Abstract
Background:
Vertebrate eye formation requires coordinated inductive interactions between different embryonic tissue layers, first described in amphibians. A network of transcription factors and signaling molecules controls these steps, with mutations causing severe ocular, neuronal, and craniofacial defects. In eyeless mutant axolotls, eye morphogenesis arrests at the optic vesicle stage, before lens induction, and development of ventral forebrain structures is disrupted.
Results:
We identified a 5-bp deletion in the rax (retina and anterior neural fold homeobox) gene, which was tightly linked to the recessive eyeless (e) axolotl locus in an F2 cross. This frameshift mutation, in exon 2, truncates RAX protein within the homeodomain (P154fs35X). Quantitative RNA analysis shows that mutant and wild-type rax transcripts are equally abundant in E/e embryos. Translation appears to initiate from dual start codons, via leaky ribosome scanning, a conserved feature among gnathostome RAX proteins. Previous data show rax is expressed in the optic vesicle and diencephalon, deeply conserved among metazoans, and required for eye formation in other species.
Conclusion:
The eyeless axolotl mutation is a null allele in the rax homeobox gene, with primary defects in neural ectoderm, including the retinal and hypothalamic primordia.
Keywords: Ambystoma, anophthalmia, eye morphogenesis, genetics, homeodomain, hypothalamus, leaky scanning, lens induction, mutation, optic vesicle, pituitary, Rax, ribosome, Rx, salamander, transcription factor, urodele
1 |. INTRODUCTION
Eye formation in vertebrates occurs via a stepwise process, involving regionalization, induction, and differentiation. Following gastrulation, the anterior neural plate is patterned into discrete regions, which are specified by eye field transcription factors (EFTFs).1 These homeodomain (HD) proteins are expressed in overlapping sectors of the prospective eye field, and orchestrate its morphogenetic transformation into bilaterally symmetric optic vesicles, which emerge from the ventral diencephalon during neurulation.2 In a series of classical experiments, first performed in urodele amphibians 120 years ago, Spemann, Lewis, and others showed that the optic vesicles induce formation of a lens placode (thickening) on each side of the head, as they contact the overlying surface ectoderm and involute to form bilayered optic cups.3–5 These ectodermal tissues subsequently differentiate to form the lens and mature retina, with inner neural and outer pigmented layers.6 Apart from critical EFTFs, such as Rax (retina and anterior neural fold homeobox) and Pax6 (paired box family), eye development is mediated by transmembrane and secreted protein factors, such as BMP4, which transmit inductive signals bidirectionally between the optic vesicle and surface ectoderm.7,8 These morphogenetic steps were defined during the past century through studies involving amphibian embryos, which are easy to maintain, monitor and manipulate; conventional genetic models, such as laboratory mice, which allow comparative molecular analysis of mutants, phenotypes, and transgenes; and more recently, 3D retinal organoid cultures.9,10
Eyeless phenotypes, including clinical anophthalmia in humans,11 may result from mutations that disrupt early patterning or partitioning of the eye fields, lens induction, migration of ocular neural crest cells, or periocular mesenchyme signaling—or disrupt the growth, separation and/or differentiation of the retina, pigmented epithelium (RPE), optic stalk or lens.6 Eye regression can also occur via positive evolutionary selection, in species adapted to life in troglobitic (caves) or subterranean environments, with a complex genetic basis.12,13
The axolotl (Nahuatl āxōlōtl), Ambystoma mexicanum, is the oldest continuously studied model organism.14 An endangered neotenic salamander, the axolotl is indigenous to Xochimilco and interconnected lakes that once surrounded Tenochtitlan (Mexico City).15 This species has an aquatic life cycle, with most individuals retaining gills after metamorphosis and sexual maturation.16,17 Axolotls have been an important model in the history of experimental embryology, and are capable of regenerating limbs and other tissues.18–20 Existing laboratory colonies and inbred lines trace their ancestry to a cohort of live specimens imported to Paris in 1863.21 Several Mendelian traits have been described, affecting organogenesis or physiology.22 However, molecular studies have been limited by the large size (C-value) of urodele genomes, which are the largest among tetrapods. The axolotl genome has 14 chromosomes and is 32 Gb, more than 12 times the size of the mouse genome.23 Recently, a chromosome-scale axolotl genome assembly was reported, and is the largest to date, providing a framework for gene discovery.23,24
Eyeless axolotls were first identified by Rufus Humphrey at Indiana University, in an inbred strain (Wistar) with a white (d/d) genetic background.25 Homozygous eyeless mutants (e/e) lacked eyes and were darkly pigmented. They had hypothalamic defects and immature gonads and were consequently sterile. The eye phenotype was initially attributed to the absence of optic vesicles,26,27 but in later studies, small or delayed optic vesicles were described, which failed to progress.28
The dark pigmentation across the dorsal head region is caused by dispersion of melanin granules within large dendritic chromatophores, and is a secondary pleiotropic effect. This phenotype can be reproduced in wild-type (WT) animals by removing both eyes, which increases the level of melanocyte stimulating hormone, MSH.29 Normal pigmentation was restored to e/e animals by grafting a single functioning eye, but these axolotls remained infertile.30 Sterility has been attributed to hypothalamic brain defects, and the absence of gonadotropin releasing hormone (GnRH). Chimeric animals with WT heads and eyeless trunks are fertile, with mature gonads, but reciprocal chimeras are sterile.26,31
Epp and van Deusen proved that the eyeless gene acts within the forebrain ectoderm and not in the surface ectoderm that forms the lens, or inner mesoderm that transiently induces the eye fields during gastrulation.26,27,32 They showed this by reciprocally grafting neural ectoderm between eyeless and WT embryos. WT grafts led to eye formation in e/e recipients, but reciprocal grafts failed. Thus, in eyeless mutants, the primary inductive interaction between surface and neural ectoderm layers, first described over a century ago,4 fails to occur. The molecular basis for this defect has been unknown. In this report, we demonstrate linkage between eyeless and the rax homeobox gene, and identify the causative molecular lesion.
2 |. RESULTS
2.1 |. eyeless mutants
The embryonic development, adult phenotypes, and transmission genetics of the eyeless (e) axolotl have been reported, with major primary defects noted in eye, hypothalamic and pituitary development.27,33 In e/e embryos, the optic vesicles fail to emerge from the ventral diencephalon or are significantly delayed; they do not progress to form bilayered optic cups or induce lens formation, and subsequently degenerate.34 Likewise, the anterior hypothalamus develops abnormally. There is no preoptic recess or extension of PAF+ neurosecretory fiber tracts; the neurohypophysis appears atrophied and the anterior pituitary gland (adenohypophysis) is dysmorphic.35 Consequently, e/e animals are sterile, lacking GnRH, and gonadotropins, and hyperpigmented, with expanded chromatophores in the absence of light perception and environmental feedback.33
To further demonstrate these phenotypes, we collected stage 42 larvae from an F2 cross. In e/e samples, anatomical defects were apparent in exterior photographs (Figure 1A), coronal immunofluorescence micrographs, stained with β3-neurotubulin (Tubb3) and Pax6 antibodies (Figure 1B), and histological sections stained with hematoxylin and eosin (H&E; Figure 1C–F). These findings are consistent with a primary patterning, identity or signaling defect in the anterior neural ectoderm. In some e/e larvae, small dysmorphic optic rudiments were observed below the surface ectoderm, with variable features suggestive of retina, lens and/or RPE (Figure 1E,F), as previously noted in eyeless axolotls at hatching34 and targeted rax mutant Xenopus.36 These rudiments do not progress and subsequently degenerate during larval life.
FIGURE 1.

eyeless phenotype at hatching (stage 42). A, E/− (wild-type) and e/e (mutant) axolotls in vitelline membranes. B, Immunofluorescence micrographs of coronal (transverse) sections through the head showing neural tracts (Tubb3, green), retinal layers and brain nuclei (Pax6, red), and absent eyes in e/e animals (right panel), with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (blue). The rabbit antibody (red) cross reacts with tissue edges, highlighting morphology. C-F, Hematoxylin and eosin (H&E) stained coronal sections showing the absence of eyes in most e/e mutants (red asterisk, right panel in C), with abortive optic rudiments in some cases (red arrowhead in F, magnified example in E). These rudiments contain variably dysmorphic retinal and lens tissues, and pigmented cells. dor, dorsal; ven, ventral; dh, dorsal hypothalamus; le, lens; on, optic nerve; ph, pharynx; ret, retina; rpe, retinal pigment epithelium; tc, tectum; vh, ventral hypothalamus; disorg ret, disorganized retina; scale bars, 200 μm
2.2 |. F2 segregation test
We initially considered two candidate genes—pax6 and rax, critical EFTFs whose spatiotemporal expression, biological functions, and mutant features in other vertebrates are consistent with e/e phenotypes.
