Abstract
Objective
To investigate the association between different smoking statuses and survival and emphysema in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Methods
This retrospective study included patients admitted from October 2014 to September 2017. Demographic, clinical, laboratory, imaging, impulse oscillometry, and traditional pulmonary function data were collected. The relationship between smoking and EI was analyzed via binary logistic regression after adjusting for other factors. Survival was analyzed using the Kaplan–Meier method and the log rank test.
Results
The patients with AECOPD (357 cases) were identified (and stratified into three groups: never smoked (NS; n=83), former smokers (FS, n=118), and current smokers (CS; n=156). Compared with CS, NS were older and predominantly female. No differences were observed in respiratory symptoms and acute exacerbation between CS and NS. NS had higher resistance and reaction in the central and peripheral airways, while CS exhibited more severe diffuse dysfunction. CS demonstrated more severe and extensive emphysema. Smoking was an independent risk factor for emphysema after adjusting for age, forced expiratory volume in the first second over predicted value, BMI, leukocyte count, and carbon monoxide transfer coefficient. No difference in 5-year survival rates between NS and CS was established.
Conclusion
CS has the worst pulmonary function, suggesting a more important destruction of the lung parenchyma, while AECOPD without smoking risk factors mostly affects the airways. Impulse oscillometry can be used for imaging airway-dominant AECOPD. There was no difference in the 5-year survival rate.
Keywords: chronic obstructive pulmonary disease, acute exacerbation, smoking, impulse oscillometry system, emphysema index
Introduction
Chronic obstructive pulmonary disease (COPD) is projected to be the third leading cause of death worldwide,1 with the health impact and economic cost of COPD posing a dual burden to society.2 COPD is a complex chronic inflammatory disease involving central airways, small airways, lung tissue, and pulmonary vessels,3 presenting heterogeneous characteristics.
COPD is associated with a variety of risk factors (the main one being smoking, but also including genetic factors, family history of COPD, indoor/outdoor air pollution, inflammatory conditions, infections, medications, diet, and snoring)3,4 though more than 20% of COPD patients have never smoked.5 The same factors are also associated with response to treatment and prognosis.3,4 There are specific etiological characteristics and risk factors for COPD in China, including an estimated 312 million Chinese who are smoking.6 Previous studies showed that cigarette smoking and second-hand smoking are important risk factors for COPD.7,8 Other risk factors include biofuel combustion, ambient air pollution, underweight, childhood chronic cough, and parental history of respiratory diseases.7 It is unclear, however, whether different risk factors result in different clinical and pathological features. Furthermore, it is unknown whether COPD caused by different pathogenic risk factors has different prognostic outcomes and these issues deserve more exploration.
Patients having an acute exacerbation of COPD (AECOPD) have a 5-year survival of 50% after hospitalization for exacerbation.3 Treatment of AECOPD requires different treatment modalities and vast medical resources. Although many studies addressed stable COPD, data about AECOPD is scarcer. While clinical and laboratory investigations, pulmonary function tests, and computed tomography (CT) can reveal some characteristics, no single modality can adequately represent the disease’s full spectrum. The COPDGene study assessed the patients in a multidimensional fashion to enhance the understanding of the complex structural and functional changes in COPD.9 In previous studies by the authors’ group,10–13 the parameters mentioned above were combined to uncover the characteristics of the AECOPD population.
Still, the impact of different lifetime smoking statuses on AECOPD requires investigation. Therefore, this study aimed to investigate the differences in clinical features, inflammatory markers, lung function indicators, imaging parameters, and survival of AECOPD in current smokers (CS), former smokers (FS), and never smokers (NS) and also attempted to identify the characteristic changes of AECOPD associated with smoking.
Methods
Study Design and Patients
This retrospective study included patients admitted to the Department of Respiratory Medicine at the authors’ hospital from October 2014 to September 2017. This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the ethics committee of Xi’an ninth hospital (#014001). The data used in the study did not involve information that can be used to identify the patients. The use of the patient data will not adversely affect patients. All data were collected from the medical charts. No new tests or measurements were performed. The patients were not contacted to obtain new data. Therefore, due to the retrospective nature of this study, informed consent of patients was waived.
The inclusion criterion was patients diagnosed with AECOPD according to the Global Initiative for Obstructive Lung Disease (GOLD) diagnostic criteria (forced expiratory volume at 1 second/forced vital capacity, FEV1/FVC <70% after inhalation of a bronchodilator).3 The exclusion criteria included 1) <40 years of age, 2) pregnancy, 3) comorbid lung diseases including lung cancer, pneumonia, active pulmonary tuberculosis, pulmonary embolism, or interstitial lung disease, 4) previous lung surgery, 5) inability to complete the pulmonary function test (PFT), 6) asthma or severe heart, liver, or kidney dysfunction, or 7) high-resolution computed tomography (CT) quality insufficient for analysis.
