Abstract
Background
COH04S1, a synthetic attenuated modified vaccinia virus Ankara vector co-expressing SARS-CoV-2 spike and nucleocapsid antigens, was tested for safety and immunogenicity in healthy adults.
Methods
This combined open-label and randomised, phase 1 trial was done at the City of Hope Comprehensive Cancer Center (Duarte, CA, USA). We included participants aged 18–54 years with a negative SARS-CoV-2 antibody and PCR test, normal haematology and chemistry panels, a normal electrocardiogram and troponin concentration, negative pregnancy test if female, body-mass index of 30 kg/m2 or less, and no modified vaccinia virus Ankara or poxvirus vaccine in the past 12 months. In the open-label cohort, 1·0 × 107 plaque-forming units (PFU; low dose), 1·0 × 108 PFU (medium dose), and 2·5 × 108 PFU (high dose) of COH04S1 were administered by intramuscular injection on day 0 and 28 to sentinel participants using a queue-based statistical design to limit risk. In a randomised dose expansion cohort, additional participants were randomly assigned (3:3:1), using block size of seven, to receive two placebo vaccines (placebo group), one low-dose COH04S1 and one placebo vaccine (low-dose COH04S1 plus placebo group), or two low-dose COH04S1 vaccines (low-dose COH04S1 group). The primary outcome was safety and tolerability, with secondary objectives assessing vaccine-specific immunogenicity. The primary immunological outcome was a four times increase (seroconversion) from baseline in spike-specific or nucleocapsid-specific IgG titres within 28 days of the last injection, and seroconversion rates were compared with participants who received placebo using Fisher's exact test. Additional secondary outcomes included assessment of viral neutralisation and cellular responses. This trial is registered with ClinicalTrials.gov, NCT046339466.
Findings
Between Dec 13, 2020, and May 24, 2021, 56 participants initiated vaccination. On day 0 and 28, 17 participants received low-dose COH04S1, eight received medium-dose COH04S1, nine received high-dose COH04S1, five received placebo, 13 received low-dose COH04S1 followed by placebo, and four discontinued early. Grade 3 fever was observed in one participant who received low-dose COH04S1 and placebo, and grade 2 anxiety or fatigue was seen in one participant who received medium-dose COH04S1. No severe adverse events were reported. Seroconversion was observed in all 34 participants for spike protein and 32 (94%) for nucleocapsid protein (p<0·0001 vs placebo for each comparison). Four times or more increase in SARS-CoV-2 neutralising antibodies within 56 days was measured in nine of 17 participants in the low-dose COH04S1 group, all eight participants in the medium-dose COH04S1 group, and eight of nine participants in the high-dose COH04S1 group (p=0·0035 combined dose levels vs placebo). Post-prime and post-boost four times increase in spike-specific or nucleocapsid-specific T cells secreting interferon-γ was measured in 48 (98%; 95% CI 89–100) of 49 participants who received at least one dose of COH04S1 and provided a sample for immunological analysis.
Interpretation
COH04S1 was well tolerated and induced spike-specific and nucleocapsid-specific antibody and T-cell responses. Future evaluation of this COVID-19 vaccine candidate as a primary or boost vaccination is warranted.
Funding
The Carol Moss Foundation and City of Hope Integrated Drug Development Venture programme.
Introduction
Since SARS-CoV-2 emerged in December, 2019, it has caused a global pandemic, with more than 300 million cases and 5·5 million fatalities (as of Jan 14, 2022).1 Preventing the incidence of COVID-19-associated morbidity and mortality while allowing a return to normal activities might best be accomplished by prophylactic vaccination. Approved COVID-19 vaccines based on mRNA and adenovirus vectors that use spike antigens have been shown to reduce the need for hospital treatment and to protect people from severe disease.2 However, as virus variants of concern arise with the capacity to evade spike-specific immune responses, there is concern that the immunity these vaccines confers might be insufficient to control disease.3, 4, 5, 6 As an alternative to the approved COVID-19 vaccines that solely use the spike protein, we developed COH04S1, a multi-antigen SARS-CoV-2 vaccine based on a synthetic version of the highly attenuated modified vaccina virus Ankara (MVA) vector.7
Research in context.
Evidence before this study
We searched PubMed from database inception to Dec 20, 2021, with no language restrictions, for clinical studies reporting the safety and immunogenicity of SARS-CoV-2 vaccine candidates based on viral vector platforms using the search terms “SARS-CoV-2”, “vaccine”, “clinical trial”, and “vector”. 15 reports were identified, all of which described studies conducted with adenovirus-based SARS-CoV-2 vaccines expressing the spike antigen (eight on ChAdOx1, two on rAd25/rAd5, two on Ad26.COV.2.S, and three on non-replicating Ad5). To our knowledge, no data from clinical trials of COVID-19 vaccines based on modified vaccinia virus Ankara (MVA) are available. We searched ClinicalTrials.gov on Dec 20, 2021, for trials of MVA-based COVID-19 vaccines; we found that, except for COH04S1, two MVA-based vaccines are being developed by the Hamburg-Eppendorf University Hospital (Hamburg, Germany), both of which are based on spike antigen (MVA-SARS-2-S [NCT04569383] and MVA-SARS-2-ST [NCT04895449]), but results are not yet available. Additionally, we searched ClinicalTrials.gov for SARS-CoV-2 vaccines expressing both spike and nucleocapsid antigens. Two vaccines were found, one based on Ad5 (hAd5-S-FusionN-ETSD) developed by ImmunityBio (Culver City, CA, USA) and one DNA-based vaccine (GX-19N) developed by Genexine (Seongnam-si, South Korea), for which preliminary reports were deposited in a preprint server.
Added value of this study
To our knowledge, this is the first study to report clinical trial data for a multi-antigenic SARS-CoV-2 vaccine based on a synthetic MVA vector platform, and the first published peer-reviewed report of a SARS-CoV-2 vaccine co-expressing spike and nucleocapsid antigens. This analysis is the first-in-human dose-ranging evaluation of COH04S1 in healthy volunteers. COH04S1 was well tolerated independent of the dose level. One immunisation was sufficient to induce robust binding antibody titres and cellular responses. Peak binding and neutralising antibody titres were induced following two doses and remained elevated up to 3 months after the second dose. Immunological parameters were equivalent among dose levels with the exception of neutralising antibody titres, which showed a dose response. However, peak neutralising antibody responses were higher in volunteers vaccinated with the lowest dose level given twice and separated by 2 months than in volunteers who were given the same dose 1 month apart, indicating that increased immunogenicity can be achieved by increased spacing of the two injections.
Implications of all the available evidence
COH04S1 was safe and immunogenic in healthy adults. Given the inclusion of both spike and nucleocapsid, COH04S1 could function as an alternative to existing vaccines to augment vaccine-elicited protective immunity to SARS-CoV-2 and its variants through heterologous immunisation. Importantly, since MVA-attenuated poxviral vectors have been shown to be highly tolerable and immunogenic even in immunosuppressed patients, COH04S1 could find crucial application in immunocompromised populations, such as patients with a haematological malignancy in whom the current EUA vaccines might not stimulate effective immune responses. Further clinical investigation is needed to evaluate the efficacy of this vaccine candidate as a primary or booster vaccination against COVID-19 in healthy adults or immunocompromised individuals. The favourable safety profile and robust immunogenicity at the lowest dose makes COH04S1 a good candidate for large-scale manufacturing for worldwide distribution.
