sequence. These heritable, dynamic marks occur naturally during tissue development and differentiation or as a result of disease or environmental exposures (National Human Genome Research Institute, 2016). Epigenomic research holds promise for the development of novel epigenetic biomarkers with the potential of identifying women at risk for negative birth outcomes (Knight et al., 2018; Knijnenburg et al., 2019; Parets et al., 2013; Wu et al., 2019). The epigenome is complex, but patterns of epigenetic signatures have been identified which alter the way a gene protein or product is expressed. Known epigenetic regulatory modifications include DNA methylation (DNAm), histone modifications, and non-coding RNA modifications (For a review of epigenetic regulatory modifications refer to Feinberg, 2018) (Figure 1). DNAm takes place on the DNA, CpG dinucleotides. Modification of histone tails on chromatin strands occurs through chemical additions by mechanisms including acetylation, methylation, phosphorylation, and ubiquitination. These modifications change how tightly DNA is wrapped around the histone and what portions of the DNA are accessible to transcription by DNA polymerase. Non-coding RNA can control gene expression through chromatin remodeling, promoting the inhibition of gene transcription (Feinberg, 2018). DNAm and subsequent gene expression is a mechanism by which the environment can affect gene expression that ultimately affects health.
Figure 1. Epigenetic Modifications.
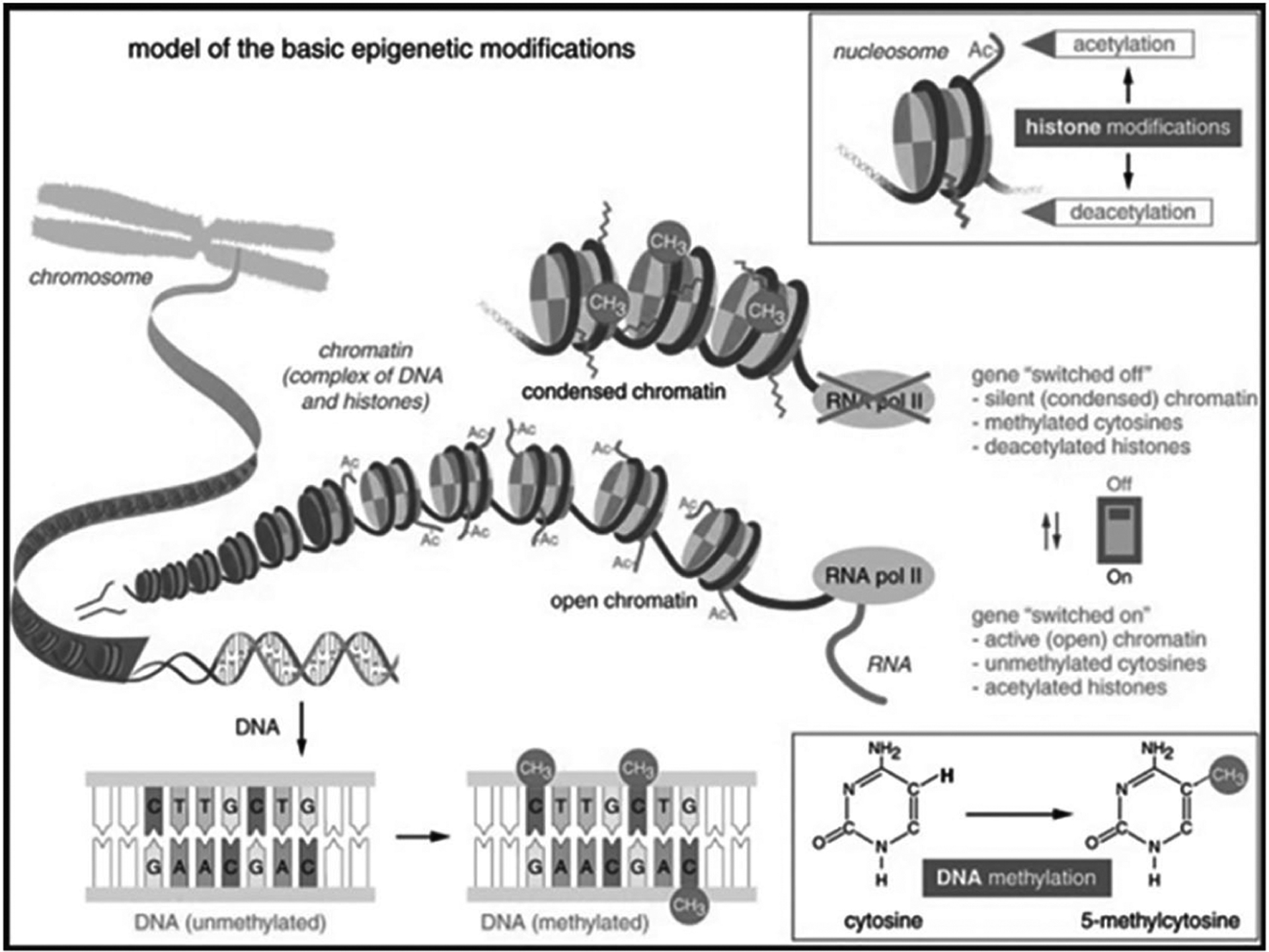
Reprinted from “The role of epigenetics in host-parasite coevolution: Lessons from the model host insects Galleria mellonella and Tribolium castaneum,” by Vilcinskas, A, 2016, Zoology, 119(4) (https://doi.10.1016/j.zool.2016.05.004). Copyright 2016 by the author; licensee Elsevier GmbH. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (BY-NC-ND) license http://creativecommons.org/licenses/by-nc-nd/4.0.
DNAm Regulatory Mechanisms
DNAm involves the covalent addition or removal (demethylation) of a methyl group (CH3) to the fifth carbon of the cytosine ring of a CpG site creating 5-Methylcytosine (5mC) (Rauluseviciute et al., 2019). DNA methyltransferases (DNMT 3a and DNMT 3b) are the enzymes responsible for catalyzing the addition of the methyl group to the CpG dinucleotides during de novo methylation (Okano et al., 1999). DNMT1 is considered to be a maintenance methyltransferase, transferring the methyl mark to the new DNA strand during replication. Another DNA methyltransferase (DNMT3L) increases the affinity of DNMT3a and 3b to co-factor, S-adenosyl-L-methionine (SAM; the universal methyl donor), (Rauluseviciute et al., 2019; Suetake, et al., 2004). Other proteins, Tet Methylcytosine Dioxygenases (TET1, TET2, and TET3), are responsible for the removal of methyl groups (Dor & Cedar, 2019). DNAm mechanisms regulate normal gene expression by providing tissue specificity essential to embryonic development and cell differentiation, X chromosome inactivation, and genomic imprinting (Moore et al., 2013). DNAm also has the potential to silence transposable and viral elements that would otherwise be harmful when integrated into the genome (Moore et al., 2013).