Data Collection
The electronic medical records were accessed to collect all data variables, including demographics (sex, age, and smoking status), body mass index (BMI), the number of hospitalizations due to AECOPD over the past 12 months, inflammatory indicators, including white blood cell count (WBC), neutrophil ratio (N%), eosinophil ratio (E%), fibrinogen(FIB), C-reactive protein (CRP), procalcitonin (PCT), arterial oxygen partial pressure (PaO2), arterial carbon dioxide partial pressure (PaCO2), and comorbidities, including coronary heart disease, hypertension, and diabetes. The routinely administered self-report questionnaires of the COPD Assessment Test (CAT) and Modified Medical Research Council Dyspnea Index (mMRC) were collected from the medical charts.10,13
Other collected variables were the ratio of forced expiratory volume occupancy in the first second (FEV1/FVC), the ratio of forced expiratory volume in 1 second to predicted (FEV1%pred), the ratio of carbon monoxide diffusion to alveolar ventilation (KCO), the ratio of residual volume to total lung volume (RV/TLC), and the ratio of maximal mid-expiratory flow to predicted (MMEF25–75%pred). The total respiratory impedance (Z5), resistance at 5 Hz (R5), resistance at 20 Hz (R20), resistance at 5Hz-resistance at 20Hz (R5-R20), reactance at 5 Hz (X5), resonant frequency (Fres), and reactance area (Ax) detected in patients without wheezing symptoms by spirometry and the impulse oscillometry system (IOS) (Master Screen IOS, Erich Jaeger, Hochberg, Germany) were collected.12,13
Patients with AECOPD with available imaging data included 65 NS, 83 FS, and 115 CS. The CT imaging parameters assessed included the percentage of the wall area (%WA), emphysema, mean emphysema density (MED), mean lung density (MLD), lung capacity (LC), and emphysema heterogeneity index (HI) of the whole lung, right lung, and left lung detected by 64-detector CT (SOMATOM Definition AS, Siemens HealthCare, Germany) and analyzed using an in-house system (FACT-Digital Lung)14 and collected as described previously.10,13 The primary outcome of this study was the 5-year survival rate. Patients were followed up until April 2021. For patients lost to follow-up, the vital status was obtained from the government.
Definitions
NS was defined as a lifetime exposure of <1 cigarette/year.15 FS was defined who had quit smoking for more than 1 year. CS was defined as individuals who still smoked. The %WA and lung volume with a CT attenuation value below −950 HU (%LAA-950) was defined as the emphysema index (EI). Emphysema (%LAA), mean emphysema density (MED), mean lung density (MLD), lung capacity (LC), and emphysema heterogeneity index (HI) were recorded for the whole lung (HI whole lung), right lung (HI right lung), and left lung (HIleft lung). The %WA distribution of the bronchus of different generations in each lung lobe was expressed as %WARUL4–7, %WARML4–7, %WARLL4–9, %WALUL4–7, and %WALLL4–9.
Statistical Analysis
All analyses were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to assess whether the data conformed to the normal distribution. Continuous data conforming to the normal distribution were displayed as mean ± standard deviation; data not conforming to the normal distribution were displayed as median (IQR). If the continuous data were normally distributed and the variances were uniform, a one-way analysis of variance was used. The Bonferroni test provided a further pairwise comparison between groups. For continuous data not normally distributed, the Kruskal–Wallis test was used to evaluate the differences among the three groups and for comparison between every two groups with a p-value of 0.016. p<0.05 was considered significant elsewhere. Categorical variables were displayed in n (%) and analyzed by chi-square test. The relationship between smoking and EI was analyzed via binary logistic regression after adjusting for other factors, with a probability value for entry (p=0.1) and removal (p=0.05). Correlation analysis between the amount of smoking and EI was done with the Spearman test. Survival curves for the two main groups (never smokers and all smokers) were constructed according to the Kaplan-Meier method and compared using the log rank test.
Results
Comparison of Demographic and Clinical Data
Age and sex-related differences were observed between the NS and smoker (both FS and CS) groups. NS were older, with a greater preponderance of females. Further comparison of the three groups revealed that the age of the NS and FS groups was higher than that of the CS group (p<0.001). The percentage of males in the NS group was lower than in the FS and CS groups (p<0.001). The mMRC of the FS group was higher than in the CS group (p<0.001). No significant differences were observed in BMI, CAT, N%, E%, platelet, FIB, CRP, PCT, PaO2, and PaCO2 (Table 1).
Table 1.