COH04S1 was designed to co-express the spike protein together with the nucleocapsid protein based on the rationale to broaden the stimulation of humoral and cellular immune responses that target multiple SARS-CoV-2 antigens.8 To our knowledge, in addition to COH04S1 only two other SARS-CoV-2 vaccines co-expressing spike and nucleocapsid are being tested in the clinic.9, 10 COH04S1 showed potent immunogenicity in mice,7 and studies in hamsters and non-human primates showed protection by COH04S1 against upper and lower respiratory tract infection following SARS-CoV-2 challenge.11 We aimed to assess the safety and immunogenicity of COH04S1 in healthy adults.
Methods
Study design and participants
We did a combined open-label and placebo-controlled, randomised, phase 1 study at the City of Hope Comprehensive Cancer Center (Duarte, CA, USA). Healthy individuals aged 18–54 years with a negative SARS-CoV-2 antibody test (SCoV-2 Detect IgG ELISA, In-Bios, Seattle, WA, USA) and a negative nasopharyngeal wash SARS-CoV-2 PCR test (Simplexa for COVID-19, DiaSorin Molecular, Cypress, CA, USA) were eligible. Other inclusion criteria were institutional normal haematology and chemistry panels, a normal electrocardiogram (ECG) and troponin concentration, negative pregnancy test if female, body-mass index of 30 kg/m2 or less, and no MVA or poxvirus vaccine in the past 12 months (appendix pp 25–28). Other exclusion criteria were based primarily on the absence of COVID-19 risk factors as outlined by the US Centers for Disease Control and Prevention in a June 25, 2020, guidance.12
The study met all ethics and regulatory requirements as determined in an external review by the Advarra institutional review board (Columbia, MD, USA), and an independent external data monitoring committee that reviewed study plans and progress. The protocol is available in the appendix (pp 2–85). Written informed consent from all participants was obtained before screening.
Randomisation and masking
In the open-label cohort, COH04S1 was first administered to sentinel participants at 1·0 × 107 plaque-forming units (PFU; low dose), 1·0 × 108 PFU (medium dose), and 2·5 × 108 PFU (high dose). After the open-label safety assessment, in a randomised dose expansion cohort, we randomly assigned additional participants (3:3:1), using a block size of seven, to receive low-dose COH04S1 injection followed by placebo (low-dose COH04S1 plus placebo group), two low-dose COH04S1 injections (low-dose COH04S1 group), or two placebo injections (placebo group). An identical randomisation procedure within medium and high doses was initially planned, but it was replaced with a separate permuted blocked randomisation between medium and high doses. When the low-dose COH04S1 cohort was one participant short of planned accrual, random assignment (1:1), using a block size of four, of participants was diverted to a medium-dose COH04S1 group and a high-dose group as the second dose expansion cohort. Emergency use authorisation (EUA) of some vaccines affected random assignment to placebo (since they had the alternative to get an EUA vaccine), and documentation for travel and other activities further affected study conduct. For the same reason, the blinding period was limited to 56 days after first vaccination, at which point participants were informed of their vaccination status. Participant receiving any vaccine regimen could request an EUA vaccine at unblinding, and an additional low dose of COH04S1 was administered after unblinding to participants in the low-dose COH04S1 plus placebo group who opted for a second dose of COH04S1. The randomisation list was generated by the study statistician using R software and supplied to the central registration office, who coordinated with Investigational Drug Services and the clinical team. COH04S1 and placebo were produced in the Investigation Drug Services Pharmacy and delivered to the clinical staff in identical unlabelled syringes to ensure masking. Group allocation was masked from participants, the principal investigator, physicians, nurses, data coordinators, and other staff who interact with patients. Participants were enrolled by the clinical team.
Procedures
The COH04S1 vaccine construct was generated using a fully synthetic MVA platform originating from chemically synthetised DNA fragments7 (appendix p 86). Before each injection, COH04S1 was thawed and diluted with sterile diluent (phosphate-buffered saline with 7·5% lactose) to the appropriate dose. Placebo consisted of phosphate-buffered saline containing 7·5% lactose. Vaccine formulations and placebo in 1·0 mL volume were administered to the upper non-dominant arm by intramuscular injection on day 0 (prime) and day 28 (boost) in both open-label and expansion cohorts. For each dose level, the first patient was observed for at least 1 week, before enrolling further sentinel patients at that dose level. Following the flexible rules of the modified queue-based design (IQ 3+3 design; appendix pp 46, 86) escalation could occur with as few as three patients (with no moderate adverse events), although the study resulted in four, seven, and six sentinel patients per dose level, respectively.
Laboratory assessments included biochemistry and haematology tests, ECG, and cardiac troponin test (appendix pp 25–26). For assessment of adverse events, solicited adverse events were recorded during the post-injection period (days 1–28 after each injection) via a phone call to the recipient on days 1–3 and 4–6 after each injection by a registered nurse using a prescribed script and by visits with a medical doctor on the day of each injection (day 0 and 28 [and day 56 for low-dose COH04S1 plus placebo and low-dose COH04S1 group]) and 1 week and 2 weeks after each injection (days 7, 14, 35, and 42, [and day 63 and 70]). Unsolicited adverse events were recorded at subsequent visits (days 56, 90, 120, and 365 [or on an equivalent shifted schedule for low-dose COH04S1 plus placebo and low-dose COH04S1 group]; appendix pp 43–44). Participants were also asked to report any intercurrent infection, and PCR testing was available in such cases.
Blood samples were collected for immunological analyses at the time of vaccination, day 14 after each vaccination, and days 56, 90, and 120 after the first injection. The same sample collection schedule applied to participants in the low-dose COH04S1 plus placebo group who were given another low-dose COH04S1 vaccination at day 56, resulting in additional samples and an extended timeline.
Serum spike-specific, receptor-binding domain (RBD)-specific, and nucleocapsid-antigen specific IgG concentrations were measured using indirect ELISA and expressed as endpoint titres. A titre of half the lower limit of quantification (LLOQ) of the assay was assigned to samples with titres below the LLOQ. Seroconversion was defined as a four times increase in spike or nucleocapsid antibody endpoint titres relative to baseline. Serum neutralising antibody titres were measured using SARS-CoV-2 pseudovirus based on the SARS-CoV-2 Wuhan spike sequence, with additional Asp614Gly substitution. WHO International Reference Panel for anti-SARS-CoV-2 immunoglobulin (UK National Institute for Biological Standards and Control code, 20/268) was analysed with ELISA and a SARS-CoV-2 pseudovirus assay. Samples from participants in the open-label cohort were also analysed using pseudoviruses representing the alpha, beta, gamma, and delta variants of concern. The serum dilution that reduced pseudovirus entry into susceptible cells by 50% was defined as 50% neutralisation titre. Absolute numbers of spike-specific, nucleocapsid-specific, and membrane-specific peripheral blood mononuclear cells secreting interferon-γ (IFNγ) and interkeukin-4 (IL-4) were measured using an enzyme-linked immunospot (ELISpot) assay. Spot forming-cells (SFCs) per 106 peripheral blood mononuclear cells were obtained after subtraction of spots in unstimulated controls from stimulated samples. The IFNγ:IL-4 ratio was used as a measure of T helper 1 (Th1) versus Th2 polarisation. Activated or cycling spike-specific and nucleocapsid-specific T cells were longitudinally evaluated in the open-label cohort using CD137 multiparameter flow cytometry assay. Full details of the immunological assays are reported in the appendix (pp 86–88).
