Abstract
Sodium dodecyl sulfate (SDS), an alkyl sulfate surfactant derived from an organic alcohol, possesses surfactant properties but also denatures and unfolds both monomeric and subunit proteins. In preliminary experiments, we demonstrated that SDS is a potent inactivator of herpes simplex virus type 2 and human immunodeficiency virus type 1 at concentrations comparable to those used for the surfactant nonoxynol-9. We hypothesized that SDS might be capable of denaturing the capsid proteins of nonenveloped viruses. In this report, we demonstrate inactivation of rabbit, bovine, and human papillomaviruses after brief treatment with dilute solutions of SDS. Effective concentrations were nontoxic to rabbit skin and to split-thickness grafts of human foreskin epithelium. This is the first report of a microbicidal surfactant that will inactivate papillomaviruses. We propose that SDS is now a candidate microbicide for formulation and testing with humans.
One popular approach to the control of transmission of sexually transmitted diseases (STDs) is the use of topically applied, female-controlled microbicides that inactivate the relevant pathogens. Most frequently, these are spermicidal preparations containing nonoxynol-9 (N-9) that inactivate enveloped viruses such as herpes simplex virus type 2 (HSV-2) and human immunodeficiency virus type 1 (HIV-1). To date, these preparations have not been effective against nonenveloped viruses such as the human papillomaviruses (HPVs) (7).
The papillomaviruses (PVs) represent a group of nonenveloped, icosahedral DNA viruses that induce benign neoplasms that can progress to cancers (for reviews, see references 9, 10, and 35). Animal papillomas occur in a large number of species, and studies have developed bovine papillomaviruses (BPVs) and the Shope cottontail rabbit papillomavirus (CRPV) into model systems. HPVs, of which there are now more than 70 types, cause warts in epithelial target tissues. Common warts of the hands (verrucae vulgaris) and feet (plantar warts) and genital condylomata all represent common clinical infections in humans. Genital warts represent a ubiquitous STD, with as many as 25% of women infected by genital HPV types and 1 to 3% of women presenting with clinically apparent warts in the genital tract (for a review, see reference 37). Genital lesions containing HPV types 16, 18, 31, 33, and 35 and others present an increased risk for progression to cervical cancer. The work of Meisels and colleagues (26, 27) clearly indicates that in cervical lesions, benign neoplasms caused by HPVs progress histologically through stages of increasing dysplasia and, without intervention, can progress to carcinoma in situ and frankly invasive carcinoma. In the United States, 15,000 women per year are diagnosed with cervical cancer, and there are about 5,000 deaths per year. In developing countries, this cancer is the number one cause of cancer-related deaths in women, causing 250,000 deaths per year.
Existing microbicides such as N-9, octoxynol-9, benzalkonium chloride, and chlorhexidine are surfactants that can disrupt the envelopes of HSV-2 and HIV-1 via their surfactant and detergent properties. These agents do not inactivate the nonenveloped PVs. A microbicidal agent that would reliably inactivate PVs could be used for prevention of transmission of animal and human virus types. N-9 is a potent inactivator of several enveloped virus types such as HSV-2 and HIV-1 (8, 12, 30). The action of N-9 is attributable to its surfactant and detergent properties on phospholipid membranes and its resultant ability to disrupt enveloped viruses. PVs, however, are not inactivated by conventional microbicidal or spermicidal formulations that include N-9 (7). Topical microbicides for inactivation of the PVs and prevention of animal or human transmission are not available.
The anionic detergent sodium dodecyl sulfate (SDS) also possesses detergent and surfactant properties but will additionally dissociate and denature proteins (16, 31, 40). SDS is effective at very low concentrations compared with many other denaturants. SDS unfolding induces α-helix formation in a number of proteins (11). SDS denaturation of proteins has been used extensively to determine the molecular weight of denatured polypeptides by polyacrylamide gel electrophoresis (25, 43). Because of these properties, we hypothesized that SDS would denature the capsid structures of nonenveloped viruses.
MATERIALS AND METHODS
Chemicals.
SDS was purchased from Bio-Rad (Richmond, Calif.), and filter-sterilized solutions were prepared with phosphate-buffered saline (PBS). N-9 was obtained from Rhone-Poulenc Rorer Pharmaceuticals Inc. (Collegeville, Pa.). C31G was obtained from Biosyn, Inc. (Philadelphia, Pa.). All additional detergents were purchased from Boehringer Mannheim (Indianapolis, Ind.).
HSV-2 inactivation assay.
HSV-2 (strain 333) virus stocks were prepared at a low multiplicity of infection with African Green monkey kidney (CV-1) cells, and subsequently, cell-free supernatants were prepared from frozen and thawed preparations of lytically infected cultures. Virus titers were determined by assay in CV-1 cell monolayers as described previously (1). Virus stocks were maintained in Dulbecco’s medium supplemented with antibiotics and 10% fetal calf serum. The protein concentration of the virus stocks was increased by the cellular proteins released by the freezing and thawing of the infected cells.
For inactivation of HSV-2, 39 μl of virus stock was mixed with 1 μl of a 40×-concentrated solution of detergent, and the mixture was then incubated at 37°C for 10 min. After inactivation, 40 μl of virus sample was diluted to 4 ml with cell culture medium, and 1 ml of the virus was adsorbed onto CV-1 monolayers for 1 h at 37°C. Following adsorption, monolayers were refed and incubated at 37°C in 5% CO2. At between 20 and 24 h postinfection, the monolayers were fixed and stained with crystal violet and the plaques were counted with a dissecting microscope. Each datum in Table 1 represents an average for two plates.
TABLE 1.
