Abstract
Traumatic brain injuries (TBI) have led to lasting deficits for an estimated 5.3 million American patients. Effective therapies for these patients remain scarce and each of the clinical trials stemming from success in experimental models has failed. We believe that the failures may be, in part, due to the lack of preclinical assessment of cognitive domains that widely affect clinical TBI. Specifically, the behavioral tasks in the TBI literature often do not focus on common executive impairments related to the frontal lobe such as cognitive flexibility. In previous work, we have demonstrated that the attentional set-shifting test (AST), a task analogous to the clinically-employed Wisconsin Card Sorting Test (WCST), could be used to identify cognitive flexibility impairments following controlled cortical impact (CCI) injury. In this study, we hypothesized that both the administration of the antidepressant drug citalopram (CIT) and exposure to a preclinical model of neurorehabilitation, environmental enrichment (EE), would attenuate cognitive performance deficits on AST when provided alone and lead to greater benefits when administered in combination. Adult, male rats were subjected to a moderate-severe CCI or sham injury. Rats were randomly divided into experimental groups that included surgical injury, drug therapy, and housing condition. We observed that both CIT and EE provided significant cognitive recovery when administered alone and reversal learning performance recovery increased the most when the therapies were combined (p<0.05). Ongoing studies continue to evaluate novel ways of assessing more clinically relevant measurements of high order cognitive TBI-related impairments in the rat model.
Keywords: controlled cortical impact (CCI), citalopram, antidepressants, environmental enrichment, executive function, attentional set-shifting
1. Introduction
Traumatic brain injury (TBI) affects approximately 2.8 million Americans each year who often experience long-term cognitive disability that impedes quality of life, work productivity, as well as mental and emotional health (E.A. Finkelstein, 2007; Langlois et al., 2006; Taylor CA, 2017). Specific impairments such as declines in executive function, attention and memory loss, inability to acquire and store new information (Bondi et al., 2008), and behavioral flexibility deficits (Gioia and Isquith, 2004) are commonly reported; however, laboratory studies aimed at identifying effective therapies often limit their cognitive testing paradigms to tasks focused on spatial learning and memory retention (Meconi et al., 2018; Radabaugh et al., 2020; Radabaugh et al., 2016; Tadepalli et al., 2020). While these tasks lead to consistent, measurable deficits following experimental TBI, they do not encompass the full syndromic experience noted in the clinical population. This lack of attention to higher-order cognitive impairments may be contributing to the translatability crisis currently plaguing TBI research. Executive function is a collection of related, yet distinct capabilities that provide for higher-order processes such as rule formation and selection in response to stimuli (attention set-shifting), and overcoming previously developed habits (reversal learning) (Bondi et al., 2008). Studies from multiple groups have uncovered that these functions require proper functioning of the prefrontal cortex (PFC) (Birrell and Brown, 2000; Bondi et al., 2014a; Clarke et al., 2007; Floresco et al., 2009). In humans, the Wisconsin Card Sorting Test (WCST) is commonly used to assess potential impairments (Stuss et al., 2000). In patients with TBI, depression, and other neuropsychiatric disorders, the WCST has been shown to sensitively characterize strategy-switching deficits thus implicating impairments in the PFC (Merriam et al., 1999; Stuss et al., 2000). Our laboratory has previously shown that rodents subjected to a CCI injury of varying depths (2.6 mm, 2.8 mm, or 3.0 mm) produced cortical depth-dependent cognitive deficits on the attentional set-shifting test (AST), a rodent correlate of the WCST (Birrell and Brown, 2000; Bondi et al., 2014a).
Moreover, one-third of TBI patients are later diagnosed with major depressive disorder (MDD) which is linked to further cognitive impairments, thus becoming a significant hindrance to recovery (Jorge et al., 2004; Rapoport et al., 2005). Citalopram (CIT) is a selective serotonin reuptake inhibitor (SSRI) prescribed on-label as an antidepressant drug to treat MDD (Rapoport et al., 2008). In clinical TBI studies, CIT improved depression outcome and remission (Luo et al., 2015; Rapoport et al., 2008). Environmental enrichment (EE) is analogous to multimodal clinical rehabilitation by promoting sensory stimulation, physical exercise, and social interaction (Bondi et al., 2014b; Kline et al., 2012; Sozda et al., 2010). EE has been shown to improve motor performance, spatial learning, and memory retention relative to standard (STD)-housed rats after experimental TBI (Bondi et al., 2014b; de la Tremblaye et al., 2017; Hicks et al., 2002; Lajud et al., 2019; Passineau et al., 2001; Radabaugh et al., 2016). To better model the clinic where drug therapies are often administered alongside neurorehabilitation, we sought to assess the combination therapy of chronic CIT and EE housing. Following our success using AST as a measure of higher order dysfunction after TBI, we hypothesized that both therapeutic interventions, CIT and EE, would attenuate AST deficits as monotherapies but that the combination of CIT and EE would lead to greater benefits than either therapy alone.
2. Materials and Methods
2.1. Animals
Eighty-six adult male Sprague-Dawley rats (Envigo, Indianapolis, IN) were housed in commercially available clear Plexiglas® individually-ventilated cages (Allentown LLC, Allentown, NJ) under a regulated light-controlled cycle (12/12-h light/dark; lights on at 7:00 AM) and kept at a constant temperature (21 ± 1 ⁰ C) with food and water ad libitum. After a week of acclimation to the housing environment, experimental manipulations began during the light phase of the cycle. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh (Pittsburgh, PA) and all efforts were made to minimize pain and discomfort and to minimize the number of rats used.
2.2. Surgery
On the day of surgery, rats weighing between 300–325g were randomly assigned to CCI or sham injury groups. All surgical procedures were performed as previously described (Cheng et al., 2012; Hoffman et al., 2008; Kline et al., 2010; Radabaugh et al., 2017; Shaw et al., 2013; Yelleswarapu et al., 2012). Isoflurane anesthesia was induced and maintained using a 2:1 N2O/O2 gas mixture at concentrations of 4% and 2%, respectively. Rats were intubated endotracheally, placed on mechanical ventilation for anesthesia maintenance, and secured in a stereotaxic frame. A heating pad was used to maintain a core temperature of 37 ° C, which was measured throughout surgery with a rectal probe. Using aseptic procedures, a craniectomy was performed in the right hemisphere with a dental drill. A TBI was produced by impacting the exposed right parietal cortex at a depth of 2.8 mm and speed of 4 m/s with a dwell time of 100ms. After impact, anesthesia was discontinued and the incision was sutured. Sham animals underwent all surgical procedures but were not subjected to the impact.
2.3. Post-surgery
After surgery, the rats were randomly assigned to four TBI groups and four sham control groups that received the same treatments as their TBI counterparts.
2.3.1. Acute Neurological Evaluation
Hind limb reflexive ability was assessed immediately after the discontinuation of anesthesia by gently squeezing the rat’s paw every 5 s and recording the latency to elicit a withdrawal response. Return of the righting reflex was determined by recording the time needed for the rat to return to the prone position after three consecutive supine placements.
2.3.2. EE Housing
After surgery, the rats were placed in their respective assigned housing conditions for 3 weeks before AST food restriction began. The EE consisted of specifically designed 36×30×20 inch stainless steel wire cages with three levels and ladders to ambulate from one level to another to interact with the various toys (i.e. balls, blocks, tubes), nesting materials (i.e. saw dust), cage mates, and ad libitum food and water. To maintain novelty, the objects were rearranged daily and changed twice weekly. Rats from TBI and sham control groups were housed together to minimize variability. Rats in STD conditions were places in standard Plexiglas cages (two rats per cage) with ad libitum food and water. At 3 weeks post-surgery, rats in EE were moved into STD housing so that mild food restriction (14 g/day, per rat) could take place for 1 week prior to AST behavior.