Dose escalation and expansion were based on the incidence of moderate toxicity in sentinel participants, with subsequent safety constraints during the expansion cohorts. Moderate toxicity was defined as grade 2 possibly, probably, or definitely attributable to the research treatment that persisted for 7 days or more, or any grade 3 treatment-related adverse event that was an expected, vaccine-associated side-effect such as fever, chills, malaise, headache, and influenza-like symptoms (myalgia and arthralgia) that resolved to grade 1 or better in less than 7 days. Treatment-related adverse events of grade 3 or worse that did not qualify as moderate toxicity would halt all accrual on all dose levels. Toxicity was graded according to standard Division of AIDS adult toxicity tables.13
Outcomes
The primary objective was to evaluate the safety and tolerability of COH04S1 vaccine in healthy participants at 1·0 × 107 PFU, 1·0 × 108 PFU, and 2·5 × 108 PFU.
The protocol-defined primary immunological endpoint was based on serum IgG against SARS-CoV-2. Specifically, a four times rise from baseline value of IgG specific for spike or nucleocapsid protein up to day 28 after the last injection was considered a positive immunogenicity response, provided the participant was not diagnosed with SARS-CoV-2. Secondary immunogenicity objectives were the longitudinal evaluation of SARS-CoV-2 spike-specific, RBD-specific, and nucleocapsid-specific IgG, IgA, and IgM in serum and saliva; neutralising antibody to ancestral and SARS-CoV-2 variants of concern; evaluation of SARS-CoV-2 spike-specific and nucleocapsid-specific T-cell levels and Th1 versus Th2 polarisation; activated or cycling phenotype markers on T cells; and durability of immune responses. Additionally, we explored the role of two versus one low-dose injections. In this analysis, we present the primary safety endpoints up to day 90 after the last injection, the primary immunogenicity endpoint evaluated up to day 28 after the last injection, and secondary immunogenicity results up to day 90 after the last injection. The exploratory endpoint was the rate of incidental COVID-19 during follow-up based on self-reporting.
Statistical analysis
Approximately four to eight patients were anticipated for each of the open-label cohorts for a total of approximately 18 patients. The initial planned sample size for the dose expansion cohorts was based on a combination of safety (eg, any adverse event with an incidence of 15% would be very likely [95% probability] to appear in at least one of 19 participants on a dose level) and humoral immune response (eg, 15 patients in the low-dose COH04S1 group and five in the placebo group provides for 82% power to detect a significant difference between low-dose COH04S1 and placebo [with a type I error of 10% using Fisher's exact test] in the primary immunological response rate with a true response rate of 82% for the low-dose COH04S1 group and 20% for the placebo group). The placebo, recommended by the US Food and Drug Administration, provided both a background level of adverse events, and a control for immunogenicity.
Safety analysis was based on the full analysis set including all enrolled participants who received at least one injection. For safety assessment, the open-label and expansion cohorts were analysed individually. Immunogenicity analysis included all enrolled participants who received at least one injection and provided samples for immunogenicity analyses. Participants who received an EUA vaccine at any time during enrolment remained on the trial, but safety and immunological analysis is presented only up to the time of EUA vaccination. For each dose level, we pooled data from the open-label and expansion cohorts for immunological analysis given that participants received the same vaccination regimens and immunogenicity was noted to be similar.
We described binding antibody and neutralising antibody titres based on seroconversion (four times increase or not) relative to baseline and on geometric mean titres (GMT) and medians with IQRs or ranges. We calculated 95% CIs for proportions using the Clopper-Pearson method. We followed previous approaches for participants with no baseline detectable antibody titres,14, 15 where half the LLOQ was used as baseline (results were not sensitive to replacing half LLOQ with the LLOQ). Additional, post-hoc immunological analyses included post-prime and post-boost (14 and 28 days after each injection and days 30 and 60 after the last injection) increase in spike-specific, RBD-specific, and nucleocapsid-specific binding and neutralising antibodies compared with baseline, and the proportion of participants who seroconverted. Cellular responses measured by ELISpot were described based on median spot values. Samples with undetectable ELISpot values were assigned a value of 1 for fold-increase calculation. To characterise Th cell polarisation, for each timepoint, the ratio of spike-specific or nucleocapsid-specific IFNγ to IL-4 T cells was calculated, and an increase over baseline was considered a Th1-polarised response. Statistical comparisons used non-parametric tests (Wilcoxon, Fisher's exact test, and Kruskal-Wallis test) and exact p values for Pearson's test. All p values are two-sided unless otherwise stated. Sentinel participants with available baseline samples were longitudinally analysed for concentrations of spike-specific and nucleocapsid-specific CD137+CD4+ and CD137+CD8+ cells in blood samples. Percentage change was measured on the natural log scale.16 Anti-SARS-CoV-2 humoral and cellular responses at day 56 (or 28 days after the second dose) were compared between participants in the COH04S1 low-dose group born before or during/after 1973 using two way ANOVA. There was no attempt to address multiple comparison issues with respect to these multiple exploratory endpoints in the context of this study. All calculations were done with R (version 4.02) or StatXact (version 12). This trial is registered with ClinicalTrials.gov, NCT04639466. The study is closed for accrual.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
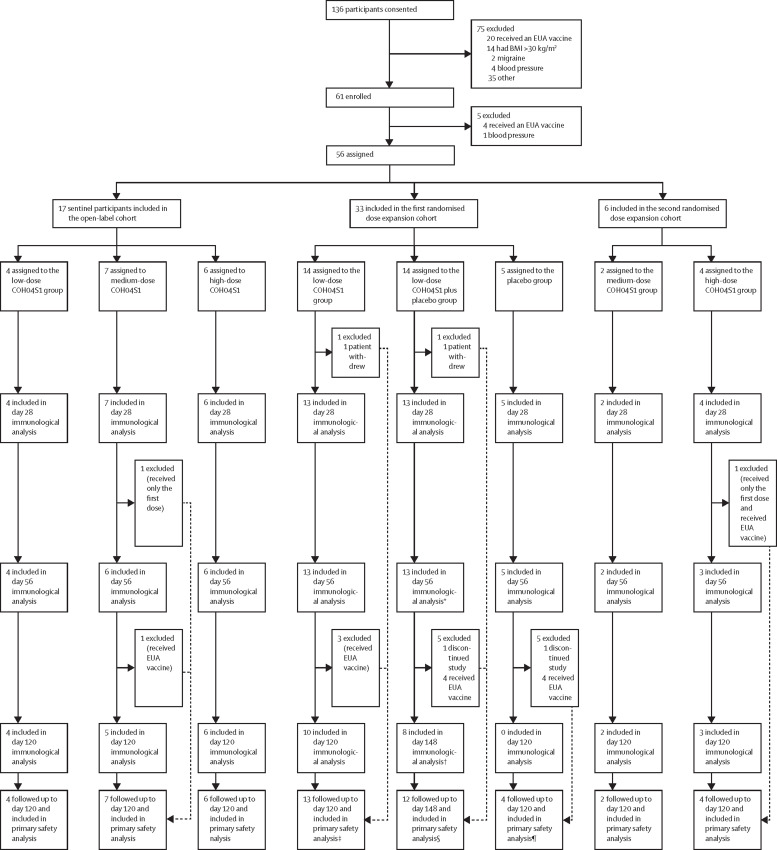
Between Dec 13, 2020, and May 24, 2021, 56 participants initiated vaccination (figure 1 ). 17 participants were included in the open-label cohort (four received low-dose, seven received medium-dose, and six received high-dose COH04S1). All sentinels received the prime vaccination on day 0 and the boost vaccination on day 28 except for one sentinel in the medium-dose COH04S1 group who received only the first dose. Additionally, one sentinel participant in the medium-dose COH04S1 group received an EUA vaccine after day 56. The remaining 39 participants were included in the two randomised dose expansion cohorts. In the first dose expansion cohort, 14 participants were randomly assigned to the low-dose COH04S1 group, 14 to the low-dose COH04S1 plus placebo group, and five to the placebo group. Two of 33 participants in the first dose expansion cohort (one in the low-dose COH04S1 group and one in the low-dose COH04S1 plus placebo group) discontinued the study (unrelated to adverse events) before the second vaccination and did not provide post-vaccination blood samples. The second dose expansion cohort closed at six participants (two were randomly assigned to the medium-dose COH04S1 group and four to the high-dose COH04S1 group) due to accrual limitations when EUA vaccine availability became widespread. One participant in the high-dose COH04S1 group did not receive the second injection.
