Abstract
Purpose:
Older patients undergoing radiation therapy (RT) for pelvic malignancies are at increased risk for pelvic fracture, which is associated with significant morbidity and mortality. RT techniques such as brachytherapy or intensity modulated RT (IMRT) allow for more conformal dose distributions, but it is not known whether the risk for pelvic fracture varies by RT modality.
Methods and Materials:
This observational cohort study involved 28,354 patients ≥65 years old, treated with RT for pelvic malignancies. We evaluated the relative risk of pelvic fracture by type of RT when accounting for baseline factors. To test for nonspecific effects, we also evaluated risk of nonpelvic fractures in the same population.
Results:
The 5-year incidence of pelvic fractures was 12.7% (95% confidence interval [CI], 11.6%–13.8%), 11.8% (10.8%–12.8%), and 3.7% (3.4%–4.0%) for patients with gastrointestinal, gynecologic, and prostate cancer, respectively. On multivariable analysis, being treated with IMRT (hazard ratio, 0.85; 95% CI, 0.73–0.99) or brachytherapy therapy alone (hazard ratio, 0.43; 95% CI, 0.34–0.54) was associated with a reduced hazard for pelvic fractures compared with 3D conformal radiation therapy in female patients. In contrast, there was no association with RT modality and the hazard for nonpelvic fractures among females. There was no significant association between pelvic fractures and IMRT or brachytherapy for male patients. White race, advanced age, and higher comorbidity were associated with an increased hazard for pelvic fracture.
Conclusions:
IMRT and brachytherapy were associated with a reduced risk of pelvic fractures in older women undergoing RT for pelvic malignancies. Pelvic insufficiency fracture risk should be considered when treating with pelvic RT.
Introduction
Pelvic radiation therapy (RT) is associated with an increased risk for insufficiency fractures.1–11 Pelvic insufficiency fractures can occur spontaneously or with minimal trauma in bones with deficient elastic resistance.12 The sacral ala and sacroiliac joint are weight-bearing areas at particular risk for insufficiency fractures.13 Among older patients, pelvic fractures can result in pain, morbidity, and considerable 1-year mortality.14,15 It is therefore of significant clinical interest how the risk for pelvic insufficiency fractures can be mitigated in older patients undergoing RT.
Technological advances with intensity modulated radiation therapy (IMRT) allow for more conformal dose distributions with sparing of adjacent organs at risk compared with standard 3D conformal RT (3DRT) techniques.16 Brachytherapy with intracavitary or interstitial implantations of radioactive sources create rapid dose fall-off, resulting in sparing of adjacent structures.17,18 Prior studies have suggested a dose-dependent relationship between radiation exposure to bony pelvic structures and the risk for fracture, but it remains unclear how the type of RT affects that risk.4,10
The objective of this population-based study is to test whether pelvic RT with IMRT or brachytherapy alone is associated with reduced risk of pelvic fractures compared with patients treated with standard 3DRT techniques. We evaluated a cohort of patients from the Surveillance, Epidemiology, and End Results (SEER) registry combined with Medicare enrollment records and utilization data (SEER-Medicare) who were treated with RT for pelvic malignancies between 2004 and 2013. Similar to the study by Baxter et al,1 we also compared nonpelvic fracture risk by pelvic RT modality to test for potential unmeasured confounding.
Methods and Materials
Data source and patient selection
We studied a cohort of patients from SEER-Medicare cancer registry that included patients ≥65 years old, diagnosed between 2004 to 2013, and treated with RT for endometrial, cervical, anal, rectal, or prostate cancer. Patients were excluded for having multiple primaries, metastatic disease, missing follow-up data, or noncontinuous Medicare coverage.
Covariates, exposures, and outcomes
Baseline patient and cancer-related factors including age, sex, race, marital status, stage, and cancer type were extracted from the SEER database. Charlson comorbidity index (CCI) was calculated from Medicare claims data using the International Classification of Diseases, Ninth Edition (ICD-9) diagnostic codes, as previously described.19 Treatment with chemotherapy, androgen deprivation therapy (ADT), or surgery was determined for each cancer type from Medicare procedural codes.1,20–22 The dates of diagnosis and death were extracted from SEER for time-to-event analysis.
The type of RTwas categorized as 3DRTwith or without Brachytherapy, IMRT with or without Brachytherapy, or Brachytherapy alone. The primary outcome was the incidence of pelvic fractures, including those of the acetabulum, pubis, sacrum, coccyx, or femoral neck (ICD 9 = 808.0–9, 805.6, 805.7, 806.6, 806.7, 820.0–9) after RT. We measured effects on the hazard scale as opposed to cumulative incidence (subdistribution hazard) scale because the former is typically preferred for measuring effects of treatments and prognostic factors.23 Owing to the observational nature of this study, patients treated with different types of RT may have differences in comorbidity and frailty that are not accounted for by the available covariates. Because the risk factors are subject to unmeasured confounding in this observational study, we compared the hazard ratios (HRs) for pelvic fractures to the HRs for nonpelvic fractures in the same population including those of the thoracic spine, arm, forearm, or wrist (805.2, 812.0–9, 813.0–9, 814.0–9).
