Abstract
Enterobacter cloacae 8009 produced an inducible class A β-lactamase which hydrolyzed cefotaxime efficiently. It also hydrolyzed other β-lactams except cephamycins and carbapenems. The activity was inhibited by clavulanic acid and imipenem. The bla gene was transferable to Escherichia coli by electroporation of plasmid DNA. The molecular mass of the β-lactamase was 29 kDa and its pI was 7.3. All of these phenotypic characteristics of the enzyme except for inducible production resemble those of some extended-spectrum class A β-lactamases like FEC-1. The gene encoding this β-lactamase was cloned and sequenced. The deduced amino acid sequence of the β-lactamase was homologous to the AmpA sequences of the Serratia fonticola chromosomal enzyme (96%), MEN-1 (78%), Klebsiella oxytoca chromosomal enzymes (77%), TOHO-1 (75%), and FEC-1 (72%). The conserved sequences of class A β-lactamases, including the S-X(T)-X(S)-K motif, in the active site were all conserved in this enzyme. On the basis of the high degree of homology to the β-lactamase of S. fonticola, the enzyme was named SFO-1. The ampR gene was located upstream of the ampA gene, and the AmpR sequence of SFO-1 had homology with the AmpR sequences of the chromosomal β-lactamases from Citrobacter diversus (80%), Proteus vulgaris (68%), and Pseudomonas aeruginosa (60%). SFO-1 was also inducible in E. coli. However, a transformant harboring plasmid without intact ampR produced a small amount of β-lactamase constitutively, suggesting that AmpR works as an activator of ampA of SFO-1. This is the first report from Japan describing an inducible plasmid-mediated class A β-lactamase in gram-negative bacteria.
Since the introduction of extended-spectrum cephalosporins into clinical use, gram-negative bacteria have reacted to them mainly by producing various new types of plasmid-mediated extended-spectrum β-lactamases, in addition to overproducing chromosomal β-lactamases. Bush’s classification of β-lactamases was updated in 1995 (5). A total of 178 kinds of different β-lactamases were included in that classification. Recently, additional extended-spectrum β-lactamases which hydrolyze oxyimino-cephalosporins have been isolated all over the world (5, 7, 12, 13, 14, 21, 27, 28). Most of them are related to the TEM and SHV families of β-lactamases. However, in Japan, extended-spectrum β-lactamases derived from these families have never been isolated. On the other hand, different types of extended-spectrum β-lactamases classified as 2be and 2e (class A) (10, 17, 20, 31), 1 (class C) (8), and 4 (class B) (11, 30) have been isolated. The differences in enzyme populations are thought to arise from the various clinical uses of antibacterial agents. We reported on plasmid-mediated oxyimino-cephalosporin-hydrolyzing class A β-lactamase FEC-1 in 1988 (20). Although it was not from a clinical isolate, the possibility of the appearance of such an enzyme was suggested. Since then, a similar type of enzyme has been identified in Japan (31).
Enterobacter cloacae is known to produce inducible chromosomal β-lactamases. Overproduction or constitutive production of this enzyme causes resistance to most β-lactams except carbapenems (16, 29) without the loss of any porin. On the other hand, an amino acid insertion into the omega loop region of the class C β-lactamase causes substrate specificity extension to extended-spectrum β-lactams (24). We found 1 ceftizoxime-susceptible strain among 12 cefotaxime-resistant E. cloacae strains isolated at Teikyo University in 1988. The strain produced an inducible enzyme which hydrolyzed cefotaxime efficiently. The phenotypic characteristics of this enzyme resembled those of FEC-1 except for the inducible production. Since induction of plasmid-mediated β-lactamase is known to be rare in gram-negative bacteria, the origin of this enzyme is of great interest. In this study the gene was cloned and was further analyzed.
MATERIALS AND METHODS
Bacterial strains.
E. cloacae 8009 was isolated clinically in 1988 and was kindly provided by K. Ubukata of Teikyo University (Tokyo, Japan). Competent Escherichia coli DH10B was obtained commercially from GIBCO-BRL. The constructed strains and plasmids are described in Table 1.
TABLE 1.
Strains used in this study
| Host | Plasmid | Phenotype | Restriction enzyme insert (length [kb]) | Vector | Markera | Nickname |
|---|---|---|---|---|---|---|
| E. cloacae 8009 | pFCX300L | AmpA AmpR | Cryptic | CTX | ||
| E. cloacae 8009 | pFCX300M | AmpA AmpR | Cryptic | CTX | ||
| E. cloacae 8009 | pFCX300S | Cryptic | ||||
| E. coli DH10B | ||||||
| E. coli DH10B | pFCX310 | AmpA | Sau3AI (2.3) | pHSG396 | CP, CTX | TF2-2 |
| E. coli DH10B | pFCX320 | AmpA | Sau3AI (2.3) | pHSG396 | CP, CTX | TF2-3 |
| E. coli DH10B | pFCX300L | AmpA AmpR | CTX | TF-L | ||
| E. coli DH10B | pFCX300M | AmpA AmpR | CTX | TF-M | ||
| E. coli DH10B | pFCX301 | AmpA AmpR | EcoRI (10) | pHSG396 | CP, CTX | TF-L7 |
| E. coli DH10B | pFCX302 | AmpA AmpR | EcoRI (10) | pHSG396 | CP, CTX | TF-M4 |
| E. cloacae 199S | AmpC− | |||||
| E. cloacae 199S | pFCX310 | AmpA | Sau3AI (2.3) | pHSG396 | CP, CTX | |
| E. cloacae 199S | pFCX320 | AmpA | Sau3AI (2.3) | pHSG396 | CP, CTX | |
| E. cloacae 199 | AmpC (inducible) | |||||
| E. cloacae 199C | AmpC (constitutive) |
CTX, cefotaxime; CP, chloramphenicol; Rif, rifampin.