Figure 1.
Trial profile
BMI=body-mass index. EUA=emergency use authorisation. *Ten participants opted to receive a second low-dose vaccination at day 56 (nine) or day 90 (one). †Day 90 post-last dose, the equivalent of day 120 for the normal 28-day interval schedule. ‡One participant received the first injection but was lost to follow-up (followed up for safety days 0-14), primary safety included up to that point. §One participant discontinued after the first dose and one subject discontinued at day 60 after the last dose (primary safety included up to that point). ¶One discontinued after day 56 so they were followed up and included in primary safety analysis up to day 56.
Following unblinding, ten of 13 participants remaining in the low-dose COH04S1 plus placebo group opted to receive a second low-dose COH04S1 vaccination at day 56 (nine participants) or day 90 (one participant). The other three participants received an EUA vaccine. In the placebo group, four of five participants received EUA vaccination at day 56 and one discontinued the study with no further follow-up. Detailed demographic characteristics of all participants are listed in table 1 .
Table 1.
Demographic characteristics
| Whole cohort (n=56) | Low-dose COH04S1 group*(n=18) | Medium-dose COH04S1 group*(n=9) | High-dose COH04S1 group*(n=10) | Placebo group (n=5) | Low-dose COH04S1 plus placebo group (n=14) | ||
|---|---|---|---|---|---|---|---|
| Age, years | 41 (21–55) | 46 (26–51) | 39 (28–55) | 32 (26–54) | 35 (24–46) | 40 (21–55) | |
| Gender | |||||||
| Female | 31 (55%) | 10 (56%) | 6 (67%) | 6 (60%) | 2 (40%) | 7 (50%) | |
| Male | 25 (45%) | 8 (44%) | 3 (33%) | 4 (40%) | 3 (60%) | 7 (50%) | |
| Body-mass index, kg/m2 | 25·0 (19·4–30·0) | 25·3 (19·7–30·0) | 25·8 (20·2–30·0) | 22·4 (19·4–29·9) | 21·3 (21·1–28·5) | 25·0 (22·2–29·9) | |
| Race | |||||||
| Asian | 10 (18%) | 2 (11%) | 1 (11%) | 1 (10%) | 2 (40%) | 4 (29%) | |
| Non-disclosed | 2 (4%) | 0 | 1 (11%) | 0 | 0 | 1 (7%) | |
| Unknown | 8 (14%) | 1 (5·6%) | 3 (33%) | 4 (40%) | 0 | 0 | |
| White | 36 (64%) | 15 (83%) | 4 (44%) | 5 (50%) | 3 (60%) | 9 (64%) | |
| Ethnicity | |||||||
| Hispanic | 13 (23%) | 4 (22%) | 4 (44%) | 3 (30%) | 0 | 2 (14%) | |
| Non-Hispanic | 40 (71%) | 14 (78%) | 5 (56%) | 5 (50%) | 5 (100%) | 11 (79%) | |
| Non-disclosed | 1 (2%) | 0 | 0 | 0 | 0 | 1 (7%) | |
| Unknown | 2 (4%) | 0 | 0 | 2 (20%) | 0 | 0 | |
Data are median (range) or n (%).
Includes participants from the open-label and two randomised dose expansions cohorts.
Laboratory assessments were within the normal range with few exceptions graded 1 in severity and observed in both the placebo group and COH04S1 groups. Adverse events were those that were expected following a vaccine injection: injection site reactions (42 [82%] of 51 non-placebo participants vs two of five placebo participants) followed by fatigue (34 [67%] of 51 vs one of five) and headache (25 [49%] of 51 vs two of five). Local injection sites reactions were all grade 1. No serious adverse events and no unanticipated problems were reported (table 2 ; appendix pp 89–90).
Table 2.
Local and systemic adverse reactions after one, two, and three vaccinations in the first randomised dose expansion cohort
|
Placebo group day 0 injection (n=5) |
Placebo group day 28 injection (n=5) |
Low-dose COH04S1 plus placebo group day 0 injection (n=14) |
Low-dose COH04S1 plus placebo group day 28 injection (n=13) |
Low-dose COH04S1 plus placebo group day 56 injection (n=10) |
Low-dose COH04S1 group day 0 injection (n=14) |
Low-dose COH04S1 group day 28 injection (n=13) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 1 | Grade 1 | Grade 3 | Grade 1 | Grade 1 | Grade 1 | Grade 2 | Grade 1 | |
| Injection site reaction | 2 (40%) | 2 (40%) | 8 (57%) | 0 | 3 (23%) | 9 (90%) | 5 (36%) | 0 | 7 (54%) |
| Fatigue | 1 (20%) | 0 | 4 (29%) | 0 | 4 (31%) | 6 (60%) | 7 (50%) | 0 | 5 (38%) |
| Headache | 1 (20%) | 2 (40%) | 2 (14%) | 0 | 2 (15%) | 5 (50%) | 5 (36%) | 0 | 4 (31%) |
| Myalgia | 0 | 1 (20%) | 2 (14%) | 0 | 0 | 2 (20%) | 3 (21%) | 0 | 5 (38%) |
| Chills | 0 | 1 (20%) | 3 (21%) | 0 | 2 (15%) | 3 (30%) | 1 (7%) | 0 | 0 |
| Insomnia | 0 | 1 (20%) | 0 | 0 | 0 | 1 (10%) | 1 (7%) | 0 | 0 |
| Nausea | 0 | 1 (20%) | 0 | 0 | 0 | 1 (10%) | 1 (7%) | 0 | 0 |
| Sore throat | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 1 (7%) | 0 | 1 (8%) |
| Cough | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 | 0 | 1 (8%) |
| Bilateral hand swelling | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 0 | 0 | 0 |
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 1 (8%) | 0 | 0 | 0 | 0 |
| Creatinine increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 |
| Diarrhoea | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 0 | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 | 0 |
| Hypocalcaemia | 1 (20%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalaemia | 0 | 0 | 1 (7%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymph node adenopathy | 0 | 1 (20%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malaise | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 |
| Nasal congestion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (8%) |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 | 0 |
| Idiopathic iritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7%) | 0 |
| Tender breasts | 0 | 0 | 0 | 0 | 0 | 1 (10%) | 0 | 0 | 0 |
Grade 2 idiopathic iritis was unrelated to treatment. Grade 3 fever was a moderate toxicity that lasted 1 day, and became grade 1, which also lasted 1 day.
In the open-label cohort, one of 7 sentinel participants in the medium-dose COH04S1 group had grade 2 anxiety and grade 2 fatigue on the first injection that lasted 2 weeks (appendix p 89). This participant did not receive a second injection. In the first randomised dose expansion cohort, adverse events were more frequent in the COH04S1 groups than in the placebo group, and one participant in the low-dose COH04S1 plus placebo group had grade 3 fever that lasted less than 24 h after the first injection (the second injection was placebo).