Prior studies have described increased utilization of IMRT over the time frame described in this cohort.24 As a secondary objective we evaluated the annual prevalence of IMRT use (vs 3DRT) by primary type from 2004 to 2013.
Statistical methods
The mean and standard deviation of continuous covariates were calculated by primary type. For nominal variables, the absolute number and percentage were reported. Cause-specific cumulative hazards for pelvic and nonpelvic fractures were estimated for gastrointestinal (anal and rectal), gynecologic (cervical and endometrial), and prostate cancer, and incidence estimates were obtained as 1 minus the Kaplan-Meier survival estimate (1-KM).25,26 Censoring events included end of follow-up and patient death. The time of diagnosis was designated as time zero. To test for potential bias, we also performed a sensitivity analysis with time zero as the start of radiation therapy.
Comparison of nominal variables was performed with a χ2 test. The 5-year incidence of pelvic fractures was estimated according to type of RT and compared using a univariable Cox proportional hazards model. We used a multivariable Cox proportional hazards model to measure the relative hazard for fractures between RT groups when accounting for baseline patient, cancer-related, and treatment factors. Models were stratified by sex, as women have a significantly higher baseline risk for insufficiency fractures.27,28 Model covariates were selected using backward selection with a P value threshold of .20. To visually represent the effect of radiation modality on the risk for pelvic fractures, we plotted adjusted 1-KM curves using the conditional method of weighted averages as described by Therneau et al29 (“survminer” package version 0.4.6). Note that although the 1-KM method can overestimate cumulative incidence in the presence of competing risks,23 this method was necessary to obtain conditional incidence plots based on the multivariable Cox model.
The proportional hazards assumption was tested using the Schoenfeld residuals test for the overall model and individual covariates.30 In the multivariable model for pelvic fractures among women, the proportional hazards assumption was violated for treatment with Brachytherapy alone (P < .001) and the global model (P = .02). We subsequently modeled the effect of treatment with Brachytherapy alone by introducing step function for the coefficient from 0 to 2 years and >2 years based on the plotted Schoenfeld residuals over time.31,32 The resultant model and all other Cox models did not significantly violate the proportional hazards assumption for individual covariates or the global model. Because treatments, including the primary effect of interest (RT type), were delivered concurrently, and other covariates were established at baseline, we considered it unlikely that immortal time bias would be a factor or that a landmark analysis would change our results. Pelvic radiation dose and spatial distribution vary by cancer type, which could lead to a differential risk for pelvic fracture by the RT modality. Accordingly, we tested for an interaction term between primary type and RT modality that was not significant among male or female patients.
Stage was missing for 5.0% (n = 1,414) of patients and imputed for multivariable regression using multivariate imputation by chained equations.33 Statistical analysis was performed with R, version 3.5.1 (Vienna, Austria).