2.4. Drug Administration
Citalopram hydrobromide was dissolved in 50% DMSO in saline, which also served as the vehicle (VEH). CIT (20 mg/kg) free base and VEH (1.0 ml/kg) were administered intraperitoneally beginning 24 h after surgery and once daily until AST was completed at 30–32 days post-surgery. On the days of behavioral testing, the injections were administered 1 h before testing to avoid behavioral symptoms associated with serotonin syndrome (i.e. flat body posture) that could affect performance. The dose and route of administration were selected from previously published work (Lapiz-Bluhm et al., 2010; Furr et al., 2012).
2.5. Attentional Set-shifting Test
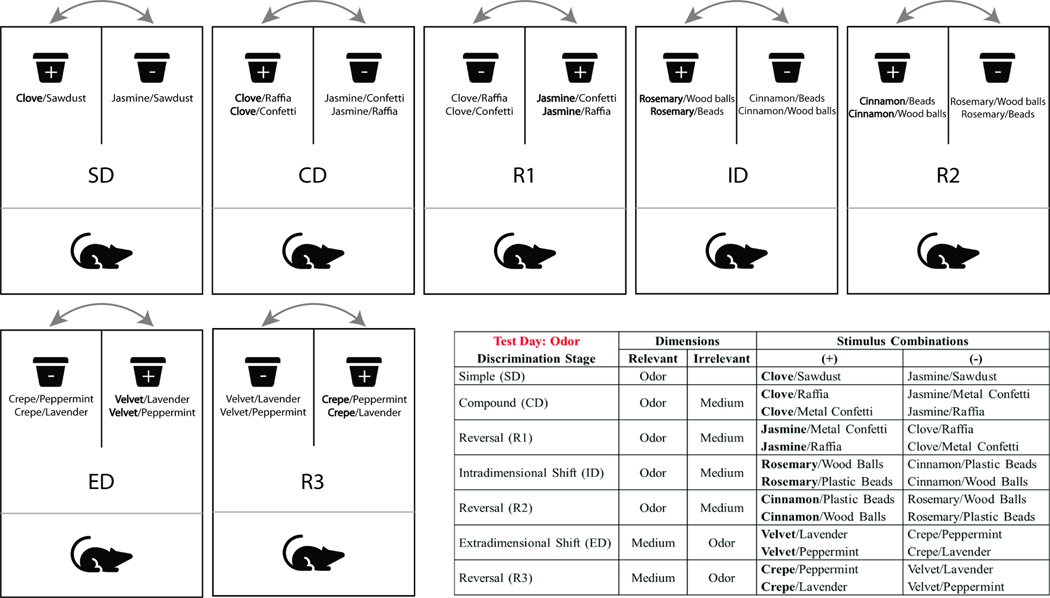
Procedures for AST were adapted from Birrell and Brown (Birrell and Brown, 2000) and have been previously described (Bondi et al., 2010; Lapiz and Morilak, 2006). One week prior to behavioral testing (day 21 post surgery), rat food intake was limited to 14g/day, with water freely available. Weight loss was measured on testing day and compared to body weight taken before food restriction to ensure that the injured rats lost weight at the same rate as the control groups. The testing arena was a custom-built, rectangular Plexiglas arena (Fig. 1). A removable divider separated the holding area (one third of the arena) from the experimental portion, which allowed the rat to be contained between sets while the experimenter changed pots and cleaned the arena. Each trial began with removing the divider and giving the rat access to the arena. A clear Plexiglas panel separated the opposite one third of the area into two separate sections with a digging pot in each section. Terracotta digging pots (internal rim diameter, 7cm; depth, 6 cm) were each defined by a pair of cues along two stimulus dimensions: the digging medium and an odor (Fig. 1). To mark each pot with a distinct odor, two drops (10 μl/drop) of scented aromatic oil (NOW Foods, Bloomingdale, IL) were applied to the inner rim at least five days before initial use. Then 1–2 μl were reapplied daily to maintain consistent odor intensity. A different pot was used for each odor and digging material combination and only one odor was applied to a given pot. The bait, one quarter of a Honey Nut Cheerio (General Mills Cereals, Minneapolis, MS), was buried 2 cm below the surface of the digging medium in the “positive” pot. A small quality of powdered Cheerio dust was sprinkled over both pots before beginning each task to prevent the rat from locating the bait by smell rather than learning the discrimination. Investigations of the rim of the pot or the surface of the digging material with paws or snout were not classified as digging. Digging was defined as a vigorous displacement of the medium to retrieve the reward buried in the pot. This definition of digging allowed for rats to explore using their somatosensory, olfactory, and visual senses. The behavioral assessment took place over three consecutive days.
Fig. 1.
Representative examples of stimulus pairs and the progression through the AST stages. This example portrays odor as the initial discriminative dimension, followed by a shift to digging medium in the ED stage. For each stage, the positive stimulus is in bold and is paired pseudorandomly across trials with the two stimuli for the irrelevant dimension. Positive (reward granting) and negative pots are placed on the left versus right sides of the arena and their position is changed throughout testing to avoid the possibility of preferred side bias.
2.5.1. Day one: Habituation
Rats were first trained to dig reliably in pots to obtain a food reward. Two unscented pots with increasing amounts of sawdust were placed in the rat’s home cage. Rats had 5 min to dig for a Cheerio food reward and with three successful digs, the amount of sawdust increased (empty, one-third full, two-thirds full, full). Once the rat was comfortable digging, they were placed in the testing arena and given three 5 min trials to dig for a Cheerio in both sawdust filled pots.
2.5.2. Day two: Training
Rats were trained with a series of simple discriminations (SD) to reach a criterion of six consecutive correct trials. First, they learned to associate a food reward with an odor cue (i.e. lemon vs. eucalyptus, both pots filled with sawdust). After reaching the criterion for the odor discrimination, rats learned to discriminate between different media (e.g. paper strips vs. felt, both pots unscented). All rats were trained using the same stimuli pairs and in the same order. The positive and negative cues for each rat were pseudorandomly determined and equally represented. The stimuli implemented during training were not used during testing. Any rat that failed to complete the training procedures was eliminated from further testing.
2.5.3. Day three: Testing
Rats were tested on a series of multiple rule-shifting stages aiming to measure aspects of cognitive flexibility, specifically via stimulus reversals and an extra-dimensional shift (Fig. 1), and six consecutive correct trials were required to move from one stage to the next. Each testing stage included a variation between the discriminative stimulus dimension and the positive cue. Rats first encountered a SD involving a single stimulus dimension (i.e., odor or medium). Half of the rats in each group discriminated between scented sawdust filled pots and the other half discriminated between pots filled with different media that were not scented.
For clarity, the following description will consider only the group of rats whose SD began with odor (Fig. 1). The second stage was a compound discrimination (CD) in which the previously learned contingency rule was required (odor), and the second, irrelevant dimension (medium) was introduced. Similar to the SD, the CD associated only one odor with a reward, while the stimulus from the irrelevant dimension was paired randomly with the two odors. The third stage introduced the reversal (R1) of the previous discrimination, in which the same odors and media were used. Odor continued to be the relevant dimension. However, the negative odor from the previous two stages became the positive, reward-granting odor, and the previously positive odor became negative. The fourth stage was an intradimensional (ID) shift with the introduction of new odors and media. Odor remained the relevant dimension and medium remained the irrelevant dimension. The fifth stage was the reversal (R2) of the ID, where the previously irrelevant odor became the positive, similar to R1. The sixth stage was the extradimensional (ED) cognitive set-shift. All new stimuli were introduced. However, the repeatedly reinforced dimension (cognitive set) became irrelevant and the previous irrelevant dimension (digging medium) became relevant. Finally, the seventh stage was the final reversal (R3), where the previously positive stimulus became negative and the negative stimulus became positive, as in the previous reversals.