Plasmids.
pHSG396 was from Takara Shuzo (Otsu, Japan).
Antibiotics.
The antibiotics used in this study were commercially available. Imipenem-cilastatin (Banyu, Tokyo, Japan), ceftazidime (Glaxo Japan, Tokyo, Japan), cefoperazone (Toyama, Tokyo, Japan), moxalactam (Shionogi, Osaka, Japan), aztreonam (Eisai, Tokyo, Japan), cefpiramide (Sumitomo, Osaka, Japan), ceftizoxime (Fujisawa, Osaka, Japan), cefotaxime (Chugai, Tokyo, Japan), cefmenoxime (Takeda, Osaka, Japan), ceftriaxone (Roche, Tokyo, Japan), cefuroxime (Glaxo), cefotiam (Takeda), cefoxitin (Banyu), cefamandole (Shionogi), cefazolin (Fujisawa), cephalothin (Shionogi), cephaloridine (Shionogi), and ampicillin (Fujisawa) were used. Cefoselis (FK037) (22), clavulanic acid, and nitrocefin were synthesized in our laboratories.
Susceptibility testing.
MICs were determined with serial dilutions of antibiotics in Mueller-Hinton medium with inoculum sizes of 104 and 106 CFU per spot. The MICs were read as the lowest concentration of antibiotic that inhibited visible growth after 18 h of incubation at 37°C.
Preparation of β-lactamase.
Exponentially growing cells of the test strains in Trypticase soy broth (BBL, Becton Dickinson Microbiology Systems, Cockeysville, Md.) were harvested, washed once, and resuspended in a 1/20 volume of 50 mM potassium phosphate buffer (pH 7.0). The suspension was sonically disrupted and the debris was removed by centrifugation (15,000 × g, 30 min). β-Lactamase was partially purified by ion-exchange chromatography with POROS 20HS and a BioCAD work station (PerSeptive Biosystems, Tokyo, Japan).
Assay of β-lactamase activity.
β-Lactamase activity was determined spectrophotometrically (UV-2200; Shimadzu) at 37°C in 0.067 M potassium phosphate buffer (pH 7.0).
β-Lactamase inhibitor susceptibility.
Susceptibility to β-lactamase inhibitors was determined as mentioned previously (20).
Analytical isoelectric focusing.
Isoelectric focusing was performed with crude extracts with an LKB Ampholine PAG plate (pH 3.5 to 9.5) and an analytical electrofocusing system (LKB Stockholm, Bromma, Sweden). β-Lactamase activity was detected by overlaying the gel with filter paper containing nitrocefin (0.5 mg/ml).
Inducibility of β-lactamase.
Imipenem (0.1 μg/ml) was added to a mid-logarithmic-phase culture, and the culture was incubated for 2 more h. The cells were harvested by centrifugation (10,000 × g, 10 min), resuspended in a 1/10 volume of 0.067 M potassium phosphate buffer (pH 7.0), and disrupted by sonication. The β-lactamase activity and protein concentration were measured, and specific activities were compared.
Transformation study.
Plasmid DNA was isolated from E. cloacae 8009 and was used to transform E. coli DH10B by electroporation. Ampicillin-resistant colonies with different plasmids were selected and named TF-L (E. coli DH10B/pFCX300L) and TF-M (E. coli DH10B/pFCX300M). E. cloacae 199S (a chromosomal β-lactamase-deficient strain) was also used as a recipient.
Cloning of β-lactamase gene.
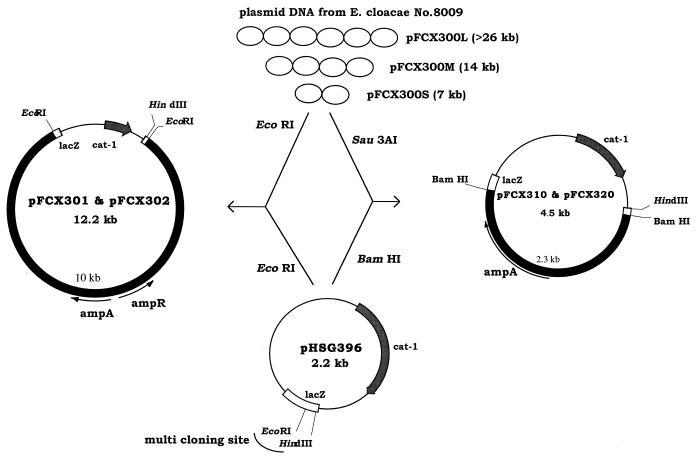
Plasmid DNA from E. cloacae 8009 was partially digested with Sau3AI and was ligated into the BamHI site of pHSG396 (see Fig. 1). The ligation mixture was used to transform E. coli DH10B. Transformants harboring plasmids which have short fragment insertions were selected (TF2-2, E. coli DH10B/pFCX310; and TF2-3, E. coli DH10B/pFCX320).
