Dear Editor,
In this Journal, Murray and colleagues recently described their validation of a commercially available indirect assay for SARS-CoV-2 neutralizing antibodies using a pseudotyped-virus assay [1]. Here, we report the use of different assays to determine the vaccine-induced antibodies ability to neutralize Variant Of Concerns (VOCs). Recently, it has been shown that Omicron is able to escape vaccine antibodies; one month after the third BNT162b2 (BioNTech-Pfizer) dose, neutralizing activity was 75% for Delta and below 50% for Omicron [2]. The better characterization of the humoral response beyond the level of antibodies is crucial.
The present study was performed on sera samples from nine healthcare workers (HCWs) from Bichat-Claude Bernard Hospital Virology department (Paris, France) vaccinated with BNT162b2. Samples were collected between first vaccine dose and up to one month after third dose. Anti-N serology was negative at all time-points (SARS-CoV-2 IgG kit, Abbott, IL, USA). All HCWs provided informed consents. Following viral supernatant production, SARS-CoV-2 was titrated by a lysis-plaque assay and live virus neutralization assay was performed as previously described [3,4]. A pseudoneutralization method, based on ACE-2 receptor binding inhibition (iFlash®−2019-nCoV Nab, YHLO, Shenzhen, China), measured the ability of antibodies to bind the RBD of the ancestral SARS-CoV-2 spike protein. The Meso-Scale-Discovery® (V-PLEX SARS-CoV-2 Panel13) assay enabled to assess pseudoneutralization activity of sera against different VOCs using a multiplexed Spike antigens technology. The CoViDiag kit (Innobiochips®, Loos, France) is a quantitative ELISA test detecting IgG against different VOCs including Omicron.
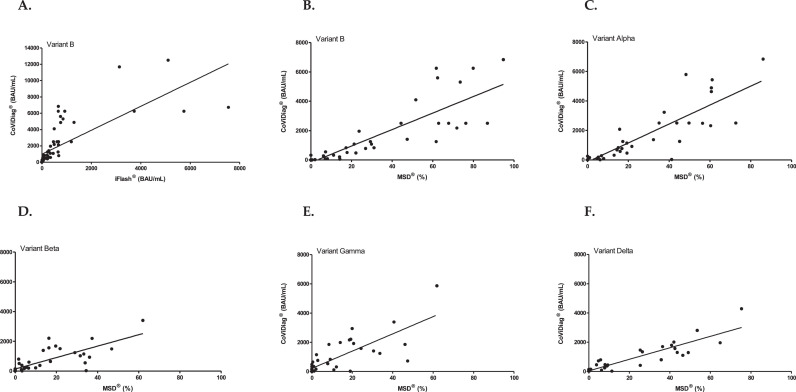
Nine BNT162b2-vaccinated HCW, were followed up to one month after the third vaccine dose, received in median 8.6 months (IQR=8.5–9.2) following the second dose. Kinetics of anti-S SARS-CoV-2 antibodies is described in Supplementary Fig. 1. We report the results of live virus neutralization for Omicron and others VOCs until one month after the third vaccine dose (Fig. 1 A), extending our previous data [3,5]. We showed that Omicron neutralization titers were in median 16- and 8-fold lower than the Delta ones, one month after the second and one month after the third dose, respectively. We also extending our previous data [3] with the iFlash® pseudoneutralization assay showing an increased median of antibody titers from 44 BAU/mL (IQR=38–57) to 3134 (IQR=758–5109) before and one month after the third dose, respectively. The median antibody titer was significantly higher after the third than after the second dose (669 vs 3134 BAU/mL, p = 0.02) (Fig. 1B, Supplementary Table 1). One month after the second dose, using Meso-Scale-Discovery® assay, we showed a median percentage of inhibition of 63% (IQR=62–80), 60% (IQR=44–61), 42% (IQR=36–54), 29% (IQR=16–37) and 21% (IQR=14–40) for B, Alpha, Delta, Beta and Gamma variants, respectively. The median percentage of inhibition at the last available sample before the third dose was 24% (IQR=17–52), 19% (IQR=14–38), 9% (IQR=6–26), 12% (IQR=3–22), and 8% (IQR=0–19) for B, Alpha, Delta, Beta and Gamma variants, respectively (Fig. 1C). Using the CoViDiag® assay, one month after the second dose, antibodies median titers were 2500 BAU/mL (IQR=2500–6250), 2500 (IQR=2500–5437), 1695 (IQR=1459–2007), 1486 (IQR=1237–2192), 1918 (IQR=1852–2942), and 603 (IQR=545–1192) for B, Alpha, Delta, Beta, Gamma and Omicron variants, respectively (Fig. 1D). Then, a decrease was observed until right before the third dose, at which point median titers were 186 BAU/mL (IQR=161–250), 169 (IQR=120–250), 90 (IQR=48–111), 103 (IQR=69–166), 124 (IQR=71–241), and 50 (IQR=35–90) for B, Alpha, Delta, Beta, Gamma and Omicron, respectively. One month after the third dose the median titers were 6250 BAU/mL (IQR=4861–6724), 6250 (IQR=4928–6250), 3189 (IQR=2659–4868), 3757 (IQR=2451–6250), 4150 (IQR=2957–6250), and 1648 (IQR=1207–2700) for B, Alpha, Delta, Beta, Gamma and Omicron, respectively. The antibody titer for Omicron after the second dose was significantly lower than the titer for Delta (median fold-change=2.8; p = 0.0008), difference no longer observed after the third dose (median fold-change=1.9; p = 0.12). The median antibody titer after the third dose was higher than the titer reached after the second for all VOCs (Omicron: p<0.0001). The Spearman's correlation coefficient between iFlash® and CoViDiag® titers for B variant was ρ=0.95 (p < 0.0001) (Fig. 2 ). The correlation coefficient between Meso-Scale-Discovery® percentages and CoViDiag® titers were ρ=0.94 (p < 0.0001), ρ=0.90 (p < 0.0001), ρ=0.87 (p < 0.0001), ρ=0.80 (p < 0.0001), and ρ=0.81(p < 0.0001) for B, Alpha, Delta, Beta and Gamma (Fig. 2).