Environmental Influences, the Epigenome, and PTB
The epigenome is not static and changes extensively, not only through the developmental years but throughout the lifespan. For example, epigenetic modifications are subject to environmental influences that include the social and physical environment (e.g., diet, neighborhood disorder) and individual psychological distress (Cao-Lei et al., 2016; Jaenisch & Bird, 2003). Researchers have focused specifically on glucocorticoid (GC; stress-related) pathway genes and their association with psychological distress and PTB (Kertes et al., 2017; Kertes et al., 2016; St.-Pierre et al., 2018; Togher et al., 2018; Togher et al., 2017). Findings from these studies suggest that environmental influences may lead to epigenomic modification of genes regulating the hypothalamic-pituitary-adrenal (HPA) axis—the body’s primary stress-response system. Maternal stress is known to affect birth outcomes (Davalos et al., 2012; Dunkel Schetter & Tanner, 2012). Thus, maternal stress leading to DNAm of HPA axis regulatory genes may be the pathway by which maternal stress leads to PTB. These premises set the stage for our ongoing research focusing DNAm and gene expression of candidate genes obtained from maternal leukocytes and gestational age at birth for 44 pregnant African American women. Methods included the collection of maternal peripheral blood for DNAm and gene expression analysis. Methods for the collection and analysis of biological data must be thoroughly assessed to assure reliable and accurate results. Our nested case-control study was a subsample of women who participated in a larger study. Forty-four African American women—22 women with preterm birth and 22 women with full-term birth (FTB)—18–45 years of age and 8–18 weeks gestation were selected for the study. The women completed questionnaires related to psychological distress and neighborhood disorder and had peripheral blood samples collected in DNA PAXgene (PreAnalytiX Qiagen/BD, Hombrechtikon, Switzerland) blood collection tubes for DNAm analysis. Our goal of identifying biomarkers for women early in pregnancy allowing for interventions for risk reduction drove the timing of blood collection at 8–18 weeks gestation. In the process of conducting our study, we determined that data were lacking in the literature for nurse researchers to gain clarity around methods for collection and analyses of epigenomic samples and specific ethical considerations for African American, pregnant women.
Methods for Methylation Measurement
There are numerous methods available for the measurement of DNAm (Table 2). These include enzyme digestion, affinity-based enrichment, and sodium bisulfite conversion. Enzyme digestion approaches (e.g., Methyl-seq and Methylation Sensitive Cut Counting) are often used to study the methylation status of CpG Islands. These approaches use restriction endonucleases, which are short strings of identical bases on both DNA strands to detect and cleave specific DNA sequences (Brahmachari & Jain, 2013). This type of methylation identification is limited to the specific sequences recognized by the selected enzymes (Brahmachari & Jain, 2013).
Table 2.
Methods for Methylation Measurement.
| Methods | Advantages | Disadvantages | |
|---|---|---|---|
| Candidate Gene Approach | |||
| Sanger Sequencing | Chain-termination sequencing using capillary gel electrophoresis | High quality | High cost; small scale sequencing |
| Pyrosequencing | Sequencing by synthesis method that detects how many complementary nucleotides have been incorporated into a strand | High quality; low cost; faster than Sanger Sequencing | Small scale sequencing |
| Methylation-Specific PCR (e.g., MethyLight) | Determines DNAm status of target loci with specific primers and probes | ||
| Mass Spectrometry (e.g., EpiTyper) | After bisulfite conversion, uses base-specific cleavage, and mass spectrometry to quantify® DNA fragments | Reliable; cost-effective | |
| Whole Genome Approach | |||
| Enzyme Digestion (e.g., Met-seq1; MSCC2) | Uses restriction endonucleases, to detect and cleave specific DNA sequences | Low cost | Limited to sequences recognized by selected enzymes |
| Affinity-Based Antibody Enrichment (e.g., Me-DIP-seq3; MBD-seq4) | Anti-5methylcytosine antibodies are used to isolate methylated DNA followed by microarray | Low cost | Low resolution |
| Microarray (e.g., Illumina 850k EPIC BeadChip Array) | Quantification of all known genes, promoter sites, and enhancer regions of bisulfite converted DNA | Single CpG site resolution with small samples | High cost; limited to known CpG sites and subject to probe-type biases |
| Whole Genome Bisulfite Sequencing (WGBS) | Uses bisulfite converted DNA for DNAm analysis across the genome | High-quality interrogation w/o bias; DNAm status of single CpG sites | Higher cost |
| Reduced Representation Bisulfite Sequencing (RRBS) | Uses restriction enzymes and bisulfite conversion for DNAm analysis of the subset of the genome | High-quality interrogation w/o bias; DNAm status of single CpG sites | Lower cost; focuses on smaller regions instead of precise CpG sites |
| Enhanced Reduced Representation Bisulfite Sequencing (ERRBS) | Uses restriction enzymes and bisulfite conversion for DNAm analysis of biologically relevant loci | High-quality interrogation w/o bias; DNAm status of single CpG sites | Lower cost; focuses on biologically relevant loci |
Methyl-Seq = methylation sequencing;
MSCC = Methylation Sensitive Cut Counting;.
Me-DIP-seq = Methylated DNA Immunoprecipitation;
MBD-seq = methylated CpG-binding.
Affinity-based, antibody enrichment methods include methylated DNA immunoprecipitation (e.g. MeDIP-seq) and methylated CpG-binding proteins (e.g., MBD-seq) to provide access to methylated regions for sequencing (Barros-Silva et al., 2018). Non-methylated portions are washed away and the remaining methylated portions are sequenced (Barros-Silva et al., 2018). This technique is generally low cost, but also has relatively low resolution, and is susceptible to copy number variation bias and bias toward observations of methylated sites (Singer, 2019).
Bisulfite conversion is the most commonly used and considered the gold standard technique for DNAm analysis (Kurdyukov & Bullock, 2016; Singer, 2019). During the bisulfite conversion process, genomic DNA is first denatured into single strands, then treated with sodium bisulfite, resulting in the deamination of unmethylated cytosine into uracil and then to thymine during polymerase chain reaction (PCR) analysis (Singer, 2019). The methylated cytosine remains unchanged, and the resulting sequences are then compared with the unconverted original sample to determine which cytosines were methylated (Singer, 2019). Bisulfite conversion methods provide single-cell resolution and require lower input than other methods. However, they are more labor-intensive because bisulfate-treated sequences are no longer complementary and must be computationally aligned (Barros-Silva et al., 2018).