Inactivation of HSV-2 infectivity by SDS
| % SDS during treatmenta | Final % SDSb | No. of plaques/platec |
|---|---|---|
| 0 | 0 | 57, 73, 343, 145, 145 |
| 1 × 10−1 | 1 × 10−3 | 0, 0, 0, 0, 0 |
| 5 × 10−2 | 5 × 10−4 | 0, 0, 0, 0, 0 |
| 2.5 × 10−2 | 2.5 × 10−4 | 0, 0, 0, 0, 0 |
| 1.25 × 10−2 | 1.25 × 10−4 | 0, 0, 0, 2, 81 |
| 2.5 × 10−3 | 2.5 × 10−5 | 28, 54, 322, 145, 104 |
Sterile SDS stocks (40 times the treatment concentration) were added to virus aliquots to achieve the treatment concentration. After mixing, the samples were incubated at 37°C for 10 min.
Following SDS treatment, virus stocks were diluted 100-fold and 1-ml aliquots were immediately adsorbed onto CV-1 cells. Plaques were counted after 20 to 24 h of infection. Each number represents an average for two plates.
Data are values from five experiments.
HIV inactivation assay.
One day prior to the assay, HeLa cells expressing CD4 on the surface and β-galactosidase (β-gal) under the control of the HIV-1 long terminal repeat were seeded into 12-well culture dishes at a concentration of 8 × 104 cells per well. A high-titer (107.17 50% tissue culture infective doses/ml) stock of HIV-1 (strain IIIB; Advanced Biotechnologies, Inc., Columbia, Md.) was diluted 1:10 with RPMI 1640 supplemented with 10% fetal bovine serum. To assess viral inactivation by C31G or SDS, 78 μl of diluted virus were mixed with 2 μl of surfactant solution, and the mixture was incubated for 10 min at 37°C. After the inactivation period, the virus and surfactant were diluted with 720 μl of RPMI 1640 supplemented with 10% fetal bovine serum and supplemented with DEAE dextran (final concentration, 20 μg/ml). Aliquots of treated virus (300 μl) were then added to duplicate wells of HeLa cells, and the plates were incubated at 37°C for 2 h. Following virus adsorption, 2 ml of fresh medium (Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 0.1 mg of G418 per ml, and 0.05 mg of hygromycin B per ml) was added to each well. After incubation at 37°C and 5% CO2 for 48 h postinfection, the cells were fixed and stained for β-galactosidase expression as described previously (13).
BPV-1 focus assay.
Cell-free stocks of BPV type 1 (BPV-1) were prepared by extraction (10% [wt/vol]) of epidermal bovine warts in PBS. In order to detect the transforming ability of BPV-1, C127 mouse cells were seeded into T-25 flasks (3 × 105 cells per flask). After 24 h of growth, subconfluent cells were infected with BPV-1. For the positive controls, stock virus (20 μl) was diluted (1:1) with PBS, incubated at 37°C for 10 min, diluted 1:1,000, and then added (100 μl) to the 5 ml of cell culture medium present on the cells. The cells were refed at 24 h and subsequently two times weekly. The presence of morphologically transformed foci was counted after 2 weeks and then again at 3 weeks. This assay was performed as described previously (5).
Virus inactivations were carried out in vitro by the addition of concentrated SDS solutions to the virus stocks (20 μl of virus plus 20 μl of detergent) and subsequent incubation at 37°C for 10 or 30 min, as indicated. Following inactivation, virus was diluted 1:1,000 to lower the detergent concentration, and the preparations were immediately used for infection as described above.
Shope papilloma induction.
Stocks of CRPV were prepared from papillomas generated in wild cottontail rabbits as described previously (20). Virus stocks were cell extracts (10% [wt/vol]) of papillomas in PBS. Shaved dorsal skin was lightly scarified with a razor blade. Virus stocks were used to inoculate domestic cottontail rabbits (Hazelton Research Products, Denver, Pa.); a 40-μl aliquot of virus was dropped onto the surface of four locations on the dorsal skin and was rubbed into the scarified skin with the tip of a 20-gauge needle. The two left sites on each rabbit received untreated virus, and the two right sites received treated virus. Inactivation of either a 10−1 or 10−2 solution of virus stock was accomplished by the addition of concentrated SDS solutions which were 40 times the final indicated concentrations. SDS and virus were incubated at 37°C for 10 min and were then used immediately for inoculation of rabbits. Virus was not further diluted following inactivation; the concentration of SDS present during inactivation and inoculation was 0.05%. Papillomas were first observed to develop in control sites at about 2 weeks after inoculation. The geometric mean diameter (GMD) of all visible lesions was measured and is equal to the cube root of the length times the width times the height of the lesions, as measured in millimeters with calipers.
Human papilloma induction.
Stocks of experimentally generated infectious HPV type 11 (HPV-11) were prepared as described previously (18, 19, 22) and represented 10% (wt/vol) cell extracts of virus in PBS. Undiluted aliquots of virus stocks (39 μl) were mixed with a 40× solution of SDS (1 μl), and the mixture was incubated at 37°C for 10 min and was immediately used to infect split-thickness grafts of newborn human foreskin epithelium. Virus was not subsequently diluted. Control grafts were infected with untreated virus stock. Virus adsorption was for 1 h at 37°C. The concentration of SDS present during the inactivation period and during virus adsorption was 0.05%. Grafts were then transplanted beneath the renal capsule of athymic mice as described previously (21). The animals were maintained in isolator bubbles in the animal colony of the Hershey Medical Center. Three months following infection, the animals were killed, their kidneys were removed, and the xenografts were grossly examined. None of the remaining organs showed any abnormalities. Portions of each graft were immediately fixed in 10% neutral-buffered formalin and were processed by standard histology techniques for staining with hematoxylin and eosin.