Demographic and Baseline Characteristics
| Characteristics | Never Smokers | Former Smokers | Current Smokers | P-value |
|---|---|---|---|---|
| (n=83) | (n=118) | (n=156) | ||
| Age, years | 71 (64~79) | 71.5 (65~79) | 63.5 (58~70) *# | <0.001 |
| Gender | ||||
| Male (%) | 49 (59%) | 114 (96.6%) * | 153 (98.1%) * | <0.001 |
| Female (%) | 34 (41%) | 4 (3.4%) * | 3 (1.9%) * | <0.001 |
| Comorbidities | 1 (0~2) | 1 (0~2) | 1 (0~1) | 0.238 |
| Exacerbations/year | 0 (0~1) | 0 (0~2) | 0 (0~1) | 0.049 |
| CAT | 19 (14~26.5) | 19 (14~25) | 20.5 (12~25) | 0.712 |
| mMRC | 1 (0~2) | 2 (1~3) | 1 (0~2) # | 0.002 |
| BMI (kg/m2) | 23.5±3.9 | 22.8±3.6 | 23.1±3.9 | 0.448 |
| WBC, (×109/L) | 6.5 (5.02~7.79) | 6.84 (5.47~9.38) | 6.84 (5.31~8.68) | 0.091 |
| Neutrophils, (%) | 71.21±12.07 | 73.25±11.19 | 70.35±11.9 | 0.124 |
| Lymphocytes, (%) | 21.3 (13.25~27.1) | 18.85 (10.8~23.9) | 20.6 (14.2~27.65) | 0.11 |
| Eosinophils, (%) | 1.4 (0.4~2.9) | 1.5 (0.5~3.3) | 1.55 (0.35~3) | 0.813 |
| PLT, (×109/L) | 163 (131.5~209.5) | 161.5 (124~227) | 167.5 (134~212) | 0.977 |
| FIB (g/L) | 3.93 (3.26~5.16) | 3.84 (3.19~5.07) | 3.95 (3.13~4.81) | 0.829 |
| D-Dimer(ug/ml) | 0.91 (0.61~1.28) | 0.9 (0.67~1.46) | 0.9 (0.66~1.28) | 0.963 |
| CRP (mg/L) | 6.99 (3.28~23.15) | 9.64 (3.28~38.2) | 6 (3.28~24.8) | 0.164 |
| PCT(ng/ml) | 0.05 (0.05~0.05) | 0.05 (0.05~0.05) | 0.05 (0.05~0.05) | 0.475 |
| PH | 7.42 (7.4~7.44) | 7.42 (7.39~7.44) | 7.41 (7.38~7.44) | 0.067 |
| PaO2 (mmHg) | 78 (67.5~89) | 73 (65~88) | 73 (64~83) | 0.123 |
| PaCO2 (mmHg) | 40 (36~44) | 42 (38~47) | 42 (37~48) | 0.074 |
Notes: *P < 0.05 compared with NS; #P < 0.05 compared with FS.
Abbreviations: BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; COPD, chronic obstructive pulmonary disease; WBC, white blood cell count; N, neutrophil; E, eosinophils; PLT, blood platelet count; FIB, fibrinogen; CRP, C-reactive protein; PCT, procalcitonin; PaO2, arterial oxygen partial pressure; PaCO2, arterial carbon dioxide partial pressure.
Comparison of PFTs
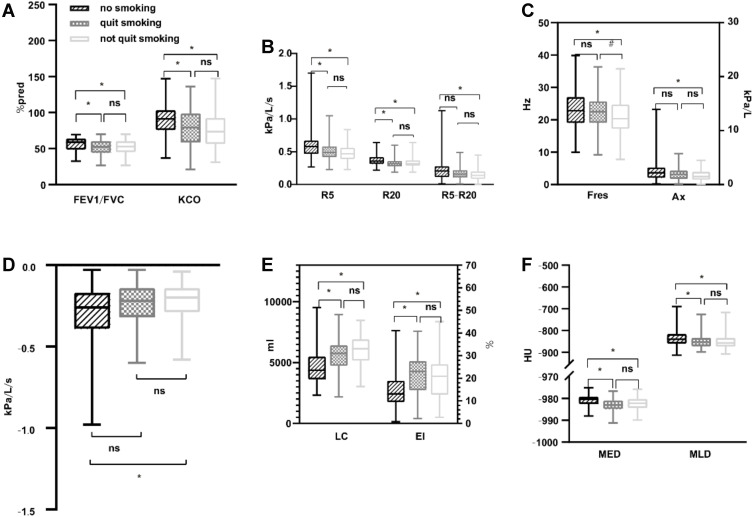
Differences were observed between the results of traditional PFTs (ie, FEV1/FVC and DLCO/VA) of the NS and smoker (including FS and CS) groups. NS exhibited higher FEV1/FVC and DLCO/VA than all smokers. There were no differences between FEV1/FVC and DLCO/VA in the FS and CS groups. No significant differences were found in FEV1%pred, MMEF25–75%, and RV/TLC among the three groups (Table 2, Figure 1A).
Table 2.