Recently, bisulfite conversion techniques have been introduced utilizing alternative cytosine modifications, for example, 5-hydroxymethylcytosine (5hmC) detection that results from oxidization of the methylated cytosine (5mc) (Barros-Silva et al., 2018; Singer, 2019). Although there are numerous methods to assess DNAm, bisulfite conversion followed by high-throughput sequencing is a cost-effective, reliable, and popular method for assessing DNAm.
The method selected for differential DNAm (DNAm profiling), subsequent to bisulfite sequencing, depends initially on whether the researcher aims to study methylation of candidate genes or whole-genome methylation. Other considerations include the amount and quality of the samples, the availability of equipment, reagents, access to bioinformatics analysis, and of course, cost (Kurdyukov & Bullock, 2016). Following bisulfite conversion, specific PCR amplification of a specific segment of DNA in a candidate gene (amplicons) can be cloned and sequenced using pyrosequencing, Sanger-based sequencing (the original “gold standard”), methylation-specific quantitative PCR (qPCR) (e.g., EpTect; MethyLight), or mass spectrometry using EpiTYPER® assay (Agena Biosciences) (Sant & Goodrich, 2019). EpiTYPER® assay uses base-specific cleavage and mass spectrometry to quantify DNA fragments (Ehrich et al., 2005). Approaches for whole-genome DNAm profiling include microarrays and next-generation sequencing (NGS) technologies. NGS options include whole-genome bisulfite sequencing (WGBS), reduced representation (RRBS), and enhanced reduced representation bisulfite sequencing (ERRBS). Each quantifies DNAm at CpG sites but varies in CpG site coverage and cost (Sant & Goodrich, 2019). After bisulfite conversion, library preparation, and sequencing, reads are mapped onto a reference genome and cytosine versus thymine is quantified at each CpG site as a ratio of methylated to unmethylated cytosines (Sant & Goodrich, 2019). WGBS is of high quality and high cost. RRBS and EERBS may be preferable NGS solutions in terms of cost and manageability of data (Sant & Goodrich, 2019). However, these solutions focus on relevant regions and the exact CpG sites may not be sequenced for each experiment making comparisons difficult (Sant & Goodrich, 2019).
Illumina Infmium® BeadChips are probe-based arrays that provide comprehensive quantification of all known human genes, promoter sites, and enhancer regions (Sant & Goodrich, 2019). Probes, small sections of DNA coupled with a fluorescent tag, are attached to a silica bead. The probes hybridize (combine) with complementary DNA sequences in the sample and fluoresce allowing for identification. The Infinium® MethylationEPIC 850K bead chip samples 95% of CpG islands, including more than 850,000 CpG sites, and utilizing 99% of RefSeq promotors covering the genome (Illumina Inc., 2016). Probes (methylated and unmethylated bead types) are used to differentiate between cytosine and uracil, a labeled dideoxynucleotide triphosphate (ddNTP) is incorporated during probe extension and stained with a fluorescent reagent (Illumina Inc., 2020b). Methylation level is determined by examining the ratio of fluorescent signals from the methylated and unmethylated sites (Illumina Inc., 2020b). BeadChip arrays have the advantage of single CpG site resolution with small (500ng) bisulfite-converted DNA samples; however, unlike NGS, CpG coverage is limited to known CpG sites and subject to probe-type biases (Sant & Goodrich, 2019).
Our study aimed to examine candidate genes. We originally planned to utilize mass spectrometry with EpiTYPER® (Agena Biosciences) assays—a cost-effective and popular method for candidate gene analyses. However, from the time of planning stages until DNAm analysis, the cost of bead chip analysis continued to decline, leading to our choice to utilize bisulfite conversion follow by Infinium® MethylationEPIC 850K bead chip analysis (Illumina Inc., 2016). In addition to the select CpG site data for the candidate genes, another benefit of the bead chip array is that it provides whole-genome methylation data for thousands of additional CpG sites for future analyses.
Measurement of Gene Expression
Once differential DNAm is identified, the effect of that difference is measured by gene expression analysis. In other words, how did DNAm of the gene change the expression of the gene product or protein? Prior to gene expression analysis, collection and storage of blood samples are of the utmost importance for the preservation of RNA. Because RNA is unstable, peripheral blood must be immediately cryopreserved in the clinic or collected in RNA preservation storage media that are commercially available, such as RNAlater Stabilization Reagent (Thermo Fisher Scientific, Waltham, MA), PAXgene Blood RNA tubes (PreAnalytiX Qiagen/BD, Hombrechtikon, Switzerland), or Tempus Blood RNA tubes (Applied Biosystems, Foster City, CA) (Rodríguez et al., 2020). The tubes are then frozen for short-term storage or cryopreserved at −80°C for later extraction. Carefully planned procedures for collection, storage, and extraction of RNA are crucial to preserving the quality and quantity of RNA for accurate gene expression quantification (Rodríguez et al., 2020) (Figure 2). Common RNA isolation methods are organic/phenol-chloroform extraction (e.g., Trizol), spin columns, and magnetic particles (Edinburgh CRF Genetics Core). Commercial kits are readily available for each of these methods. Isolated RNA quantity and quality are traditionally measured with Ultraviolet (UV) spectrophotometry. Fluorometry is another way to measure the quantity and quality of RNA. Fluorometers measure the intensity of fluorescent excitation (Ex) and emission (Em) wavelengths. UV and fluorescent light absorbance of RNA samples are measured between 260 and 280 nanometers (nm) (ThermoFisher Scientific, n.d.). A linear change in absorbance ratio (A260/A280) is calculated, and a 1.8 to 2.1 ratio is considered to be pure (ThermoFisher Scientific, n.d.).
Figure 2.

Gene Expression Analysis.
Two methods for analyzing gene expression from isolated, purified complementary strands of DNA (cDNA) include real-time, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and RNA sequencing (RNA seq). qRT-PCR is the “gold standard” for gene expression quantification (Adamski et al., 2014). RNA-seq is used for analyzing gene expression utilizing a sequencing library and a high-throughput platform (Stark et al., 2019). Because RNA and RNA polymerase are unable to withstand thermocycler conditions, mRNA is used as a transcript to create cDNA which is then used as an indirect measure of RNA expression. Sybr Green fluorescent dye or TaqMan® (Applied Biosystems, Foster City, CA) probes attach to cDNA template (primers) and the amount of cDNA is measured in real-time after each amplification cycle by an increased fluorescent signal proportionate to the number of replicated DNA fragments, or amplicons, generated (Applied Biosystems, 2014). The signal is plotted against the cycle number and it represents the accumulation of amplicons over the course of the experiment (Applied Biosystems, 2014). Threshold is the point at which a fluorescent signal is significantly above background fluorescence, and a detectable amount of amplicon product has been generated (Applied Biosystems, 2014). The threshold cycle is directly proportional to the original relative expression level of the selected gene. Mean +/− SEM for the relative amount of target genes in each group is calculated by the 2-ΔΔCT method (Livak & Schmittgen, 2001). Housekeeping genes are those required basic cellular functions, and their expression is normally very stable. They are used in qRT-PCR (and other biological techniques) as reference genes to correct for variation between samples.