In the second randomised dose expansion cohort, one participant in the high-dose COH04S1 group had grade 2 bronchospasm that occurred 2 weeks after the first injection during a seasonal asthma attack that was judged to be unrelated to the research injection of this known asthmatic person (appendix p 90). This participant also had a cornea tear (grade 1) associated with a history of dry eye (grade 1) and was put on steroids, which made them ineligible for a second injection.
Local and systemic adverse events did not appear to be dose related. In the first 120 days, there were no reports of intercurrent SARS-CoV-2 infections.
54 participants provided immunological samples following vaccination. Two of the 54 participants did not get their second injection. Five received two placebo injections 28 days apart, 17 received two low-dose COH04S1 injections 28 days apart (four in the open-label cohort and 13 in the randomised dose expansion cohorts), eight received two medium-dose COH04S1 injections 28 days apart (six in the open-label cohort and two in the randomised dose expansion cohorts), and nine received two high-dose injections 28 days apart (six in the open-label cohort and three in the randomised dose expansion cohorts). Therefore, 34 participants had two COH04S1 injections and five had two placebo injections on the planned schedule for the primary immunological comparisons.
The prespecified primary immunogenicity response (IgG higher than four times increase from baseline of either spike or nucleocapsid within 56 days of the first injection [or within 28 days of the second injection]) was observed in all 34 vaccinated participants and none of the placebo participants. For spike-specific IgG, all 34 participants, compared with no participants in the placebo group, responded (p<0·0001), and for nucleocapsid-specific IgG 32 (94%) of 34 participants responded (15 of 17 in the low-dose COH04S1 group, all eight in the medium-dose COH04S1 group, and all nine in the high-dose COH04S1 group; with p<0·0001 vs placebo; appendix p 91).
Seroconversion was achieved in 13 (76·5%, 95% CI 50·1–93·2) of 17 participants after one low-dose COH04S1 injection, and 17 (100·0%, 80·5–100·0) participants after two low-dose COH04S1 injections. Seroconversion was achieved in eight (88·9%, 95% CI 51·8–99·7) of nine participants after one medium-dose COH04S1 injection, and all eight (100·0%, 63·1–100·0) participants after two medium-dose COH04S1 injections. Seroconversion was achieved in seven (70·0%, 34·8–93·3) of ten participants after one high-dose COH04S1 injections, and all nine (100·0%, 66·4–100·0) participants after two high-dose COH04S1 injections. Additionally, in the participants vaccinated with low-dose COH04S1 followed by placebo and a late boost with low-dose COH04S1, seroconversion was achieved in 12 (92·3%, 95% CI 64·0–99·8) of 13 participants after one injection, and all ten (100·0%, 69·2–100·0) participants after a delayed second injection (appendix p 100).
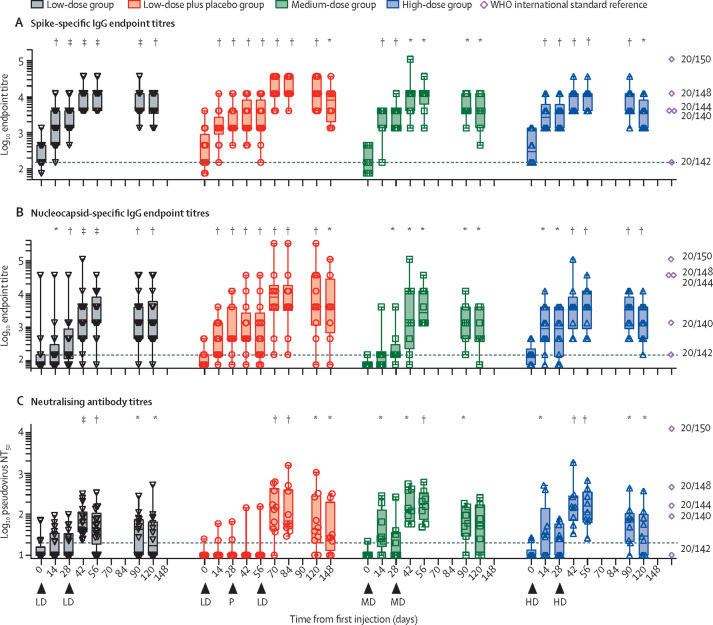
Spike-specific binding antibody GMT was 241·5 (95% CI 165·3–352·8) at day 0, 2748·3 (1689·1–4471·6) at day 28, and 9382·4 (6447·2–13 653) at day 56 for low-dose COH04S1; 171·7 (94·1–313·3) at day 0, 2808·1 (1545·6–5102·0) at day 28, and 9232 (4090·0–20 838) at day 56 for medium-dose COH04S1; and 361·2 (175·4–743·8) at day 0, 3251·1 (1749·0–6043·1) at day 28, and 9518·1 (5420·6–16 712·8) at day 56 for high-dose COH04S1. For RBD, binding antibody GMT was 99·8 (76·2–130·6) at day 0, 176·0 (123·7–250·4) at day 28, and 6366·7 (3745·3–10 823) at day 56 for low-dose COH04S1; 81·0 (67·8–96·7) at day 0, 531·9 (212·4–1331·8) at day 28, and 12 150 (5191·4–28 436) at day 56 for medium-dose COH04S1; and 118·4 (78·1–179·5) at day 0, 560·6 (229·7–1368·1) at day 28, and 6599·5 (2323·8–18 742) at day 56 for high-dose COH04S1. Nucleocapsid-specific binding antibody GMT were 150·6 (68·3–332·1) at day 0, 314·9 (138·5–716·1) at day 28, and 2318·6 (1072·6–5012·1) at day 56 for low-dose COH04S1; 81·0 (67·8–96·7) at day 0, 226·3 (91·8–558·0) at day 28, and 4050·0 (1351·1–12 139) at day 56 for medium-dose COH04S1; and 151·8 (95·0–242·4) at day 0, 678·5 (217·1–2120·7) at day 28, and 3584·6 (1132·7–11 344) at day 56 for high-dose COH04S1 (figure 2 , appendix p 101). All comparisons between days 0 and 28, days 0 and 56, and days 28 and 56 were significant on paired testing (Wilcoxon paired test p<0·05, which removed one patient in medium-dose and one patient in high-dose for day 56 pairwise comparisons who were missing values after day 28; appendix pp 92, 93, 95).
Figure 2.
Serum SARS-CoV-2-specific binding and neutralising antibodies following COH04S1 vaccination with different dose levels and schedules
Spike (A) and nucleocapsid (B) IgG endpoint titres following COH04S1 vaccination were quantified by ELISA. SARS-CoV-2-specific neutralising antibody titres (C) were evaluated using a SARS-CoV-2 pseudovirus based on the Wuhan spike sequence with Asp614Gly substitution. Box plots extend from the 25th to the 75th percentiles, median values are shown as a line (key geometric mean titres are discussed in the text), whiskers extend from minimum to maximum values. Individual values are superimposed. Reported are statistical testing results using Wilcoxon rank-sum paired test and comparing each timepoint to baseline (day 0). Exact p values are shown in appendix pp 92–94. Dashed lines represent the lower limit of quantification. Arrowheads represent time of vaccination. LD represents the low dose of COH04S1 (1·0 × 107 PFU), MD represents the medium dose (1·0 × 108 PFU), and HD represents the high dose (2·5 × 108 PFU). P represents placebo. Indicated are spike and nucleocapsid endpoint titres and pseudovirus NT50 titres measured in WHO reference panel 20/268 (ranked based on SARS-CoV-2 antibody titres: 20/150 indicates high, 20/148 indicates mid, 20/144 indicates low spike and high nucleocapsid, 20/140 indicates low, and 20/142 indicates negative). WHO assigned values are shown in appendix p 103). NT50=50% neutralisation titre. PFU=plaque-forming unit. *p<0·05. †p<0·01. ‡p<0·001. §p<0·0001.