Pax6 is required for eye and brain development in diverse metazoans, and has deeply conserved structure, expression, and developmental functions.37,38 It contains paired and homeobox DNA-binding domains, acts near the top of a hierarchy for eye development, and is notably sufficient to induce ectopic eyes in Drosophila and some vertebrates.39,40 Loss-of-function mutations have been reported in humans, mice, zebrafish and fruit flies with anophthalmia and major brain malformations, including defects in the cortex, hypothalamus and pituitary41–44 or cognate structures.45,46 Null mutations are typically lethal in vertebrates, owing to defects in the brain and pancreatic islets, but hypomorphic and regulatory alleles are known.47–50 Heterozygotes have milder phenotypes (haploinsufficiency). Pax6 mRNA is expressed throughout the optic primordia. In mutant mice, eye development arrests at the optic cup stage, before lens induction.51 Previous in situ hybridization analyses show reduced cephalic pax6 mRNA in axolotl e/e embryos—a potential cause or effect of the eyeless phenotype.52
The Rax (also Rx) homeobox gene is likewise required for eye and ventral brain development in chordates.53–56 It acts earlier than Pax6, but has a more limited pattern and phenotype.57,58 Rax is strongly and broadly expressed in the diencephalon, within the anlagen giving rise to the hypothalamus, pineal, optic vesicles and stalk. Spontaneous or engineered Rax mutations cause eyeless phenotypes in medaka and zebrafish, frogs, mice, and humans,36,55,59–63 with limited progression past the optic vesicle stage. Homozygous Rax-null mice die at birth with brain and craniofacial malformations64; however, inbred eyeless mice carrying a hypomorphic allele (ey1, RaxM10L) are viable and fertile.65 There are two vertebrate Rax families, which arose through ancestral whole genome duplications, WGD.66,67 In partially tetraploid species (Xenopus laevis, teleost fish), these paralogs are further duplicated. In zebrafish, for example, rx3 is expressed in the anterior neural plate, optic vesicles, and forebrain, and corresponds to mouse Rax, whereas rx1 and rx2 are mainly localized to the neuroretina.68 The orthologous gene (raxL, RxL, RAX2) promotes photoreceptor differentiation in chick, Xenopus and humans,69–73 but was lost in at least five mammalian lineages, including rodents.67 RAX2 coding mutations cause retinal degeneration in humans74,75 and an rx1 regulatory deletion underlies the large natural variation in cone opsin expression among Lake Malawi cichlid species with diverse visual sensitivities.76
To test these candidates, we intercrossed carriers and prepared genomic DNA from F2 larvae, of which approximately 25% were eyeless. We then genotyped a subset of eyeless (e/e) and WT (E/−) offspring by PCR sequencing. We amplified pax6 and rax 3′UTRs and scored single-nucleotide polymorphisms (SNPs). For pax6, we scored a G/A variant, heterozygous in each parent, and observed 1:2:1 genotype ratios in both phenotypic classes (Table 1). pax6 thus segregates independently of the eyeless trait and is excluded (P = 0.33, χ2 = 2.22, df = 2, n = 23). Consistent with these data, pax6 cDNAs from e/e and E/E embryos have identical sequence (TG and RL Maas, unpublished).
TABLE 1.
eyeless F2 screen (male E/e × female E/e cross)
| (A) pax6 3′ UTR SNP (G/A × G/A) | ||
|---|---|---|
| F2 offspring | ||
| Genotype | e/e | WT |
| GG | 3 | 6 |
| GA | 12 | 7 |
| AA | 8 | 8 |
| sum | 23 | 21 |
| (B) rax 3′UTR SNP (insG/+ × +/+) | ||
| F2 offspring | ||
| Genotype | e/e | WT |
| insG/+ | 0 | 51 |
| +/+ | 55 | 35 |
| sum | 55 | 86 |
Abbreviation: SNP, single-nucleotide polymorphism.
For rax, we scored a 1-bp insertion (insG) that was heterozygous in the male parent only. In this case, we observed a 2:1 genotype ratio among 86 WT larvae, and no heterozygotes among 55 eyeless larvae (Table 1), with 1:1 ratios expected in each class for independent assortment. rax is thus tightly linked to eyeless (P = 3.30 × 10−13, χ2 = 53.02, df = 1, n = 55) and maps within 1.5 cM (95% CI).
2.3 |. rax frameshift mutation in eyeless axolotls
To identify the causative mutation, we amplified the three rax exons and four conserved noncoding elements (CNEs 0–3) from archived E/− and e/e samples, with flanking PCR primers designed using the 32 Gb genome assembly from wild axolotl stocks23,24 as a guide. Exons 1 to 3 and internal CNE2 (intron 2) span 16.1 kb of contig AMEXG_74394 (50.7 kb) on chromosome 6p (Figure 2A), and were identified through BLAST homology searches with known vertebrate rax sequences as query. This contig maps inside AMEXG_0030000955 (6.5 Mb), within a 51 kb assembly gap, flanked by CNE1 and CNE3 on opposite sides. This scaffold encompasses seven genes (pmaip1, ccbe1, lman1, cplx4, rax, grp, sec11c), with conserved synteny and order among tetrapods (Figure 2A). The rax CNEs are well characterized as cis regulatory enhancers in mice and frogs.77–79 These seven features, which comprise the rax gene and regulatory unit, span about 500 kb in axolotls, compared to 15 kb in mice, consistent with the enormous evolutionary expansion of salamander (urodele) gene models.80 In this case, the expansion is greater than average (33-fold vs 12-fold genome-wide).
FIGURE 2.

rax gene structure and eyeless mutation. A, Layered maps of rax exons and CNEs, with relevant chr 6p scaffolds in assembly Amex_PQ.v4, showing flanking genes, coding sequence, Δ5bp mutation (*) and E′ transposon insertion site (arrowhead). B, Sanger chromatograms of genomic PCR products, showing exon 2 profiles from e/e, E/e, and E/E genotypes. The reading frame and Δ5 mutation are indicated. C, Magnified view of exon 2, showing diagnostic PCRs, and results from E′/e genomic DNA (right column). The agarose gel shows ex2 PCR products amplified with a wild-type (WT)-specific primer overlying codon 155 (amplicon DWT). The large product in E′/e lanes contains a transposon insertion
The rax coding sequence extends from exons 1 to 3, and includes conserved octapeptide (OP), paired-class homeobox, RX (PPXY), and OAR (orthopedia, aristaless, rax) domains. In the exon 2 sequence chromatograms, we observed a 5-bp deletion (ΔCGGAC) in e/e samples, and mixed profiles in E/e samples, compared to the WT reference (Figure 2B). The eyeless deletion causes a translational frameshift at codon Pro154, in the HD. It is predicted to truncate the protein prematurely in exon 3, after 35 nonsense codons (P154fs35X).
2.4 |. Genetic linkage between eyeless and rax
To further establish linkage between rax and eyeless, we genotyped 100 F2 larvae from cross 13673 for the HD deletion in exon 2 and UTR variant in exon 3. To our surprise, 51 offspring appeared homozygous for the Δ5bp mutation in exon 2; these include 29 eyeless larvae, lacking the insG UTR allele (as noted above), and 22 WT larvae, heterozygous for the insG allele, with well-developed eyes (Table 2). Reduced penetrance is theoretically possible, as an explanation for this discordance, and occurs in mice with the hypomorphic RaxM10L mutation65; however, we expected these 22 axolotl larvae to exhibit some disruption of eye morphogenesis, if they were truly homozygous for a rax deletion allele.