Results
Patient and treatment characteristics
A total of 28,354 patients (cervical = 1025, endometrial = 4954, rectal = 4816, anal = 998, and prostate = 16,561) were included in the study (Fig. E1, available at https://doi.org/10.1016/j.ijrobp.2019.10.006). The mean age at diagnosis was 74.3 years (Table 1). The majority of patients were male (68.6%), white (82.9%), and had a CCI of 0 (60.5%).
Table 1.
Patient and treatment characteristics by tumor type
| Cervical | Endometrial | Rectal | Anal | Prostate | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| N = 1025 | N = 4954 | N = 4816 | N = 998 | N = 16,561 | |
| Age (mean [SD]) | 75.84 (6.93) | 75.06 (6.56) | 75.84 (6.69) | 76.12 (7.21) | 73.44 (4.88) |
| Gender (%) | |||||
| Female | 1025 (100.0) | 4954 (100.0) | 2194 (45.6) | 736 (73.7) | 0 (0.0) |
| Male | 0 (0.0) | 0 (0.0) | 2622 (54.4) | 262 (26.3) | 16,561 (100.0) |
| Race (%) | |||||
| Black | 157 (15.3) | 438 (8.8) | 313 (6.5) | 68 (6.8) | 1741 (10.5) |
| White | 718 (70.0) | 4273 (86.3) | 4091 (84.9) | 889 (89.1) | 13,546 (81.8) |
| Other | 150 (14.6) | 243 (4.9) | 412 (8.6) | 41 (4.1) | 1274 (7.7) |
| Married (%) | 310 (30.2) | 2223 (44.9) | 2570 (53.4) | 389 (39.0) | 12,005 (72.5) |
| CCI (%) | |||||
| 0 | 575 (56.1) | 2889 (58.3) | 2731 (56.7) | 545 (54.6) | 10,438 (63.0) |
| ≥ 1 | 450 (43.9) | 2065 (41.7) | 2085 (43.3) | 453 (45.4) | 6123 (37.0) |
| Stage (%) | |||||
| I | 255 (24.9) | 2655 (53.6) | 1023 (21.2) | 165 (16.5) | ≤10 (0.0) |
| II | 318 (31.0) | 772 (15.6) | 1655 (34.4) | 402 (40.3) | 14,364 (86.7) |
| III | 358 (34.9) | 1082 (21.8) | 1939 (40.3) | 221 (22.1) | 1276 (7.7) |
| IV | 42 (4.1) | 47 (0.9) | 0 (0.0) | 0 (0.0) | ≤400 (≤3%) |
| Unknown | 52 (5.1) | 398 (8.0) | 199 (4.1) | 210 (21.0) | 555 (3.4) |
| Surgery (%) | 196 (19.1) | 4543 (91.7) | 3895 (80.9) | 375 (37.6) | 3648 (22.0) |
| RT (%) | |||||
| 3DRT | 695 (67.8) | 2060 (41.6) | 3567 (74.1) | 452 (45.3) | 2647 (16.0) |
| IMRT | 330 (32.2) | 966 (19.5) | 1249 (25.9) | 546 (54.7) | 13,084 (79.0) |
| Brachytherapy Alone | 0 (0.0) | 1928 (38.9) | 0 (0.0) | 0 (0.0) | 830 (5.0) |
| Brachytherapy (%) | 676 (66.0) | 3646 (73.6) | 22 (0.5) | ≤10 (≤1) | 7933 (47.9) |
| Chemotherapy (%) | 635 (62.0) | 1385 (28.0) | 2788 (57.9) | 682 (68.3) | 41 (0.2) |
| ADT (%) | NA | NA | NA | NA | 7575 (45.7) |
| Treatment year (%) | |||||
| 2004–2008 | 515 (50.2) | 2296 (46.3) | 2429 (50.4) | 411 (41.2) | 10,057 (60.7) |
| 2009–2013 | 510 (49.8) | 2658 (53.7) | 2387 (49.6) | 587 (58.8) | 6504 (39.3) |
Abbreviations: SD, standard deviation; CCI, Charlson Comorbidity Index; RT, Radiation Therapy; 3DRT, 3-Dimensional Conformal RT; IMRT, Intensity Modulated RT.
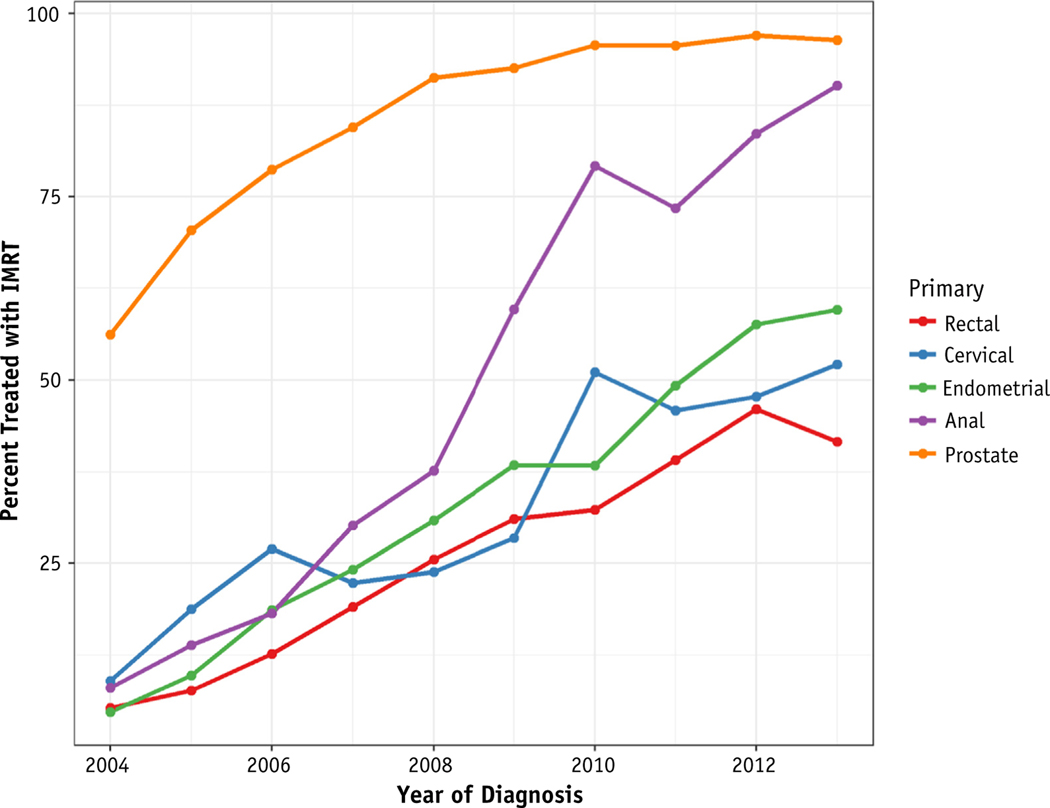
Among the entire cohort, 16,175 (57%) were treated with IMRT, 9421 (33%) were treated with 3DCT, and 2758 (10%) were treated with Brachytherapy alone. For those treated with external beam RT (EBRT, ie, not Brachytherapy alone), 63.2% were treated with IMRT. Prostate cancer had the highest rates of IMRT utilization at 83.2% of patients treated over this period. Rectal cancer had the lowest rate of utilization at 25.9%. The rate of IMRT utilization increased for all primary types over the period of this study (Fig. 1). Anal cancer had the largest increase in IMRT utilization with 8.1% of patients treated with IMRT in 2004 compared with 90.2% in 2013.