A four times increase in neutralising antibody titres against Asp614Gly pseudovirus within 56 days of the first injection (or within 28 days of the second injection) was measured in nine of 17 participants in the low-dose COH04S1 group, all eight participants in the medium-dose COH04S1 group, eight of nine participants in the high-dose COH04S1 group, and no participants in the placebo group. This finding was statistically distinct from the placebo group for the medium and high-dose levels (p=0·0537 for the low group, p=0·0008 for the medium dose, and p=0·0030 for the high dose, Fisher's exact test) and indicated a dose effect (p=0·0232, Fisher's exact test on the three dose levels, excluding placebo).
Asp614Gly pseudovirus neutralising antibody GMT was 13·8 (10·1–19·0) at day 0, 17·8 (12·2–25·8) at day 28, 43·4 (26·0–72·4) at day 56 for low-dose COH04S1; 10·9 (9·2–13·0) at day 0, 20·6 (10·0–42·5) at day 28, and 166·9 (95·1–293·2) at day 56 for medium-dose COH04S1; and 12·1 (9·4–15·4) at day 0, 23·7 (14·1–39·7) at day 28, and 136·6 (72·3–258·0) at day 56 for high-dose COH04S1. Significant increases in neutralising antibody GMT compared with baseline were measured at day 14 for medium-dose COH04S1 (p=0·0225) and high-dose COH04S1 (p=0·0223) but not low-dose COH04S1 (p=0·068). At day 56, all dose level cohorts showed significant increases in neutralising anti-body titres compared with baseline (p=0·0027 for low dose, p=0·0078 for medium dose, and p=0·0039 for high dose), which seemed less pronounced at day 120 (p=0·030, p=0·0591, and p=0·0360; appendix p 94). Among ten participants in the low-dose COH04S1 plus placebo group who received low-dose COH04S1 on or after day 56, neutralising antibody GMT was 10·7 (9·4–12·3) at day 0, 12·4 (9·1–16·3) at day 28, 12·3 (8·2–18·4) at day 56, and 110·5 (53·9–226·6) at day 84. The increase in neutralising antibody titres from 28 days after the prime to 28 days after the boost injection was significantly higher in participants in the COH04S1 plus placebo group who received delayed low-dose COH04S1 than in the low-dose COH04S1 group (Wilcoxon test p=0·0290) and did not differ from the medium-dose and high-dose COH04S1 groups (Kruskal-Wallis p=0·71). Blood samples from the open-label cohort were longitudinally evaluated for the presence of neutralising antibodies against variants of concern (appendix p 102). Neutralising antibodies recognising SARS-CoV-2 variants of concern were lower in sentinels in the low-dose COH04S1 group than in those in the medium-dose and high-dose COH04S1 groups. COH04S1 induced similar neutralising antibody titres to the different variants, although antibodies to the beta (B.1.351) and delta (B.1.617.2) variants were uniformly lower. SARS-CoV-2-specific binding antibody and neutralising antibody titres in participants vaccinated with COH04S1 were within the range of WHO reference panel members with mid-to-low titres (figure 2; appendix pp 101–03).
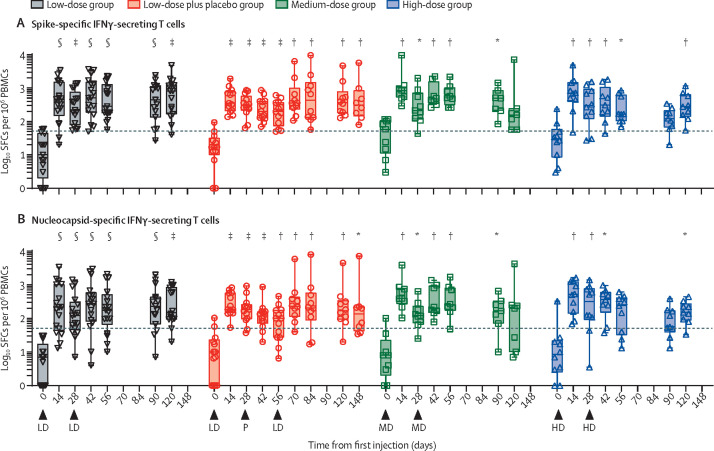
At day 56, median spike-specific IFNγ-secreting T cells were 13·3 (IQR 6·67–23·50) in the placebo group, 283·3 (182·5–966·5) in the low-dose COH04S1 group, 535·0 (425·0–871·1) in the medium-dose COH04S1 group, and 167·0 (103·3–566·6) in the high-dose COH04S1 group, whereas median nucleocapsid-specific IFNγ-secreting T cells were 3·33 (0·00–6·67) in the placebo group, 173·5 (95·0–420·0) in the low-dose COH04S1 group, 246·5 (196·8–738·9) in the medium-dose COH04S1 group, 240·0 (40·0–376·6) in the high-dose COH04S1 group (p<0·0001, p=0·0043, p=0·0010 vs placebo for spike-specific in low, medium, and high-dose groups, and p=0·0017, p=0·0043, p=0·0051 vs placebo for nucleocapsid specific in low, medium and high-dose groups). In the ten participants in the low-dose COH04S1 plus placebo group who received an injection at or after day 56, medians were 432·0 (p<0·0020 vs placebo) spike-specific IFNγ-secreting T cells and 205·0 (p<0·0020 vs placebo) nucleocapsid-specific IFNγ-secreting T cells 28 days after the low-dose boost (figure 3 ; appendix pp 96–97). A four times increase in spike-specific or nucleocapsid-specific IFNγ-secreting T cells was measured in 48 (98%, 95% CI 89–100) of 49 participants across all doses post-prime and post-boost within 28 days of the prime vaccination. 90 days after the last injection, 31 (82%, 66–92) of 38 participants across all groups had a sustained four times increase in spike-specific or nucleocapsid-specific IFNγ-secreting T cells. IL-4-secreting T cells were also induced at all doses, although to a much lower concentration than IFNγ-secreting T cells (appendix pp 98–99, 104). At day 56, there was a median increase of 174% (IQR 53–307) for the spike-specific and of 211% (80–358) for the nucleocapsid-specific Th1-to-Th2 (IFNγ:IL-4) ratio that was significant compared with baseline across all doses (p<0·0001; appendix p 105). No longitudinal increase in membrane-specific cellular responses occurred in any of the groups (appendix p 106). Additionally, no significant changes in any immunological parameter were measured in the placebo group (appendix p 107).
Figure 3.