A second set of control grafts was exposed only to identical concentrations of SDS and no virus. These grafts were harvested on days 1, 5, 11, and 20 following transplantation in order to follow the viability and growth of the grafts after exposure to SDS.
RESULTS
Inactivation of infectivity of HSV-2 by SDS.
Because it is known that N-9 can effectively inactivate enveloped viruses such as HSV-2, inactivation of this virus by SDS was tested. In five separate experiments, treatment concentrations of SDS as low as 0.0125 to 0.025% were effective in eliminating the ability of the virus to induce plaques in a monolayer of monkey kidney cells (Table 1). Total HSV-2 inactivation was achieved with SDS concentrations of between 0.0125 and 0.025%. These effective concentrations are similar to the concentrations of N-9 needed for the destruction of HSV infectivity (data not shown).
Inactivation of infectivity of HIV-1 by SDS and the amphoteric surfactant C31G.
It is established that N-9 can also inactivate HIV-1. We compared inactivation of HIV-1 by a second surfactant, C31G (3, 4, 41), to that of SDS. High-titer stocks of HIV-1 were incubated with either C31G or SDS and were then assayed on indicator cells expressing CD4 on the surface and β-gal under the control of the HIV-1 long terminal repeat. After 48 h the cells were stained and the number of cells with increased levels of β-gal expression was counted. Both of these surfactants were highly effective in the inactivation of HIV-1 (Table 2). Total inactivation of HIV-1 was achieved with C31G concentrations as low as 0.0125% and with SDS concentrations as low as 0.025%.
TABLE 2.
Inactivation of HIV-1 by treatment with C31G and SDS
| Microbicide and concn (%) during treatment or cell | % Cells expressing β-gal genea | No. of cells counted |
|---|---|---|
| C31G | ||
| 5 × 10−2 (toxic) | 0, 0 | >5 × 104 |
| 2.5 × 10−2 | 0, 0 | >5 × 104 |
| 1.25 × 10−2 | 0, 0 | >5 × 104 |
| 6.25 × 10−3 | 19 ± 6.1, 19 ± 6.4 | 1,080, 805 |
| 2.5 × 10−3 | 22 ± 7.4, 29 ± 8.1 | 1,620, 1,820 |
| SDS | ||
| 5 × 10−2 | 0, 0 | >5 × 104 |
| 2.5 × 10−2 | 0, 0 | >5 × 104 |
| 1.25 × 10−2 | 24 ± 3.2, 24 ± 10 | 2,810, 2,190 |
| 6.25 × 10−3 | 10 ± 1.7, 15 ± 2.1 | 2,390, 2,290 |
| 2.5 × 10−3 | 9 ± 5.5, 11 ± 3.5 | 1,940, 1,910 |
| Mock infected cells | 0, 0 | >5 × 104 |
| HIV-1-infected cells | 17 ± 4.8, 24 ± 5.4 | 2,680, 1,480 |
Data are for duplicate wells. Five random fields of cells were counted in each plate displaying blue cells. Duplicate plates were assayed for each sample; individual numbers are plus or minus the standard deviation within five fields of one plate.
Destruction of ability of BPV-1 to induce morphologically transformed foci in monolayers of C127 mouse cells.
Although SDS could effectively reduce HSV-2 and HIV-1 infectivity, it remained likely that this destruction was mediated by envelope removal. Because PVs are nonenveloped, the possibility remained that SDS would fail to inactivate these viruses. We used BPV-1 as a prototype PV because of its ability to rapidly (within 2 weeks) form multilayered transformed foci in mouse fibroblasts in an in vitro assay. Table 3 describes the results of two separate experiments in which stocks of BPV-1 were incubated at 37°C with various concentrations of SDS (5 to 5 × 10−4%) for either 10 or 30 min, diluted in cell culture medium to lower the SDS concentration (to avoid cell toxicity), and then used to infect C127 cells. Following incubation of control or infected cultures, foci were counted at 14 and 17 days after infection. Results indicate that SDS concentrations as low as 0.05 or 0.005% can totally inactivate BPV-1 transforming ability after treatment of the virus at 37°C for 10 or 30 min, respectively. Inactivation of BPV-1 by the lower concentration of 0.005% after 30 min indicated that inactivation is proportional to time as well as to surfactant concentration. Several other commercially available detergents were tested for possible inactivation of BPV-1. These included N-9, C31G, 3-[(3-choloamidopropyl)-dimethylammonio]-2-hydroxy-1-propane sulfonate, N-dodecyl-N,N-dimethyl-3-ammonio-1-propane sulfonate, 3-[(3-cholamidopropyl)dimethylammonio]-1-propane sulfonate, isotridecylpoly(ethylene-glycolether)n, octanoyl-N-methyl-glucamide, Triton X-100, and Thesit. None of these detergents (at a 1% final concentration) inactivated the morphologic transforming properties of BPV-1 after 10 min of incubation at 37°C.
TABLE 3.
Inhibition of BPV-1 morphologic transformation of C127 cells by SDS treatment of virus
| % SDS during treatmenta | Final % SDSb | No. of foci/platec
|
||
|---|---|---|---|---|
| Expt 1 (day 12) | Expt 2
|
|||
| Day 14 | Day 17 | |||
| 0 | 0 | 266 | 255 | 153 |
| 5 | 5 × 10−3 | 0 | ND | ND |
| 5 × 10−1 | 5 × 10−4 | 0 | ND | ND |
| 5 × 10−2 | 5 × 10−5 | 0 | 0 | 0 |
| 5 × 10−3 | 5 × 10−6 | 0 | 271 | 150 |
| 2.5 × 10−3 | 2.5 × 10−6 | ND | 273 | 162 |
| 5 × 10−4 | 5 × 10−7 | ND | 229 | 151 |
Sterile SDS stocks (40 times the treatment concentration) were added to virus aliquots to achieve the treatment concentration.