TABLE 2.
rax—eyeless linkage (male E′/e x female E/e cross)
| rax genotype | F2 offspring | |||||
|---|---|---|---|---|---|---|
| L1 ins | ex2 Δ5 | 3′UTR insG | e/e | WT | Interpretation | |
| L1/+ | +/Δ5 | insG/+ | 0 | 22 | E′/e | |
| +/+ | Δ5/Δ5 | +/+ | 29 | 0 | e/e | |
| L1/+ | +/+ | insG/+ | 0 | 16 | E′/E | |
| +/+ | +/Δ5 | +/+ | 0 | 33 | E/e | |
| sum | 29 | 71 | ||||
Alternatively, the male parent may carry a different WT allele (E′) that failed to amplify with ex2 primers and is coupled in cis to the ex3 insG variant (E′ haplotype). To test this hypothesis, we amplified ex2 segments from discordant F2 offspring using multiple PCR primer pairs (Figure 2C). By moving the 5′ primer closer to exon 2, we were able to amplify both alleles (WT and Δ5). In this way, we mapped a 1 kb transposon insertion 102 bp upstream from ex2 in the E′ haplotype. This L1 retroelement81 inhibits amplification of E′ products in a competitive genotyping PCR. Using a WT-specific primer spanning codon 155, we amplified long E′ products from E′/e carriers spanning the insertion site (Figure 2C). After regenotyping all available F2 samples with informative PCRs (Table 2), we found that the rax Δ5bp mutation and eyeless trait are fully concordant, with 29 eyeless (Δ5/Δ5, e/e) offspring and 71 WT offspring, of which 16 were +/+ (E′/E) and 55 were Δ5/+ (33 E/e, 22 E′/e). This final linkage result is highly significant (P = 1.69 × 10−20, χ2 = 95.18, df = 3, n = 100, with LOD = 19.8). The eyeless trait is thus caused by a rax frameshift mutation.
2.5 |. Effects on rax mRNA and protein
Premature translation termination typically causes mRNA degradation through the nonsense-mediated decay pathway, unless the premature stop codon is encountered by the pioneer ribosome in the last 1–2 exons.82 The eyeless P154fs35X mutation in exon 2 and premature stop in exon 3 are thus not expected to alter rax mRNA stability. To test this prediction, we performed quantitative fluorescence RT-PCR on pooled heterozygous (E/e) stage 22 to 25 embryos with primers in exons 1 and 3, and directly compared the abundance of Δ5bp and WT transcripts (Figure 3). At this stage, rax is highly expressed in eye and brain rudiments, which develop normally in E/e heterozygotes. The molar ratio of RT-PCR products was 1.14 (eyeless to WT). We conclude that mutant rax mRNA is normally expressed, spliced and stable.
FIGURE 3.

rax mRNA analysis. A, RT-PCR design. B, Capillary electrophoresis profile showing equimolar wild-type (+) and eyeless mutant (Δ5) products coamplified from E/e cDNA in relation to ROX-500 size standards (n = 3 pooled embryos)
Since axolotl eyeless mRNA is abundant, it should be translated, producing a truncated RAX protein with an abnormal C-terminal peptide (Figure 4A). This predicted protein should not bind DNA, since the HD is disrupted (Figure 4B), and may have a relatively short half-life. However, the altered polypeptide does not elicit significant toxicity, since E/e heterozygotes are phenotypically similar to WT. Therefore, the eyeless mutation is most likely a rax null allele.
FIGURE 4.

Protein effects of the eyeless mutation. A, RAX polypeptide maps showing octapeptide (OP), homeodomain (HD), RX, and OAR domains, dual start codons, exon junctions (arrowheads), the predicted eyeless protein with Δ5 frameshift mutation and C-terminal nonsense peptide (red). B, Vertebrate RAX homeobox amino acid alignment, showing sequence identities, three critical α-helices for DNA binding, and eyeless HD
In the HD amino acid alignment, we also show axolotl Rax2 protein (Figure 4B). This paralog, on chromosome 1p, was identified in our original BLAST search. It maps on scaffold AMEXG_0030005363 (2.7 Mb), between matk and apba3, and is likely to activate transcription in the developing retina and ciliary marginal zone during photoreceptor differentiation.72,73 Like other tetrapod Rax2 proteins, it has a truncated N-terminus and no OP motif to mediate groucho repression.66,72,73
Interestingly, axolotl Rax shares a unique N-terminal feature with all other gnathostome orthologs—dual AUG start codons separated by 8 to 10 triplets (Figure 5), with translation initiating via a conserved leaky scanning mechanism.65,83 This pattern was also found among Rax2 proteins in jawed fish. In each case, the first AUG occurs in a poor context, with mismatched nucleotides at core positions −3 (purine) and +4 (guanine), whereas the second AUG conforms well to the consensus sequence for optimal translation initiation in eukaryotes.84
FIGURE 5.
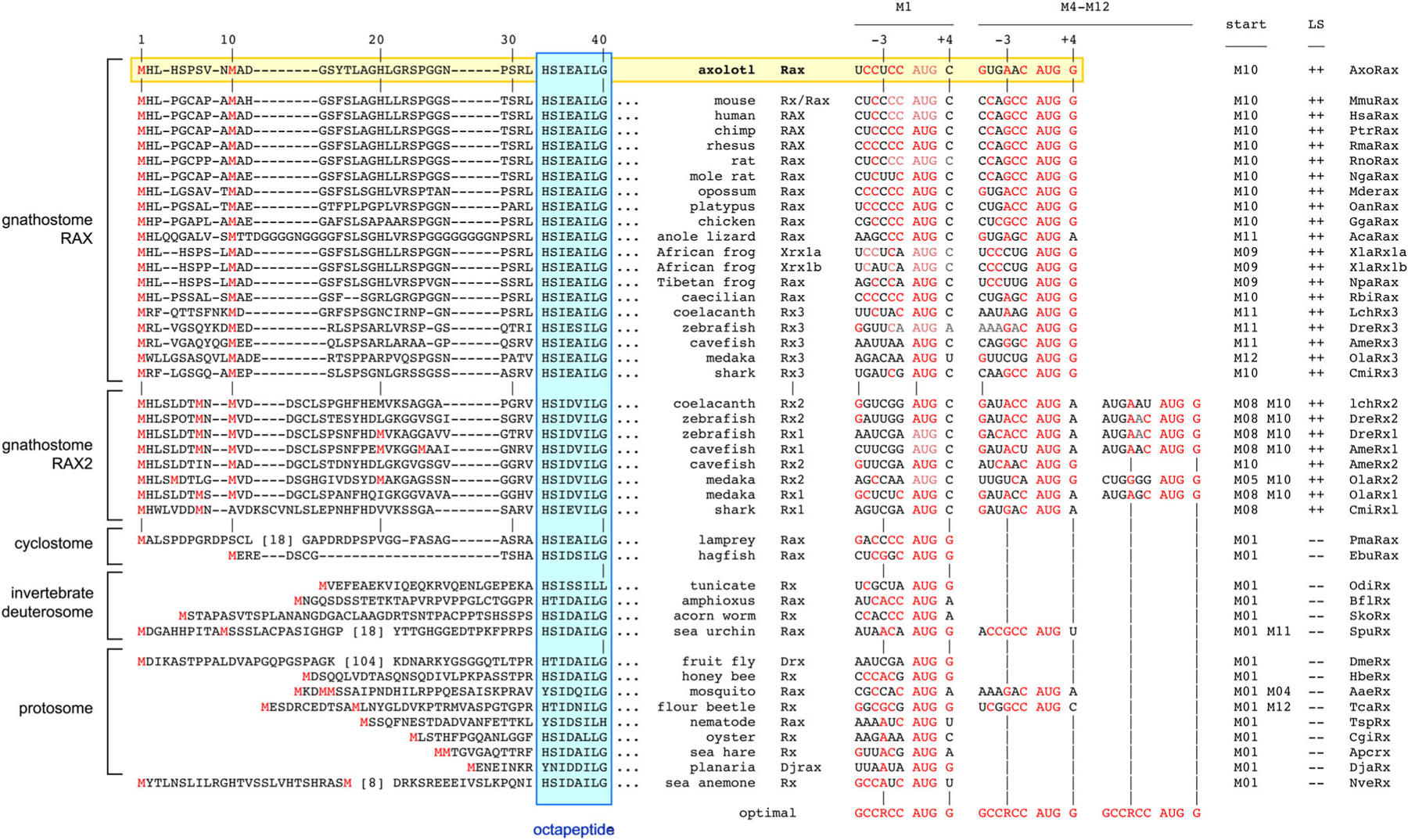
Conserved dual start codons with leaky scanning. Left: N-terminal alignment of Rax proteins, numbered in relation to axolotl (mouse), with methionines (red) and gaps (−) indicated. The presence of a methionine at positions 8 to 12 is a conserved feature among gnathostome Rax proteins containing an OP motif, including axolotl Rax. Right: Comparison of M1 and M4–12 start sites in Rax mRNAs. The optimal sequence for translation initiation84 is shown below each alignment, with nucleotide matches indicated in red. For scanning ribosomes to initiate, AUG sites must have a −3 purine (R) or +4 guanine (G). In every case, the M1 site is weaker than the downstream AUG. Predicted leaky scanning (LS) and initiation codons (start) are indicated
3 |. DISCUSSION
The linkage and molecular data in this report establish rax (P154fs35X) as the causative mutation for eyeless axolotls. This study was sped by the assembly of the Ambystoma mexicanum genome.23,24 Likewise, a small number of classical Mendelian loci, involving axolotl pigmentation, limb and cardiac development, were recently mapped or identified using the emerging genome database as a framework.23,85 Our finding of two rax haplotypes may reflect admixture, at the AGSC, of Wistar (E,e) and divergent (E′) laboratory populations.14,86 Individuals in the AGSC cohort also harbor a significant fraction of North American tiger salamander (Ambystoma tigrinum) DNA in their genomes (5.8 ± 1%), which were cointroduced with the albino trait,87 but these introgressed segments do not overlap the rax 1p region.85
3.1 |. Molecular hindsight
Our results are consistent with studies involving Rax mutants in other species, and allow us to reinterpret older work on eyeless with molecular hindsight. First, the ocular, brain, pigmentation and fertility defects in e/e axolotls closely resemble the malformations reported in spontaneous rx3 mutant (chokh) medaka63 and zebrafish,59,60,62 and Xenopus tropicalis with CRISPR engineered rax mutations.36 In these mutants, notably, anterior forebrain territories do not segregate, and the eye fields are transfated into an expanded telencephalon and diencephalon.88 However, unlike eyeless axolotls, rax mutant Xenopus tropicalis die at metamorphosis.36 Among mammals, Rax knockout mice have no eyes or pituitary, and die at birth with craniofacial, oropharyngeal and brain defects that interfere with breathing and feeding.64,89 In contrast, mice homozygous for a hypomorphic allele (RaxM10L) are viable and fertile, but lack eyes65; they have disturbed circadian rhythms and abnormal hypothalamic morphology,90,91 with expression of these traits dependent on modifier loci. Humans with biallelic RAX mutations are generally anophthalmic but viable, as their alleles allow partial activity.61,92–95 In each case, the inheritance is recessive, but heterozygous phenotypes have been suggested.96,97 Likewise, severe eye malformations have been reported in heterozygous (E/e) axolotls carrying the renal insufficiency (r/r) mutation, which modifies the penetrance of eyeless.27,34