IFNγ-secreting T-cell responses following COH04S1 vaccination with different dose levels and schedules
Spike-specific (A) and nucleocapsid-specific (B) IFNγ-secreting T-cell responses were quantified at the indicated timepoints by IFNγ:IL-4 ELISpot upon PBMC stimulation with spike and nucleocapsid peptide libraries in participants vaccinated with COH04S1. Box plots extend from the 25th to the 75th percentiles, median values are shown as a line, whiskers extend from minimum to maximum values. Individual values are superimposed. Reported are statistical testing results using Wilcoxon rank-sum paired test and comparing each timepoint to baseline (day 0). Exact p values are shown in appendix (pp 96–97). Dashed lines represent the arbitrary threshold for positive response (50 SFCs per 106 PBMCs). Arrowheads represent time of vaccination. LD represents the low dose of COH04S1 (1·0 × 107 PFU), MD represents the medium dose (1·0 × 108 PFU), and HD represents the high dose (2·5 × 108 PFU). P represents placebo. ELISpot=enzyme-linked immunospot. IFNγ=interferon-γ. IL-4=interleukin-4. PFU=plaque-forming unit. PBMCs=peripheral blood mononuclear cells. SFCs=spot-forming cells. *p<0·05. †p<0·01. ‡p<0·001. §p<0·0001.
Expression of T-cell activation marker CD137 following stimulation with spike and nucleocapsid peptides was evaluated in sentinel participants (appendix p 108). There was a significant increase in both spike-specific (290% median increase [IQR 205–336], p=0·0025), and nucleocapsid-specific (211% median increase [18–325], p=0·0064) CD137+CD4+ cell concentrations from day 0 to day 14, which remained significantly different from baseline up to day 120. Spike-specific CD137+CD8+ cells had a median transient increase of 41% (0–113, p=0·0414) from day 0 to day 14. Phenotypic analysis revealed a preponderance of central memory phenotype for spike-specific and nucleocapsid-specific CD137+CD4+ T cells and a marked effector memory phenotype for spike-specific and nucleocapsid-specific CD137+CD8+ T cells (appendix p 109), consistent with T-cell phenotypes observed in SARS-CoV-2 convalescent individuals.17 Finally, COH04S1 immunogenicity was similar in low-dose group participants born before or after 1973, the year of the end of the smallpox eradication campaign (appendix p 110).
Discussion
In this first-in-human phase 1 trial with randomised expansion cohorts, vaccination with COH04S1 elicited robust humoral and cellular immunity to both spike and nucleocapsid vaccine antigens with no indication of serious safety concerns. Adverse events were predominantly mild with few events moderate in severity and no apparent difference in adverse events between the first and second doses and among the three dose levels. COH04S1 induced spike-specific and nucleocapsid-specific binding antibody titres following the first vaccine dose, which were further boosted after the second dose so that by 1 month after the second dose 100% of the participants at all dose levels had reached the primary immunological endpoint.
Consistent with what has been observed in clinical trials evaluating other COVID-19 vaccines,18, 19, 20 maximal induction of neutralising antibodies by COH04S1 was achieved after two doses. COH04S1 stimulated neutralising antibody responses that showed activity against pseudoviruses specific for Asp614Gly and several variants of concern, indicating that COH04S1 has the capacity to elicit cross-reactive neutralising antibody responses that are effective against ancestral SARS-CoV-2 and its emerging variants. Although COH04S1-induced binding antibody and cellular responses were similar among dose levels, a dose effect was observed for neutralising antibodies, with higher neutralising antibody titres measured in all participants who received medium-dose or high-dose COH04S1 than in those who received low-dose COH04S1. Notably, in participants immunised with low-dose COH04S1, we observed that a delay of 1 or 2 months in the administration of a second dose significantly increased, albeit temporarily, peak neutralising antibody titres post-boost in comparison to participants vaccinated following the standard 28-day interval schedule. An improvement in vaccine-induced immune responses with longer intervals between doses has been recently confirmed with other viral vector vaccines.21 As described for mRNA vaccines,22 a decline in neutralising antibody titres was observed at 2–3 months post-boost at all dose levels, although neutralising antibody titres were still detectable at day 90 post-second COH04S1 injection in most vaccinated participants. Further timepoints are needed to identify whether the neutralising antibody titres will continue to decline or plateau at a lower level. Nonetheless, spike-specific and nucleocapsid-specific binding antibody and cellular responses were still elevated at day 90 post-second COH04S1 injection. Given that COH04S1 was equally well tolerated and immunogenic at all dose levels, we concluded that COH04S1 could be safely used to induce robust SARS-CoV-2 specific humoral and cellular responses even when used at the lowest dose level, thus providing easy scalability to mass production.
COH04S1 differs from approved COVID-19 vaccines because of its dual antigen design combining in a single vector the spike and nucleocapsid antigens, which was chosen primarily to broaden the stimulation of T-cell responses.23 Given the conserved nature of T-cell epitopes, vaccines that elicit strong T-cell immunity appear particularly valuable to sustain protection in the face of the decline of humoral immunity in the wake of mutations causing virus escape from neutralisation.24, 25 Additionally, T-cell responses to SARS-CoV-2 can be present in convalescent individuals even in the absence of detectable antibody responses26 and contribute to survival in patients with COVID-19 and haematological malignancies.27 Beside spike, nucleocapsid has been suggested as a strong candidate antigen,24, 28 and has recently been shown to confer spike-independent protective immunity in rodents.29, 30 We show that COH04S1-induced nucleocapsid-specific T-cell responses were of similar magnitude and phenotype compared with spike-specific T-cell responses, thus supporting the addition of nucleocapsid in the COH04S1 vaccine formulation. Additionally, considering that both spike-specific and nucleocapsid-specific T-cell responses reached maximum levels already after the first dose, COH04S1 could be used to generate nucleocapsid-specific T-cell responses even in the context of a booster immunisation to a previous spike-only vaccine, thus extending the breadth of the cellular response to an immune-dominant antigen that is less prone to viral escape than spike. Finally, we predominantly measured Th1-biased cellular responses, suggesting low risk of vaccine-associated enhanced respiratory disease.
In addition to their proven safety record,31 MVA-based vaccines are known for inducing long-lasting humoral and cellular protective immune responses against several infectious diseases, in both healthy and immunocompromised individuals using homologous and heterologous vaccination regimens.32, 33 In particular, in haematopoietic stem cell transplant recipients, MVA has been shown to induce vaccine-mediated cellular immunity early post-transplant at a time of maximal susceptibility to viral infections.32 Based on this premise, immunocompromised patient populations might benefit from the safety and immunogenicity profile of COH04S1. This hypothesis is being currently evaluated in a phase 2, randomised, blinded, EUA vaccine-controlled trial in patients with blood cancer who have received stem cell transplant or cellular therapy (NCT04977024).
There is a concern that immunogenicity of viral vector-based vaccines might be blunted in the presence of pre-existing vector immunity or in the context of a homologous prime/boost immunisation.34 We have previously shown that pre-existing vaccinia immunity does not affect the immunogenicity of MVA vaccines.35 Additionally, comparison of COH04S1-induced humoral and cellular immunity between individuals with or without possible pre-existing smallpox immunity did not show evidence of a weakened response to COH04S1 in participants who were probably vaccinated with a smallpox vaccine. Furthermore, in the current study we showed that homologous prime/boost vaccination with an MVA-based vaccine promotes the induction of neutralising antibodies without negatively affecting the magnitude of the cellular immune response to the antigens. Nonetheless, use of COH04S1 in a heterologous vaccination setting might result in improved immunogenicity, similar to what has been shown for other SARS-CoV-2 vaccines36 and is currently being evaluated in a phase 2 clinical trial using COH04S1 as a booster vaccination to authorised COVID-19 vaccines (NCT04639466).