The assignment of positive and negative to each stimuli pair, as well as the positioning within the arena was determined pseudorandomly in advance. All rats were tested using the same stimuli pairs and in the same order, with half starting on odor-based discriminations, and half starting on medium-based discriminations. The dependent measure was the number of trials to reach criterion of six consecutive correct responses (trials to criterion) for each stage of the test. We also assessed the total number of errors that were recorded for each stage prior to reaching criterion. Moreover, set loss errors for each rat on every stage were calculated as the errors occurring after 50% or more of the contingency acquisition has been achieved (i.e., an error after three, four, or five correct responses), parallel to the WCST (Stuss et al., 2000). The rats were given 10 min to make a choice and received an error if a choice was not made in the allotted time. Complete testing occurred within 2–4 h per rat, depending on its own speed and propensity to engage in a timely fashion. Rats were removed from testing if they failed to make a choice in six consecutive trials or failed to complete a stage within 50 trials.
2.6. Histology: Cortical Lesion Volumes
Following the completion of behavioral testing, rats were anesthetized at 5 weeks post-surgery with Fatal-Plus (Henry Schein Animal Health, Columbus, OH; 0.3 ml intraperitoneally) and then perfused transcardially with 200 ml of 0.1M phosphate-buffered saline (pH 7.4), followed by 300 ml of 4% paraformaldehyde (PFA). Brains were extracted, post-fixed in 4% PFA for 1 week, dehydrated with alcohols, and embedded in paraffin. Seven μm-thick coronal slices were cut at 1-mm intervals though the lesion on a rotary microtome and mounted on Superfrost/Plus glass microscope slides (Fisher Scientific, Pittsburgh PA). After drying at room temperature, sections were deparaffinized through xylenes, rehydrated, and stained with Cresyl violet. Cortical lesion volumes (mm3) were assessed by an observer blinded to experimental conditions using a Nikon Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan). The area of the lesion was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7; Nikon NIS-Elements AR 3.22.14 Software; Nikon) and then by summing the lesions measured as previously reported (Hoffman et al., 2008; Kline et al., 2012; Kline et al., 2002; Njoku et al., 2019; Radabaugh et al., 2016).
2.7. Statistical Analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using Statistica 64 version 13 Academic software (Dell Inc., Tulsa, OK). Righting reflex, hind limb reflexive ability, and weight gain were analyzed by multivariate analysis of variance (MANOVA) with Injury, Drug, and Housing as factors to ensure that no surgery biases were reflected in subsequent drug assignment groups. Histological data were analyzed using ANOVAs (Drug × Housing). For analysis of AST testing behavior, the total trials to criterion, total response errors, and set loss errors, were recorded for each test stage. These data were analyzed by repeated measures MANOVAs (Injury × Drug × Housing × Stage), with Stage as the repeated measures. When significant main effects or interactions were indicated, post-hoc one-way ANOVA analyses were performed for each stage followed by Fisher’s Least Significant Difference (LSD) tests, as appropriate. Results are expressed as the mean ± standard error of the mean (S.E.M.), and significance for all analyses was determined as p<0.05.
3. Results
A total of 25 rats were excluded from all statistical analyses. Three injured rats were eliminated due to lack of thriving or death post-surgery. The other exclusions occurred due to an inability to either complete the habituation stage (i.e., 1 SHAM), the training protocol (i.e., 8 TBI and 4 SHAM), inability to complete testing (i.e., 6 TBI and 2 SHAM), or inability to complete the required criterion within 50 trials per stage (i.e., 1 TBI). Therefore, final groups were TBI+VEH+STD (n=10), TBI+CIT+STD (n=7), TBI+VEH+EE (n=10), TBI+CIT+EE (n=9), Sham+VEH+STD (n=7), Sham+CIT+STD (n=6), Sham+VEH+EE (n=6), and Sham+CIT+EE (n=6).
3.1. Acute neurological evaluation
There was a statistically significant effect of TBI compared to sham rats with respect to withdrawal latency after a brief paw pinch was administered to either limb (left paw: TBI range = 150.5±1.87 s, SHAM range = 19.04±2.248 s, F(1,59)= 2018.203, p<0.001; right paw: TBI range= 145.639±1.875 s, SHAM range = 14,32±2.249 s, F(1,59)= 2011.232, p<0.001) after cessation of anesthesia. Moreover, there were no significant differences observed between groups randomly pre-assigned to separate housing paradigms (EE or STD; left paw: F(1,58)= 0.572, p=0.453, right paw: F(1,58)= 0.561, p=0.457) or daily injections (CIT or VEH; left paw: F(1,58)= 0.008, p=0.929, right paw: F(1,58)= 0.008, p=0.927). ANOVA analyses also detected significant differences between TBI and SHAM groups for return to righting ability (F(1,59)= 675.009, p < 0.001; TBI range: 368.222±6.105 s; SHAM range: 120.44±7.327 s), again with no bias reflective of subsequent housing (F(1,58)= 0.686, p=0.411) or drug group (F(1,58)= 0.074, p=0.787) pre-assignment.
3.2. Weight gain
Multifactorial ANOVAs were then used to assess any differences in body weight at surgery prior to group designation and on the day of AST testing. On the day of surgery, there were no weight differences among animals designated to receive TBI or SHAM (F(1,53)= 2.07, p=0.156), EE or STD housing (F(1,53)= 0.22, p=0.643), as well as CIT or VEH administration (F(1,53)= 3.62, p=0.063). On the day of AST testing, there was a significant housing effect (F(1,53)= 6.51, p<0.05), but no effects of surgery (F(1,53)= 3.32, p=0.074) or drug administration (F(1,53)= 0.56, p=0.456) were detected. Rats housed in EE weighed in on average at approximately 3% more than rats housed in the STD condition (EE range: 344.242±2.747 g, STD range: 334.293±2.767 g), which is not considered robust enough to constitute a bias towards being differently affected by the mild food restriction or Cheerio reward intake during testing (data not shown).