Fig. 1.
Annual utilization of IMRT by cancer type among patients treated with external beam radiation therapy for pelvic malignancies. Abbreviation: IMRT = intensity modulated radiation therapy.
Incidence of pelvic fractures
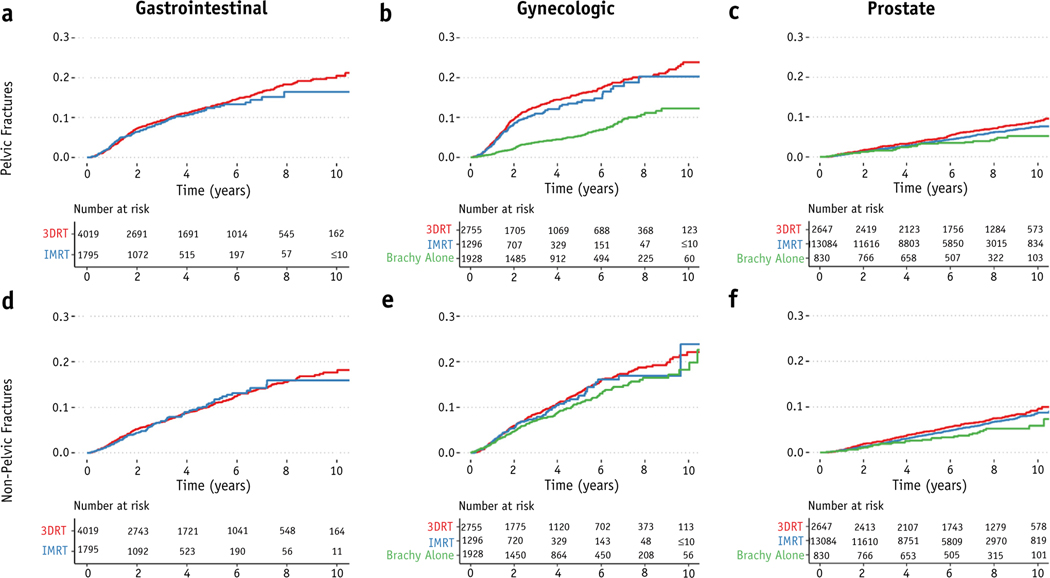
The 5-year incidence of pelvic fractures was 12.7% (11.6–13.8%), 11.8% (10.8–12.8%), and 3.7% (3.4–4.0%) for patients with gastrointestinal, gynecologic, and prostate cancer, respectively (Fig. 2). On univariable analysis, IMRT was not associated with a statistically significant decrease in pelvic fractures for patients with gastrointestinal (HR, 0.93; 95% CI, 0.77–1.12) or gynecologic cancer (HR, 0.90; 95% CI, 0.74–1.11). Among patients with prostate cancer, however, IMRT was associated with a decrease in the hazard for pelvic fractures compared with 3DRT on univariable analysis (HR, 0.82; 95% CI, 0.69–0.98). IMRT was not associated with the hazard for nonpelvic fractures for patients with gastrointestinal (HR, 0.99; 95% CI, 0.80–1.20), gynecologic (HR, 0.96; 95% CI, 0.76–1.20), or prostate cancer (HR, 0.88; 95% CI, 0.74–1.03).
Fig. 2.
Incidence of pelvic and nonpelvic fractures by type of RT. Pelvic fractures (a-c) and nonpelvic fractures (d-f) for gastrointestinal (a,d), gynecologic (b,e), and prostate (c,f) cancer by type of radiation therapy. Abbreviations: 3DRT = 3D conformal radiation therapy; brachy = brachytherapy; IMRT = intensity modulated radiation therapy; RT = radiation therapy.
Patients treated with Brachytherapy alone had a lower hazard for pelvic fractures for both gynecologic (HR, 0.36; 95% CI, 0.29–0.45) and prostate cancer (HR, 0.61; 95% CI, 0.42–0.90). Being treated with Brachytherapy alone was also significantly associated with a lower hazard for nonpelvic fractures in patients with prostate (HR, 0.65; 95% CI, 0.45–0.94) but not gynecologic cancer (HR, 0.83; 95% CI, 0.69–1.01).