While qRT-PCR has been the gold standard for the measurement of gene expression for many years, as costs continue to decline, RNA seq is quickly becoming the new standard (Stark et al., 2019). RNA seq is a high-throughput, massively parallel sequencing technique. Briefly, RNA is extracted and purified from the tissue of interest. The mRNA is converted to cDNA and fragmented into similarly sized pieces (Kukurba & Montgomery, 2015). Adaptors are added so that the fragments can bind to the sequencer, and a cDNA library is created. The library is analyzed on the sequencer, and the sequenced reads are then mapped onto the genome and quantified (Kukurba & Montgomery, 2015). Neither qRT-PCR nor RNA seq is without biases; however, there are several advantages with RNA seq technology. For example, with qRT-PCR, probes and primers are selected to interrogate known sequences for differential gene expression (Wang et al., 2009). RNA seq analyses uses digitally aligned read counts to detect RNA transcripts giving a very broad look at both known and novel sequences (Xiong et al., 2017). For targeted studies, RNA seq can provide a greater depth of sequencing to reveal gene expression in very small amounts (Illumina Inc., 2020a).
Research involving biological specimens must carefully consider numerous ethical factors of the study prior to determining methods for collection and analysis. Consideration for the study population is particularly important and requires that all aspects of responsible and ethical conduct of research are met.
Ethical Considerations in Omics Research
Conducting research with underrepresented groups presents unique challenges based on historical ethical violations. These issues must be kept in mind so that they are recognized and addressed. As we examine the universal principles of ethical treatment of human subjects in research, considerations for African American participants must include an understanding of the history of racism and systemic injustice in biomedical research, medical communities, and institutions. Two well-known examples are the Tuskegee Study of Untreated Syphilis and the injustices befalling Henrietta Lacks (Centers for Disease Control and Prevention (CDC, 2021; Skloot, 2010). Over a period of 40 years, the United States Public Health Service conducted the Tuskegee syphilis study to observe the effects of untreated syphilis (Centers for Disease Control and Prevention (CDC, 2021). Hundreds of African American men who were affected with syphilis were included in the study. They were not told of their diagnosis, nor were they treated for this curable disease. Instead, the men unknowingly spread syphilis to their partners, and the disease was allowed to progress to end-stage neurosyphilis until the study was finally halted in 1972 (Centers for Disease Control and Prevention (CDC, 2021; Skloot, 2010). In 1951, a segregated Johns Hopkins Hospital treated Henrietta Lacks, an African American woman, for cervical cancer (Skloot, 2010). During the course of treatment, Henrietta Lack’s malignant cervical cells were collected without her consent or knowledge and unexpectantly continued to multiply in vitro (Skloot, 2010). These cells were the start of the first immortal human cell line—“HeLa” cells. The value of the cell line in scientific research and discovery is immeasurable. Moreover, sales of the cells resulted in millions of dollars in profit from which neither Henrietta Lacks nor her family benefitted (Skloot, 2010). The Belmont Report and subsequent codification of the Common Rule arose from such atrocities.
The Belmont Report
The Belmont report requires that three ethical principles be followed when conducting research with human subjects—respect for persons, beneficence, and justice (U.S. Department of Health & Human Services (HHS), 1979). Respect for persons requires recognizing that people must be autonomous in their choices and that some groups require additional protections based on the risk of personal harm versus benefits (U.S. Department of Health & Human Services (HHS), 1979). Beneficence encapsulates the principle to first do no harm, and second, to maximize overall benefits while minimizing harm (U.S. Department of Health & Human Services (HHS), 1979). Justice is based on the fairness of distribution and relates to exclusion of participation in studies due to financial barriers or benefits of publicly funded research (e.g., therapies or procedures) given only to those who can afford them (U.S. Department of Health & Human Services (HHS), 1979). Despite advances in the area of bioethics, many African Americans remain understandably distrustful. At the same time, amid the distrust about genomic research, African Americans believe participation in genomic/omic research is critical (Scherr et al., 2019). Researchers conducting omics research must pay particular attention to health inequalities and disparities that are born of the inequitable distribution of resources and proceed with respect for the community and with an understanding of specific sociocultural needs and ethical considerations for performing omics research (Taylor et al., 2018; Williams & Anderson, 2018).
The Common Rule
In 2017, the revised Common Rule (45 U.S.C. § 46) (U.S. Department of Health & Human Services [HHS], 2017) was adopted to enhance protections for human subjects research in light of changes in the nature of research. The use of omics technologies must align with principles set forth by the Belmont Report (Williams & Anderson, 2018).
The Common Rule revisions (45 CFR §46) for pregnant women and fetuses require several components for consideration depending upon whether the study involves the pregnant woman and/or the fetus and whether the study is categorized as minimal risk or greater than minimal risk. In addition, a risk-benefit analysis must be included along with other specific informed consent requirements for this protected group.
Informed Consent.
Other requirements under the revised Common Rule (HHS, 2017) relate to the content, organization, and presentation within consent forms including the requirement that explanations involving genomic research must be understandable and facilitate the participant’s decision-making process. Specifically, when genomic information is collected, participants must be advised as to whether identifiers will be removed, and if so, the process for their removal from the data. In addition, a statement must be included that specifies if whole-genome sequencing will be performed and whether biospecimens (de-identified or not) may be used for commercial profit. Informed consent is also required to state whether research results, including individual results, will be disclosed to participants (HHS, 2017). Difficulty in understanding the informed consent document is recognized in the literature and is further enhanced with the addition of omics data (Alexa-Stratulat et al., 2018; Williams & Anderson, 2018). Thus, informed consent must state the purpose of the omics research and its risks and benefits in language that is understandable to potential participants and whether the biospecimens will be de-identified and whether study results will be disclosed to participants (HHS, 2017). The internal review board (IRB) for this study required that consent forms be written at no more than an eighth-grade reading level. The consent form states specifically that biospecimens will be de-identified, the precise de-identification process utilized, that study results will not be disclosed, nor will publications resulting from the research contain any identifying information.
Broad Consent.