3.3. Attentional set-shifting test
3.3.1. ED shift: odor-to-medium versus medium-to-odor
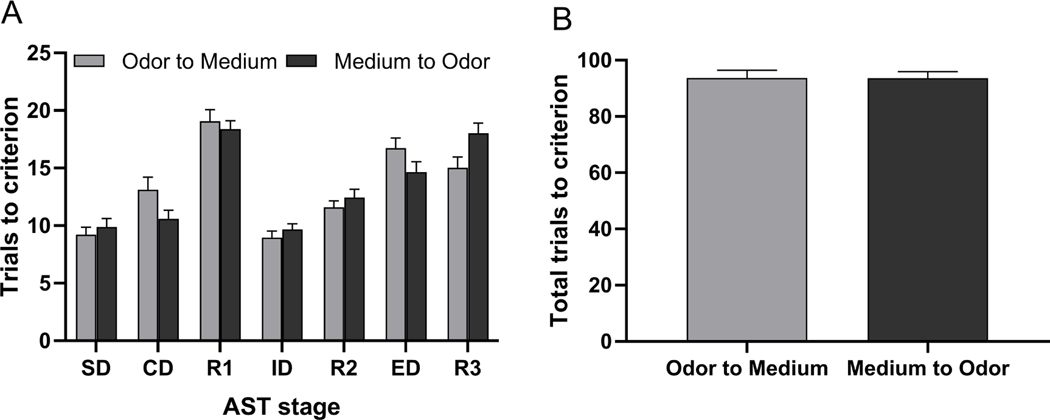
Fig. 2 shows a comparison between rats subjected to an odor-to-medium versus medium-to-odor ED set-shift on the trials to reach the criterion for each task of the AST regardless of injury, treatment, or housing group. There was no significant effect of ED shift type (F(1,59)= 0.001, p=0.98) on AST performance across stages, however there was a significant ED shift type × Stage interaction (F(6,354)= 3.08, p<0.01, Fig. 2A). Fisher’s LSD post hoc analyses revealed a significant difference on CD and R3 (p<0.05), suggesting that rats subjected to the odor-to-medium relevant dimension switch required more trials to complete CD than rats subjected to an odor-to-medium switch, whereas the opposite occurred on R3. Odor-based discrimination learning taking more trials than medium-based at the CD and R3 stages is likely due to the stimulus pairings used at those stages, rather than a discrepancy between odor and medium-based learning per se. No other stage comparisons were significantly different regardless of the dimension to which the contingency belonged, such as odor or medium. Furthermore, when a t-test was performed to compare total trials across the test for rats assigned to either of the two perceptual stimuli set-shifts, no differences were unveiled by means of an independent t-test (t60= −0.03, p=0.98, Fig. 2B).
Fig. 2.
Progression through the attentional set-shifting test reveals a significant difference on CD and R3 (p<0.05), suggesting that rats subjected to a odor-to-medium relevant dimension switch required more trials to complete CD than rats subjected to an odor-to-medium switch, where as the opposite occurred on R3 (panel A). However, there was no injury-related bias against a sensory modality necessary for contingency rule acquisition. No other stage comparisons were significantly different regardless of the dimension to which the contingency belonged. Further, there were no significant differences in total trials to criterion across the AST (panel B). Data are expressed as Mean (± S.E.M.), n=27–34/group.
3.3.2. Trials to criterion
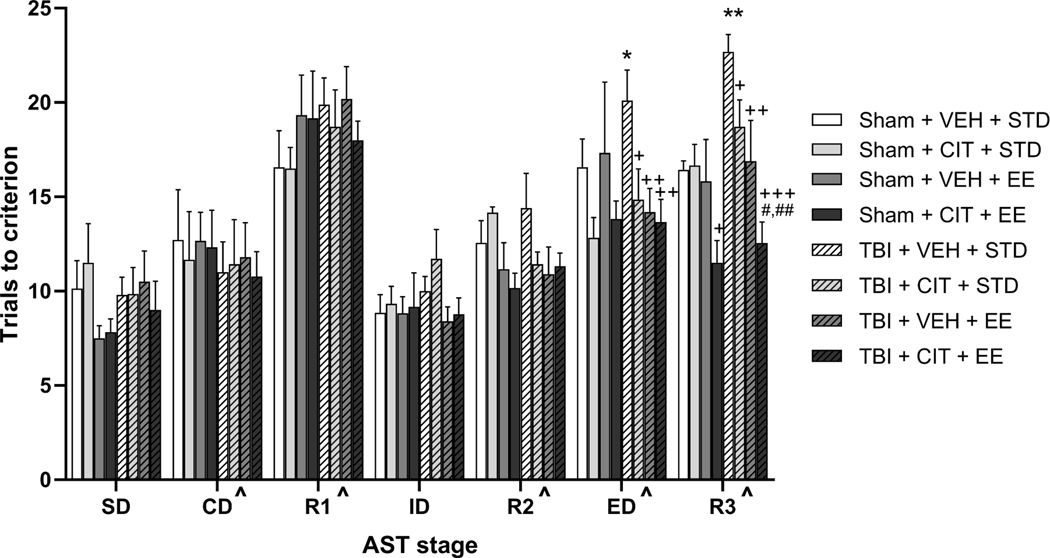
Fig. 3 shows the effects of CCI injury, chronic CIT administration, and EE exposure on the trials to criterion for each stage of the AST at 4 weeks post-surgery. A repeated measures multivariate ANOVA (Injury × Drug × Housing × Stage) revealed significant main effects of Drug (F(1,53)= 5.445, p<0.05), Housing (F(1,53)= 8.586, p<0.001), Stage (F(6, 318)= 41.137, p<0.0001), and a significant Housing × Stage interaction (F(6, 318)= 2.671, p<0.05). For the main effect of Stage, post-hoc comparisons across all groups collapsed regardless of group designation showed that significantly more trials were required to reach criterion during R1, R3, and ED than all other stages, as well as more trials during CD and R2 compared to SD and ID (Fig. 3). As previously reported, this effect particularly validates the inherent difficulty of the stimulus reversals and ED set-shifting stage compared to the other stages of the AST (Bondi et al., 2014a; Njoku et al., 2019). Further, post-hoc one-way ANOVA comparisons for individual stages revealed significant group effects on ED set-shifting (F(7,53)= 2.265, p<0.05) and third reversal (F(7,53)= 5.965, p<0.001) stages. Post-hoc analyses using Fisher’s LSD tests also determined that on the ED stage, rats subjected to CCI with no treatment interventions (TBI+VEH+STD) required significantly more trials to complete the stage as compared to Sham+CIT+STD and Sham+CIT+EE (p<0.05), but not to Sham+VEH+STD. All three treatment groups [TBI+CIT+STD (p<0.05), TBI+VEH+EE (p<0.01), TBI+CIT+EE, (p<0.01)] significantly improved performance on the ED stage compared with TBI+VEH+STD rats (Fig. 3). On the R3 stage, Sham+CIT+EE rats performed significantly better than Sham+VEH+STD and Sham+CIT+STD rats (p’s<0.05), which did not differ from each other. Additionally, rats in the TBI+VEH+STD group performed significantly worse on R3 than Sham+VEH+STD (p<0.01), requiring more trials to finish the stage. Individual treatments after TBI significantly improved performance at 4 weeks post injury (p=0.05 for CIT alone, p<0.01 for EE alone). Moreover, the combined treatment (TBI+CIT+EE) showed a more robust effect in reducing trials to reach criterion on R3 than individual treatments in the TBI+CIT+STD (p<0.01) and TBI+VEH+EE (p<0.05) groups, which support our hypothesis (Fig. 3). No other main effects or interactions were revealed on any other test stages.
Fig. 3.
Mean (± S.E.M.) trials to reach criterion on AST. Rats in TBI+VEH+STD displayed significant cognitive impairments on the ED stage as compared to Sham+CIT+STD and Sham+CIT+EE. Combined treatment effects were seen in sham rats on R3, with the Sham+CIT+EE group requiring significantly less trials to reach criterion than SHAM+VEH+STD and Sham+CIT+STD. Rats in TBI+VEH+STD group displayed significant cognitive impairments on ED as compared to Sham+CIT+STD and Sham+CIT+EE, and on R3 as compared to Sham+VEH+STD, while CIT, EE, and the combined treatment significantly attenuated the injury-induced set-shifting performance deficit. Additionally, the combined treatment produced a more robust effect in reducing trials to criterion on the R3 as compared to individual treatments alone. +p<0.05 Sham+CIT+EE vs Sham+VEH+STD and Sham+CIT+STD; *p<0.05 vs Sham+CIT+STD, Sham+CIT+EE; **p<0.05 vs Sham+STD+VEH; +p=0.05 TBI+CIT+STD vs TBI+VEH+STD; ++p<0.01, +++p<0.001 vs TBI+VEH+STD; #p<0.05 vs TBI+VEH+EE; ##p<0.01 vs TBI+CIT+STD; ^p<0.05 for CD and R2 vs SD and ID; ^p<0.05 for R1, ED, R3 vs all other stages. n=6–10/group.