PFT Parameters
| Parameters | Never Smokers | Former Smokers | Current Smokers | P-value |
|---|---|---|---|---|
| n=83 | n=118 | n=156 | ||
| FEV1 (L) | 1.14 (0.77~1.56) | 1.18 (0.84~1.59) | 1.37 (0.95~1.77) *# | 0.005 |
| FEV1%pred | 49.6 (37~66.35) | 45.75 (31.2~57) | 45.4 (35.6~58.4) | 0.089 |
| FVC% pred | 70.2 (55.5~86.3) | 68.3 (51.8~79.5) | 67.05 (56.4~79.9) | 0.376 |
| FEV1/FVC (%) | 58.78 (48.63~63.74) | 52.38 (44.23~60.27) * | 53.68 (45.07~60.32) * | 0.007 |
| PEF %pred | 39.2 (28.25~51.2) | 37.5 (28~46.1) | 39 (29.85~48.8) | 0.599 |
| MMEF25–75% | 19.4 (14~28.9) | 18.6 (12.8~25) | 19.25 (13.7~27) | 0.598 |
| DLCO/VA (%) | 89.5±22.27 | 78.29±25.86* | 75.63±23.98* | <0.001 |
| RV/TLC (%) | 57.09±10.48 | 58.44±11.2 | 55.14±10.84 | 0.051 |
| Z5%pred | 182.1 (144.5~219.6) | 175.6 (143.1~207.4) | 169.6 (142.8~205.8) | 0.483 |
| R5 (kPa/L/s) | 0.58 (0.48~0.67) | 0.49 (0.42~0.58) * | 0.47 (0.39~0.56) * | <0.001 |
| R20 (kPa/L/s) | 0.36 (0.32~0.42) | 0.32 (0.28~0.36) * | 0.32 (0.29~0.37) * | <0.001 |
| R5-R20 (kPa/L/s) | 0.21 (0.12~0.28) | 0.16 (0.11~0.22) | 0.14 (0.09~0.2) * | 0.001 |
| X5 | −0.26 (−0.38~-0.17) | −0.22 (−0.32~-0.14) | −0.2 (−0.29~-0.14) * | 0.009 |
| Fres (Hz) | 23.34±5.79 | 22.73±5.07 | 21±5.23*# | 0.002 |
| Ax (kPa/L) | 2.18 (1.27~3.09) | 1.88 (1.06~2.57) | 1.49 (0.89~2.36) * | 0.003 |
Notes: *P < 0.05 compared with NS group; #P < 0.05 compared with FS group. Abbreviations: PFT pulmonary function test, FEV1 forced expiratory volume in 1 second, FVC forced vital capacity, FEV1/FVC forced expiratory volume in 1 sec/forced vital capacity, MMEF25–75% maximal mid expiratory flow, RV/TLC residual volume/total lung capacity, DLCO/VA ratio of carbon monoxide diffusion capacity to alveolar ventilation, %Pred, of the predicted value, Z5 total respiratory impedance, R5 resistance at 5 Hz, R20 resistance at 20 Hz, X5 reactance at 5 Hz, Fres response frequency, Ax reactance area.
Figure 1.
Comparison of two lung function test parameters and imaging parameters. (A) Comparison of FEV1/FVC and DLCO/VC. (B) Comparison of R5, R20, R5-R20. (C) Comparison of Fres and Ax. (D) Comparison of X5. (E) Comparison of LC and EI of whole lungs. (F) Comparison of MED and MLD of whole lungs. Notes: *P < 0.05 compared with NS (no smoking); #P < 0.05 compared with FS (quit smoking).
Abbreviation: FEV1/FVC the ratio of forced expiratory volume occupancy in the first second, KCO the ratio of carbon monoxide diffusion to alveolar ventilation, R5 resistance at 5 Hz, R20 resistance at 20 Hz, R5-R20 resistance at 5Hz-resistance at 20Hz, X5 reactance at 5 Hz, Fres resonant frequency, AX reactance area, EI emphysema index, MED mean emphysema density, MLD mean lung density, LC lung capacity, NS, no significance.
The resistance at R5, R20, R5-R20, X5, Fres, and Ax on the IOS test differed between the NS and smokers (former and current smokers) groups (Table 2, Figure 1B–D). NS with AECOPD had a higher resistance and reactance in the central airway and lung periphery compared with smokers with AECOPD, particularly with CS.
Comparison of CT Imaging Features Among the Three Groups
The LC whole lung, LC Right lung, and LC Left lung of smokers (including FS and CS) was higher than in NS (p < 0.001) (Table 3), but these parameters did not differ between FS and CS (Figure 1E).
Table 3.
Imaging Characteristics
| Parameters | Never Smokers | Former Smokers | Current Smokers | P-value |
|---|---|---|---|---|
| n=65 | n=83 | n=115 | ||
| LC Whole (ml) | 4594.28±1332.85 | 5663.81±1305.35* | 6004.53±1252.95* | <0.001 |
| LC Right lung (ml) | 2495.24±712.45 | 3033.31±700.95* | 3201.49±655.4* | <0.001 |
| LC Left lung (ml) | 2099.03±643.76 | 2630.5±644.48* | 2824.56±605.5* | <0.001 |
| %LAA−950 Whole lung | 14.94±7.93 | 21.45±9.25* | 20.54±9.5* | <0.001 |
| %LAA−950 Right lung | 13.89 (9.26~18.75) | 21.93 (15.17~28.45) * | 20.73 (13.53~26.48) * | <0.001 |
| %LAA−950 Left lung | 13.67 (8.96~19.85) | 22.11 (14.33~27.57) * | 21.35 (12.29~26.83) * | <0.001 |
| MED Whole lung (HU) | −980.44 (−982.54~-979.23) | −983 (−984.8~-981.15) * | −982.23 (−984.3~-980.35) * | <0.001 |
| MED Right lung (HU) | −980.06 (−982.64~-978.72) | −982.84 (−984.71~-980.875) * | −981.69 (−984.24~-980.07) * | <0.001 |
| MED Left lung (HU) | −980.78 (−982.94~-979.9) | −983.3 (−984.74~-981.2) * | −982.6 (−984.79~-980.21) * | 0.001 |
| MLD Whole lung (HU) | −839.68 (−856.24~-817.42) | −852.92 (−871.94~-835.07) * | −857.25 (−873.4~-834.18) * | 0.005 |
| MLD Right lung (HU) | −841.27 (−856.14~-818.25) | −855.27 (−871.74~-835.4) * | −855.12 (−873.67~-834.98) * | 0.004 |
| MLD Left lung (HU) | −838.08 (−864.02~-815.32) | −852.12 (−870.95~-831.11) | −858.67 (−874.93~-836.64) * | 0.005 |
| HI Whole lung | 0.08±0.25 | 0.14±0.28 | 0.13±0.27 | 0.398 |
| HI Right lung | 0.08±0.29 | 0.16±0.3 | 0.17±0.29 | 0.16 |
| HI Left lung | −0.1±0.29 | −0.09±0.28 | −0.12±0.32 | 0.75 |
Note: *P < 0.05 compared with NS group.