Multivariable regression
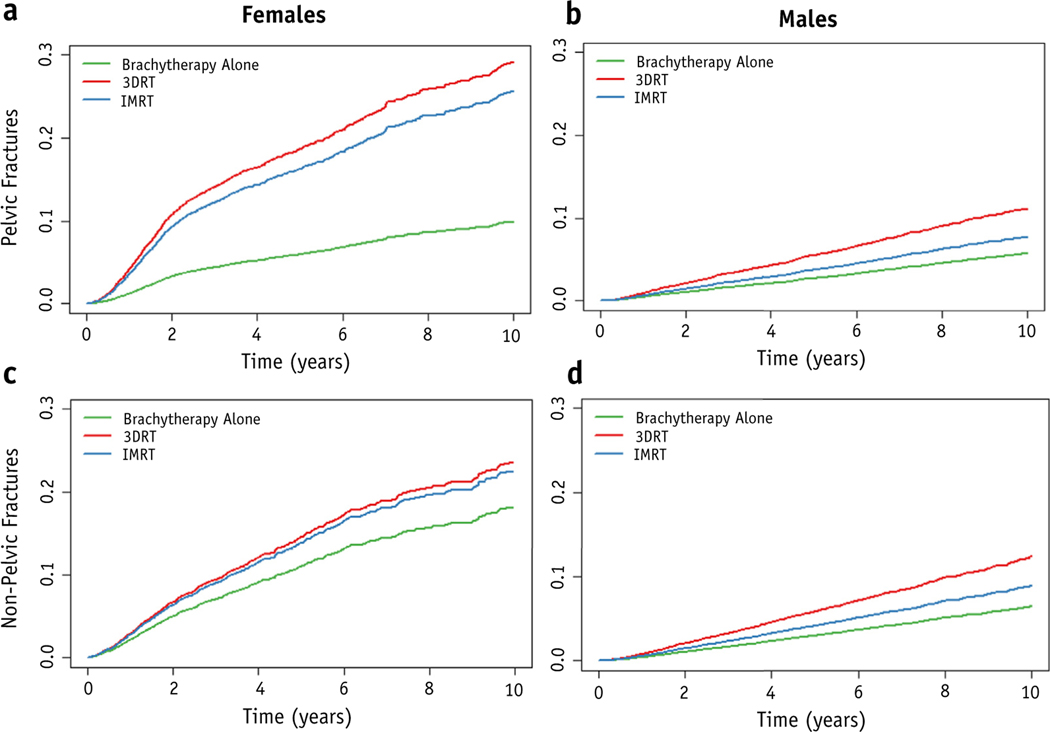
Compared with 3DRT, both IMRT (HR, 0.85; 95% CI, 0.73–0.99) and Brachytherapy alone (HR, 0.43; 95% CI, 0.34–0.54) were associated with a significant decrease in adjusted hazard for pelvic fractures among women (Table 2, Fig. 3). When stratified by time, the HR for treatment with Brachytherapy alone compared with 3DRT was 0.28 (95% CI, 0.20–0.39) for time 0 to 2 years and 0.63 (95% CI, 0.47–0.84) for time >2 years (Table E1, available at https://doi.org/10.1016/j.ijrobp.2019.10.006). In contrast, there was no association between nonpelvic fractures and IMRT (HR, 0.99; 95% CI, 0.83–1.18) or Brachytherapy alone (HR, 0.87; 95% CI, 0.71–1.05) in women. Among male patients, being treated with Brachytherapy alone (HR, 0.92; 95% CI, 0.78–1.08) or IMRT (HR, 0.75; 95% CI, 0.51–1.1) was not significantly associated with the hazard for pelvic fracture.
Table 2.
Multivariable Cox proportional hazards model
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Pelvic fracture HR (95% CI) | Nonpelvic fracture HR (95% CI) | Pelvic fracture HR (95% CI) | Nonpelvic fracture HR (95% CI) | |||||
| RT (ref: 3DRT) | ||||||||
| Brachytherapy alone | 0.43* | (0.34–0.54) | 0.87 | (0.71–1.05) | 0.92 | (0.78–1.08) | 0.91 | (0.78–1.07) |
| IMRT | 0.85* | (0.73–0.99) | 0.99 | (0.83–1.18) | 0.75 | (0.51–1.10) | 0.72 | (0.50–1.04) |
| Age (per 10 years) | 1.94* | (1.77–2.12) | 1.56* | (1.41–1.72) | 2.82* | (2.5–3.18) | 1.64* | (1.46–1.86) |
| Race (ref: white) | ||||||||
| Black | 0.46* | (0.34–0.61) | 0.28* | (0.18–0.41) | 0.56* | (0.42–0.77) | 0.55* | (0.42–0.73) |
| Other | 0.57* | (0.43–0.77) | 0.87 | (0.66–1.15) | 0.54* | (0.4–0.73) | 0.59* | (0.45–0.78) |
| Primary (ref: rectal) | ||||||||
| Cervical | 1.38* | (1.14–1.68) | 1.02 | (0.81–1.3) | NA | NA | ||
| Endometrial | 0.75* | (0.64–0.88) | 0.80* | (0.68–0.96) | NA | NA | ||
| Anal | 1.23 | (0.99–1.52) | 0.83 | (0.63–1.08) | 1.01 | (0.54–1.87) | 0.92 | (0.49–1.7) |
| Prostate | NA | NA | 0.73* | (0.53–1) | 0.59* | (0.44–0.79) | ||
| Chemotherapy | 0.91 | (0.79–1.05) | 0.88 | (0.75–1.03) | 1.04 | (0.74–1.46) | 0.85 | (0.62–1.17) |
| ADT† | NA | NA | 1.28* | (1.1–1.48) | 1.3* | (1.12–1.5) | ||
| Stage (ref: I-II) | ||||||||
| III–IV | 1.29* | (1.13–1.47) | 1.02 | (0.87–1.19) | 1.13 | (0.92–1.39) | 1.16 | (0.96–1.41) |
| CCI (ref: 0) | ||||||||
| ≥ 1 | 1.17* | (1.03–1.32) | 1.33* | (1.16–1.51) | 1.35* | (1.18–1.55) | 1.32* | (1.16–1.5) |
| Diagnosis year | ||||||||
| (ref: 2003–2008) | ||||||||
| 2009–2013 | 0.91 | (0.79–1.04) | 0.94 | (0.81–1.09) | 0.84 | (0.71–1.01) | 1.00 | (0.85–1.18) |
Abbreviations: 3DRT = 3-dimensional conformal RT; ADT = androgen deprivation therapy; CCI = Charlson comorbidity index; CI = confidence interval; HR = hazard ratio; RT = radiation therapy; IMRT = intensity modulated RT; SD = standard deviation.