In addition to informed consent, broad consent was added as an option to researchers in the 2017 revisions to the Common Rule 45 CFR §46.116(d) (HHS, 2017). Broad consent for future use of identifiable biological specimens must include: (1) a description of the types of research that may be conducted in the future research, (2) a description of how the private information or identifiable biospecimens will be shared and the types of institutions where the research might take place, (3) a description of the period of time that the samples will be stored and maintained, and (4) that participants will not receive study results (Maloy & Bass, 2020; Williams & Anderson, 2018).
Privacy
Privacy is an ethical concern tied to the principle of beneficence and the duty to do no harm. The Health Insurance Portability and Accountability Act of 1996 (HIPAA; United States, 2004) is legislation that provides for data privacy for personal health information (PHI) and security provisions for safeguarding medical information. Privacy becomes more confusing for omics specimens. The Genetic Information Nondiscrimination Act (GINA; United States, 2009) provides protections against using genetic information to discriminate in health insurance and employment. Research participants are provided some protection under GINA so that if employers and health insurance companies somehow obtain genetic information, they cannot use it to discriminate (Braverman et al., 2018). However, GINA does not apply to life insurance or long-term care insurance. Even when data are de-identified, a legitimate argument can be made that genetic data is never truly de-identified and can feasibly be traced back to an individual (Braverman et al., 2018). Yet, biospecimens have not been deemed inherently identifiable. Informed consent for research involving biospecimens should provide a statement of protections that are or are not provided under GINA in addition to the required HIPAA disclosure.
Discussion
The components of precision health are to predict, prevent, and cure (Minor & Rees, 2020). These goals are driven by advances in science and technology, and they are based on our understanding of biological processes and the application of that knowledge to health challenges (Minor & Rees, 2020). “Precision medicine seeks to redefine our understanding of disease onset and progression, treatment response, and health outcomes through the more precise measurement of molecular, environmental, and behavioral factors that contribute to health and disease.” (PMI Working Group, 2015). Identifying women at risk for PTB through environmentally influenced epigenetic biomarkers and the translation into interventions that prolong pregnancy or prevent PTB in African American women represents a clear example of the “promise” of precision medicine (National Institutes of Health, 2019). The complexity of PTB will require a multidisciplinary approach that includes not only expertise across health disciplines, but also omics, informatics, and the fundamental sciences (Minor & Rees, 2020). Stevenson, et al., 2020, suggests a “transdisciplinary research enterprise” to integrate and address the complex risks and determinants of PTB. Omics studies are an essential component of precision health. Approaches, designs, and methods vary with the research question, the phenotype of interest, the feasibility of data management and analysis, collection and storage capability, and financial limitations. Careful consideration of sample collection and methods for nucleic acid extraction and analysis is required to protect the integrity of the samples.
Our study was designed to examine maternal peripheral leukocytes early in pregnancy and analyze DNAm differences in six glucocorticoid candidate genes among women with PTB and those with FTB. Although patterns of differential DNAm may be identified, mRNA expression analysis is needed to determine whether the DNAm differences result in actual changes in gene expression. Changes in expression of gene protein products as a result of environmental stressors that modify DNAm and modify gene expression may shed light on the pathways that lead from maternal stress to PTB in African American women.
Biomedical research requires additional considerations for research involving African Americans given the systematic historical and present-day implicit and systemic racism that has resulted in social inequities (e.g., redlining, segregation, mortgage discrimination) and health disparities (Richardson, 2020). There remains a “steep social gradient that characterizes who becomes sick and who dies” (Bayer & Galea 2015, p. 500). Health equity defined by the World Health Organization (WHO, 2019) states that every person should have an equal opportunity to obtain health care and that no one should be prevented from attaining their full health potential. Sadly, this is not the case. In fact, the Centers for Disease Control (CDC, 2017) reports that African Americans die younger from all causes than their White counterparts. Black women experience higher rates of death from heart disease, breast cancer, and cervical cancer (Kaiser Family Foundation, 2020) and over twice the death rates for pregnancy-related causes than non-Hispanic White women (37.1 and 14.7 per 100,000 live births, respectively) (CDC, 2020). Thus, study design and protocol require a thorough examination of both practical and ethical concerns particularly when researching African American populations. Researchers must be mindful of both the historical as well as present-day oppression and injustices. Recent Black Lives Matter movements against police violence and disproportionately high COVID-19 death rates among African Americans provide examples of current disparate impacts on African American communities. Social and physical environments in which we are born and live in shape the trajectory of diseases and are important factors that must be considered in biomedical research and precision medicine (Gillespie et al., 2019).
Study procedures must include considerations of trust and privacy. Transparency in all study procedures is a priority for building trust within the study population. For example, in addition to the content of the consent form and oral consent procedures, study brochures and flyers were posted to help to fully explain and reiterate the study goals and procedures, steps for confidentiality, assurance that participation is optional, and participants may withdraw at any time. In addition, the women were consented to in the exam rooms to promote privacy.
Community-based, participatory research (CBPR) is another approach for researchers to gain awareness, assess the needs, and build trust within the community. CBPR involves working with community members, groups, or organizations and engaging those individuals in shared decision-making in research study aspects from development through data analysis and dissemination (Israel et al., 2010; Israel et al., 1998). These partnerships help to build trust, increase engagement, and create social change (Wallerstein & Duran, 2010).