Abbreviations: LC, lung capacity; %LAA−950, the extent of emphysema of CT attenuation value below −950 HU; MED, mean emphysema density; MLD, mean lung density; HI, emphysema heterogeneity index, when emphysema is equally distributed among the lobes or the full extent in the whole lung is < 1%, HI is near zero; otherwise, HI = (%LAA upper -%LAA lower)/(%LAA upper+%LAA lower)*100.
The EI and MED reflect the extent and severity of emphysema. The MLD reflects overall lung parenchymal damage. The EI, MED, and MLD of smokers (both former and current) exhibited more critical changes than those of NS in the whole lung, right lung, and left lung (p < 0.001) (Table 3). However, there were no differences between FS and CS (Figures 1E, F and 2).
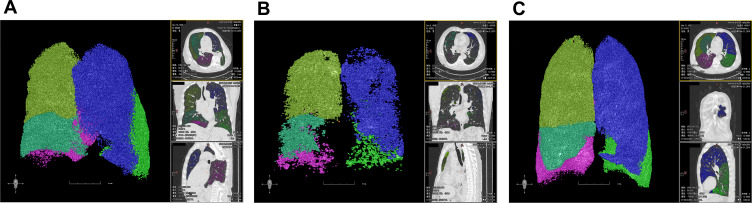
Figure 2.
(A) A 65 years old male of NS: FEV1=1.65L, FEV1/FVC=65.36%, KCO=100.6%, Z5%pred=99.7%, R5=0.28, R20=0.22, R5-R20=0.06, X5=−0.11, Fres=15.38, Ax=0.49, EI=13.68%, MLD=−841.91HU, MED=−979.35HU, LC=4823mL. (B) A 66 years old male of FS: FEV1=0.92L, FEV1/FVC=44.37%, KCO=96.5%, Z5%pred=174.3%, R5=0.46, R20=0.31, R5-R20=0.15, X5=−0.27, Fres=20.07, Ax=1.62, EI=26.85%, MLD=−850.85HU, MED=−988.4HU, LC=6004mL. (C) A 65 years old male of CS: FEV1=1.16L, FEV1/FVC=46.03%, KCO=71.9%, Z5%pred=242.5%, R5=0.64, R20=0.31, R5-R20=0.33, X5=−0.38, Fres=27.17, Ax=3.63, EI=25.19%, MLD=−874.26HU, MED=−981.88HU, LC=6905mL.
There were no significant differences between the HI whole lung, HI Right lung, and HI Left lung (HI represents emphysema distribution) of never smokers and smokers (including former and current smokers; p > 0.016) (Table 3). There were no significant differences in the %WARUL4–7, %WARML4–7, %WARLL4–9, %WALUL4–7, and %WALLL4–9 between the three groups (p > 0.05) (Supplementary Table S1).
Analysis of the Relationship Between Smoking and EI
Spearman’s test was performed on 263 patients with smoking AECOPD. No correlation was observed between the frequency of smoking and %LAA-950Whole lung, LCWhole lung, MED whole lung, or MLDwhole lung.
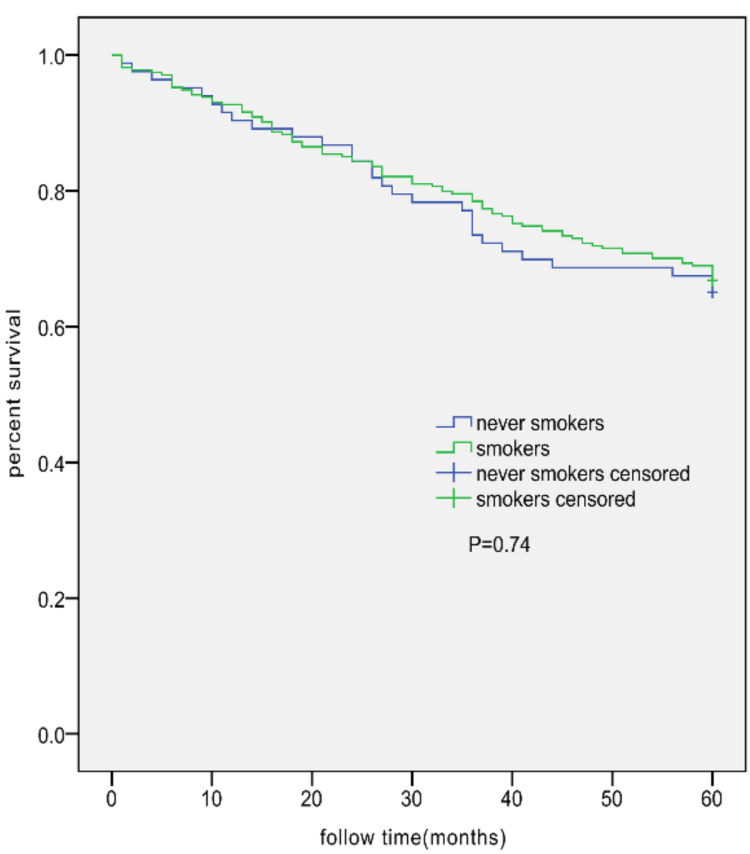
A model was established to elucidate the dependence of EI>20% on smoking and other factors. Clinical practice and literature,9,10,13 outline the following factors which were included in the univariable logistic regression analysis: smoking (yes), age (>65 years), FEV1%pred (<50%), BMI (<21), PaO2 (<60%), PaCO2 (>50%), CAT (>10), mMRC (>2), KCO (<80% and ≥60%, <60%), complications (yes), and acute exacerbation hospitalization in the past 1 year (yes). A multicollinearity analysis was performed to ensure