3.3.3. Total errors
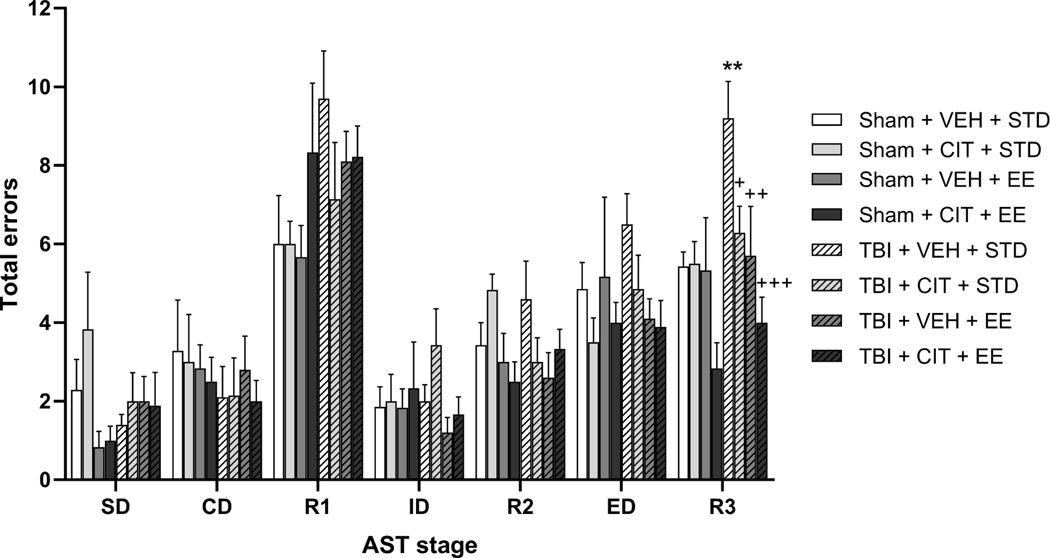
Fig. 4 depicts the effects of CCI, chronic CIT treatment, and EE housing on the total errors for each test stage at 4 weeks post-surgery. A repeated measures multivariate ANOVA (Injury × Drug × Housing × Stage) revealed significant main effects of Housing (F(1,53)= 9.684, p<0.01), Stage (F(6, 318)= 44.235, p<0.0001), as well as significant Surgery × Housing (F(1,53)= 8.652, p<0.01), Stage × Housing (F(6, 318)= 2.313, p<0.05), and Stage × Surgery × Drug (F(6, 318)= 3.339, p<0.01) interactions. Post-hoc one way ANOVA comparisons for individual stages revealed significant group effects on the third reversal (R3) stage (F(7,53)= 4.333, p<0.001). Post-hoc analyses revealed that TBI+VEH+STD rats displayed significantly more errors than Sham+VEH+STD rats while completing the third reversal stage (p<0.01). Furthermore, CIT-treated injured rats (TBI+CIT+STD) performed significantly better than their VEH-treated counterparts (p<0.05), while EE-housed injured rats (TBI+VEH+EE) performed better than their STD-housed counterparts (p<0.01). The combined treatment group also displayed significantly less total errors than the TBI+VEH+STD group (p<0.001) by reducing the number of total errors to a level comparable to Sham groups, although there was only a trend towards statistical differences compared to an individual treatment (p=0.08 compared with TBI+CIT+STD). No other main effects or interactions were revealed on any other test stage.
Fig. 4.
Mean (± S.E.M.) total response error rates on AST. CCI significantly increased the total errors on R3 as compared to Sham+VEH+STD rats. Therapies given individually as well as the combined treatment provided complete and robust cognitive recovery on the R3, with a trend for the combined paradigm to reach statistical significance as compared to TBI+CIT+STD (p=0.08). *p<0.05 vs Sham+VEH+STD; +p<0.05, ++p<0.01, +++p<0.001 vs TBI+VEH+STD. n=6–10/group.
3.3.4. Set loss errors
Set loss errors (Table 1) for each rat on every stage were calculated as the errors occurring after 50% or more of the contingency acquisition has been achieved (i.e., an error after 3, 4, or 5 correct choices) (Bondi et al., 2014a; Lapiz et al., 2007; Njoku et al., 2019). A repeated measures multivariate ANOVA (Injury × Drug × Housing × Stage) revealed significant main effects of Drug (F(1,53)= 12.498, p<0.001) Housing (F(1,53)= 6.676, p<0.05), Stage (F(6, 318)= 6.767, p<0.0001), and a significant Surgery × Stage interaction (F(6, 318)= 2.196, p<0.05). Post-hoc one-way ANOVA comparisons for individual stages revealed significant group effects on first reversal (F(7,53)= 3.21, p<0.01), ED set-shifting (F(7,53)= 2.912, p<0.05), and third reversal (F(7,53)= 4.011, p<0.01) stages. Post-hoc analyses showed that EE paradoxically increased set-loss errors on R1 in Sham+VEH+EE rats compared to all other Sham groups (p<0.01), although trials to criterion and total errors were similar among all control groups (Figs. 3 and 4). On the ED stage, significantly more set loss errors were made by the TBI+STD+VEH group as compared to Sham+VEH+EE (p<0.05), Sham+CIT+STD, and Sham+CIT+EE (p’s<0.01), but not Sham+VEH+STD. CIT and EE significantly reduced set-loss errors in TBI rats (p<0.05) when administered alone, while the combined treatment led to a more pronounced reduction compared to TBI+VEH+STD rats (p<0.001), although it was not statistically different than individual treatments. On the R3 stage, TBI+VEH+STD rats displayed more set loss errors than Sham+VEH+STD rats (p<0.01). Additionally, CIT and EE alone reduced set-loss errors in TBI rats (p<0.01), while the combined paradigm facilitated stage completion without any set-loss errors in injured rats, thus providing significant improvements compared to TBI+VEH+STD (p<0.001) rats and to the EE group alone (TBI+VEH+EE, p<0.05). No other main effects or interactions were revealed on any other stages.
Table 1.
Mean set loss errors across AST stages, mirroring error analysis in the Wisconsin Card Sorting Test (i.e. after 50% or more of the criterion of six consecutive responses has been achieved). EE paradoxically increased set-loss errors on R1 in Sham+VEH+EE rats compared to all other Sham groups (bp<0.01), although trials to criterion and total errors were similar among all control groups (Figs. 3 and 4). More set loss errors were made by the TBI+STD+VEH on the ED stage as compared to Sham+VEH+EE (ap<0.05), Sham+CIT+STD, and Sham+CIT+EE (both bp<0.01), but not Sham+VEH+STD. CIT and EE reduced set-loss errors in TBI rats (cp<0.05) when given individually, while the combined treatment led to a more pronounced reduction compared to TBI+VEH+STD rats (ep<0.001). On the R3 stage, TBI+VEH+STD rats displayed more set loss errors than Sham+VEH+STD rats (bp<0.01). Additionally, CIT and EE alone reduced set-loss errors in TBI rats (dp<0.01), while the combined paradigm rendered injured rats to complete the stage without set-loss errors, thus providing significant improvements compared to TBI+VEH+STD (ep<0.001) and TBI+VEH+EE (fp<0.05) groups.