Our study has several limitations. The sample size was originally small but appropriate for a phase 1 trial. However, accrual was negatively affected by the availability of authorised SARS-CoV-2 vaccines, which resulted in unanticipated necessary changes in the study design. Another limitation is that the analysis of SARS-CoV-2-specific neutralising antibodies was performed using a pseudovirus instead of authentic virus. However, we have previously shown that neutralising antibody titres measured with lentiviral pseudovirus correlate with neutralising antibody titres measured using SARS-CoV-2.7 Additionally, analysis of cross-reactive neutralising antibody responses to variants of concern was only performed with samples from sentinel participants. Finally, in this report we assessed durability of COH04S1-induced immune responses up to day 120, or 3 months after the booster injection. Long-term immune responses will be evaluated up to 1 year and will be presented in a follow-up report.
In conclusion, we demonstrated that COH04S1 was tolerable and immunogenic in healthy adults aged 18–54 years at all dose levels tested. Humoral and cellular responses against SARS-CoV-2 were measured after the first dose of the vaccine and seroconversion was achieved in 100% of the participants after two doses. These findings support the ongoing phase 2 evaluation of COH04S1 in immunocompromised individuals as a primary vaccine and in healthy adults as a booster vaccination.
Data sharing
We support data sharing of the individual de-identified participant data that underlie the results reported in this Article. Data will be available when the trial is completed. Clinical study reports and study protocol with any amendments will be shared upon request to the corresponding author.
Declaration of interests
DJD and FW are co-inventors on a patent application covering the design and construction of the synthetic modified vaccinia Ankara platform (PCT/US2021/016247). DJD, FW, and FC are co-inventors on a patent application covering the development of a COVID-19 vaccine (PCT/US2021/032821). FC, JAZ, PHF, RS, JD, BW, AMA, KFr, RAT, JKD, SD, AGP, DDN, PA, YC, HC, CLR, KT, YP, JM, AI, QZ, VK, DJ, KFa, TK, JN, MK, VHN, SOF, AW, FW, and DJD are employees of City of Hope National Medical Center (Duarte, CA, USA), which developed the vaccine and funded the trial. AS is a consultant for Gritstone, Flow Pharma, Merck, Epitogenesis, Gilead, and Avalia. La Jolla Institute for Immunology has filed for patent protection for various aspects of T-cell epitope and vaccine design work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Funding for this study was provided by the Carol Moss Foundation and the City of Hope Integrated Drug Development Venture programme. We thank all the participants who volunteered in the study and all the investigators and study site personnel who assisted in the clinical trial completion. Sandra Thomas (Department of Hematology & HCT, City of Hope, Duarte, CA, USA) is warmly acknowledged for her remarkable commitment to these efforts. We acknowledge and thank Christoph Pittius and Yuriy Shostak (Research Business Development, City of Hope) for excellent project management. We thank Karen Gutierrez and Christina Ulloa (Department of Hematology & HCT, City of Hope) for excellent support of investigators and meeting coordination.
Contributors
JAZ is the principal investigator of the trial. JAZ, RS, PHF, and DJD designed the trial and study protocol. FC, JAZ, PHF, DJD, and FW drafted the manuscript. COH04S1 was developed by DJD, FW, and FC. JM, AI, JN, MK, and VHN supported vaccine development. KT provided regulatory oversight. JD, BW, AMA, KFr, RAT, JKD, SD, AGP, DDN, and PA collected study data and oversaw participants visits. PHF and YC did the statistical analysis. Immunogenicity testing was done by FC, HC, YP, QZ, VK, DJ, KFa, SOF, and AW. Immunogenicity analysis was performed by FC and CLR. Study material was provided by TK, AG, and AS. Data were verified by FC, PHF, and YC. All authors contributed to the reviewing and editing of the report and approved the final version of the manuscript.
Supplementary Material
References
- 1.WHO WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Haas EJ, McLaughlin JM, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00566-1. published Sept 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause PR, Fleming TR, Longini IM, et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385:179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edara VV, Pinsky BA, Suthar MS, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Liu J, Xia H, et al. BNT162b2-elicited neutralization against new SARS-CoV-2 spike variants. N Engl J Med. 2021;385:472–474. doi: 10.1056/NEJMc2106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiuppesi F, Salazar MD, Contreras H, et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni A, Sidney J, Vita R, et al. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe. 2021;29:1076–1092. doi: 10.1016/j.chom.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn JY, Lee J, Suh YS, et al. Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: an interim analysis of two open-label, non-randomised, phase 1 trials in healthy adults. Lancet Microbe. 2022 doi: 10.1016/S2666-5247(21)00358-X. published online Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieling P, King T, Wong R, et al. Prime hAd5 spike + nucleocapsid vaccination induces ten-fold increases in mean T-cell responses in phase 1 subjects that are sustained against spike variants. medRxiv. 2021 doi: 10.1101/2021.04.05.21254940. published online June 9. (preprint). [DOI] [Google Scholar]
- 11.Chiuppesi F, Nguyen VH, Park Y, et al. Synthetic multiantigen MVA vaccine COH04S1 protects against SARS-CoV-2 in Syrian hamsters and non-human primates. npj Vaccines. 2022 doi: 10.1038/s41541-022-00436-6. published online Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention CDC updates, expands list of people at risk of severe COVID-19 illness. June 25, 2020. https://www.cdc.gov/media/releases/2020/p0625-update-expands-covid-19.html [PubMed]
- 13.US Department of Health and Human Services. National Institutes of Health. National Institute of Allergy and Infectious Diseases. Division of AIDS Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. July 2017. https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables
- 14.Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect Dis. 2021;21:1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falsey AR, Frenck RW, Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tornqvist L, Vartia P, Vartia YO. How should relative changes be measured. Am Stat. 1985;39:43–46. [Google Scholar]
- 17.Neidleman J, Luo X, Frouard J, et al. SARS-CoV-2-specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 19.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pegu A, O'Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373:1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarke A, Sidney J, Methot N, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redd AD, Nardin A, Kared H, et al. CD8+ T-cell responses in COVID-19 convalescent individuals target conserved epitopes from multiple prominent SARS-CoV-2 circulating variants. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferretti AP, Kula T, Wang Y, et al. Unbiased screens show CD8+ T cells of COVID-19 patients recognize shared epitopes in SARS-CoV-2 that largely reside outside the spike protein. Immunity. 2020;53:1095–1107. doi: 10.1016/j.immuni.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matchett WE, Joag V, Stolley JM, et al. Cutting edge: nucleocapsid vaccine elicits spike-independent SARS-CoV-2 protective immunity. J Immunol. 2021;207:376–379. doi: 10.4049/jimmunol.2100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trimpert J, Herwig S, Stein J, et al. Deciphering the role of humoral and cellular immune responses in different COVID-19 vaccines— a comparison of vaccine candidate genes in Roborovski dwarf hamsters. Viruses. 2021;13 doi: 10.3390/v13112290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volz A, Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldoss I, La Rosa C, Baden LR, et al. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: a phase 2, randomized clinical trial. Ann Intern Med. 2020;172:306–316. doi: 10.7326/M19-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page K, Melia MT, Veenhuis RT, et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N Engl J Med. 2021;384:541–549. doi: 10.1056/NEJMoa2023345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altenburg AF, van Trierum SE, de Bruin E, et al. Effects of pre-existing orthopoxvirus-specific immunity on the performance of modified vaccinia virus Ankara-based influenza vaccines. Sci Rep. 2018;8 doi: 10.1038/s41598-018-24820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Rosa C, Longmate J, Martinez J, et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood. 2017;129:114–125. doi: 10.1182/blood-2016-07-729756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuart ASV, Shaw RH, Liu X, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We support data sharing of the individual de-identified participant data that underlie the results reported in this Article. Data will be available when the trial is completed. Clinical study reports and study protocol with any amendments will be shared upon request to the corresponding author.