Second, the constellation of defects in e/e axolotl embryos matches the rax mRNA expression patterns in mice, frogs and fish, beginning in the anterior neural plate, and continuing in the diencephalon and optic vesicles, which give rise to the neural retina, RPE, optic stalk, hypothalamus, and some cortical regions.53,57,68,98,99 Likewise, mesenchymal cell disruptions in e/e axolotls100 are reminiscent of neural crest migratory defects in zebrafish rx3 mutants.101 In the mouse hypothalamus, Rax is most strongly expressed in the rostral mediobasal region (preoptic, ventromedial, arcuate, and suprachiasmatic nuclei), associated with GnRH secretion, metabolic regulation and circadian rhythmicity,102–104 consistent with the hypogonadal phenotype in e/e animals.
Third, the stage of eye developmental arrest in e/e mutants fits the timing of rax action in neural ectoderm. In e/e embryos, optic vesicles form, but fail to progress, similar to mouse and Xenopus Rax mutants.36,55 However, rax endocrine phenotypes differ significantly between species. e/e axolotls lack gonadotropins (LH, FSH), but retain other pituitary functions and anatomy.27,35 They grow normally, develop limbs and are hyperpigmented—reflecting the action of hormones secreted by somatotropes (GH), thyrotropes (TSH), and corticotropes, respectively, although as facultative paedomorphs, WT and e/e axolotls are relatively hypothyroid.17,105,106 In contrast, Rax-null mice lack all pituitary lobes—through a combination of cell-autonomous and indirect effects.89 In Rax −/− mouse embryos, neurogenesis and dorsoventral patterning of the hypothalamus is profoundly disrupted107; consequently, the diencephalon does not extend an infundibulum (neurohypophysis), so Rathke’s pouch is not induced to form the anterior pituitary gland (adenohypophysis). These disparate phenotypes may reflect fundamental differences in pituitary ontogeny between taxa,108 or a smaller role for Rax in patterning the hypothalamus of urodeles compared to mammals.
Fourth, older studies of e/e embryos, involving blastomere chimeras,26 transplantation,27,109,110 ex vivo tissue culture34 (Cuny and Malacinski) and parabiosis,26,111 showed that the eyeless defect is cell-autonomous and intrinsic to the anterior neural ectoderm—consistent with a nuclear transcription factor, such as Rax, which acts to define the retinal fields and repress alternative forebrain identities.36 In reciprocal embryo grafts, neural plate tissue from WT donors could induce lens formation in the head ectoderm of e/e recipients, but not vice versa.27 Other grafting studies, however, suggested that an inhibitory signal from head epidermis is responsible for the eyeless phenotype.112 Importantly, WT eyes transplanted into e/e heads developed optic nerves with topographic axon projections to the tectum, and restored functional vision with behavioral and pigmentary responses to light.30,109 In retrospect, this developmental plasticity is not surprising, considering the remarkable ability of axolotls to regenerate limb and eye tissues.20 Knowledge of the eyeless gene may help future studies investigating the molecular basis for ocular regeneration in urodeles.
3.2 |. Dual start codons
While their ultimate effects on expression (kinetics and levels) and evolutionary fitness are unknown, the dual Rax start codons are separated by the footprint width113,114 of one 40S subunit (24–30 nucleotides) and may interact alternately with ribosomes, in two different modes via leaky scanning. As ribosomes bypass the first AUG codon and engage the second (favorable) AUG, forming an initiation complex, they would impede progress of later ribosomes scanning from the 5′ mRNA cap.115 These queued ribosomes would then be forced to pause over the first (unfavorable) AUG and initiate. This tiered mechanism may allow higher levels of translation when rax mRNA is limiting and precise tuning of protein output.116 It would also create long and short Rax isoforms, with a variable N-terminal protein extension, and may thus modulate docking of the nearby OP (eh1, engrailed homology) domain to groucho/TLE1 corepressors.117,118 Apart from tetrapod Rax2 paralogs, which lack an OP motif,73 every gnathostome Rax protein we examined has dual AUG start codons in a “leaky” configuration—reflecting strong evolutionary conservation (with emergence before the 2R WGD) or convergence. The surrounding amino acid residues are notably dissimilar.
3.3 |. Recurrent mutations
Rax is commonly mutated in eyeless laboratory models, which share a singular striking phenotype. In a similar way, convergent mutations in one gene, encoding tyrosinase, are responsible for sporadic cases of true albinism in most vertebrate species, including axolotls.85 Accordingly, RAX mutations underlie a portion of clinical anophthalmia cases,11 and rx3 cosegregates with an ocular regression QTL (quantitative trait locus) under positive evolutionary selection in Astyanax cavefish,12 suggesting the workings of a shared developmental pathway.
4 |. EXPERIMENTAL PROCEDURES
4.1 |. Animals
Axolotl embryos were provided by the AGSC (Ambystoma Genetic Stock Center) at Indiana University (in 1992) and the University of Kentucky (in 2013 and 2020). Eyeless and WT embryos (spawn nos. 8146, 8209, 13673, 15428) were generated from F2 crosses between heterozygous carriers of the eyeless gene (e/+) that descended from Rufus Humphrey’s original colony.25 The eyeless mutation was considered lost in 2009, but reappeared in 2013 in another AGSC stock. Embryos were maintained in modified ×0.2 Holfretter’s solution at 4°C to 19°C and staged using developmental landmarks.119 For F2 linkage analysis, eye phenotypes were scored at hatching (stage 42).
4.2 |. Genomic DNA analysis
DNA was extracted from embryos (degelled in 3% cysteine), adult tail clips, hatchling larvae, or deparaffinized tissue sections by digestion in TNE (100 mM NaCl 10 mM Tris 1 mM EDTA pH 7.4) with 0.5% Na dodecyl sulfate and 250 μg/mL proteinase K at 55°C for 16 hours with agitation, isopropanol precipitation, and resuspension in TE.120 We initially amplified 3′ UTR segments of pax6 (accession AF169414.1) and rax from F2 samples with PCR primers designed from cDNA data (Table 3), and scored polymorphisms by sequencing the products.
TABLE 3.