| Group | SD | CD | R1 | ID | R2 | ED | R3 |
|---|---|---|---|---|---|---|---|
| Sham+VEH+STD | 0.286 | 0.429 | 0.429 | 0.000 | 0.286 | 0.571 | 0.429 |
| Sham+CIT+STD | 0.167 | 0.333 | 0.167 | 0.000 | 0.167 | 0.167 | 0.667 |
| Sham+VEH+EE | 0.000 | 0.667 | 1.500 b | 0.000 | 0.000 | 0.333 | 0.500 |
| Sham+CIT+EE | 0.000 | 0.667 | 0.167 | 0.000 | 0.167 | 0.167 | 0.167 |
| TBI+VEH+STD | 0.400 | 0.400 | 0.400 | 0.100 | 0.600 | 1.100 a,b | 1.200 b |
| TBI+CIT+STD | 0.286 | 0.429 | 0.857 | 0.286 | 0.286 | 0.429 c | 0.429 d |
| TBI+VEH+EE | 0.200 | 0.300 | 0.400 | 0.100 | 0.500 | 0.500 c | 0.500 d |
| TBI+CIT+EE | 0.000 | 0.556 | 0.500 | 0.333 | 0.333 | 0.333 e | 0.000 e,f |
3.4. Histology: Cortical lesion volume
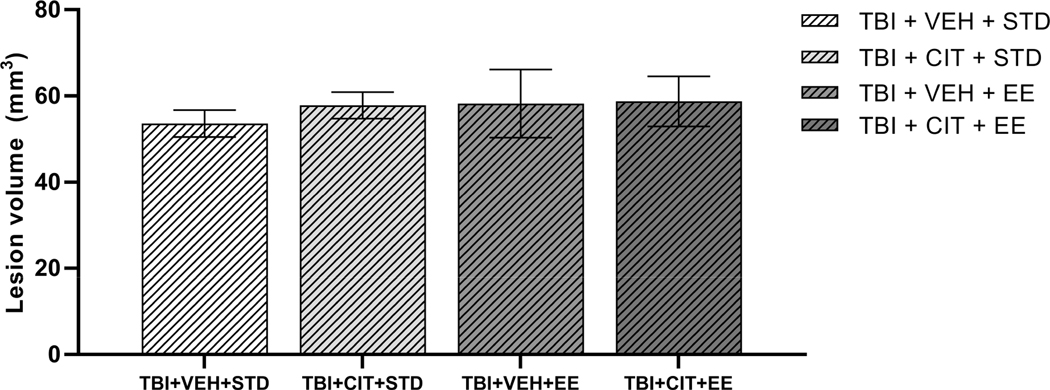
At 5 weeks post-surgery, mean cortical lesion volume in the TBI+VEH+STD group was 53.6 mm3, in line with previous reports (Bondi et al., 2014a), where the injured group (2.8 mm deformation depth) had a 49.9 mm3 average lesion volume. Furthermore, a one-way ANOVA found there was no significant difference among lesion sizes for the various TBI groups in this study (F(3, 15)= 0.225, p=0.877, Fig. 5).
Fig. 5.
Mean (± S.E.M.) cortical lesion volumes (mm3) 5 weeks after controlled cortical impact (CCI) injury at a cortical deformation depth of 2.8 mm. There is no significant difference between different TBI groups post injury and treatments. n=4–5/group.
4. Discussion
In this study, we assessed whether the chronic administration of the antidepressant drug, CIT, a well-validated rodent model of neurorehabilitation, EE, or the combination of both therapies would attenuate TBI-induced executive function deficits measured by the AST. We found that, in replication of our previous findings (Bondi et al., 2014a; Njoku et al., 2019), CCI resulted in attentional set-shifting and behavioral flexibility impairments, as indicated by increased trials to reach criterion, higher total errors, and increased set loss errors on the AST. Moreover, in support of our hypotheses, administering CIT or EE as monotherapies rendered significant improvements on various components of the AST post-injury. Further, the combined treatment paradigm conferred the most robust recovery of cognitive function post-CCI in rats.
Birrell and Brown developed this analogous behavioral test to measure attentional set shifting performance in rats, and demonstrated the pivotal role played by the rodent PFC in mediating complex cognitive processes, such as attentional set-shifting and stimulus reversal learning (Birrell and Brown, 2000). Rodents are required to form and maintain an attentional set, or shift from one stimulus dimension to another, which previously served as an overlapping distractor (Birrell and Brown, 2000; Bondi et al., 2014a; Bondi et al., 2008; Lapiz and Morilak, 2006). Associations with the food reward must be made between the salient stimulus dimensions, which are associations based on predetermined pseudorandomized order of stimulus presentation and overlapping secondary dimension distractors, while facing rule changes as in the WCST upon learning the contingency (Bondi et al., 2014a; Bondi et al., 2008; Njoku et al., 2019). Tests that measure higher order cognitive processes, specifically executive function, cognitive flexibility, and other impairments indicative of frontal lobe dysfunction, are a necessary addition to the behavioral arsenal utilized in TBI laboratories.
For these experiments, the impactor was placed over the right-parietal lobe, thus resulting in a focal displacement of the underlying neural tissue and initiating a series of pathophysiological cascades that lead to further cell death and dysfunction in adjacent brain areas. In the rat brain, this region lies directly over the dorsal hippocampus and behind the PFC. Previous work from our group has reported that this injury model leads to significant and quantifiable executive function deficits measured by the AST (Bondi et al., 2014a; Njoku et al., 2019). Executive function is thought to be a distributed neural modulatory system that involves many brain regions including the ventral hippocampus, medial PFC, orbitofrontal cortex (OFC), nucleus accumbens, basal forebrain, and the mediodorsal nucleus of the thalamus (Brooks et al., 2012; Floresco et al., 2009; Tseng et al., 2009). Further, numerous studies have attributed specific AST performance deficits to damage in several of these regions (Clarke et al., 2007; Floresco et al., 2009; McAlonan and Brown, 2003).
In addition to executive function deficits, the risk for developing MDD following TBI remains elevated from 1 year post-injury (Jorge et al., 2004) to decades later (Anstey et al., 2004). MDD is often treated by SSRIs such as CIT. In fact, clinical studies have demonstrated that patients prescribed CIT for MDD following a mild to moderate TBI, showed statistically significant reduction of depressive symptoms (Rapoport et al., 2008). Given the link between development of MDD following mild-moderate TBI and cognitive impairment (Rapoport et al., 2005), the improvements seen on AST in injured groups that received CIT could be due, in part, to a decrease in depressive symptoms that were affecting cognitive performance. Alternatively, serotonin (5-HT) transmission impacts behaviors relevant to cognitive flexibility through both direct influences over impulse control (Robbins, 2000) and indirect influences through the modulation of the dopaminergic and noradrenergic systems (Hatcher et al., 2005; Rodefer et al., 2008). Importantly, each monoamine neurotransmitter has an overall modulatory effect within the PFC and their synergetic interactions lead to normal PFC function and disruption leads to PFC dysfunction (Morilak and Frazer, 2004). There is a body of literature using AST to measure the cognitive effects following the administration of pharmacological agents in various pathologies. Attentional set-shifting involves dopamine (DA) (Egerton et al., 2005; Rodefer et al., 2005) and norepinephrine (NE) innervations in the mPFC (Merriam et al., 1999; Tait et al., 2007). Furthermore, the OFC role in reversal learning is most likely mediated through 5-HT (Anstey et al., 2004). CIT was previously reported to improve reversal learning in rats exposed to chronic intermittent cold stress (Lapiz-Bluhm et al., 2010; Furr et al., 2012), an effect due at least in part to modulatory influence of 5-HT2A receptors in the OFC (Furr et al., 2012). Reduction or destruction of cortical serotonergic projections also causes deficits in reversal learning (Clarke et al., 2007; McAlonan and Brown, 2003). In this study, TBI+VEH+STD rats made more set loss errors on the ED and R3 as compared to TBI groups that received CIT treatment; we can speculate that increased serotonergic activity may have improved executive functioning.