Following virus treatment, treated virus stocks were further diluted 1:1,000 in order to dilute the detergent; the cells in all plates remained viable.
In experiment 1, virus and SDS were mixed and incubated at 37°C for 30 min; in experiment 2, virus and SDS were mixed and incubated at 37°C for 10 min. ND, not done. In control plates without BPV-1, no foci appeared.
Effect of SDS inactivation of CRPV on formation of Shope papillomas in rabbits.
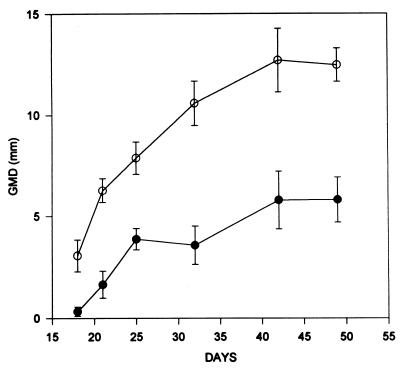
To extend the observation of PV inactivation by SDS to an in vivo animal model system, we used the CRPV model system that is well established in our laboratories. A standard CRPV stock known to form papillomas with 100% efficiency was used. The 50% infectious dose for the virus stock corresponds to 50 μl of a 10−3 dilution of the stock virus. In our experiments, 40 μl of a 10−1 dilution and subsequently 40 μl of a 10−2 dilution of the virus stock solution were used. Both of these concentrations exceeded the 50% infectious dose, by 100- and 10-fold, respectively. SDS was mixed with virus to a final concentration of 0.05%, and the mixture was subsequently incubated at 37°C for 10 min. Immediately following incubation, virus was inoculated by scarification of the skin on the backs of the rabbits. Inoculated sites contained two untreated (left) and two treated (right) virus samples on the same rabbit. Figure 1 demonstrates the average GMD for six lesions inoculated with normal CRPV (10−1 dilution) and six lesions inoculated with SDS-treated CRPV. GMDs were measured and compared on postinoculation days 18, 21, 25, 32, 42, and 50. The results indicate that a 10−1 dilution of virus stock was substantially inactivated by a 10-min, 0.05% SDS treatment at 37°C. It should be noted that the development of papillomas was delayed at each of the six sites that received SDS-treated preparations, indicating a substantial inactivation of virus (data not shown). Once papillomas developed, however, the growth rate of the lesions appeared similar to the ones that developed from the untreated inoculum.
FIG. 1.
Inactivation of CRPV by SDS. Aliquots of CRPV (10−1 dilution) were mixed with concentrated SDS to a final concentration of 0.05% as described in Materials and Methods. Untreated virus (○) or treated virus (•) was inoculated at two sites each per animal, and papilloma production was measured as the GMD of visible lesions on the indicated days. Data represent the average GMD for six lesions resulting from treated virus or from untreated virus inoculation.
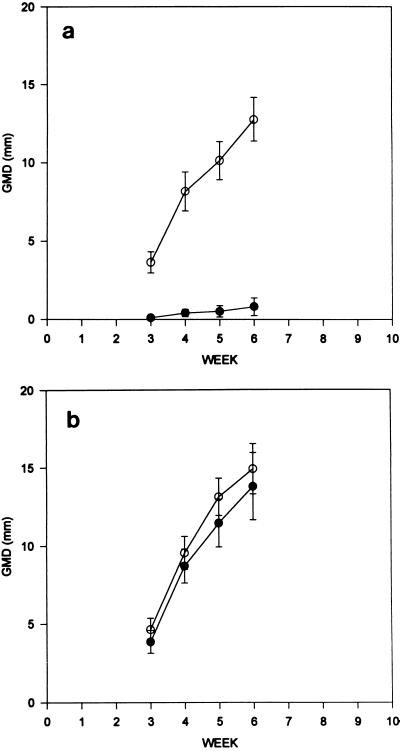
In a subsequent experiment (Fig. 2a and b), a 10−2 dilution of CRPV virus stock was also incubated at 37°C for 10 min with either 0.05% SDS or 0.05% N-9. This dilution of the stock virus not only contained less virus but also contained a lower total protein concentration. Following incubation, detergent-treated and control virus samples were inoculated onto five rabbits for the N-9 samples and five rabbits for the SDS-treated samples. Untreated virus samples were also inoculated onto the same rabbits at different sites. This experiment was undertaken for two purposes: to observe the inactivation of a smaller amount of CRPV by SDS and to directly compare the inactivation achieved by SDS treatment to that achieved by N-9 treatment. As in the previous experiment, the left inoculation sites (two per animal) received untreated virus and the right inoculation sites (two per animal) received treated virus. Figure 2a shows the GMD for 10 inoculation sites that received SDS-treated virus compared to that for 10 inoculation sites that received normal virus. GMDs were measured at 3, 4, 5, and 6 weeks after virus inoculation. At 8 of 10 sites inoculated with SDS-treated virus, papillomas failed to develop; at the remaining 2 sites very small papillomas developed 4 weeks after inoculation. Although quantitative measurements were not performed, the SDS-inoculated sites did not exhibit any irritation during the experiment. Papillomas developed at all 10 sites inoculated with normal CRPV within 2 weeks after inoculation and grew progressively.
FIG. 2.