Axolotl PCR primers and amplicons
| (A) Initial UTR screen | |||
|---|---|---|---|
| Amplicon | WT (bp) | FORWARD primer | REVERSE primer |
| pax6 | 885 | pax6 UTR for 5′-CAGTGAACCTGACATGTCTCAATAC | pax6 UTR rev 5′-TATAGCTAAGAACAAGGGACGTCTG |
| rax | 562 | rax UTR for 5′-AGACTATTTCAAAGCCTGGCATAC | rax UTR rev 5′-TGACTGCAGAGTAACCCAAAAACA |
| (B) Rax genomic analysis (with eyeless deletion indicated) | |||
| Amplicon | WT (bp) | FORWARD primer | REVERSE primer |
| CNE0 | 155 | axorax CNE0 for 5′-AAGCTCAGGCTGTCATCCCA | axorax CNE0 rev 5′-TGACTGCACACAAGAGGCTG |
| CNE1 | 732 | axorax CNE1 for 5′-AGACAAGTGAGTGTGGGTCG | axorax CNE1 rev 5′-TTGCTAATTAGTGCCGGCCA |
| CNE2 | 720 | axorax CNE2 for 5′-TCCACCGTTTTACCAAGCGA | axorax CNE2 rev 5′-TTTCCAGCACTGCTCAGGAG |
| CNE3 | 283 | axorax CNE3 for 5′-ATGGAGAGTGCCTTCCGACA | axorax CNE3 rev 5′-TGGCTGTAAACGTGGACTCT |
| 3′UTR1 | 892 | axorax 3′UTR1 for 5′-TTCCCAGTAGCTACACCCCA | axorax 3′UTR1 rev 5′-AGAGTCTGCCACCAATGCTG |
| 3′UTR2 | 787 | axorax 3′UTR2 for 5′-TGGTTGCACCCACTTGTTCT | axorax 3′UTR2 rev 5′-TCCACAGCATCACCACTCAC |
| 5′UTR | 705 | axorax 5′ UTR for 5′-TGTGGTGTCAGGAGTGGGTA | axorax 5′UTR rev 5′-AAGATGGCCTCGATGCTGTG |
| ex1 | 467 | axorax ex1 for 5′-GTAGTTCAGCCGACTGCACT | axorax ex1 rev 5′-GCACTCACCTTCGAAGCTCT |
| ex2 (A) | 602 | axorax ex2.0 for 5′-AAGGCGCTGGTTTGTGACTA | axorax ex2.0 rev 5′-GCTACTGCCAGGCTCTGAAA |
| ex2 (B) | 613 | axorax ex2.1 for 5′-ATCATTCAAACGGCAGGCAG | axorax ex2.2 rev 5′-TTCGGGCAGGTTGACCTTGA |
| ex2 (C) | 448 | axorax ex2.3 for 5′-TGCCTCTTGTGTCAGTGCAC | axorax ex2.1 rev 5′-ATGCTAAGCTATGCCGGTGT |
| ex2 (Dwt) | 426 (E) 1354 (E′) | axorax ex2.0 for 5′-AAGGCGCTGGTTTGTGACTA | axorax WTspec rev 5′-GTACACGTCCGGGTAGTGGGA |
| ex2 (Dmut) | 429 (e) | axorax ex2.0 for 5′-AAGGCGCTGGTTTGTGACTA | axorax MUspec rev 5′-TCGCGGCTGTACAC|GGTAGTG |
| ex3 | 784 | axorax ex3 for 5′-TCAGTGTCCGCATTGCTCTT | axorax ex3 rev 5′-AATGCCTGTCCAGTTACCCG |
| (C) Rax cDNA analysis (with *fluorescent end-label) | |||
| Amplicon | WT (bp) | FORWARD primer | REVERSE primer |
| cDNA ex 1–3 | 497 | rax cDNA ex1 for 5′-ACAGCATCGAGGCCATCTTG | rax cDNA ex3 rev *5′-TGAGTCCTGCAGCTTCATTG |
We identified three rax exons and four associated CNEs on contigs AMEXG_74394 and AMEXG_0030000955 of the assembled axolotl genome23,24 using vertebrate rax orthologs as query in BLAST homology searches. Segments were amplified from archived e/e and E/− samples using flanking primers (Table 3), GoTaq polymerase (Promega, Madison, Wisconsin) and a touchdown PCR protocol: 5 minutes initial denaturation at 94° C; followed 15 cycles of 20 seconds denaturation at 94° C, 30 seconds annealing at 62° C (decreasing 0.5° per cycle to 55° C), and 60 seconds extension at 70° C; followed by 28 further cycles with 55° C annealing; followed by final 7 minute extension at 70° C. Products were assessed by agarose gel electrophoresis and Sanger sequencing. To score the eyeless deletion in F2 offspring and map the E′ insertion, exon 2 segments were amplified using a combination of primers, including some specific to WT or mutant alleles (Table 3).
4.3 |. Statistics
Linkage between eyeless and rax was assessed using the chi-squared test, with independent assortment as the null hypothesis. Confidence limits for linkage distance were calculated as described for the special case of complete concordance.121
4.4 |. mRNA analysis
Total RNA was isolated from whole stage 22 to 24 embryos by affinity chromatography (RNA miniprep spin columns, Zymo Research, Irvine, California). To compare the abundance of rax transcripts, we performed reverse transcriptase (RT) PCR on pooled E/e embryos, which were genotyped using 2 mm of tail tissue. For each sample, we synthesized cDNA from 500 ng RNA template using iScript reagents (Bio-Rad, Hercules, California) with blended oligo-dT/random dN6 priming, and amplified PCR products using primers in exons 1 and 3 (Table 3), with a 6-FAM (carboxyfluorescein) end-label on the reverse primer. The ratio of WT and eyeless (Δ5bp) rax products in the E/e pool was determined from fluorometric peak areas using Peak Scanner software (Thermo Fisher) after capillary electrophoresis.
4.5 |. Histology and immunostaining
Degelled embryos or larvae were rinsed in phosphate-buffered saline (PBS) and fixed overnight in 4% paraforaldehyde 0.1M NaPO4 pH7.4 at 4° C. Fixed embryos were cryopreserved stepwise in 10% to 30% sucrose PBS at 4° C and frozen in optimal cutting temperature compound (Thermo Fisher) or were dehydrated through a graded ethanol series, equilibrated with xylenes, and embedded in paraffin (Paraplast Plus, Thermo Fisher). Paraffin sections (10 μm) were stained for histology with H&E, stabilized in Permount and imaged at ×20 magnification as stitched composites using a Zeiss ApoTome (White Plains, New York).
Cryosections (10 μm) were blocked in Tris-buffered saline 0.1% Tween-20 (TBST) with 3% bovine serum albumin, 1% normal goat serum, and 1% normal donkey serum, processed for indirect immunofluorescence, and counterstained with 1μg/μL 4′,6-diamidino-2-phenylindole as described.122 Primary antibodies were mouse anti-Tubb3 (clone 2G10, Sigma, 1:500) and rabbit anti-PAX6 (PRB278P, Covance, 1:50, and secondary antibodies were anti-mouse IgG2a and anti-rabbit IgG (AlexaFluor 488 and 647 conjugates, Jackson Immuno-Research, 1:500). After mounting in Prolong Gold (Thermo Fisher), sections were imaged with fluorescence optics using a Leica SPE confocal microscope (Wetzler, Germany) and displayed using RGB indexed color.