no collinearity among outcomes. The variance inflation factors (VIF) were 1.131 for age, 1.117 for smoking, 1.201 for the number of complications, 1.376 for acute exacerbation hospitalizations in the past 12 months, 1.117 for the CAT score, 1.435 for the mMRC score, 1.260 for BMI, 1.108 for WBC, 1.057 for PaO2, 1.330 for PaCO2, 1.417 for FEV1%PRED, and 1.343 for DLCO/VA (KCO). The results indicate that the VIF of all variables was <5, meaning that there is no collinearity among variables. Statistically different parameters were then included in multivariable logistic regression analysis, including smoking (yes), FEV1%pred (<50%), BMI (<21), PaCO2 (>50%), and KCO (<80% and ≥60%, <60%). The results showed a significant difference between smoking and EI after adjusting for other factors (Table 4). No difference was found in the 5-year survival rate between smokers and NS (Figure 3).
Table 4.
Logistic Regression Analysis of Smoking and EI
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Smoking | 4.185 (2.179~8.04) | <0.001 | 3.052 (1.342~6.938) | 0.008 |
| BMI, kg/m2 | 3.448 (1.967~6.044) | <0.001 | 2.429 (1.192~4.949) | 0.015 |
| FEV1%pred | 2.694 (1.63~4.451) | <0.001 | 1.954 (1.014~3.765) | 0.045 |
| KCO (60%≤KCO<80%) | 4.22 (2.235~7.967) | <0.001 | 2.828 (1.386~5.769) | 0.004 |
| KCO (<60%) | 18.739 (8.459~41.513) | <0.001 | 10.975 (4.716~25.544) | <0.001 |
| PaCO2 | 2.332 (1.151~4.725) | 0.019 | ||
| Comorbidities | 0.88 (0.538~1.44) | 0.611 | ||
| CAT | 0.967 (0.596~1.572) | 0.894 | ||
| Exacerbations/year | 1.181 (0.713~1.957) | 0.518 | ||
| mMRC | 1.257 (0.77~2.053) | 0.361 | ||
| Age | 0.978 (0.601~1.591) | 0.927 | ||
| PaO2 | 1.116 (0.574~2.17) | 0.747 | ||
Notes: After adjusting age (>65 years), FEV1%pred (<50%), BMI (<21), and DLCO/VA (<80%), the factor of smoking was related to emphysema formation EI (≥20%).
Figure 3.
Kaplan–Meier survival curves for AECOPD patients in the NS group (n=83; 29 deaths) and all smokers AECOPD group (n=274; 91 deaths). No significant difference between the two groups was observed (log rank test, 0.113; P=0.74).
Discussion
COPD is a heterogeneous disease, and its occurrence in NS has not been widely recognized. Similarly, differences in characteristics of COPD in NS and smokers are not fully understood in part due to the scarcity of relevant clinical studies. Therefore, the purpose of this study was to identify the characteristics of AECOPD under different smoking exposures and assess 5-year survival among smoking status groups and provide more objective evidence of the etiological heterogeneity of AECOPD.