Inactivation of CRPV by SDS and comparison with inactivation by N-9. Aliquots of CRPV (10−2 dilution) were mixed with concentrated SDS or concentrated N-9 to a final concentration of 0.05% as described in Materials and Methods. Untreated virus (○) or treated virus (•) was inoculated at two sites each per animal, and papilloma production was measured as the GMD of visible lesions on the indicated days. (a) Comparison of results for 10 SDS-treated and 10 untreated virus inoculation sites. (b) Comparison of 10 N-9-treated and 10 untreated virus inoculation sites. Papilloma production was measured as the average GMD of visible lesions on the indicated days.
Figure 2b demonstrates the comparative growth of papillomas at 10 sites that received normal CRPV compared to that at 10 sites that received CRPV treated with N-9. The GMD for each papilloma was measured at 3, 4, 5, and 6 weeks after virus inoculation. There were no differences in lesion growth after inoculation with these two virus preparations. In addition, the growth rates of control and experimental papillomas for the N-9-treated animals did not differ from the growth rates of control lesions for the SDS-treated animals (data not shown).
Effect of SDS inactivation on ability of HPV-11 to induce experimental condylomata in human foreskin epithelial xenografts.
In order to extend the usefulness of SDS inactivation to HPVs, we used a model system developed in our laboratories for the infection and transformation of human epithelial tissues with HPV-11. This system fully recapitulates the life cycle of HPV-11 and produces infectious virions, and the papillomas that develop in the infected tissues are identical in every observable way to the clinical papillomas seen in patients. Prevention of HPV-11 infection of human epithelium in this model system would be highly predictive of prevention of natural HPV infection. Standard stocks of HPV-11 were used as undiluted virus. These virus stocks normally induce condylomata in 90 to 100% of infected xenografts when the stocks are diluted 1:1,000. In this experiment, 39 μl of undiluted HPV-11 stock was mixed with 1 μl of SDS to a final concentration of 0.05% SDS, and the mixture was then incubated at 37°C for 10 min. Infection was then carried out for 1 h and the grafts were subsequently transplanted in vivo. Eight animals (16 kidneys) received grafts infected with SDS-treated virus, and nine animals (17 kidneys) received grafts infected with normal virus. Table 4 shows the results for the harvested grafts. In the animals with untreated HPV-11 infections, 17 of 17 grafts survived, and of these, 14 were transformed morphologically upon histologic examination and had a typical papillomatous appearance. In animals receiving SDS-treated virus, 13 of 16 xenografts showed viable tissue at the time of harvest, and histologic examination of the grafts revealed normal, viable, differentiating human epithelium. We concluded that the SDS had effectively prevented virus infection by inactivation of the virus. The results for animals receiving SDS-treated virus are compatible with our previous observations with uninfected grafts in that normal grafts are occasionally resorbed in the mice and do not survive for three months. This conclusion is based on our observations of hundreds of control foreskin grafts implanted in the renal capsules of athymic mice over a period of more than 10 years. Further studies to more carefully define the toxicity limits of SDS in epithelial xenografts are in progress.
TABLE 4.
Inhibition of HPV-11-induced papillomas in experimental xenografts of human foreskin following SDS treatment of virus
| % SDS during treatmenta | Final % SDS | Total no. of papillomas | No. of surviving grafts/no. of transplanted grafts |
|---|---|---|---|
| 0 | 0 | 14 | 17/17 |
| 0.025 | 0.025 | 0 | 13/16 |
Sterile SDS stocks (40 times the treatment concentration) were added to virus aliquots to achieve the treatment concentration.
Effect of SDS exposure on viability of human foreskin xenografts.
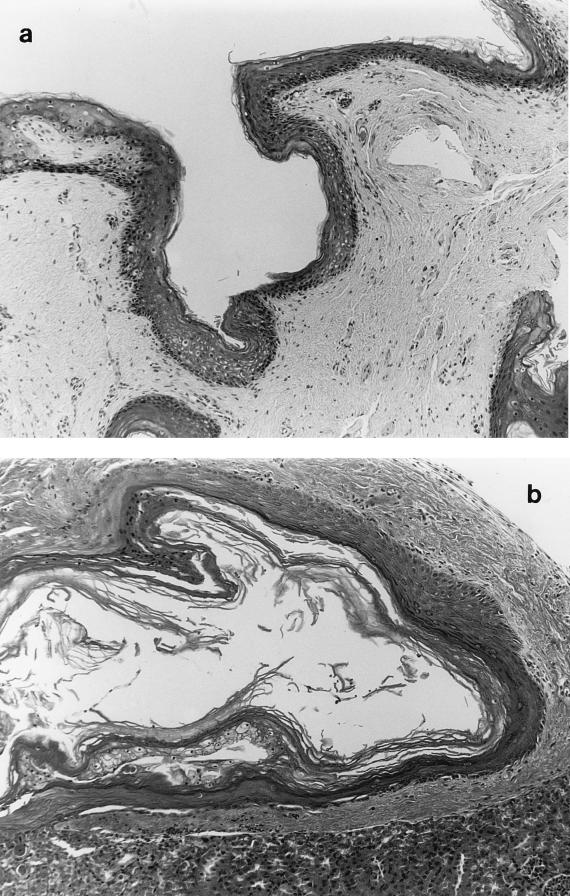
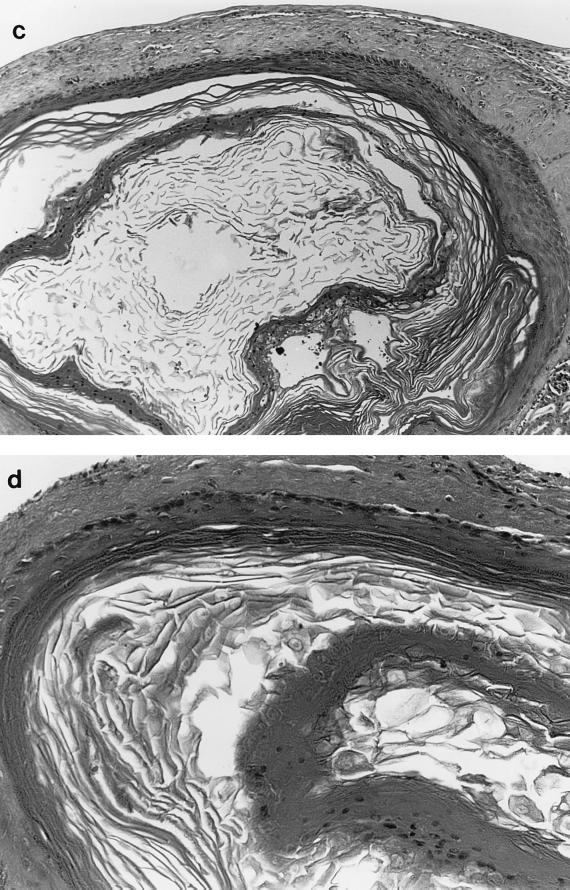
Because of concern about the potential for SDS to kill human epithelium, control experiments were performed. In those experiments split-thickness grafts of neonatal foreskin were exposed to 0.05% SDS alone and were then subsequently grafted. All conditions in this experiment were identical to those used for the HPV-11 infections with treated virus, except that virus was not present. SDS-exposed grafts (two animals at each time) were harvested, fixed, and sectioned immediately after exposure and on days 1, 5, 11, and 20 after treatment. Examination of the tissues demonstrated fully viable epithelium on all days and no apparent necrosis associated with detergent exposure (Fig. 3). The original split-thickness grafts were approximately 1 by 1 by 1 mm; in addition, they were punctured many times with the tip of a needle in order to allow entrance of the HPV-11 and/or the SDS into the epithelial layers. These punctures can be seen in Fig. 3a. Although it is possible that some epithelial cells may have been damaged or killed during SDS exposure, damage was minimal and epithelial growth in the grafts was normal.