4.6 |. Bioinformatics
For HD and AUG translation start site alignments, sequences were deduced from nucleotides of Rx/Rax cDNAs with accession numbers as follows—Aedes aegypti (mosquito): AaeRx, XM_021841460; Anolis carolinensis (anolis lizard); AcaRax, XM_008118598; Apis mellifera (honey bee): HbeRx, XM_001119966; Aplysia californica (sea hare): ApcRx, XM_013086395; Astyanax mexicanus (cavefish): AmeRx1, AF264703; AmeRx2, XM_022678823; AmeRx3, XM_007246033; Branchiostoma floridae (amphioxus): BflRx, JX101655; Callorhinchus milii (elephant shark): CmiRx3, XM_007903126; CmiRx1, XM_007908006; Crassostrea gigas (oyster): CgiRx, XM_011429408; Danio rerio (zebrafish): DreRx1, AF001907; DreRx2, AF001908; DreRx3, NM_131227; Drosophila melanogaster (fruit fly): DmeRx, NM_166413; Dugesia japonica (planaria): DjaRx, AM942442; Eptatretus burgeri (hagfish): EbuRax, ENSEBUT00000011203.1; Gallus gallus (chicken): GgaRax, NM_001243724; GgaRax2, NM_204104; Homo sapiens (human): HsaRax, NM_013435; HsaRax2, NM_032753; Latimeria chalumnae (coelacanth): LchRx2, XM_014490274; LchRx3, XM_006005788; Macaca mulatta (rhesus macaque): RmaRax, XM_015122061; Monodelphis domestica (opossum): MdoRax, XM_007487510; MdeRax2, XM_001373844; Mus musculus (mouse): MmuRax, NM_013833; Nannospalax galili (mole rat): NgaRax, XM_008840033; Nanorana parkeri (Tibetan frog): NpaRax, XM_018570456; Nematostella vectensis (sea anemone): NveRx, XM_001634160; Oikopleura dioica (tunicate): OdiRx, AY705677; Ornithorhynchus anatinus (platypus): OanRax, XM_007659835; OanRax2, XM_001516307; Oryzias latipes (medaka fish): OlaRx1, XM_004068380; OlaRx2, NM_001104903; OlaRx3, NM_001104904; Pan troglodytes (chimpanzee): PtrRax, XM_001142510; Petromyzan marinus (lamprey): PmaRax, PIZI01000102v1; Rattus norvegicus (rat): RnoRax, NM_053678; Rhinatrema bivittatum (caecilian): RbiRax, XM_029586501; Saccoglossus kowalevskii (acorn worm): SkoRx, NM_001164903; Strongylocentrotus purpuratus (sea urchin): SpuRx, XM_777214; Tribolium castaneum (flour beetle): TcaRx, XM_968375; Trichinella spiralis (nematode): TspRx, XM_003371992; Xenopus laevis (African frog): XlaRx1a, NM_001088218; XlaRx1b, DQ360108;XlaRax2; NM_001088220.
ACKNOWLEDGMENTS
The authors are grateful to the Ambystoma Genetic Stock Center for providing embryos; Nadean Brown, Nick Marsh-Armstrong, and Marilyn Kozak for helpful advice; and teacher Ann Moriarty (Davis High School) for encouragement. The project was supported by grants from the NIH to T. G. (EY19497) and S. R. V. (OD10435, OD21479, OD19794), and from the NSF to S. R. V. (DBI-0951484). This paper is dedicated to Panagiotis (Takis) Tsonis.
Funding information
National Institutes of Health, Grant/Award Numbers: EY19497, OD10435, OD21479, OD19794; National Science Foundation, Grant/Award Number: DBI-0951484
REFERENCES
- 1.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130(21):5155–5167. 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 2.Grainger RM. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992;8(10):349–355. 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- 3.Lewis WH. Experimental studies on the development of the eye in amphibia. I. On the origin of the lens. Rana palustris. Am J Anat. 1904;3:505–536. [Google Scholar]
- 4.Spemann H Ueber Korrelationen in der Entwicklung des Auges. Verb Anat Ges. 1901;15:61–79. [Google Scholar]
- 5.Spemann H Embryonic Development and Induction. New Haven, CT: Yale University Press; 1938. [Google Scholar]
- 6.Fuhrmann S Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61–84. 10.1016/B978-0-12-385044-7.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12(23):3764–3775. 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Liu Y, Filas B, Gunhaga L, Beebe DC. Negative and positive auto-regulation of BMP expression in early eye development. Dev Biol. 2015;407(2):256–264. 10.1016/j.ydbio.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda H, Osakada F, Watanabe K, et al. Generation of Rx +/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102(32):11331–11336. 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 11.Williamson KA, FitzPatrick DR. The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur J Med Genet. 2014;57(8):369–380. 10.1016/j.ejmg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 12.McGaugh SE, Gross JB, Aken B, et al. The cavefish genome reveals candidate genes for eye loss. Nat Commun. 2014;5: 5307. 10.1038/ncomms6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partha R, Chauhan BK, Ferreira Z, et al. Subterranean mammals show convergent regression in ocular genes and enhancers, along with adaptation to tunneling. Elife. 2017;6: 25884. 10.7554/eLife.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiss C, Olsson L, Hossfeld U. The history of the oldest self-sustaining laboratory animal: 150 years of axolotl research. J Exp Zool B Mol Dev Evol. 2015;324(5):393–404. 10.1002/jez.b.22617. [DOI] [PubMed] [Google Scholar]
- 15.Voss SR, Woodcock MR, Zambrano L. A tale of two axolotls. Bioscience. 2015;65(12):1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould SJ. Ontogeny and Phylogeny. Cambridge, MA: Harvard University Press; 1977:501. [Google Scholar]
- 17.Crowner A, Khatri S, Blichmann D, Voss SR. Rediscovering the axolotl as a model for thyroid hormone dependent development. Front Endocrinol (Lausanne). 2019;10:237. 10.3389/fendo.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kragl M, Knapp D, Nacu E, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460(7251):60–65. 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 19.Suetsugu-Maki R, Maki N, Nakamura K, et al. Lens regeneration in axolotl: new evidence of developmental plasticity. BMC Biol. 2012;10:103. 10.1186/1741-7007-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joven A, Elewa A, Simon A. Model systems for regeneration: salamanders. Development. 2019;146(14):167700. 10.1242/dev.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith HB. Discovery of the axolotl and its early history in biological research. In: Armstrong JB, Malacinski GM, eds. Developmental Biology of the Axolotl. New York, NY: Oxford University Press; 1989:3–12. [Google Scholar]
- 22.Malacinski GM. Developmental genetics. In: Armstrong JB, Malacinski GM, eds. Developmental Biology of the Axolotl. New York, NY: Oxford University Press; 1989:102–109. [Google Scholar]
- 23.Smith JJ, Timoshevskaya N, Timoshevskiy VA, Keinath MC, Hardy D, Voss SR. A chromosome-scale assembly of the axolotl genome. Genome Res. 2019;29(2):317–324. 10.1101/gr.241901.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowoshilow S, Schloissnig S, Fei JF, et al. The axolotl genome and the evolution of key tissue formation regulators. Nature. 2018;554(7690):50–55. 10.1038/nature25458. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey RR. A recently discovered mutant “eyeless” in the Mexican axolotl Ambystoma mexicanum. Anat Rec. 1969;163:306. [Google Scholar]
- 26.van Deusen E Experimental studies on a mutant gene (e) preventing the differentiation of eye and normal hypothalamus primordia in the axolotl. Dev Biol. 1973;34(1):135–158. 10.1016/0012-1606(73)90344-8. [DOI] [PubMed] [Google Scholar]
- 27.Epp LG. A review of the eyeless mutant in the Mexican axolotl. Am Zool. 1978;18(2):267–272. 10.1093/icb/18.2.267. [DOI] [Google Scholar]
- 28.Brun RB. The eyeless mutant axolotl (Ambystoma mexicanum) produces small and delayed optic vesicles. Am Zool. 1983;23:998. [Google Scholar]
- 29.Pietsch P, Schneider CW. Vision and the skin camouflage reactions of Ambystoma larvae: the effects of eye transplants and brain lesions. Brain Res. 1985;340(1):37–60. 10.1016/0006-8993(85)90772-3. [DOI] [PubMed] [Google Scholar]
- 30.Harris WA. The transplantation of eyes to genetically eyeless salamanders: visual projections and somatosensory interactions. J Neurosci. 1982;2(3):339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epp LG. Development of pigmentation in the eyeless mutant of the Mexican axolotl, Ambystoma mexicanum, Shaw. J Exp Zool. 1972;181(2):169–180. 10.1002/jez.1401810204. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson AG. Inductive processes in embryonic development. Science. 1966;152(3718):25–34. 10.1126/science.152.3718.25. [DOI] [PubMed] [Google Scholar]
- 33.Cuny R, Malacinski GM. The eyeless (e) gene: effects on embryonic development. In: Armstrong JB, Malacinski GM, eds. Developmental Biology of the Axolotl. Oxford, UK: Oxford University Press; 1989. chap 13:132–142. [Google Scholar]