Like CIT, EE alone resulted in significant benefits in the R3 and ED stages of the AST. In a study by Saland and Rodefer (2011), EE resulted in similar improvements on the reversal and ED stages in enriched rats in a model of schizophrenia using phencyclidine-induced behavioral deficits. Following injury, both the neurobehavioral and neuroanatomical benefits of EE are wide-ranging as are the mechanisms implicated as underlying the reported benefits. In fact, numerous studies have indicated that exposure to EE following TBI produces significant improvements in motor deficits and spatial learning for both male and female rats (Bondi et al., 2014b; de la Tremblaye et al., 2017; Hicks et al., 2002; Kline et al., 2010; Passineau et al., 2001; Radabaugh et al., 2016; Radabaugh et al., 2017). Our current findings further support EE as a therapy capable of producing neurobehavioral benefits, specifically in reversal learning, following CCI injury.
Also common in EE exposure are histological changes that include changes to neuroanatomy such as increased brain weight, dendritic arborization, and cortical and hippocampal synaptogenesis (Frick and Fernandez, 2003; Lajud et al., 2019; van Praag et al., 2000). More work will be warranted to further determine the extent to which these mechanisms may be linked to improved higher-order cognition. The lesion volumes analysis of this study showed no significant differences between the enriched and standard protocols. The disconnect between behavior and histology has been seen in prior studies (Lippert-Gruener et al., 2007). However, EE typically shows decreases in lesion volume size (Maegele et al., 2005; Monaco et al., 2014; Monaco et al., 2013). Our current findings may have been resulted from terminating the enrichment process at three weeks after injury in order to begin the AST protocol which called for mild food restriction. Testing occurred one week after this housing shift, with sacrifice being performed at five weeks post-surgery, after all rats finished testing. This led to 14 days of additional STD housing for the EE groups. It is feasible to assume that secondary damage processes were accelerated during this period in STD housing or that the metabolic demands of the healing brain were not met during food restriction. Further studies must be performed to better understand the impact that the termination of EE and mild food restriction have on tissue recovery following CCI.
Interestingly, on the R3 stage, sham control rats that received the combined treatment of CIT and EE required significantly less trials than sham rats that received individual treatments. Typically, improvements on the AST do not occur in shams given that they already perform proficiently. This effect suggests that for reversal learning, the combined treatment may optimize neurological basis for cognitive flexibility even in a non-injured state given that similar cognitive findings have been noted with early exposure to EE improving spatial learning in both sham and injured rats (Giza et al., 2005). While R1 and R2 were not affected by TBI or treatments for either total trials or errors, the results for R3 were similar aside from lack of additive treatment effects for total errors. ED set-shifting did not render differences on total errors and this type of dissimilar findings between outcome measures has been reported before by others (Neuwirth et al., 2019) and ourselves (Bondi et al., 2014), but it is not considered to detract from overall interpretations. However, a paradoxical effect of set loss errors was observed on the R1 stage with uninjured and drug-free rats (Sham+EE+VEH) making more set loss errors on the R1 compared to TBI+EE+VEH and TBI+STD+VEH. This effect occurred without any differences in total trials to criterion or total errors being distinguishable among the Sham groups. Given the effort put forth to minimize the number of rats subjected to animal research, we limited the number of rats in the sham groups to the minimum number reported in the literature and appropriately powered as sufficient to highlight group effects in the AST task (i.e., n=6–7) (Bondi et al., 2014a; Bondi et al., 2010). These uncommon results in the sham groups may be an artifact of the small group numbers.
Given the inherent complexity of TBI, it is unlikely that a “silver-bullet” treatment affording full recovery will be identified. It is therefore urgent that combinations of treatments, such as the one tested in these experiments (CIT + EE), that may lead to additive benefits greater than any monotherapy continue to be evaluated (Kline et al., 2016). Furthermore, treatments that have previously been identified as effective in the laboratory have failed to be translated to the clinic. This may be due, in part, to a failure to measure executive function deficits in the laboratory given their high incidence in the patient population. Our results show that both CIT and EE result in significant executive function benefits when administered alone. Furthermore, combining the treatments showed the greatest improvement in reversal learning. Ongoing studies are working to identify molecular markers relevant to dysfunction in discrete brain regions involved in AST and to further characterize behavioral paradigms that can better quantify deficits of cognitive flexibility in the preclinical setting.
Highlights:
Chronic citalopram restored traumatic brain injury-induced attentional deficits
Continuous enriched environment also improved executive function after brain trauma
Combined drug and rehabilitation paradigm augmented reversal learning recovery
Neither treatment managed to reduce lesion volumes despite behavioral improvements
Acknowledgements
This work was supported by the National Institutes of Health (grants NS095950, NS099683, NS110609), and University of Pittsburgh Physicians/University of Pittsburgh Medical Center Academic Foundation, and University of Pittsburgh Rehabilitation Institute (C.O.B.).
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anstey KJ, Butterworth P, Jorm AF, Christensen H, Rodgers B, Windsor TD, 2004. A population survey found an association between self-reports of traumatic brain injury and increased psychiatric symptoms. J Clin Epidemiol 57, 1202–1209. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ, 2000. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Cheng JP, Tennant HM, Monaco CM, Kline AE, 2014a. Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. Journal of neurotrauma 31, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA, 2010. Beneficial effects of desipramine on cognitive function of hopharmacol Biol Psychiatry 34, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Klitsch KC, Leary JB, Kline AE, 2014b. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. Journal of neurotrauma 31, 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA, 2008. Chronic Unpredictable Stress Induces a Cognitive Deficit and Anxiety-Like Behavior in Rats that is Prevented by Chronic Antidepressant Drug Treatment. Neuropsychopharmacology 33, 320–331. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Pershing ML, Thomsen MS, Mikkelsen JD, Sarter M, Bruno JP, 2012. Transient inactivation of the neonatal ventral hippocampus impairs attentional set-shifting behavior: reversal with an α7 nicotinic agonist. Neuropsychopharmacology 37, 2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE, 2012. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. Journal of neurotrauma 29, 2684–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC, 2007. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex 17, 18–27. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm DMS, Morilak DA, 2010. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. Int J Neuropsychopharmacol 13, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Tremblaye PB, Bondi CO, Lajud N, Cheng JP, Radabaugh HL, Kline AE, 2017. Galantamine and Environmental Enrichment Enhance Cognitive Recovery after Experimental Traumatic Brain Injury But Do Not Confer Additional Benefits When Combined. Journal of neurotrauma 34, 1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, C PS, Miller TR, 2007. Incidence and economic burden of injuries in the United States. J Epidemiol Community Health 61, 926–926. [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA, 2005. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology (Berl) 179, 77–84. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T, 2009. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res 204, 396–409. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, 2003. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging 24, 615–626. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA, 2012. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. Int J Neuropsychopharmacol 15, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, 2004. Ecological Assessment of Executive Function in Traumatic Brain Injury. Developmental Neuropsychology 25, 135–158. [DOI] [PubMed] [Google Scholar]