FIG. 1.
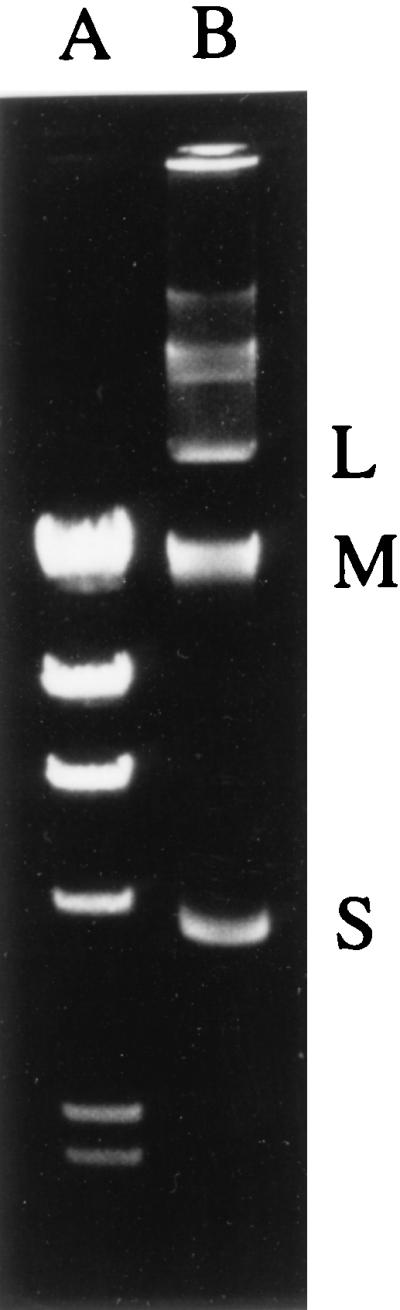
Agarose gel electrophoresis of plasmid DNAs from E. cloacae 8009. Lane A, HindIII-digested bacteriophage λ DNA; lane B, E. cloacae 8009. L, large plasmid; M, medium-size plasmid; S, small plasmid.
Alternatively, the DNAs of two plasmids, pFCX300L and pFCX300M, were each digested with EcoRI and were cloned into pHSG396 to get TF-L7 (E. coli DH10B/pFCX301) and TF-M4 (E. coli DH10B/pFCX302).
DNA sequence analysis.
DNA sequence analysis was performed by the PCR cycle sequencing method with the Sequthermo kit (Epicenter Technology) and the thermosequenase kit (Amersham) and with the LI-COR 4000LS system. For determination of the sequence of ampA, pFCX310 and pFCX320 were used as templates. The AmpR gene and franking region were sequenced by using pFCX301 and pFCX302 and their deletion plasmids as templates and primers synthesized from the sequences of pFCX310 and pFCX320. The ABI PRISM 310 Genetic Analyzer (Applied Biosystems) was also used. Homology was analyzed by the FASTA program of the DNA Data Base of Japan (DDBJ).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB003148.
RESULTS
Characteristics of β-lactamase from E. cloacae 8009.
E. cloacae 8009 was resistant to various β-lactam antibiotics including extended-spectrum cephalosporins such as cefotaxime, cefoperazone, cefoselis, and monobactam (aztreonam) but excluding carbapenems (Table 2). However, strain 8009 was not as resistant to such oxyimino-cephalosporins (ceftizoxime and ceftazidime) as the high-level producers of chromosomal β-lactamase (Table 2). Also, E. cloacae 8009 was not resistant to the other antibiotics tested (Table 3). The β-lactamase was partially purified from E. cloacae 8009 by ion-exchange chromatography. The molecular mass of this enzyme was 29 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and its pI was 7.3. Values of kinetic parameters were determined (Table 4). The enzyme hydrolyzed oxyimino-cephalosporins, in addition to cephaloridine and ampicillin. Ceftazidime was the most stable among the cephalosporins tested. The hydrolysis of cephamycin (cefoxitin), oxacephem (moxalactam), and carbapenem (imipenem) by this enzyme was not detectable (data not shown). The enzyme activity was inhibited by clavulanic acid and imipenem (Table 5) but was not inhibited by EDTA and P-chloromercuribenzoate (data not shown). These characteristics resembled those of FEC-1 (20) and some other class A enzymes (5) reported previously.
TABLE 2.