Nurse researchers are well-positioned to advance health through collaborative, interdisciplinary research of biomedical sciences that are inclusive of social, environmental, and behavioral influences. Biomedical research is evolving at a precipitous pace, and nursing research must advance in step with its rapid movement. Developing an understanding of biological pathways is important to the discovery of biomarkers and the advancement of precision health initiatives.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ruth L. Kirschstein National Research Service Awards (F31NR018363; T32NR014225), the Association of Women’s Health, Obstetric and Neonatal Nurses, the International Society of Nurses in Genetics, The Ohio State University Alumni Graduate School, The Ohio State University College of Nursing, the Midwest Nursing Research Society, and Sigma Theta Tau International, Epsilon Chapter.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adamski MG, Gumann P, & Baird AE (2014). A method for quantitative analysis of standard and high-throughput qPCR expression data based on input sample quantity. PLoS One, 9(8), e103917. 10.1371/joumal.pone.0103917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa-Stratulat T, Neagu M, Neagu AI, Alexa ID, & Ioan BG (2018). Consent for participating in clinical trials - is it really informed? Developing World Bioethics, 18(3), 299–306. 10.1111/dewb.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applied Biosystems. (2014). Real-time PCR handbook. ThermoFisher Scientific, https://www.thermofisher.com/content/dam/LifeTech/global/Forms/PDF/real-time-pcr-hand-book.pdf [Google Scholar]
- Barros-Silva D, Marques CJ, Henrique R, & Jeronimo C (2018). Profiling DNA methylation based on next-generation sequencing approaches: New insights and clinical applications. Genes, 9(9), 429. 10.3390/genes9090429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R, & Galea S (2015). Public health in the precision-medicine era. The New England Journal of Medicine, 373(6), 499–501. 10.1056/NEJMp1506241 [DOI] [PubMed] [Google Scholar]
- Beligere N, Perumalswamy V, Tandon M, Mittal A, Floora J, Vijayakumar B, & Miller MT (2015). Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Seminars in Fetal & Neonatal Medicine, 20(5), 346–353. 10.1016/j.siny.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Bird A (2002). DNA methylation patterns and epigenetic memory. Genes & Development, 16(1), 6–21. 10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- Brahmachari V, & Jain S (2013). Methylation-sensitive restriction endonucleases. In Dubitzky W, Cho KH, & Yokota H (Eds.), Encyclopedia of systems biology (pp. 1300–1301). Springer. 10.1007/978-1-4419-9863-7_852 [DOI] [Google Scholar]
- Braverman G, Shapiro ZE, & Bernstein JA (2018). Ethical issues in contemporary clinical genetics. Mayo Clinic Proceedings: Innovations, Quality & Outcomes, 2(2), 81–90. 10.1016/j.mayocpiqo.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lei L, de Rooijb SR, Kinga SG, Matthew SC, Metzd GAS, Roseboome TJ, & Szyff M (2016). Prenatal stress and epigenetics. Neuroscience and Biobehavioral Reviews, 117,198–210. 10.1016/j.neubiorev.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2017). African American health: Creating equal opportunities for health. Centers for Disease Control and Prevention, https://www-cdc-gov.proxy.lib.ohio-state.edu/vitalsigns/pdf/2017-05-vitalsigns.pdf [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2020). First data released on maternal mortality in over a decade. National Center for Health Statistics, https://www-cdc-gov.proxy.lib.ohio-state.edu/nchs/pressroom/nchs_press_releases/2020/202001_MMR.htm [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2021). U.S. Public health service syphilis study at Tuskegee. U.S. Department of Health & Human Services, https://www-cdc-gov.proxy.lib.ohio-state.edu/tuskegee/index.html [Google Scholar]
- Conesa A, & Beck S (2019). Making multi-omics data accessible to researchers. Scientific Data, 6, 251. 10.1038/s41597-019-0258-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump C (2020). Preterm birth and mortality in adulthood: A systematic review. Journal of Perinatology, 40(6), 833–843. 10.1038/s41372-019-0563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis G, Arabin B, Antsaklis A, & Roura LC (2019). Preterm labor: Up to date. BioMed Research International, 2019, 1–2. 10.1155/2019/4870938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Yadon C, & Tregellas H (2012). Untreated prenatal maternal depression and the potential risks to offspring: A review. Archives of Women’s Mental Health, 15(1), 1–14. 10.1007/s00737-011-0251-1 [DOI] [PubMed] [Google Scholar]
- Dor Y, & Cedar H (2019). Principles of DNA methylation and their implications for biology and medicine. Lancet, 392(10149), 777–786. 10.1016/S0140-6736(18)31268-6 [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, & Tanner L (2012). Anxiety, depression and stress in pregnancy implications for mothers, children, research, and practice. Current Opinion in Psychiatry, 25(2), 141–148. 10.1097/YCO.0b013e3283503680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, Cantor CR, Field JK, & van den Boom D (2005). Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proceedings of the National Academy of Sciences - PNAS, 102(44), 15785–15790. 10.1073/pnas.0507816102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP (2018). The key role of epigenetics in human disease prevention and mitigation. The New England Journal of Medicine, 378(14), 1323–1334. 10.1056/NEJMra1402513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Macias R, Figueras F, Martinez-Portilla RJ, & Palacio M (2019). A systematic review and meta-analysis of randomized controlled trials comparing 17-alpha-hydroxypro-gesterone caproate versus placebo for the prevention of recurrent preterm birth. International Journal of Gynecology and Obstetrics, 147(2), 156–164. 10.1002/ijgo.12940 [DOI] [PubMed] [Google Scholar]
- García-Giménez JL, Seco-Cervera M, Tollefsbol TO, Romá-Mateo C, Peiró-Chova L, Lapunzina P, & Pallardó FV (2017). Epigenetic biomarkers: Current strategies and future challenges for their use in the clinical laboratory. Critical Reviews in Clinical Laboratory Sciences, 54(7–8), 529–550. 10.1080/10408363.2017.1410520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Hardy LR, & Anderson CM (2019). Patterns of DNA methylation as an indicator of biological aging: State of the science and future directions in precision health promotion. Nursing Outlook, 67(4), 337–344. 10.1016/j.outlook.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, & Romero R (2008). Epidemiology and causes of preterm birth. The Lancet, 371(9606), 75–84. http://www.scopus.com/inward/record.url?eid=2-s2.0-37449004386&partnerID=40&md5=24ce927d7922593db7d65abbffcce548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, & Osterman MJK (2020). Births: Provisional data for 2019. Vital statistics rapid release. National Center for Health Statistics; Retrieved from https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf [PubMed] [Google Scholar]
- Illumina Inc. (2016). Feld guide to methylation methods. Illumina Inc. https://www.illumina.com/content/dam/illumina-marketing/documents/products/other/field_guide_methylation.pdf [Google Scholar]