FIG. 3.
Human foreskin epithelium xenografts grown in the renal capsule of athymic mice following in vitro exposure to SDS. Human foreskin xenografts were exposed to SDS as described in the text. The day 0 graft was not transplanted; this tissue was fixed in 10% buffered formalin immediately following SDS exposure. All other samples were grafted to the renal capsule of athymic mice immediately after exposure to detergent and were harvested on the following days: 0 (a), 5 (b), 11 (c), and 20 (d). Hematoxylin and eosin stain was used. Magnification, ×100.
DISCUSSION
Several studies have reported that N-9 can inactivate HSV-2 and HIV-1 under defined conditions (8, 12, 30). However, an inability to inactivate PVs makes N-9 an inadequate virucide for the prevention of PV transmission. In addition, chronic use of N-9 was recently associated with increased seroconversion for positivity for HIV-1 antibodies in a group of prostitutes, raising the possibility that N-9 may erode and therefore expose vaginal epithelium (24). Frequent use of N-9 has been positively correlated with bacterial vaginosis (28), genital ulcers and vulvitis (24), vaginal candidiasis (33), toxic shock syndrome (34), and epithelial disruption of the cervix and vagina (29, 32). A recent study indicated that the use of condoms containing N-9 more than once per week increases a woman’s odds of a urinary tract infection by more than threefold (6). However, N-9 is the prevalent microbicide and spermicide in a large number of commercially available products, and there is strong experimental evidence of its virucidal activity in vitro against enveloped viruses. Weir and colleagues (44) reported that N-9 use was not associated with genital ulcers and may have been protective against the formation of lesions. In this study the frequency of administration or dose of N-9 may have been below that which would have caused a risk of ulceration. Experiments to identify additional microbicides were undertaken in our laboratories with the specific goal of extending microbicidal activity to the PV group.
We found that SDS is a potent virucide with activity against the PVs as well as against HSV-2 and HIV-1. In the experiments presented in this paper, very low concentrations of SDS completely inactivated HSV-2 and HIV-1, as well as three separate PV types, after brief exposures to surfactant at physiologic temperatures. In all cases, 0.1% concentrations were well above those exhibiting complete inactivation of all the microbes tested. Further formulation studies are needed to determine the effective concentrations of SDS for topical application in humans. Studies are in progress to examine the ability of SDS to inactivate additional agents, including those causing other genital infections.
Early studies of the interactions of detergents and animal viruses were directed to the preparation of tissue- or allantoic fluid-derived suspensions of virus for possible use as vaccine preparations. Several commercial anionic and cationic detergents, as well as soaps, could inactivate the enveloped influenza virus (2, 14, 15, 39). Inactivation was dependent on the detergent used and the ratio of virus to detergent. Influenza virus that had been purified by ultracentrifugation was also inactivated (17). Vaccinia virus, an enveloped pox virus, was also inactivated by SDS and other detergents (15, 36), and the electrophoretic mobility of the elementary bodies of vaccinia virus was altered after exposure to Duponol, a mixture of homologues of SDS (36). Detergents have also been shown to inactivate lymphocytic choriomeningitis virus of mice, an enveloped arenavirus (38).
Disruption of the envelope of the viruses described above via the surfactant properties of SDS would be sufficient to destroy their infectivity. In our experiments, the PVs represent a group of nonenveloped viruses that are not destroyed by a wide range of surfactants that are capable of destroying the infectivity of enveloped viruses. We attribute the effectiveness of SDS to its denaturing capability and recognize that when enveloped viruses are inactivated with SDS, both envelope disruption and denaturation of virus structural proteins are occurring simultaneously.