Susceptibilities of E. cloacae 8009 and various transformants to β-lactams
| Strain | Presence of ampR | MIC (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefuroxime | Cefotaxime | Ceftizoxime | Cefoperazone | Ceftazidime | Cefoselis | Cefoxitin | Moxalactam | Aztreonam | Imipenem | ||
| E. cloacae 8009 | + | >100 | 100 | 6.25 | >100 | 3.13 | 12.5 | >100 | 0.78 | 12.5 | 0.78 |
| E. coli DH10B | − | 6.25 | 0.05 | ≦0.025 | 0.1 | 0.2 | ≦0.025 | 3.13 | 0.1 | 0.1 | 0.2 |
| E. coli DH10B/pFCX310 | − | >100 | 50 | 1.56 | 50 | 3.13 | 3.13 | 3.13 | 0.2 | 25 | 0.39 |
| E. coli DH10B/pFCX320 | − | >100 | 50 | 1.56 | 100 | 3.13 | 6.25 | 3.13 | 0.2 | 25 | 0.2 |
| E. coli DH10B/pFCX300L | + | 50 | 0.78 | 0.05 | 0.78 | 0.2 | 0.1 | 3.13 | 0.1 | 0.78 | 0.2 |
| E. coli DH10B/pFCX300M | + | >100 | 6.25 | 0.2 | 6.25 | 1.56 | 0.78 | 6.25 | 0.2 | 6.25 | 0.39 |
| E. coli DH10B/pFCX301 | + | >100 | 25 | 0.78 | 25 | 3.13 | 3.13 | 6.25 | 0.39 | 25 | 0.2 |
| E. coli DH10B/pFCX302 | + | >100 | 25 | 1.56 | 50 | 3.13 | 3.13 | 6.25 | 0.39 | 25 | 0.2 |
| E. cloacae 199Sa | 3.13 | 0.05 | ≦0.025 | 0.2 | 0.1 | ≦0.025 | 0.78 | 0.05 | ≦0.025 | 0.05 | |
| E. cloacae 199S/pFCX310 | >100 | 25 | 0.39 | 50 | 1.56 | 1.56 | 0.78 | 0.05 | 6.25 | 0.05 | |
| E. cloacae 199S/pFCX320 | >100 | 50 | 1.56 | >100 | 3.13 | 6.25 | 1.56 | 0.1 | 12.5 | 0.05 | |
| E. cloacae 199b | 6.25 | 0.2 | 0.1 | 0.39 | 0.2 | 0.05 | >100 | 0.1 | 0.1 | 0.39 | |
| E. cloacae 199Cc | >100 | 50 | 50 | 25 | 50 | 1.56 | >100 | 3.13 | 25 | 0.2 | |
| E. coli DH5α | − | 3.13 | ≦0.025 | ≦0.025 | ≦0.025 | 0.05 | ≦0.025 | 3.13 | 0.1 | ≦0.025 | 0.2 |
| E. coli DH5α/FEC-1 | − | >100 | >100 | 6.25 | >100 | 12.5 | 100 | 3.13 | 0.39 | 50 | 0.2 |
| E. coli KU3290d | + | >100 | 25 | 50 | 50 | 100 | 1.56 | >100 | 12.5 | 25 | 0.2 |
β-Lactamase nonproducer.
Inducible β-lactamase producer (original strain).
Constitutive β-lactamase producer.
Transformant producing cloned P. vulgaris β-lactamase.
TABLE 3.
Susceptibilities of E. cloacae 8009 and various transformants to other antibiotics
| Strain | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| Gentamicin | Amikacin | Kanamycin | Streptomycin | Chloramphenicol | Ofloxacin | STa | |
| E. cloacae 8009 | 0.39 | 1.56 | 1.56 | 3.13 | 3.13 | 0.05 | 0.39 |
| E. coli DH10B | 0.2 | 0.78 | 1.56 | >100 | 3.13 | <0.025 | 0.39 |
| E. coli DH10B/pFCX310 | 0.1 | 0.2 | 0.78 | >100 | >100 | <0.025 | 0.39 |
| E. coli DH10B/pFCX320 | 0.2 | 0.78 | 1.56 | >100 | >100 | <0.025 | 0.39 |
| E. coli DH10B/pFCX300L | 0.2 | 1.56 | 1.56 | >100 | 3.13 | <0.025 | 0.39 |
| E. coli DH10B/pFCX300M | 0.2 | 0.78 | 1.56 | >100 | 3.13 | <0.025 | 0.39 |
| E. coli DH10B/pFCX301 | 0.39 | 0.78 | 1.56 | >100 | >100 | <0.025 | 0.39 |
| E. coli DH10B/pFCX302 | 0.2 | 0.78 | 1.56 | >100 | >100 | <0.025 | 0.39 |
| E. cloacae 199Sb | 0.2 | 0.78 | 0.78 | 1.56 | 12.5 | 0.05 | 1.56 |
| E. cloacae 199S/pFCX310 | 0.2 | 0.39 | 0.78 | 1.56 | >100 | 0.05 | 1.56 |
| E. cloacae 199S/pFCX320 | 0.2 | 0.39 | 0.78 | 1.56 | >100 | 0.05 | 1.56 |
ST, sulfamethoxazole-trimethoprim.
β-Lactamase nonproducer.
TABLE 4.