P < .05.
Regression among patients with prostate cancer only.
Fig. 3.
Covariate-adjusted incidence of pelvic and nonpelvic fractures by type of RT. Conditionally adjusted incidence curves for pelvic fractures (a,b) and nonpelvic fractures (c,d) for female (a,c) and male (b,d) patients. Abbreviations: 3DRT = 3D conformal radiation therapy; brachy = brachytherapy; IMRT = intensity modulated radiation therapy; RT = radiation therapy.
Black race (compared with white) was associated with a decreased hazard for pelvic fractures in both women (HR, 0.46; 95% CI, 0.34–0.77) and men (HR, 0.56; 95% CI, 0.42–0.77). Having a racial designation other than white or black was also associated with a decreased hazard for pelvic fractures (compared with white) in both women (HR, 0.57; 95% CI, 0.43–0.77) and men (HR, 0.54; 95% CI, 0.40–0.73). Higher comorbidity (CCI ≥1 vs 0) was associated with an increased hazard for pelvic fractures for both women (HR, 1.17; 95% CI, 1.00–1.32) and men (HR, 1.35; 95% CI, 1.18–1.55). Among female patients, having a cervical primary (compared with rectal cancer; HR, 1.38; 95% CI, 1.14–1.68) or advanced stage (American Joint Committee on Cancer stage III-IV vs I-II; HR, 1.29; 95% CI,1.13–1.47) was correlated with increased hazard for pelvic fractures. Among male patients, those treated with ADT had an increased hazard for pelvic (HR, 1.28; 95% CI, 1.10–1.48) and nonpelvic fractures (HR, 1.30; 95% CI, 1.12–1.50). Treatment with chemotherapy was not associated with increased hazard for pelvic or nonpelvic fracture for either gender. Covariate estimates did not differ significantly when the start of RT was used as time zero (Fig. E2 and Table E2, available at https://doi.org/10.1016/j.ijrobp.2019.10.006).
Discussion
In this population-based cohort study, we found that being treated with IMRT or Brachytherapy alone compared with 3DRT was associated with a decreased adjusted risk for pelvic fractures in female patients. Interestingly, there was a significant change in the proportional hazard associated with Brachytherapy over time, with the greatest risk reduction prior to 2 years. There was no significant association between IMRT or Brachytherapy and nonpelvic fractures. Among male patients, there was no significant association between being treated with IMRT or Brachytherapy alone and pelvic fracture risk on multivariable analysis.
The association between ionizing radiation and insufficiency fracture risk has been shown in numerous clinical and translational studies, including a seminal paper by Baxter et al.1–11 The Baxter study evaluated a cohort of older women in the SEER-Medicare database diagnosed with pelvic malignancies from 1986 to 1999 and found that those treated with pelvic RT had an increased risk of fractures (HR, 1.65–3.16).1 Ionizing radiation is thought to induce bone injury via free radical formation that damages vasculature and the mesenchymal stem cell microenvironment.2,6 RT can result in focal osteopenia within the treated field that is at risk for insufficiency fractures, especially in weight bearing regions.4 Prospective clinical trials have used dose constraints for femoral heads including max dose <50 Gy and the volume receiving 30 Gy to less than 50% for fractionated pelvic RT, although there are limited data to support these guidelines.34–37 It remains unclear how the dose and volume of RT relates to the risk of pelvic fractures and how different RT modalities (ie, 3DRT vs IMRT vs Brachytherapy) affect this risk.
Technological advances with IMRT allow for precise and highly conformal radiation delivery that can spare critical structures through inverse planning.16 Brachytherapy is delivered via intracavitary or interstitial implants with sparing of adjacent structures owing to rapid dose fall-off, according to the inverse square law.17,18 Observational data from single institutions have produced conflicting data as to whether IMRT reduces the risk of pelvic fractures compared with 3DRT.8,11 A prior study of prostate cancer patients by Sheets et al10 found that IMRT was associated with a decreased risk of hip fractures (HR, 0.78) on propensity weighted analysis. Our study included patients with additional pelvic primaries (rectal and anal) and used different methods to adjust for confounding variables. Although we did not find a statistically significant reduction in pelvic fractures with IMRT versus 3DRT in prostate cancer, our observed HR (0.75; 95% CI, 0.51–1.10) was similar to that reported in the Sheets study. Note, however, this was similar to the HR observed for nonpelvic fractures (HR, 0.72; 95% CI, 0.50–1.04).