Differences were noted in gender, with males comprising 59% NS while comprising 96.6% of the FS group and 98.1% of the CS group. The male sex is a recognized risk factor for COPD.3 The gender-related risk exposure status was different between smoking and non-smoking COPD.16 This phenomenon is widespread, particularly in developed and developing Asian countries, such as Japan and India,17 where most smokers are men, which is often related to social and cultural norms. The current study showed that NS was significantly older than the CS group, suggesting that smoking is more significant in the etiology of COPD than other non-smoking factors in China. Interestingly, an Indian study showed that biomass-COPD was comprised of younger than the smokers COPD group,17 while the Canadian Cohort of Obstructive Lung Disease study (CanCOLD) also showed that NS was younger and included more women.16
Reports on the proportion of non-smokers with COPD in the total COPD population are inconsistent with studies showing that non-smokers COPD accounted for 25–45%.17,18 The COPDGene study reported that non-smoking COPD accounted for 27% of all COPD patients.9,19 In this study, NS accounted for 23% of the total AECOPD. Regardless of whether smoking is involved or not, the AECOPD did not differ in assessing the key indicators of the disease, such as CAT, mMRC, BMI, and the history of acute exacerbations in the past 12 months. This study found that all smokers (CS and FS) had a significantly lower ratio of carbon monoxide diffusion to alveolar ventilation (DLCO/VC or KCO) than NS. This suggests that lung diffusion dysfunction (oxygen translation problems from the alveolar membrane into the blood) in the smokers was more obvious, inferring that lung parenchymal damage such as alveolar tissue was more serious. Similar results were reported in the CanCOLD study in the COPD stable population, where results showed that smoking COPD was significantly lower in DLCO/VA than that in non-smoking COPD.16
Although the current diagnosis and evaluation of COPD are mainly based on symptoms, frequency of exacerbations, and lung function, chest CT scans can provide a wealth of information related to diagnosis and individualized treatment.9 Therefore, finding reliable imaging markers is vital in COPD research. Quantitative CT measurement of EI was a more accepted imaging parameter and a clear predictor of COPD, given its reflection of both pathological and functional impairments.20,21 The COPDGene study found that for COPD with an EI of 35%, an additional increase in EI of 5% would increase the COPD deterioration rate 1.18 times.19 In this study, a CT scan revealed that smokers had more extensive and severe emphysema than NS. Like the pulmonary function index DLCO/VA mentioned above, this suggests lung parenchymal damage from a pathophysiological point, while EI also means severer lung parenchymal and lung diffusion damage from a pathological point of view. Similarly, other imaging parameters such as MED, MLD, and LC reflected the same pathological changes and clinical significance. This study showed that smoking was a risk factor for emphysema (EI=20%), which is consistent with other studies that have recognized that smoking can damage lung parenchymal tissues through mechanisms including inflammation, oxidative stress, and elevated protease activity.22 The inflammatory process involves numerous cells such as macrophages, neutrophils, different subgroups of T and B lymphocytes, and dendritic cells. Research between smoking and the autoimmune origin of COPD is also attracting more interest, opening new research prospects for both the cause of COPD and new potential targets for treatment.
Emphysema is one of the hallmark imaging features of COPD. Only 40% of smokers would develop COPD, and it was found that genetic susceptibility may be involved in the occurrence of COPD.22 Therefore, the relationship between smoking and emphysema is not a simple linear relationship.23 Similarly, in this study, no correlations were found between the amount of smoking (pack-years) and the EI, supporting the above viewpoints and reflecting the heterogeneity and complexity of COPD pathogenesis.
Airway wall thickening is a result of inflammatory changes and airway remodeling.24,25 More accurate and reliable airway imaging markers might provide vital information for early diagnosis and evaluation of COPD. The COPDGene early study showed that a thickening of the bronchus by 1 mm, led to an acute exacerbation rate of COPD increasing 1.84 times per year.19 In this study, there were no differences in airway measurements expressed as WA% of a specific segment and sub-segment bronchus between the smoker and NS groups, which might be due to the method for determining thickness not being sensitive enough and the measured bronchial levels concentrated in larger airways, which could not fully reflect the thickening of the small airway wall. In another study, a Pil0 was used (expressed as the square root of the wall area of a hypothetical airway with an internal perimeter of 10 mm) to measure 2000 smokers and 46 NS who were followed for 5 years; the results found that Pil0 decreased in the FS subjects and increased in subjects that began smoking again.24
Interestingly, it was observed that the IOS parameters R5, R20, R5-R20, X5, Fres, and Ax were significantly higher in the NS compared with smokers, indicating that the NS showed higher resistance and blockage in the central and peripheral airway and implying this group might require more diastolic airway treatment. Previous studies have also found that IOS parameters have a certain correlation with traditional lung function parameters with complementary value for assessing pathophysiological changes of COPD.12 This study showed that NS displayed more obvious characteristics of airway obstruction, which comport with similar conclusions of an Indian study that showed that R5, Ax, and resonant frequency values were significantly greater in NS than smoking, but no differences were found in R5-R20, R5-R20/R5 and X5 between the groups.17 In order to assess differences between the two studies, the present study focused on the exacerbation of COPD, in which more mucosal edema and inflammatory cell infiltration are observed, leading to more obvious airway obstruction. Therefore, IOS parameters possess certain application values in AECOPD, superior to airway obstruction.
This study implies that NS suffer from the “airway predominant phenotype” more than smokers. These observations provide insights into phenotypic differences in potential pathogenic factors. This study looking at differences in clinical subtypes under different exposure factors is valuable in providing clinicians with better evidence for treatment decisions. Hence, smoking was a risk factor for emphysema in AECOPD. Similarly, FEV1%pred <50%, BMI <21, KCO (60%≤KCO<80%) were also independent risk factors. Therefore, when taken together, the results suggest that AECOPD is not worse in NS and smokers since the survival outcome was similar among the groups since COPD with pathologic changes involving airway and emphysema have high morbidity and mortality.3 On the other hand, NS and smokers have different etiologies of AECOPD, suggesting that NS and smokers with AECOPD could benefit from different therapeutic approaches. Studies and trials should be designed to examine such therapies.
The present study had some limitations. It was not a longitudinal or a multicenter study. Due to the nature of the retrospective study of the current study, there may be information bias, and a large sample size prospective study is needed for further verification. Despite that, we present one of the few studies on the etiological heterogeneity of the AECOPD, with its value being for the different populations and ethnic groups, which resulted in similar findings in different periods.