Kinetic parameters of SFO-1 and FEC-1 for β-lactam antibiotics
| Substrate | SFO-1
|
FEC-1
|
||||
|---|---|---|---|---|---|---|
| Km (μM) | Relative Vmaxa | Relative Vmax/Kma | Km (μM) | Relative Vmaxa | Relative Vmax/Kma | |
| Cephaloridine | 110 | 100 | 100 | 152 | 100 | 100 |
| Cephalothin | 60 | 234 | 428 | 134 | 198 | 225 |
| Cefamandole | 171 | 241 | 154 | 122 | 125 | 156 |
| Cefotiam | 27 | 50 | 203 | 38 | 43 | 170 |
| Cefoperazone | 6.6 | 15 | 246 | 2.8 | 2.6 | 139 |
| Cefpiramide | 26 | 71 | 297 | 33 | 36 | 167 |
| Cefuroxime | 13 | 23 | 195 | 27 | 32 | 179 |
| Ceftizoxime | 217 | 12 | 5.8 | 821 | 12 | 2.3 |
| Cefotaxime | 2.2 | 16 | 790 | 61 | 23 | 59 |
| Cefmenoxime | 21 | 20 | 108 | 84 | 61 | 110 |
| Ceftriaxone | 19 | 15 | 84 | 27 | 14 | 80 |
| Ceftazidime | 785 | 0.47 | 0.065 | 393 | 0.13 | 0.05 |
| Ampicillin | 40 | 19 | 51 | 30 | 17 | 89 |
Values relative to that for hydrolysis of cephaloridine, which was set equal to 100.
TABLE 5.
Susceptibility of SFO-1 to β-lactamase inhibitors
| Enzyme | Bush type | IC50 (μg/ml)a
|
|
|---|---|---|---|
| Clavulanic acid | Imipenem | ||
| SFO-1 | 2e | 0.1 | 0.35 |
| FEC-1 | 2e | 0.0022 | 0.13 |
| Chromosomal β-lactamase of P. vulgaris | 2e | 0.6 | 0.62 |
| TEM-1 | 2b | 1.0 | 14 |
| Chromosomal β-lactamase of E. cloacae | 1 | 12 | 0.24 |
| Chromosomal β-lactamase of E. coli | 1 | 7.8 | 1.4 |
IC50, concentration that inhibits 50% of activity after 10 min of incubation at 37°C; nitrocefin was used as the substrate.
Effect of inducer on the β-lactamase production in E. cloacae 8009.
Imipenem induced cefotaxime-hydrolyzing β-lactamase production in E. cloacae 8009, and the specific cefotaxime-hydrolyzing activity was about 10 times higher when it was induced (Table 6).
TABLE 6.
β-Lactamase inducibility in E. cloacae 8009 and various transformants
| Expt. no. and strain | Phenotype | Inducera | β-Lactamase activityb
|
Inducibility and inducibility ratioc | |
|---|---|---|---|---|---|
| μM/min · mg of protein | Ratiod | ||||
| Expt 1 | |||||
| E. cloacae 8009 | AmpA AmpR | − | 340 | 1.0 | Inducible |
| + | 2,400 | 7.1 | 7.1 | ||
| E. coli DH10B | − | <1 | <0.003 | Constitutive | |
| + | <1 | <0.003 | 1.0e | ||
| E. coli DH10B/pFCX300L (TF-L) | AmpA AmpR | − | 22 | 0.064 | Inducible |
| + | 210 | 0.64 | 9.6 | ||
| E. coli DH10B/pFCX300M (TF-M) | AmpA AmpR | − | 119 | 0.35 | Inducible |
| + | 1,720 | 5.1 | 14.5 | ||
| E. coli DH10B/pFCX301 (TF-L7) | AmpA AmpR | − | 700 | 2.1 | Inducible |
| + | 6,500 | 19 | 9.2 | ||
| E. coli DH10B/pFCX302 (TF-M4) | AmpA AmpR | − | 620 | 1.9 | Inducible |
| + | 7,400 | 22 | 11.9 | ||
| Expt 2 | |||||
| E. cloacae 8009 | AmpA AmpR | − | 260 | 1.0 | Inducible |
| + | 2,500 | 9.6 | 9.3 | ||
| E. coli DH10B/pFCX310 (TF2-2) | AmpA | − | 280 | 1.1 | Constitutive |
| + | 280 | 1.1 | 1.0 | ||
| E. coli DH10B/pFCX320 (TF2-3) | AmpA | − | 350 | 1.3 | Constitutive |
| + | 350 | 1.3 | 1.0 | ||
The inducer was imipenem-cilastatin at 0.1 μg/ml.
Cefotaxime (100 μM) was used as a substrate.
Induced activity/uninduced activity for each set.
Activity of extract/uninduced activity of E. cloacae 8009.
Nitrocefin was used as the substrate for calculation of the inducibility ratio for this strain.
Transformation of E. coli.
E. cloacae 8009 had plasmids of three different sizes (Fig. 1). E. coli DH10B was transformed by electroporation with total plasmid DNA isolated from E. cloacae 8009 and was selected on plates containing cefotaxime at 1 μg/ml. The largest plasmid, pFCX300L, was detected in every transformant (TF-L) except one (TF-M), in which middle-size plasmid pFCX300M was detected (Fig. 1). Transconjugation from E. cloacae 8009 to E. coli was unsuccessful, suggesting that the gene is on a nonconjugable R plasmid.