SDS is of low intrinsic toxicity both to skin and to mucous membranes. Preparations such as shampoos and detergents that contact both skin and mucous membranes contain dodecyl sulfate derivatives (sodium or ammonium dodecyl sulfate) at concentrations exceeding 10%. In addition, products that are routinely used in the oral cavity, such as toothpaste, have very high (5 to 8%) concentrations of these compounds and apparently are not acutely toxic to the oral mucosa.
Walker and colleagues (42) examined the low order of toxicity of SDS in a study comparing detergent alcohols derived from natural sources with alcohol-derived synthetic surfactants. Acute oral toxicity studies were performed with groups of five male and five female rats (weight range, 150 to 250 g). Animals that were fasted overnight received a single intragastric dose of SDS and were subsequently fed and watered ad libitum for a 10-day observation period. The acute oral 50% lethal dose was 1,288 mg/kg of body weight (95% confidence limits), a dose corresponding to 0.1288% of total body weight. Thirteen-week feeding studies were also performed. Groups of 12 male and 12 female, individually caged rats (age, 5 weeks) were fed dietary levels of SDS ranging from 40 to 5,000 ppm of active material. The health, behavior, body weight, and food intake, as well as hematological (hemoglobin and packed cell volume) and urinary findings, for animals with SDS-supplemented diets remained unchanged over the course of 13 weeks. SDS did not affect the organ weights of male animals in any of the groups of animals, while a slight increase in liver weight was observed in female animals with the highest dose (5,000 ppm). This absolute organ weight increase was statistically significant (P < 0.05), but corresponding increases in relative organ weights were not statistically significant due to a nonsignificant increase in body weights. Additionally, at autopsy, no SDS-associated pathological changes were observed.
In our studies, effective concentrations of SDS were nontoxic to rabbit skin and human newborn foreskin. Further studies, however, are in progress to examine the interaction of SDS with dissociated and intact vaginal epithelium. Models for examination of potential toxicity for both the rabbit and the human vagina exist in our laboratories.
We propose that SDS is now a candidate microbicide for formulation and testing with humans. Because cervical cancer is the number one cause of cancer-related mortality in women in developing countries, effective prevention of HPV transmission should have a significant impact on world health.
ACKNOWLEDGMENTS
The work reported here was supported by program project grant PHS 1 PO1 AI37829 and by funds from the Jake Gittlen Memorial Golf Tournament. HeLa cells expressing CD4 on the surface and β-gal under the control of the HIV-1 long terminal repeat were obtained through Michael Emerman of the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Adelman S F, Howett M K, Rapp F. Quantification of plasminogen activator activity associated with herpesvirus-transformed cells. J Gen Virol. 1980;50:101–110. doi: 10.1099/0022-1317-50-1-101. [DOI] [PubMed] [Google Scholar]
- 2.Burnet F M, Lush D. The action of certain surface-active agents on viruses. Aust J Exp Biol Med Sci. 1940;18:141–150. [Google Scholar]
- 3.Calis S, Yulug N, Summu M, Ayhan A, Hincal A A. A non-antibiotic antimicrobial mixture (C31G): evaluation of the antimicrobial efficiency of C31G on vaginal cultures. Boll Chim Farmaceut. 1992;131:335–338. [PubMed] [Google Scholar]
- 4.Corner A M, Dolan M M, Yankell S L, Malamud D. C31G, a new agent for oral use with potent antimicrobial and antiadherence properties. Antimicrob Agents Chemother. 1988;32:350–353. doi: 10.1128/aac.32.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvoretzky I, Shober R, Chattopadhyay S K, Lowy D R. A quantitative in vitro focus assay for bovine papillomavirus. Virology. 1980;103:369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- 6.Fihn S D, Boyko E J, Normand E H, Chen C, Grafton J R, Hunt M, Yarbro P, Scholes D, Stergachis A. Association between use of spermicide-coated condoms and Escherichia coli urinary tract infection in young women. Am J Epidemiol. 1996;144:512–520. doi: 10.1093/oxfordjournals.aje.a008958. [DOI] [PubMed] [Google Scholar]
- 7.Hermonat P L, Daniel R W, Shah K V. The spermicide nonoxynol-9 does not inactivate papillomavirus. Sex Transm Dis. 1992;19:203–205. doi: 10.1097/00007435-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Hicks D R, Martin L S, Getchell J P, Heath J L, Francis D P, McDougal J S S, Curran J W, Voeller B. Inactivation of HTLV-III/LAV-infected cultures of normal human lymphocytes by nonoxynol-9 in vitro. Lancet. 1985;ii:1422–1423. doi: 10.1016/s0140-6736(85)92584-x. [DOI] [PubMed] [Google Scholar]
- 9.Howley P M. Papillomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2045–2076. [Google Scholar]
- 10.Isom H C, Wigdahl B, Howett M K. Molecular pathology of human oncogenic viruses. In: Sirica A E, editor. Cellular and molecular pathogenesis. New York, N.Y: Raven Press; 1996. pp. 341–388. [Google Scholar]
- 11.Jirgensons B. Optical rotator dispersion of non-helical proteins. J Biol Chem. 1966;241:147–152. [PubMed] [Google Scholar]
- 12.Judson F N, Ehret J M, Bodin G F, Levin M J, Reitmeijer C A M. In vitro evaluations of condoms with and without nonoxynol 9 as physical and chemical barriers against Chlamydia trachomatis, herpes simplex virus type 2 and human immunodeficiency virus. Sex Transm Dis. 1989;16:51–56. doi: 10.1097/00007435-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein M, Stevens D A. In vitro and in vivo activity of synthetic detergents against influenza A virus. J Immunol. 1945;50:265–273. [Google Scholar]