To our knowledge, this is the largest study investigating the association between the type of RT used and fracture risk in patients with pelvic malignancies. Prior studies omitted patients treated with Brachytherapy alone, which can be an alternative to EBRT for some patients being treated for definitive prostate or postoperative endometrial cancer.38,39 There was no association between the risk of nonpelvic fractures and pelvic RT modality on multivariable analysis, which may suggest the correlation seen with pelvic fractures is less likely to be the result of nonspecific confounding. It is important to note, however, that this does not prove the absence of confounding, and standard model assumptions for a retrospective study apply. Interestingly, IMRT was associated with a reduced risk of pelvic fractures in women, although it is unclear whether there was intentional sparing of bony structures of the pelvis. The greatest reduction in risk associated with Brachytherapy alone occurred in the first 2 years, which could suggest a partial reversal of deleterious radiation changes over time in patients treated with 3DRT.
Additional factors associated with the risk for pelvic fracture were identified. Older age and white race were correlated with an increased risk for pelvic fractures, which is consistent with previous population-based studies that have also linked age and race with osteoporosis.40,41 Increased comorbidity has also been associated with osteoporosis risk and was found to correlate with both pelvic and nonpelvic fracture risk in our study.42 Advanced stage was associated with an increased risk of pelvic fractures among female patients. This may be related to more intensive radiation or chemotherapy treatment regimens among these patients. Patients with an elevated baseline risk may especially benefit from intentional sparing radiation dose to bony pelvic structures.
It is not fully understood why IMRT and Brachytherapy were associated with a decreased risk of pelvic fractures compared with 3DRT among women but not men. Women have a higher baseline risk for pelvic insufficiency fractures owing to higher rates of osteoporosis and osteopenia.43 It is possible that IMRT and Brachytherapy were correlated with lower rates of pelvic fractures versus 3DRT in women but not men because of the higher event rate and thus greater statistical power to detect an effect in women. It is also plausible that differences in radiation fields and dose distributions between gynecologic, gastrointestinal, and prostate cancer treatments resulted in differences in fracture risk. Radiation for prostate cancer, for example, which comprised the vast majority of the male cohort, may have been less likely to receive radiation to elective nodal fields adjacent to bone. Brachytherapy was associated with a decreased risk of nonpelvic fractures on univariable but not multivariable regression. This may be explained in part by the lower rates of ADT used in men treated with brachy-monotherapy compared with EBRT (26.7 vs 46.7%, P < .01).
There are potential limitations with this study. Although we controlled for available covariates, there may be residual confounding owing to the retrospective nature of the analysis. Some known confounders for osteoporosis, including body mass index, were not available in the SEER-Medicare database.8,44 Although differences in start times for different therapies could potentially result in immortal time bias, the primary effect of interest (RT modality) and treatments such as chemotherapy and ADT were delivered concurrently. Moreover, key covariates (eg, CCI, stage, age, etc) examined in this analysis were established at baseline, mitigating this concern. The cause of the pelvic fractures is not known and may include some pathologic fractures in addition to insufficiency fractures. Confounding from pathologic fractures should be limited, however, by excluding metastatic patients at baseline and controlling for disease stage in multivariable regression. This study is also limited by the lack of dosimetric data that could better characterize the relationship between the dose and distribution of radiation and fracture risk. It is also notable that there is collinearity between the utilization of IMRT and the date of diagnosis in this cohort. Improved medical management of osteoporosis over this period could distort the observed association with IMRT.45
In conclusion, IMRT and Brachytherapy were associated with a reduced risk of pelvic fractures in older women undergoing RT for pelvic malignancies in this population-based cohort. Further studies are needed to characterize the relationship between the type of RT, pelvic dose distribution, and fracture risk to optimize management.