- Illumina Inc. (2020a). Coverage depth recommendation: Learn how to estimate the depth of sequencing coverage needed for your research. Illumina Inc. https://www.illumina.com/science/technology/next-generation-sequencing/plan-experiments/coverage.html [Google Scholar]
- Illumina Inc. (2020b). Infinium methylation assay. Illumina, Inc. https://www.illumina.com/science/technology/microarray/infinium-methylation-assay.html [Google Scholar]
- Institute of Medicine. (2012). Omics-based clinical discovery: Science, technology, and applications. National Academies Press. https://www.ncbi.nlm.nih.gov/books/NBK202165/ [Google Scholar]
- Isayama T, Lewis-Mikhael AM, O’Reilly D, Beyene J, & McDonald SD (2017). Health services use by late preterm and term infants from infancy to adulthood: A meta-analysis. Pediatrics, 140(1), e20170266. 10.1542/peds.2017-0266 [DOI] [PubMed] [Google Scholar]
- Israel BA, Coombe CM, Cheezum RR, Schulz AJ, McGranaghan RJ, Lichtenstein R, Reyes AG, Clement J, & Burris A (2010). Community-based participatory research: A capacity-building approach for policy advocacy aimed at eliminating health disparities. American Journal of Public Health, 100(11), 2094–2102. 10.2105/AJPH.2009.170506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, & Becker AB (1998). Review of community-based research: Assessing partnership approaches to improve public health. Annual Review of Public Health, 19(1), 173–202. 10.1146/annurev.publ-health.19.1.173 [DOI] [PubMed] [Google Scholar]
- Jaenisch R, & Bird A (2003). Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nature Genetics, 33, 245–254. 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. (2020). Health status indicators: Deaths. Kaiser Family Foundation, https://www.kff.org/state-category/health-status/deaths-health-status/ [Google Scholar]
- Kertes DA, Bhatt SS, Kamin HS, Highes DA, Rodney NC, & Milligan C (2017). Bdnf methylation in mothers and newborns is associated with maternal exposure to war trauma. Clinical Epigenetics, 9(1), 68. 10.1186/s13148-017-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, & Mulligan CJ (2016). Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic republic of Congo. Child Development, 87(1), 61–72. 10.1111/cdev.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AK, Conneely KN, Kilaru V, Cobb D, Payne JL, Meilman S, Corwin EJ, Kaminsky ZA, Dunlop AL, & Smith AK (2018). Slc9b1 methylation predicts fetal intolerance of labor. Epigenetics, 13(1), 33–39. 10.1080/15592294.2017.1411444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijnenburg TA, Vockley JG, Chambwe N, Gibbs DL, Humphries C, Huddleston KC, Klein E, Kothyal P, Tasseff R, Dhankani V, Bodian DL, Wong WSW, Glusman G, Maulden DE, Miller M, Slagel J, Elasady S, Roach JC, Kramer R, … Neiderhuber JE (2019). Genomic and molecular characterization of preterm birth. Proceedings of the National Academy of Sciences - PNAS, 116(12), 5819–5827. 10.1073/pnas.1716314116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukurba KR, & Montgomery SB (2015). Rna sequencing and analysis. Cold Spring Harbor Protocols, 2015(11), 951–969. 10.1101/pdb.top084970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyukov S, & Bullock M (2016). DNA methylation analysis: Choosing the right method. Biology (Basel), 5(1), 3. 10.3390/biology5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon SK, Albert PS, Leishear K, & Mendola P (2014). The NICHD consecutive pregnancies study: Recurrent pre-term delivery by subtype. American Journal of Obstetrics and Gynecology, 210(2), 131.e131–131.e138. 10.1016/j.ajog.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-δδct method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lowe JR, Fuller JF, Do BT, Vohr BR, Das A, Hintz SR, Watterberg KL, & Higgins RD (2019). Behavioral problems are associated with cognitive and language scores in toddlers born extremely preterm. Early Human Development, 128, 48–54. 10.1016/j.earlhumdev.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy JW, & Bass PF (2020). Understanding broad consent. The Ochsner Journal, 20(1), 81–86. 10.31486/toj.19.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, & Osterman MJK (2020). Births in the United States, 2019. National Center for Health Statistics Data Brief; [PubMed] [Google Scholar]
- McGowan EC, & Vohr BR (2019). Neurodevelopmental follow-up of preterm infants. The Pediatric Clinics of North America, 66(2), 509–523. 10.1016/j.pcl.2018.12.015 [DOI] [PubMed] [Google Scholar]
- Minor L, & Rees M (2020). Discovering precision health: Predict, prevent, and cure to advance health and well-being. John Wiley & Sons, Ltd. [Google Scholar]
- Moore LD, Le T, & Fan G (2013). DNA methylation and its basic function. Neuropsychopharmacology, 38(1), 23–38. 10.1038/npp.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Human Genome Research Institute. (2016). Epigenomics fact sheet. National Human Genome Research Institute, https://www.genome.gov/27532724/ [Google Scholar]
- National Institutes of Health. (2019). The promise of precision medicine. National Institutes of Health, https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/promise-precision-medicine [Google Scholar]
- National Institutes of Health. (2020). Epigenomic fact sheet. National Human Genome Research Institute. https://www.genome.gov/about-genomics/fact-sheets/Epigenomics-Fact-Sheet [Google Scholar]
- Norman JE (2020). Progesterone and preterm birth. International Journal of Gynecology and Obstetrics, 150(1), 24–30. 10.1002/ijgo.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JE, Marlow N, Messow C, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ, Lavender T, Whyte S, & Norrie J (2018). Does progesterone prophylaxis to prevent preterm labour improve outcome? A randomised double-blind placebo-controlled trial (OPPTIMUM). Health Technology Assessment, 22(35), 1–304. 10.3310/hta22350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak AL, & Giurgescu C (2017). The built environment and birth outcomes: A systematic review. MCN, The American Journal of Maternal Child Nursing, 42, 14–20. 10.1097/NMC.0000000000000299 [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, & Li E (1999). DNA methyltransferases dnmt3a and dnmt3b are essential for de novo methylation and mammalian development. Cell, 99(3), 247–257. 10.1016/S0092-8674(00)81656-6 [DOI] [PubMed] [Google Scholar]
- Parets SE, Conneely KN, Kilaru V, Fortunato SJ, Syed TA, Saade G, Smith AK, & Menon R (2013). Fetal DNA methylation associates with early spontaneous preterm birth and gestational age. PLoS One, 8(6), e67489. 10.1371/journal.pone.0067489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PMI Working Group. (2015). The precision medicine initiative cohort program - building a research foundation for 21st century medicine. https://acd.od.nih.gov/documents/reports/PMI_WG_report_2015-09-17-Final.pdf
- Polettini J, Cobo T, Kacerovsky M, Vinturache AE, Laudanski P, Peelen MJCS, Helmer H, Lamont RF, Takeda J, Lapointe J, Torloni MR, Zhong N, & Menon R (2017). Biomarkers of spontaneous preterm birth: A systematic review of studies using multiplex analysis. Journal of Perinatal Medicine, 45(1), 71–84. 10.1515/jpm-2016-0097 [DOI] [PubMed] [Google Scholar]