Fig. 1.
Longitudinal follow-up of the humoral response of nine BNT162b2-vaccinated healthcare workers using: A. Live virus neutralization assay, B. iFlash® pseudoneutralization assay, C. Meso-Scale-Discovery® pseudoneutralization assay, and D. CoViDiag® variant-specific ELISA assay. The arrows represent the time of each vaccine dose.
Fig. 2.
Relationship between iFlash® and CoViDiag® assays for the historical B variant (Panel A), between Meso-Scale-Discovery® and CoViDiag® assays for the historical B variant (Panel B), Alpha variant (Panel C), Beta variant (Panel D), Gamma variant (Panel E), and Delta variant (Panel F) among nine BNT162b2-vaccinated healthcare workers.
In this study, among nine BNT162b2-vaccinated HCW, the lowest neutralizing antibodies titers, after the second and third doses, were observed with Omicron as well with live virus neutralization as with a variant-specific ELISA assay. Furthermore, neutralizing antibodies titers were reduced with Delta and Omicron, responsible of the two last COVID-19 waves, in agreement with previous studies [6,7]. The present study showed that the humoral response level was significantly higher after the third dose than after the second dose, whatever the assay used, confirming the boosting effect of the third dose [8]. We showed a very good correlation between Meso-Scale-Discovery® pseudoneutralization and CoViDiag® variant-specific ELISA assays for all VOCs. We also showed a very good correlation for B variant between iFlash® pseudoneutralization and CoViDiag® assay. Furthermore, for Omicron, CoViDiag® titers agree with live virus neutralization, showing that Omicron is the variant that escapes the most to the post-vaccine humoral immunity.
To our knowledge, these are the first data comparing various serological assays, pseudoneutralization or variant-specific ELISA assays, to the reference method, live virus neutralization. All these assays, despite using different technologies and measuring different variables, showed the same trend in terms of humoral response magnitude and of VOC neutralizing ability levels. Beta VOC, already known to escape to vaccine-induced immunity [9], is indeed weakly neutralized by post-vaccine antibodies with the different assays used. This low neutralizing ability is also observed for Omicron, confirming first data observed with the current circulating VOC [6,7]. These data showed that the nature of humoral response can be characterized using more easy-to-use or automated assays than the live virus neutralization. Indeed, these assays could be interesting to monitor highly immunocompromised patients, populations for whom a serological follow-up is recommended by French Health Authorities [10].
In conclusion, since the good correlation observed with the live virus neutralization assay, pseudoneutralization or variant-specific ELISA assays could be useful to monitor the humoral response.
Funding
The research leading to these results has received funding from the Agence Nationale de recherche sur le SIDA et les hépatites virales (ANRS)-Maladies Infectieuses Emergentes (ANRS | MIE) and EMERGEN consortium.
Declaration of Competing Interest
The authors have no commercial or other associations that might pose a conflict of interest.
Acknowledgements
We want to thank all the technicians of the virology lab of Bichat-Hospital and the biological studies technicians for their helpful technical assistance.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.02.031.
Appendix. Supplementary materials
References
- 1.Murray M.J., McIntosh M., Atkinson C., Mahungu T., Wright E., Chatterton W., et al. Validation of a commercially available indirect assay for SARS-CoV-2 neutralising antibodies using a pseudotyped virus assay. J Infect. 2021;82:170–177. doi: 10.1016/j.jinf.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muik A., Lui B.G., Wallisch A.K., Bacher M., Mühl J., Reinholz J., et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 MRNA vaccine–elicited human sera. Science. 2022 doi: 10.1126/science.abn7591. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferré V.M., Lebourgeois S., Menidjel R., Collin G., Chenane H.R., Guindi M.O., et al. Decreasing humoral response among healthcare workers up to 4 months after two doses of BNT162b2 vaccine. J Infect. 2021:S0163–S4453. doi: 10.1016/j.jinf.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebourgeois S., Menidjel R., Chenane H.R., Ferré V.M., Collin G., Damond F., et al. Alpha (B.1.1.7) and delta (B.1.617.2 - AY.40) SARS-CoV-2 variants present strong neutralization decay at M4 post-vaccination and a faster replication rates than D614G (B.1) lineage. J Infect. 2021 doi: 10.1016/j.jinf.2021.11.012. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021 doi: 10.1038/s41586-021-04389-z. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Khan K., Karim F., Cele S., San J.E., Lustig G., Tegally H., et al. Omicron infection enhances neutralizing immunity against the delta variant. medRxiv. 2021 2021.12.27.21268439. [Google Scholar]
- 8.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022 doi: 10.1001/jama.2022.0470. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., et al. Sensitivity of infectious SARS-CoV-2 B1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 10.https://solidarites-sante.gouv.fr/IMG/pdf/cosv_-_recommandations_pour_la_protection_des_personnes_severement_immunodeprimees_-_19_novembre_2021.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.