The antibiotic susceptibility patterns of these transformants were similar to those of FEC-1-producing E. coli DH5α except for cefoselis susceptibility, although TF-M was more susceptible than E. coli DH5α/FEC-1 (Table 2). The MICs differed for TF-M and TF-L, perhaps due to a difference in copy numbers.
Cloning and characterization of the β-lactamase gene.
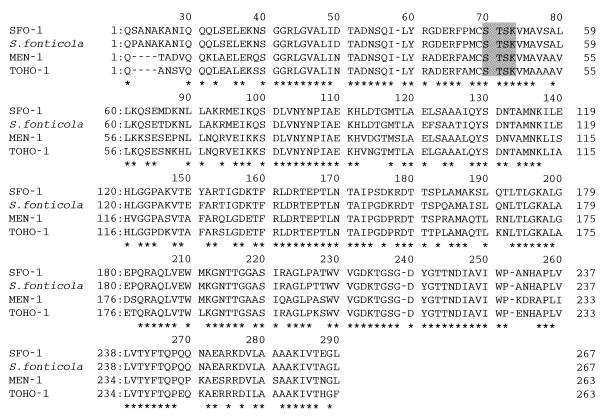
The plasmid-mediated β-lactamase gene of E. cloacae 8009 was cloned into vector plasmid pHSG396 and was used to transform E. coli DH10B (Fig. 2). Two transformants that have plasmids (pFCX310 and pFCX320) recombined with a partially digested Sau3AI fragment and that had an insertion of nearly 2.2 kb were selected. The sequence obtained was analyzed by a search of the GenBank database (GENETYX-MAC/CD) for a homologous sequence, and the search revealed the existence of a β-lactamase gene similar to that for a Klebsiella oxytoca chromosomal enzyme (2). The initiation codon and stop codon were designated on the basis of the homologies of their sequences with those for the initiation codon and stop codon of the K. oxytoca β-lactamase gene. Following a search for a homologous amino acid sequence, the highest degree of homology was seen with the β-lactamase from Serratia fonticola. We named the enzyme SFO-1. The alignments of the deduced amino acid sequence of SFO-1 with those of homologous enzymes are shown in Fig. 3. Sequence identities were 96, 78, 77, and 75% with S. fonticola β-lactamase (P80545), MEN-1 (3), K. oxytoca chromosomal enzymes (2), and TOHO-1 (11), respectively. The homology between SFO-1 and FEC-1 was 72%. The conserved sequences of class A β-lactamases including the 70S-X(T)-X(S)-K motif in the active site were all conserved in this enzyme (1). It is noteworthy that there was an ampR-related sequence that inversely followed the upstream region of ampA. However, the ampR-like sequence was not intact in pFCX310 or pFCX320. The sequence of SFO-1 also had 70% homology with the sequences of the inducible chromosomal β-lactamases of Citrobacter diversus (25) and Proteus vulgaris (24).
FIG. 2.
Construction of recombinant plasmids.
FIG. 3.
Alignment of deduced amino acid sequence of SFO-1 (AmpA) with the sequences of homologous enzymes. The amino acid numbering for the class A β-lactamase is used (1). The S-X(T)-X(S)-K motif with the active-site serine is shaded. Asterisks indicate the conserved amino acid residues among these class A sequences.
Characterization of ampR of SFO-1.
To obtain the full length of ampR with ampA on the same fragment, EcoRI-restricted fragments from pFCX300L and pFCX300M were cloned into pHSG396 (Fig. 2). Two kinds of plasmids, pFCX301 from pFCX300M and pFCX302 from pFCX300L, were obtained. They seemed to have the same fragment in different directions from the results of restriction mapping and sequencing of both ends. By using these transformants as a template, the sequence of the flanking region of ampA was read as described in Materials and Methods. From the homology with the ampR gene of P. vulgaris (6), a complete ampR gene was identified upstream from ampA. The deduced amino acid sequence of AmpR had 80, 68, 60, and 56% similarities with the AmpR sequences of the chromosomal β-lactamases from C. diversus (15), P. vulgaris (6), Pseudomonas aeruginosa (19), and S. marcescens (23), respectively. The alignments of these AmpR sequences are shown in Fig. 4.
FIG. 4.
Alignment of deduced amino acid sequence of AmpR of SFO-1 with homologous AmpR sequences. Asterisks indicate the conserved amino acid residues.
Role of ampR gene.
The inducibility of β-lactamase production in each E. coli transformant was determined as described in Materials and Methods (Table 6, experiment 2). In E. coli strains harboring pFCX310 and pFCX320 with an incomplete ampR gene, β-lactamase was not induced by imipenem, and the amount of β-lactamase produced in them stayed low. On the other hand, in E. coli strains harboring pFCX301 and pFCX302 with a complete ampR gene on the same vector, β-lactamase production was induced by the addition of imipenem. E. coli DH10B harboring native pFCX300L and pFCX300M also produced β-lactamase inducibly, although specific activities were lower than those for E. cloacae 8009.