- 15.Klein M, Kalter S S, Mudd S. The action of synthetic detergents upon certain strains of bacteriophage and virus. J Immunol. 1945;51:389–396. [PubMed] [Google Scholar]
- 16.Klotz I M, Hunston D L. Protein interactions with small molecules. Relationships between stoichiometric binding constants, site binding constants and empirical binding parameters. J Biol Chem. 1975;250:3001–3009. [PubMed] [Google Scholar]
- 17.Knight C C, Stanley W M. The effect of some chemicals on purified virus. J Exp Med. 1944;79:291–300. doi: 10.1084/jem.79.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreider J W, Howett M K. Proceedings of the IX International Congress on Infectious Diseases. 1986. Morphological transformation in vivo of human uterine cervix, skin and larynx with papillomavirus from condylomata acuminata; pp. 142–145. [Google Scholar]
- 19.Kreider J W, Howett M K. Human papillomavirus-11 infection of xenografted human tissues. UCLA Symp Mol Cell Biol New Ser. 1987;43:371–385. [Google Scholar]
- 20.Kreider J W, Pickel M D. Influence of schedule and mode of administration on effectiveness of podofilox treatment of papillomas. J Invest Dermatol. 1993;101:614–618. doi: 10.1111/1523-1747.ep12366071. [DOI] [PubMed] [Google Scholar]
- 21.Kreider J W, Howett M K, Wolfe S A, Bartlett G L, Zaino R J, Sedlacek T V, Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985;317:639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- 22.Kreider J W, Howett M K, Lill N L, Bartlett G L, Zaino R J, Sedlacek T, Mortel R. In vivo transformation of human skin with human papillomavirus type 11 from condylomata acuminata. J Virol. 1986;59:369–376. doi: 10.1128/jvi.59.2.369-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreider J W, Howett M K, Leure-Dupree A E, Zaino R J, Weber J A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987;61:590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts P L, Ruminjo I, Sajibi R, Kimata J, Fleming T R, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Meisels A, Fortin R. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 1976;20:505–509. [PubMed] [Google Scholar]
- 27.Meisels A, Fortin R, Roy M. Condylomatous lesions of the cervix. II. Cytologic, colposcopic and histopathologic study. Acta Cytol. 1977;21:379–390. [PubMed] [Google Scholar]
- 28.Mengel M B, Davis A B. Institution recurrent bacterial vaginosis: association with vaginal sponge use. Fam Pract Res J. 1992;12:283–288. [PubMed] [Google Scholar]
- 29.Niruthisard S, Roddy R E, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18:176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Rapp F, Wrzos H. Synergistic effect of human leukocyte interferon and nonoxynol-9 against herpes simplex virus type 2. Antimicrob Agents Chemother. 1985;28:449–451. doi: 10.1128/aac.28.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds J A, Herbert S, Polet H, Steinhardt J. The binding of diverse detergent anions to bovine serum albumin. Biochemistry. 1967;6:937–947. doi: 10.1021/bi00855a038. [DOI] [PubMed] [Google Scholar]
- 32.Roddy R E, Cordero M, Cordero C, Fortney J A. A dosing study of nonoxynol 9 and genital irritation. Int J STD AIDS. 1993;4:165–170. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg M J, Rojanapithayakorn W, Feldblum P J, Higgins J E. Effect of the contraceptive sponge on chlamydial infections, gonorrhea and candidiasis. A comparative clinical trial. JAMA. 1987;257:2308–2312. [PubMed] [Google Scholar]
- 34.Schwartz, B., S. Gaventa, C. V. Broome, A. L. Reingold, A. W. Hightower, J. A. Perlman, and P. H. Wolf. 1989. Nonmenstrual toxic shock syndrome associated with barrier contraceptives: report of a case-control study. Rev. Infect. Dis. 11(Suppl. 1):S43–S48. [DOI] [PubMed]
- 35.Shah K V, Howley P M. The papillomaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2077–2110. [Google Scholar]
- 36.Shedlovsky T, Smadel J E. Electrophoretic studies on elementary bodies of vaccinia. J Exp Med. 1940;72:511–521. doi: 10.1084/jem.72.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sipjänen K J. Natural history of genital human papillomavirus infections. In: Lacey C, editor. Papillomavirus reviews: current research on papillomaviruses. Leeds, United Kingdom: Leeds University Press; 1996. pp. 189–206. [Google Scholar]
- 38.Stock C C, Francis T., Jr The inactivation of the virus of lymphocytic choriomeningitis by soaps. J Exp Med. 1943;77:323–336. doi: 10.1084/jem.77.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stock C C, Francis T., Jr The inactivation of the virus of epidemic influenza by soaps. J Exp Med. 1940;71:661–681. doi: 10.1084/jem.71.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- 41.Thompson K A, Malamud D, Storey B T. Assessment of the antimicrobial agent, C31G, as a spermicide: comparison with nonoxynol-9. Contraception. 1996;53:313–318. [PubMed] [Google Scholar]
- 42.Walker A I T, Brown V K H, Ferrigan L W, Pickering R G, Williams D A. Toxicity of sodium lauryl sulphate, sodium lauryl ethoxysulphate and corresponding surfactants derived from synthetic alcohols. Food Cosmet Toxicol. 1967;5:763–769. doi: 10.1016/s0015-6264(67)83275-9. [DOI] [PubMed] [Google Scholar]
- 43.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 44.Weir S S, Roddy R E, Zekeng L, Feldblum P J. Nonoxynol-9 use, genital ulcers, and HIV infection in a cohort of sex workers. Genitourin Med. 1995;71:78–81. doi: 10.1136/sti.71.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]