- Rauluseviciute I, Drabløs F, & Rye MB (2019). DNA methylation data by sequencing: Experimental approaches and recommendations for tools and pipelines for data analysis. Clinical Epigenetics, 11(1), 193. 10.1186/s13148-019-0795-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B (2020). Redlining’s legacy of inequality: Low homeownership rates, less equity for black households. Forbes Media, LLC. https://www.forbes.com/sites/brendarichardson/2020/06/11/redlinings-legacy-of-inequality-low-homeownership-rates-less-equity-for-black-households/#653980ca2a7c [Google Scholar]
- Rodríguez A, Duyvejonck H, Van Belleghem JD, Gryp T, Simaey LV, Vermeulen S, Van Mechelen E, & Vaneechoutte M (2020). Comparison of procedures for RNA-extraction from peripheral blood mononuclear cells. PLoS One, 15(2), e0229423. 10.1371/journal.pone.0229423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, & Fisher SJ (2014). Preterm labor: One syndrome, many causes. Science, 345(6198), 760–765. 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, & Goodrich JM (2019). Methods for analysis of DNA methylation. In Mccullough SD & Dolino D (Eds.), Toxicoepigenetics: Core principles and applications (pp. 347–377). Elsevier. 10.1016/B978-0-12-812433-8.00015-0 [DOI] [Google Scholar]
- Scherr CL, Ramesh S, Marshall-Friker C, & Perera MA (2019). A review of African Americans’ beliefs and attitudes about genomic studies: Opportunities for message design. Frontiers in Genetics, 10, 548. 10.3389/fgene.2019.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy-Jefferson S, Giurgescu C, Helmkamp L, Misra D, & Osypuk T (2015). Perceived physical and social residential environment and preterm delivery in African-American women. American Journal of Epidemiology, 182(6), 485–493. 10.1093/aje/kwv106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy-Jefferson S, Giurgescu C, Slaughter-Acey J, Caldwell C, & Misra D (2016). Neighborhood context and preterm delivery among African American women: The mediating role of psychosocial factors. Journal of Urban Health, 93(6), 984–996. 10.1007/s11524-016-0083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BD (2019). A practical guide to the measurement and analysis of DNA methylation. American Journal of Respiratory Cell and Molecular Biology, 61(4), 417–428. 10.1165/rcmb.2019-0150TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Bonney E, McElrath T, & Lamont RF (2020). Prevention of preterm birth: Proactive and reactive clinical practice-are we on the right track? Placenta, 98, 6–2. 10.1016/j.placenta.2020.07.021 [DOI] [PubMed] [Google Scholar]
- Skloot R (2010). The immortal life of Henrietta Lacks. Crown Publishers. [Google Scholar]
- St.-Pierre J, Laplante DP, Guillaume E, Dawson P, Kildea S, King S, & Viaillancourt C (2018). Natural disaster-related prenatal maternal stress is associated with alterations in placental glucocorticoid system: The qf2011 Queensland flood study. Psychoneuroendocrinology, 94, 38–48. 10.1016/j.psyneuen.2018.04.027 [DOI] [PubMed] [Google Scholar]
- Stark R, Grzelak M, & Hadfield J (2019). Rna sequencing: The teenage years. Nature Reviews. Genetics, 20(11), 631–656. 10.1038/s41576-019-0150-2 [DOI] [PubMed] [Google Scholar]
- Stevenson DK, Wong RJ, Aghaeepour N, Marie I, Angst MS, Contrepois K, Darmstadt GL, Druzin ML, Eisenberg ML, Gaudilliere B, Gibbs RS, Gotlib IH, Gould JB, Lee HC, Ling XB, Mayo JA, Moufarrej MN, Quaintance CC, Quake SR, …. Katz M (2020). Towards personalized medicine in maternal and child health: Integrating biologic and social determinants. Pediatric Research, 89, 252–258. 10.1038/s41390-020-0981-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetake I, Shinozaki F, Miyagawa J, Takeshima H, & Tajima S (2004). Dnmt31 stimulates the DNA methylation activity of dnmt3a and dnmt3b through a direct interaction. The Journal of Biological Chemistry, 279(26), 27816–27823. 10.1074/jbc.M400181200 [DOI] [PubMed] [Google Scholar]
- Taylor JY, Barcelona de Mendoza V (2018). Improving-omics-based research and precision health in minority populations: Recommendations for nurse scientists -omics research and minorities. Journal of Nursing Scholarship, 50(1), 11–19. 10.1111/jnu.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThermoFisher Scientific. (2021). RNA quantitation is an important and necessary step prior to most RNA analysis methods. https://www.thermofisher.com/us/en/home/references/ambion-tech-support/ma-isolation/tech-notes/quantitating-rna.html
- Togher KL, O’Keeffe GW, Khashan AS, Clarke G, & Kenny LC (2018). Placental fkbp51 mediates a link between second trimester maternal anxiety and birthweight in female infants. Scientific Reports, 8(1), 1–12. 10.1038/s41598-018-33357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togher KL, Treacy E, O’Keeffe GW, & Kenny LC (2017). Maternal distress in late pregnancy alters obstetric outcomes and the expression of genes important for placental glucocorticoid signalling. Psychiatry Research, 255, 17–26. 10.1016/j.psychres.2017.05.013 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services (HHS). (1979). The Belmont Report: Ethical principles and guidelines for the protection of human subjects research, https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
- U.S. Department of Health & Human Services (HHS). (2017). Revised common rule, https://www.hhs.gov/ohrp/regulations-and-policy/regulations/finalized-revisions-common-rule/index.html
- United States. (2004). The Health Insurance Portability and Accountability Act of 1996 (HIPAA). [PubMed]
- United States. (2009). The Genetic Information Nondiscrimination Act of 2008 (GINA).
- Wallerstein N, & Duran B (2010). Community-based participatory research contributions to intervention research: The intersection of science and practice to improve health equity. American Journal of Public Health, 100, S40–S46. 10.2105/AJPH.2009.184036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gerstein M, & Snyder M (2009). Rna-seq: A revolutionary tool for transcriptomics. Nature Reviews. Genetics, 10(1), 57–63. 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, & Anderson CM (2018). Omics research ethics considerations. Nursing Outlook, 66(4), 386–393. 10.1016/j.outlook.2018.05.003 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2019). Health equity. World Health Organization, https://www.who.int/topics/health_equity/en/ [Google Scholar]
- Wu W, Wisherspoon DJ, Fraser A, Clark EAS, Rogers A, S. GJ, & Jorde LB (2015). The heritability of gestational age in a two-million member cohort: Implications for spontaneous preterm birth. Human Genetics, 134(7), 803–808. 10.1007/s00439-015-1558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lin X, Lim IY, Chen L, Teh AL, Maclsaac JL, Tan KH, Kobor MS, Chong YS, Gluckman PD, & Karnani N (2019). Analysis of two birth tissues provides new insights into the epigenetic landscape of neonates born pre-term. Clinical Epigenetics, 11(1), 26. 10.1186/S13148-018-0599-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Soumillon M, Wu J, Hansen J, Hu B, van Hasselt JGC, Jayaraman G, Lim R, Bouhaddou M, Ornelas L, Bochicchio J, Lenaeus L, Stocksdale J, Shim J, Gomez E, Sareen D, Svendsen C, Thompson LM, Mahajan M, … Birtwistle MR (2017). A comparison of mRNA sequencing with random primed and 3′-directed libraries. Scientific Reports, 7(1), 1–12. 10.1038/s41598-017-14892-x [DOI] [PMC free article] [PubMed] [Google Scholar]