Susceptibilities of transformants to various antibiotics.
The patterns of susceptibility to various agents for the transformants that were obtained are presented in Tables 2 and 3. The MICs were higher for E. cloacae 8009 than those for any of the other transformants tested. Moreover, E. cloacae 8009 was also resistant to cefoxitin and moxalactam, suggesting that the strain produces a fair amount of chromosomal cephalosporinase, although it was not detectable by analytical isoelectric focusing. The MICs for transformants harboring pHSG396-derived plasmids were higher than the MICs for transformants harboring low-copy-number original plasmids. The effect of copy number was larger than the effect of the AmpR activator on the SFO-1 producer.
DISCUSSION
The SFO-1 β-lactamase isolated from E. cloacae 8009 seems to be more threatening than the common TEM-1- or TEM-2-type β-lactamase because it has a broad spectrum of activity and hydrolyzes various β-lactam antibiotics but not cephamycin, oxacephem, or carbapenem. However, E. cloacae is known to gain resistance to β-lactams easily as a result of mutations of the regulatory genes for chromosomal inducible β-lactamase, making it a high-level β-lactamase producer (4, 6, 16, 18). In fact, the level of resistance of E. cloacae 8009 to ceftizoxime, ceftazidime, and moxalactam was lower than those for strains that constitutively produce large amounts of chromosomal β-lactamase (Table 2). If E. cloacae 8009 was a high-level producer of chromosomal β-lactamase, it would be almost impossible to predict the presence of a β-lactamase like SFO-1 on the basis of patterns of susceptibility to various β-lactams. Moreover, the strain would have no need to acquire SFO-1 genes for defense. E. cloacae 8009 was also resistant to cefoxitin, in contrast to the SFO-1-producing transformant derived from β-lactamase-negative E. cloacae 199S. The cefoxitin resistance of E. cloacae 8009 is considered to come from a porin change or small amounts of chromosomal β-lactamase produced by the strain, although it was difficult to detect chromosomal β-lactamase by isoelectric focusing. SFO-1 seems to have an important role in the β-lactam resistance of E. cloacae 8009. SFO-1 could be an effective weapon that various gram-negative bacteria could use to resist β-lactams.
β-Lactamase is usually produced constitutively in E. coli, which has no ampR gene (4). SFO-1 was produced constitutively in TF2-2 and TF2-3, as shown in Table 6.
Are there any merits to the coexistence of ampR with ampA? In gram-negative bacteria, virtually all of the known plasmid-mediated β-lactamases are produced constitutively, although SFO-1 was produced inducibly. The presence of ampR seems to be a disadvantage for the host strain, because E. cloacae becomes highly resistant to β-lactams when β-lactamase production is changed from inducible to constitutive. However, the amount of β-lactamase was larger in the strain which has both ampR and ampA on the plasmid, which indicates that AmpR acts as an activator as well as a repressor (4). The coexistence of ampR with ampA is considered to aid in the progression of resistance. In fact, after induction TF-L7 and TF-M4, which had ampR, produced SFO-1 in amounts more than 10 times larger than the amounts produced by TF2-2 and TF2-3, which did not have ampR, although they have SFO-1 on the same vector (Table 6). However, the MICs of the cephalosporins for the transformants were not dramatically influenced by the presence of ampR. The low level of resistance of TF-L was considered to come from the low copy number of plasmid pFCX300L.
Concerning the origin of the plasmid-mediated β-lactamase, it is most possible that the ampA of SFO-1 is from S. fonticola (8), because the AmpA sequences of both E. cloacae 8009 and S. fonticola had a high degree of homology (95.5%), although there is no information about the AmpR of S. fonticola. The AmpA sequence of E. cloacae 8009 also had a high degree of homology with those of the enzyme from K. oxytoca and MEN-1, although the latter two enzymes are constitutively produced. C. diversus is another possible source because its AmpA and AmpR sequences were 70 and 80% homologous to those of E. cloacae 8009, respectively. In the PCR study with primers designed to detect ampA and ampR of SFO-1, no strains of ordinary gram-negative organisms produced both amplified fragments, although we have not examined S. fonticola (data not shown). S. fonticola, a kind of flora usually isolated from well waters and springs, is not well known because it is not a pathogenic organism (8). However, because it is a possible source of a plasmid-mediated resistance factor, it is necessary to pay attention to minor species as possible resistance gene donors, even if they are uncommon.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frére J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal beta-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bennett P M, Chopra I. Molecular basis of β-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Jacoby G A, Medeiros A A. A functional scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datz M, Joris B, Azab E A, Galleni M, Van Beeumen J, Frere J M, Martin H H. A common system controls the induction of very different genes. The class-A beta-lactamase of Proteus vulgaris and the enterobacterial class-C beta-lactamase. Eur J Biochem. 1994;226:149–157. doi: 10.1111/j.1432-1033.1994.tb20036.x. [DOI] [PubMed] [Google Scholar]