Conclusion
This study revealed heterogeneity among patients with AECOPD regarding the pathogenic risk factors. The results suggest that the lung diffusion dysfunction (oxygen diffusion problems through the alveolar membrane) in the smokers was more obvious, inferring that lung parenchymal damage such as alveolar tissue was more serious than in NS. On the other hand, AECOPD in NS appeared to involve airway resistance more than alveolar damage. IOS had application value in airway dominant AECOPD with no difference in 5-year survival being uncovered.
Acknowledgments
We thank our generous funders, which undergirded this research. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Funding Statement
This research was supported by the Social Development Science Research Project of Shaanxi Province (No. 2016SF-151) and the Xi’an Science and Technology Projects (No. 2016045SF/YX01 and No. XA2020-YXYJ-0026).
Abbreviations
AECOPD, acute exacerbation of chronic obstructive pulmonary disease; NS, never smoked; FS, former smokers; CS, current smokers; COPD, chronic obstructive pulmonary disease; CT, computed tomography; GOLD, Global Initiative for Obstructive Lung Disease; BMI, body mass index; WBC, white blood cell count; CRP, C-reactive protein; PCT, procalcitonin; CAT, COPD Assessment Test; MED, mean emphysema density; MLD, mean lung density; LC, lung capacity; HI, heterogeneity index.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the ethics committee of Xi’an Ninth hospital (#014001). The data used in the study did not involve information that can be used to identify the patients. The use of the patient data will not adversely affect patients. All data were collected from the medical charts. No new tests or measurements were performed. The patients were not contacted to obtain new data. Therefore, due to the retrospective nature of this study, informed consent of patients was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117 [PubMed]
- 2.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed]
- 3.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed]
- 4.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 5.Labaki WW, Han MK. Improving detection of early chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15(Supplement_4):S243–S248. doi: 10.1513/AnnalsATS.201808-529MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y, Yi N, Mengwu T, et al. Major finding of 2015 China adults tobacco survery. Chin J Health Manage. 2016;10(2):85–87. doi: 10.3760/cma.j.issn.1674-0815.2016.02.002 [DOI] [Google Scholar]
- 7.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health CPH study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 8.Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic S, Chronic Obstructive Pulmonary Disease Committee of Chinese Association of Chest P. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Chin J Tuberc Respir Dis. 2021;44(3):170–205. doi: 10.3760/cma.j.cn112147-20210109-00031 [DOI] [PubMed] [Google Scholar]
- 9.Maselli DJ, Bhatt SP, Anzueto A, et al. Clinical epidemiology of COPD. Chest. 2019;156(2):228–238. doi: 10.1016/j.chest.2019.04.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei X, Yu N, Ding Q, et al. The features of AECOPD with carbon dioxide retention. BMC Pulm Med. 2018;18(1):124. doi: 10.1186/s12890-018-0691-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Ding Q, Yu N, et al. Imaging features of chronic bronchitis with Preserved Ratio and Impaired Spirometry (PRISm). Lung. 2018;196(6):649–658. doi: 10.1007/s00408-018-0162-2 [DOI] [PubMed] [Google Scholar]
- 12.Wei X, Shi Z, Cui Y, et al. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicine. 2017;96(46):e8543. doi: 10.1097/MD.0000000000008543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Ma Z, Yu N, et al. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:121–129. doi: 10.2147/COPD.S152826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu N, Wei X, Li Y,et al. Computed tomography quantification of pulmonary vessels in chronic obstructive pulmonary disease as identified by 3D automated approach. Medicine. 2016;95(40):e5095. doi: 10.1097/MD.0000000000005095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourbeau J, Tan WC, Benedetti A, et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11(2):125–132. doi: 10.3109/15412555.2012.665520 [DOI] [PubMed] [Google Scholar]
- 16.Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822–829. doi: 10.1136/thoraxjnl-2015-206938 [DOI] [PubMed] [Google Scholar]
- 17.Salvi SS, Brashier BB, Londhe J, et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res. 2020;21(1). doi: 10.1186/s12931-020-1310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 19.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782–787. doi: 10.1136/thx.2010.145995 [DOI] [PubMed] [Google Scholar]
- 21.Xie M, Wang W, Dou S,et al. Quantitative computed tomography measurements of emphysema for diagnosing asthma-chronic obstructive pulmonary disease overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:953–961. doi: 10.2147/COPD.S104484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corlăţeanu A, Odajiu I, Botnaru V,et al. From smoking to COPD–current approaches. Pneumologia. 2016;65(1):20–23. [PubMed] [Google Scholar]
- 23.Silverman EK, Vestbo J, Agusti A, et al. Opportunities and challenges in the genetics of COPD 2010: an International COPD Genetics Conference report. COPD. 2011;8(2):121–135. doi: 10.3109/15412555.2011.558864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charbonnier JP, Pompe E, Moore C, et al. Airway wall thickening on CT: relation to smoking status and severity of COPD. Respir Med. 2019;146:36–41.doi: 10.1016/j.rmed.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30(1):134–155. doi: 10.1183/09031936.00146905 [DOI] [PubMed] [Google Scholar]