- 34.Cuny R, Malacinski GM. Axolotl retina and lens development: mutual tissue stimulation and autonomous failure in the eyeless mutant retina. J Embryol Exp Morphol. 1986;96:151–170. [PubMed] [Google Scholar]
- 35.Eagleson GW, Malacinski GM. A scanning electron micros-copy and histological study on the effects of the mutant eyeless (e/e) gene upon the hypothalamus in the Mexican axolotl Ambystoma mexicanum Shaw. Anat Rec. 1986;215(3):317–327. 10.1002/ar.1092150314. [DOI] [PubMed] [Google Scholar]
- 36.Fish MB, Nakayama T, Fisher M, et al. Xenopus mutant reveals necessity of rax for specifying the eye field which otherwise forms tissue with telencephalic and diencephalic character. Dev Biol. 2014;395(2):317–330. 10.1016/j.ydbio.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31(5):351–376. 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Cvekl A, Callaerts P. PAX6: 25th anniversary and more to learn. Exp Eye Res. 2017;156:10–21. 10.1016/j.exer.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267(5205):1788–1792. 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 40.Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Development. 1999; 126(19):4213–4222. [DOI] [PubMed] [Google Scholar]
- 41.Hill RE, Favor J, Hogan BL, et al. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354(6354):522–525. [DOI] [PubMed] [Google Scholar]
- 42.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7(4):463–471. [DOI] [PubMed] [Google Scholar]
- 43.Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265(5173):785–789. 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 44.Kleinjan DA, Bancewicz RM, Gautier P, et al. Sub-functionalization of duplicated zebrafish pax6 genes by cisregulatory divergence. PLoS Genet. 2008;4(2):e29. 10.1371/journal.pgen.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callaerts P, Leng S, Clements J, et al. Drosophila Pax-6/eyeless is essential for normal adult brain structure and function. J Neurobiol. 2001;46(2):73–88. [DOI] [PubMed] [Google Scholar]
- 46.Kronhamn J, Frei E, Daube M, et al. Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development. 2002;129(4):1015–1026. [DOI] [PubMed] [Google Scholar]
- 47.Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T. 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci U S A. 2000;97(25):13755–13759. 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatia S, Bengani H, Fish M, et al. Disruption of autoregulatory feedback by a mutation in a remote, ultra-conserved PAX6 enhancer causes aniridia. Am J Human Genet. 2013;93(6):1126–1134. 10.1016/j.ajhg.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carbe C, Garg A, Cai Z, Li H, Powers A, Zhang X. An allelic series at the paired box gene 6 (Pax6) locus reveals the functional specificity of Pax genes. J Biol Chem. 2013;288(17): 12130–12141. 10.1074/jbc.M112.436865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan TH. Variability of eyeless. In: Sturtevant AH, Bridges CB, Morgan TH, Morgan LV, Li JC, eds. Contributions to the Genetics of Drosophila simulans and Drosophila melanogaster. Washington, DC: Carnegie Institute of Washington; 1929:139–168. [Google Scholar]
- 51.Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121(5):1433–1442. [DOI] [PubMed] [Google Scholar]
- 52.Eagleson GW, Gerlach LM, Platz TA. The eyeless mutant gene (e) in the Mexican axolotl (Ambystoma mexicanum) affects pax-6 expression and forebrain axonogenesis. Int J Dev Biol. 2001;45(4):653–660. [PubMed] [Google Scholar]
- 53.Casarosa S, Andreazzoli M, Simeone A, Barsacchi G. Xrx1, a novel Xenopus homeobox gene expressed during eye and pineal gland development. Mech Dev. 1997;61(1–2):187–198. 10.1016/s0925-4773(96)00640-5. [DOI] [PubMed] [Google Scholar]
- 54.Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci U S A. 1997;94(7): 3088–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387(6633):603–607. [DOI] [PubMed] [Google Scholar]
- 56.D’Aniello S, D’Aniello E, Locascio A, et al. The ascidian homolog of the vertebrate homeobox gene Rx is essential for ocellus development and function. Different Res Biol Diver. 2006;74(5):222–234. [DOI] [PubMed] [Google Scholar]
- 57.Mathers PH, Jamrich M. Regulation of eye formation by the Rx and pax6 homeobox genes. Cell Mol Life Sci. 2000;57(2): 186–194. 10.1007/PL00000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28(3–4):135–142. [PubMed] [Google Scholar]
- 59.Loosli F, Staub W, Finger-Baier KC, et al. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003; 4(9):894–899. 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy BN, Stearns GW, Smyth VA, et al. Zebrafish rx3 and mab21l2 are required during eye morphogenesis. Dev Biol. 2004;270(2):336–349. 10.1016/j.ydbio.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 61.Voronina VA, Kozhemyakina EA, O’Kernick CM, et al. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Human Mol Genet. 2004;13 (3):315–322. [DOI] [PubMed] [Google Scholar]
- 62.Rojas-Munoz A, Dahm R, Nusslein-Volhard C. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev Biol. 2005;288(2):348–362. 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 63.Loosli F, Winkler S, Burgtorf C, et al. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128(20):4035–4044. [DOI] [PubMed] [Google Scholar]
- 64.Voronina VA, Kozlov S, Mathers PH, Lewandoski M. Conditional alleles for activation and inactivation of the mouse Rx homeobox gene. Genesis. 2005;41(4):160–164. 10.1002/gene.20109. [DOI] [PubMed] [Google Scholar]
- 65.Tucker P, Laemle L, Munson A, et al. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis. 2001;31(1):43–53. [DOI] [PubMed] [Google Scholar]
- 66.Orquera DP, de Souza FSJ. Evolution of the Rax family of developmental transcription factors in vertebrates. Mech Dev. 2017;144(pt B):163–170. 10.1016/j.mod.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Kon T, Furukawa T. Origin and evolution of the Rax homeobox gene by comprehensive evolutionary analysis. FEBS Open Bio. 2020;10(4):657–673. 10.1002/2211-5463.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84(1–2):195–198. [DOI] [PubMed] [Google Scholar]
- 69.Ohuchi H, Tomonari S, Itoh H, Mikawa T, Noji S. Identification of chick rax/rx genes with overlapping patterns of expression during early eye and brain development. Mech Dev. 1999;85(1–2):193–195. 10.1016/s0925-4773(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 70.Chen CM, Cepko CL. The chicken RaxL gene plays a role in the initiation of photoreceptor differentiation. Development. 2002;129(23):5363–5375. 10.1242/dev.00114. [DOI] [PubMed] [Google Scholar]
- 71.Wang QL, Chen S, Esumi N, et al. QRX, a novel homeobox gene, modulates photoreceptor gene expression. Human Mol Genet. 2004;13(10):1025–1040. 10.1093/hmg/ddh117. [DOI] [PubMed] [Google Scholar]
- 72.Pan Y, Nekkalapudi S, Kelly LE, El-Hodiri HM. The Rx-like homeobox gene (Rx-L) is necessary for normal photoreceptor development. Invest Ophthalmol Vis Sci. 2006;47(10):4245–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu HY, Perron M, Hollemann T. The role of Xenopus Rx-L in photoreceptor cell determination. Dev Biol. 2009;327(2): 352–365. 10.1016/j.ydbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 74.Yang P, Chiang PW, Weleber RG, Pennesi ME. Autosomal dominant retinal dystrophy with electronegative waveform associated with a novel RAX2 mutation. JAMA Ophthalmol. 2015;133(6):653–661. 10.1001/jamaophthalmol.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van de Sompele S, Smith C, Karali M, et al. Biallelic sequence and structural variants in RAX2 are a novel cause for autosomal recessive inherited retinal disease. Genet Med. 2019;21(6): 1319–1329. 10.1038/s41436-018-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schulte JE, O’Brien CS, Conte MA, O’Quin KE, Carleton KL. Interspecific variation in Rx1 expression controls opsin expression and causes visual system diversity in African cichlid fishes. Mol Biol Evol. 2014;31(9):2297–2308. 10.1093/molbev/msu172. [DOI] [PubMed] [Google Scholar]