- 7.Du Bois S K, Marriott M S, Amyes S G B. TEM- and SHV-derived extended-spectrum β-lactamases: relationship between selection, structure and function. J Antimicrob Chemother. 1995;35:7–22. doi: 10.1093/jac/35.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Gavini F, Ferragut C, Izard D, Trinel P A, Leclerc H, Lefebvre B, Mossel D A A. Serratia fonticola, a new species from water. Int J Syst Bacteriol. 1979;29:92–101. [Google Scholar]
- 9.Horii T, Arakawa Y, Ohta M, Ichiyama S, Wacharotayankun R, Kato N. Plasmid-mediated AmpC-type beta-lactamase isolated from Klebsiella pneumoniae confers resistance to broad-spectrum beta-lactams, including moxalactam. Antimicrob Agents Chemother. 1993;37:984–990. doi: 10.1128/aac.37.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Arakawa Y, Ohsuka S, Wacharotayankun R, Kato N, Ohta M. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob Agents Chemother. 1995;39:824–829. doi: 10.1128/aac.39.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacoby G A, Sutton L. Properties of plasmids responsible for production of extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 15.Jones M E, Avison M B, Damdinsuren E, MacGowan A P, Bennett P M. Heterogeneity at the beta-lactamase structural gene ampC amongst Citrobacter spp. assessed by polymerase chain reaction analysis: potential for typing at a molecular level. J Med Microbiol. 1994;41:209–214. doi: 10.1099/00222615-41-3-209. [DOI] [PubMed] [Google Scholar]
- 16.Korfmann G, Wiedemann B. Genetic control of β-lactamase production in Enterobacter cloacae. Rev Infect Dis. 1988;10:793–799. doi: 10.1093/clinids/10.4.793. [DOI] [PubMed] [Google Scholar]
- 17.Kunugita C, Higashitani F, Hyodo A, Unemi N, Inoue M. Characterization of a new plasmid-mediated extended-spectrum beta-lactamase from Serratia marcescens. J Antibiot. 1995;48:1453–1459. doi: 10.7164/antibiotics.48.1453. [DOI] [PubMed] [Google Scholar]
- 18.Lindberg F, Lindquist S, Normark S. Genetic basis of induction and overproduction of chromosomal class I β-lactamase in nonfastidious gram-negative bacilli. Rev Infect Dis. 1988;10:782–785. doi: 10.1093/clinids/10.4.782. [DOI] [PubMed] [Google Scholar]
- 19.Lodge J M, Minchin S D, Piddock L J, Busby J W. Cloning, sequencing and analysis of the structural gene and regulatory region of the Pseudomonas aeruginosa chromosomal ampC beta-lactamase. Biochem J. 1990;272:627–631. doi: 10.1042/bj2720627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Ikeda F, Kamimura T, Yokota Y, Mine Y. Novel plasmid-mediated β-lactamase from Escherichia coli that inactivates oxyimino-cephalosporins. Antimicrob Agents Chemother. 1988;32:1243–1246. doi: 10.1128/aac.32.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generation of β-lactam antibiotics. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 22.Mine Y, Watanabe Y, Sakamoto H, Hatano K, Kuno K, Higashi Y, Kamimura T, Matsumoto Y, Tawara S. In vitro antibacterial activity of FK037, a novel parenteral broad-spectrum cephalosporin. J Antibiot. 1993;46:71–87. doi: 10.7164/antibiotics.46.71. [DOI] [PubMed] [Google Scholar]
- 23.Naas T, Livermore D M, Nordmann P. Characterization of LysR family protein SmeR from Serratia marcescens S6, its effect on expression of the carbapenem-hydrolyzing β-lactamase Sme-1, and comparison of this regulator with other β-lactamase regulators. Antimicrob Agents Chemother. 1995;39:629–637. doi: 10.1128/AAC.39.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nukaga M, Taniguchi K, Washio Y, Sawai T. Effect of amino acid insertion into the omega loop region of a class C β-lactamase on its substrate specificity. Biochemistry. 1998;37:10461–10468. doi: 10.1021/bi980184i. [DOI] [PubMed] [Google Scholar]
- 25.Peduzzi J, Reynaud A, Baron P, Barthelemy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing beta-lactamase of Proteus vulgaris RO104 belongs to Ambler’s class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 26.Perilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frere J M. Cloning and nucleotide sequence of the gene encoding the beta-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;83:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 27.Philippon A, Labia R, Jacoby G. Extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1989;33:1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirot, D. 1995. Extended-spectrum plasmid-mediated beta-lactamases. J. Antimicrob. Chemother. 36(Suppl. A):19–34. [DOI] [PubMed]
- 29.Vu H, Nikaido H. Role of β-lactam hydrolysis in the mechanism of resistance of a β-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum β-lactams. Antimicrob Agents Chemother. 1985;27:393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–151. doi: 10.1128/aac.35.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe Y, Yokota T, Higashi Y, Wakai Y, Mine Y. In vitro and in vivo transferable β-lactam resistance due to a new plasmid-mediated oxyiminocephalosporinase from a clinical isolate of Proteus mirabilis. Microbiol Immunol. 1991;35:87–97. doi: 10.1111/j.1348-0421.1991.tb01537.x. [DOI] [PubMed] [Google Scholar]