- Giza CC, Griesbach GS, Hovda DA, 2005. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res 157, 11–22. [DOI] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DN, 2005. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology (Berl) 181, 253–259. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Zhang L, Atkinson A, Stevenon M, Veneracion M, Seroogy KB, 2002. Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience 112, 631–637. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Malena RR, Westergom BP, Luthra P, Cheng JP, Aslam HA, Zafonte RD, Kline AE, 2008. Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci Lett 431, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt S, 2004. Major depression following traumatic brain injury. Arch Gen Psychiatry 61, 42–50. [DOI] [PubMed] [Google Scholar]
- Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO, 2016. Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Progress in neurobiology 142, 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, McAloon RL, Henderson KA, Bansal UK, Ganti BM, Ahmed RH, Gibbs RB, Sozda CN, 2010. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. Journal of neurotrauma 27, 2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP, 2012. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. Journal of neurotrauma 29, 1960–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Yu J, Massucci JL, Zafonte RD, Dixon CE, 2002. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci Lett 333, 179–182. [DOI] [PubMed] [Google Scholar]
- Lajud N, Díaz-Chávez A, Radabaugh HL, Cheng JP, Rojo-Soto G, Valdéz-Alarcón JJ, Bondi CO, Kline AE, 2019. Delayed and Abbreviated Environmental Enrichment after Brain Trauma Promotes Motor and Cognitive Recovery That Is Not Contingent on Increased Neurogenesis. Journal of neurotrauma 36, 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM, 2006. The Epidemiology and Impact of Traumatic Brain Injury: A Brief Overview. The Journal of Head Trauma Rehabilitation 21. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Bondi CO, Morilak DA, 2007. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology 32, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA, 2006. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Lippert-Gruener M, Maegele M, Garbe J, Angelov DN, 2007. Late effects of enriched environment (EE) plus multimodal early onset stimulation (MEOS) after traumatic brain injury in rats: Ongoing improvement of neuromotor function despite sustained volume of the CNS lesion. Experimental neurology 203, 82–94. [DOI] [PubMed] [Google Scholar]
- Luo L, Chai Y, Jiang R, Chen X, Yan T, 2015. Cortisol Supplement Combined with Psychotherapy and Citalopram Improves Depression Outcomes in Patients with Hypocortisolism after Traumatic Brain Injury. Aging Dis 6, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegele M, Lippert-Gruener M, Ester-Bode T, Garbe J, Bouillon B, Neugebauer E, Klug N, Lefering R, Neiss WF, Angelov DN, 2005. Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. Eur J Neurosci 21, 2406–2418. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ, 2003. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146, 97–103. [DOI] [PubMed] [Google Scholar]
- Meconi A, Wortman RC, Wright DK, Neale KJ, Clarkson M, Shultz SR, Christie BR, 2018. Repeated mild traumatic brain injury can cause acute neurologic impairment without overt structural damage in juvenile rats. PLOS ONE 13, e0197187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA, 1999. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry 156, 780–782. [DOI] [PubMed] [Google Scholar]
- Monaco CM, Gebhardt KM, Chlebowski SM, Shaw KE, Cheng JP, Henchir JJ, Zupa MF, Kline AE, 2014. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. Journal of neurotrauma 31, 1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco CM, Mattiola VV, Folweiler KA, Tay JK, Yelleswarapu NK, Curatolo LM, Matter AM, Cheng JP, Kline AE, 2013. Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Experimental neurology 247, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Frazer A, 2004. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 7, 193–218. [DOI] [PubMed] [Google Scholar]
- Neuwirth LS, Kim Y, Barrerra ED, Jo C, Chrisphonte JM, Hameed N, Rubi S, Dacius TF Jr, Skeen JC, Bonitto JR, Khairi E, Iqbal A, Ahmed I, Masood S, Tranquilee B, Thiruverkadu V, 2019. Early neurodevelopmental exposure to low lead levels induces fronto-executive dysfunctions that are recovered by taurine co-treatment in the rat attention set-shift test: implications for taurine as a psychopharmacotherapy against neurotoxicants. Advances in Experimental Medicine and Biology 1155, 821–846. [DOI] [PubMed] [Google Scholar]
- Njoku I, Radabaugh HL, Nicholas MA, Kutash LA, O’Neil DA, Marshall IP, Cheng JP, Kline AE, Bondi CO, 2019. Chronic treatment with galantamine rescues reversal learning in an attentional set-shifting test after experimental brain trauma. Experimental neurology 315, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passineau MJ, Green EJ, Dietrich WD, 2001. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Experimental neurology 168, 373–384. [DOI] [PubMed] [Google Scholar]
- Radabaugh H, Bonnell J, Schwartz O, Sarkar D, Dietrich WD, Bramlett HM, 2020. Operation Brain Trauma Therapy (OBTT): the use of machine learning to re-assess patterns of multivariate functional recovery following fluid percussion injury. Journal of neurotrauma. doi: 10.1089/neu.2020.7357 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh HL, Carlson LJ, O’Neil DA, LaPorte MJ, Monaco CM, Cheng JP, de la Tremblaye PB, Lajud N, Bondi CO, Kline AE, 2016. Abbreviated environmental enrichment confers neurobehavioral, cognitive, and histological benefits in brain-injured female rats. Experimental neurology 286, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh HL, LaPorte MJ, Greene AM, Bondi CO, Lajud N, Kline AE, 2017. Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury. Experimental neurology 294, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport MJ, Chan F, Lanctot K, Herrmann N, McCullagh S, Feinstein A, 2008. An open-label study of citalopram for major depression following traumatic brain injury. J Psychopharmacol 22, 860–864. [DOI] [PubMed] [Google Scholar]
- Rapoport MJ, McCullagh S, Shammi P, Feinstein A, 2005. Cognitive impairment associated with major depression following mild and moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci 17, 61–65. [DOI] [PubMed] [Google Scholar]
- Robbins TW, 2000. From arousal to cognition: the integrative position of the prefrontal cortex. Prog Brain Res 126, 469–483. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Murphy ER, Baxter MG, 2005. PDE10A inhibition reverses subchronic PCP-induced deficits in attentional set-shifting in rats. Eur J Neurosci 21, 1070–1076. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J, 2008. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology 33, 2657–2666. [DOI] [PubMed] [Google Scholar]
- Saland SK, Rodefer JS, 2011. Environmental enrichment ameliorates phencyclidine-induced cognitive deficits. Pharmacol Biochem Behav 98, 455–461. [DOI] [PubMed] [Google Scholar]
- Shaw KE, Bondi CO, Light SH, Massimino LA, McAloon RL, Monaco CM, Kline AE, 2013. Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. Journal of neurotrauma 30, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE, 2010. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. Journal of neurotrauma 27, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D, 2000. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38, 388–402. [DOI] [PubMed] [Google Scholar]
- Tadepalli SA, Bali ZK, Bruszt N, Nagy LV, Amrein K, Fazekas B, Büki A, Czeiter E, Hernádi I, 2020. Long-term cognitive impairment without diffuse axonal injury following repetitive mild traumatic brain injury in rats. Behavioural Brain Research 378, 112268. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW, 2007. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci 25, 3719–3724. [DOI] [PubMed] [Google Scholar]
- Taylor CA BJ, Breiding MJ, Xu L, 2017. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ 66(No. SS-9), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK, 2009. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res 204, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 2000. Neural consequences of environmental enrichment. Nat Rev Neurosci 1, 191–198. [DOI] [PubMed] [Google Scholar]
- Yelleswarapu NK, Tay JK, Fryer WM, Shah MA, Garcia AN, Cheng JP, Kline AE, 2012. Elucidating the role of 5-HT(1A) and 5-HT(7) receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci Lett 515, 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]