- 77.Danno H, Michiue T, Hitachi K, Yukita A, Ishiura S, Asashima M. Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci U S A. 2008;105(14):5408–5413. 10.1073/pnas.0710954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez-de Luna RI, Moose HE, Kelly LE, Nekkalapudi S, El-Hodiri HM. Regulation of retinal homeobox gene transcription by cooperative activity among cis-elements. Gene. 2010;467 (1–2):13–24. 10.1016/j.gene.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelly LE, Martinez-De Luna RI, El-Hodiri HM. Autoregulation of retinal homeobox (rax) gene promoter activity through a highly conserved genomic element. Genesis. 2016;54(11):562–567. 10.1002/dvg.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith JJ, Putta S, Zhu W, et al. Genic regions of a large salamander genome contain long introns and novel genes. BMC Genom. 2009;10:19. 10.1186/1471-2164-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110(1–4):462–467. 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- 82.Kurosaki T, Popp MW, Maquat LE. Quality and quantity control of gene expression by nonsense-mediated mRNA decay. Nat Rev Mol Cell Biol. 2019;20(7):406–420. 10.1038/s41580-019-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozak M Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc Natl Acad Sci U S A. 1995;92(15):7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kozak M Interpreting cDNA sequences: some insights from studies on translation. Mamm Genome. 1996;7(8):563–574. [DOI] [PubMed] [Google Scholar]
- 85.Woodcock MR, Vaughn-Wolfe J, Elias A, et al. Identification of mutant genes and introgressed Tiger salamander DNA in the laboratory axolotl, Ambystoma mexicanum. Sci Rep. 2017; 7(1):6. 10.1038/s41598-017-00059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malacinski GM, Brothers AJ. Mutant genes in the Mexican axolotl. Science. 1974;184(4142):1142–1147. 10.1126/science.184.4142.1142. [DOI] [PubMed] [Google Scholar]
- 87.Humphrey RR. Albino axolotls from an albino tiger salamander through hybridization. J Hered. 1967;58(3):95–101. 10.1093/oxfordjournals.jhered.a107572. [DOI] [PubMed] [Google Scholar]
- 88.Stigloher C, Ninkovic J, Laplante M, et al. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development. 2006;133 (15):2925–2935. 10.1242/dev.02450. [DOI] [PubMed] [Google Scholar]
- 89.Medina-Martinez O, Amaya-Manzanares F, Liu C, et al. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS One. 2009;4(2):e4513. 10.1371/journal.pone.0004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silver J Abnormal development of the suprachiasmatic nuclei of the hypothalamus in a strain of genetically anophthalmic mice. J Comp Neurol. 1977;176(4):589–606. [DOI] [PubMed] [Google Scholar]
- 91.Laemle LK, Ottenweller JE. Daily patterns of running wheel activity in male anophthalmic mice. Physiol Behav. 1998;64(2): 165–171. [DOI] [PubMed] [Google Scholar]
- 92.Lequeux L, Rio M, Vigouroux A, et al. Confirmation of RAX gene involvement in human anophthalmia. Clin Genet. 2008;74 (4):392–395. 10.1111/j.1399-0004.2008.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abouzeid H, Youssef MA, Bayoumi N, et al. RAX and anophthalmia in humans: evidence of brain anomalies. Mol Vis. 2012;18:1449–1456. [PMC free article] [PubMed] [Google Scholar]
- 94.Chassaing N, Causse A, Vigouroux A, et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet. 2014;86(4):326–334. 10.1111/cge.12275. [DOI] [PubMed] [Google Scholar]
- 95.Huang XF, Huang ZQ, Lin D, et al. Unraveling the genetic cause of a consanguineous family with unilateral coloboma and retinoschisis: expanding the phenotypic variability of RAX mutations. Sci Rep. 2017;7(1):9064. 10.1038/s41598-017-09276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.London NJ, Kessler P, Williams B, Pauer GJ, Hagstrom SA, Traboulsi EI. Sequence alterations in RX in patients with microphthalmia, anophthalmia, and coloboma. Mol Vis. 2009; 15:162–167. [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez-Rodriguez J, Pelcastre EL, Tovilla-Canales JL, et al. Mutational screening of CHX10, GDF6, OTX2, RAX and SOX2 genes in 50 unrelated microphthalmia-anophthalmia-coloboma (MAC) spectrum cases. Br J Ophthalmol. 2010;94 (8):1100–1104. 10.1136/bjo.2009.173500. [DOI] [PubMed] [Google Scholar]
- 98.Klimova L, Lachova J, Machon O, Sedlacek R, Kozmik Z. Generation of mRx-Cre transgenic mouse line for efficient conditional gene deletion in early retinal progenitors. PLoS One. 2013;8(5):e63029. 10.1371/journal.pone.0063029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bosze B, Moon MS, Kageyama R, Brown NL. Simultaneous requirements for Hes1 in retinal neurogenesis and optic cup-stalk boundary maintenance. J Neurosci. 2020;40(7):1501–1513. 10.1523/JNEUROSCI.2327-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ulshafer RJ, Hibbard E. An SEM AND TEM study of suppression of eye development in eyeless mutant axolotls. Anat Embryol. 1979;156(1):29–35. 10.1007/BF00315713. [DOI] [PubMed] [Google Scholar]
- 101.Langenberg T, Kahana A, Wszalek JA, Halloran MC. The eye organizes neural crest cell migration. Dev Dyn. 2008;237(6): 1645–1652. 10.1002/dvdy.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wataya T, Ando S, Muguruma K, et al. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci U S A. 2008;105(33):11796–11801. 10.1073/pnas.0803078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shimogori T, Lee DA, Miranda-Angulo A, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13(6):767–775. 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu F, Kar D, Gruenig N, et al. Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci. 2013;33(1):259–272. 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A. 1997;94(24): 13011–13016. 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laudet V The origins and evolution of vertebrate metamorphosis. Curr Biol. 2011;21(18):R726–R737. 10.1016/j.cub.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 107.Orquera DP, Nasif S, Low MJ, Rubinstein M, de Souza FSJ. Essential function of the transcription factor Rax in the early patterning of the mammalian hypothalamus. Dev Biol. 2016; 416(1):212–224. 10.1016/j.ydbio.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubois PM, Elamraoui A. Embryology of the pituitary gland. Trends Endocrinol Metab. 1995;6(1):1–7. 10.1016/1043-2760(94)00090-q. [DOI] [PubMed] [Google Scholar]
- 109.Hibbard E, Ornberg RL. Restoration of vision in genetically eyeless axolotls (Ambystoma mexicanurn). Exp Neurol. 1976; 50:113–123. [DOI] [PubMed] [Google Scholar]
- 110.Brun RB. Bilateral eye formation in the eyeless mutant Mexican salamander following unilateral, partial excision of neural fold tissues: a quantitative study. J Exp Zool. 1993;265(5):541–548. 10.1002/jez.1402650510. [DOI] [Google Scholar]
- 111.Harris WA. Axonal pathfinding in the absence of normal pathways and impulse activity. J Neurosci. 1984;4(4):1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brun RB. Experimental analysis of the eyeless mutant in the Mexican axolotl (Ambystoma mexicanum). Am Zool. 1978;18 (2):273–279. 10.1093/icb/18.2.273. [DOI] [Google Scholar]
- 113.Wolin SL, Walter P. Ribosome pausing and stacking during translation of a eukaryotic mRNA. EMBO J. 1988;7(11):3559–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Archer SK, Shirokikh NE, Beilharz TH, Preiss T. Dynamics of ribosome scanning and recycling revealed by translation complex profiling. Nature. 2016;535(7613):570–574. 10.1038/nature18647. [DOI] [PubMed] [Google Scholar]
- 115.Kozak M Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1990;87(21):8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ivanov IP, Shin BS, Loughran G, et al. Polyamine control of translation elongation regulates start site selection on antizyme inhibitor mRNA via ribosome queuing. Mol Cell. 2018; 70(2):254–264 e6. 10.1016/j.molcel.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jennings BH, Pickles LM, Wainwright SM, Roe SM, Pearl LH, Ish-Horowicz D. Molecular recognition of transcriptional repressor motifs by the WD domain of the Groucho/TLE corepressor. Mol Cell. 2006;22(5):645–655. 10.1016/j.molcel.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 118.Buescher JL, El-Hodiri HM. The C-terminus of the retinal homeobox (rax) gene product modulates transcription in a context-dependent manner. Mol Vis. 2019;25:165–173. [PMC free article] [PubMed] [Google Scholar]
- 119.Bordzilovskaya NP, Dettlaff TA, Duhon ST, Malacinski GM. Developmental-stage series of axolotl embryos. In: Armstrong JB, Malacinski GM, eds. Developmental Biology of the Axolotl. New York, NY: Oxford University Press; 1989: 201–219. [Google Scholar]
- 120.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19(15):4293. 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Silver LM, Appendix D. Statistics. Confidence limits and median estimates of linkage distance. Mouse Genetics. Concepts and Applications. New York, NY: Oxford University Press; 1995:294–304. [Google Scholar]
- 122.Prasov L, Glaser T. Pushing the envelope of retinal ganglion cell genesis: context dependent function of Math5 (Atoh7). Dev Biol. 2012;368(2):214–230. 10.1016/j.ydbio.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