Supplementary Material
Footnotes
Disclosures: J.D.M. receives compensation for consulting from the Boston Consulting Group.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.10.006
References
- 1.Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA 2005;294:2587–2593. [DOI] [PubMed] [Google Scholar]
- 2.Cao X, Wu X, Frassica D, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA 2011;108:1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott SP, Jarosek SL, Alanee SR, et al. Three-dimensional external beam radiotherapy for prostate cancer increases the risk of hip fracture. Cancer 2011;117:4557–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: Analysis of risk factors. Int J Radiat Oncol 2008;70:1183–1188. [DOI] [PubMed] [Google Scholar]
- 5.Igdem S‚, Alco G, Ercan T, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol 2010; 77:818–823. [DOI] [PubMed] [Google Scholar]
- 6.Nicolay NH, Sommer E, Lopez R, et al. Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol 2013;87:1171–1178. [DOI] [PubMed] [Google Scholar]
- 7.Ramlov A, Pedersen EM, Røhl L, et al. Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy. Int J Radiat Oncol 2017;97: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 8.Shih KK, Folkert MR, Kollmeier MA, et al. Pelvic insufficiency fractures in patients with cervical and endometrial cancer treated with postoperative pelvic radiation. Gynecol Oncol 2013;128:540–543. [DOI] [PubMed] [Google Scholar]
- 9.Kallogjeri D, Gaynor SM, Piccirillo ML, et al. Comparison of comorbidity collection methods. J Am Coll Surg 2014;219:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheets NC, Goldin GH, Meyer A-M, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012;307:1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ioffe YJM, Hillen TJ, Zhou G, et al. Postradiation damage to the pelvic girdle in cervical cancer patients. Int J Gynecol Cancer 2014; 24:806–812. [DOI] [PubMed] [Google Scholar]
- 12.Moreno A, Clemente J, Crespo C, et al. Pelvic insufficiency fractures in patients with pelvic irradiation. Int J Radiat Oncol 1999;44:61–66. [DOI] [PubMed] [Google Scholar]
- 13.Cooper KL, Beabout JW, Swee RG. Insufficiency fractures of the sacrum. Radiology 1985;156:15–20. [DOI] [PubMed] [Google Scholar]
- 14.Mears SC, Berry DJ. Outcomes of displaced and nondisplaced pelvic and sacral fractures in elderly adults. J Am Geriatr Soc 2011;59:1309–1312. [DOI] [PubMed] [Google Scholar]
- 15.Taillandier J, Langue F, Alemanni M, et al. Mortality and functional outcomes of pelvic insufficiency fractures in older patients. Jt Bone Spine 2003;70:287–289. [DOI] [PubMed] [Google Scholar]
- 16.Mell LK, Roeske JC, Mehta N, Mundt AJ. Gynecologic cancer: overview. In: Mundt AJ, Roeske JC, (eds). Intensity modulated radiation therapy: a clinical perspective. Ontario: BC: Decker; 2004. pp. 492–505. [Google Scholar]
- 17.Pötter R, Haie-Meder C, Limbergen Van E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer Brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 18.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate Brachytherapy. Brachytherapy 2012;11:6–19. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 20.Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. JNCI J Natl Cancer Inst 2010;102: 1780–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahinian VB, Kuo Y-F, Freeman JL, et al. Risk offracture after and rogen deprivation for prostate cancer. N Engl J Med 2005;352:154–164. [DOI] [PubMed] [Google Scholar]
- 22.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care 2002. Aug;40(8 Suppl): IV-55–61. [DOI] [PubMed] [Google Scholar]
- 23.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. ClinCancerRes 2012;18:2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol 2011;29:1517–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming TR, Harrington DP. Nonparametric estimation of the survival distribution in censored data. Commun Stat - Theory Methods 1984; 13:2469–2486. [Google Scholar]
- 26.Zhang Z. Statistical description for survival data. Ann Transl Med 2016;4:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelsey JL, Prill MM, Keegan THM, et al. Risk factors for pelvis fracture in older persons. Am J Epidemiol 2005;162:879–886. [DOI] [PubMed] [Google Scholar]
- 28.Chien L-C, Cheng H-M, Chen W-C, et al. Pelvic fracture and risk factors for mortality: A population-based study in Taiwan. Eur J Trauma Emerg Surg 2010;36:131–137. [DOI] [PubMed] [Google Scholar]
- 29.Therneau TM, Crowson CS, Atkinson EJ. Adjusted survival curves. 2015. Available at: https://cran.r-project.org/web/packages/survival/vignettes/adjcurve.pdf. Accessed October 22, 2019
- 30.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 31.Therneau T, Crowson E, Atkinson C. Using time dependent covariates and time dependent coefficients in the cox model. Survival Vignettes 2017. [Google Scholar]
- 32.Buyse M, Piedbois P. On the relationship between response to treatment and survival time. Stat Med 1996;15:2797–2812. [DOI] [PubMed] [Google Scholar]
- 33.Buuren van S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 34.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: A Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012;83:e353–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawton CAF, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol 2009;74:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol 2013;86:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alliance for Clinical Trials in Oncology. PROSPECT: Chemotherapyalone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery - full text view ClinicalTrials.gov. Available at: ClinicalTrials.gov. 2012. Accessed June 17, 2019.
- 38.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- 39.Koh WJ, Abu-Rustum NR, Bean S, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2018;16:170–199. [DOI] [PubMed] [Google Scholar]
- 40.Barrett JA, Baron JA, Karagas MR, et al. Fracture risk in the U.S. Medicare population. J Clin Epidemio 1999;52:243–249. [DOI] [PubMed] [Google Scholar]
- 41.Bollet AJ, Engh G, Parson W. Epidemiology of osteoporosis. Arch Intern Med 1965;116:191. [DOI] [PubMed] [Google Scholar]
- 42.David C, Confavreux CB, Mehsen N, et al. Severity of osteoporos is: What is the impact of co-morbidities? Jt Bone Spine 2010;77:S103–S106. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor TJ, Cole PA. Pelvic insufficiency fractures. Geriatr Orthop Surg Rehabil 2014;5:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrera G, Bunout D, Gattás V, et al. A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects. Nutrition 2004;20:769–771. [DOI] [PubMed] [Google Scholar]
- 45.Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician 2015;92:261–268. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.