Abstract
Background and Aims
Over 404 million people worldwide have been infected with coronavirus disease–2019 (COVID-19), 145 million in the United States (77 million) and Europe (151 million) alone (as of February 10, 2022). This paper aims to analyze data from studies reporting gastrointestinal bleeding (GIB) and/or endoscopic findings in COVID-19 patients in Western countries.
Methods
We conducted a systematic review of articles on confirmed COVID-19 cases with GIB in Western countries published in PubMed and Google Scholar databases from June 20, 2020, to July 10, 2021.
Results
A total of 12 studies reporting GIB and/or endoscopic findings in 808 COVID-19 patients in Western countries were collected and analyzed. Outcomes and comorbidities were compared with 18,179 non-GIB COVID-19 patients from Italy and the United States. As per our study findings, the overall incidence of GIB in COVID-19 patients was found to be 0.06%. When compared to the non-GIB cohort, the death rate was significantly high in COVID-19 patients with GIB (16.4% vs 25.4%, P < .001, respectively). Endoscopic treatment was rarely necessary, and blood transfusion was the most common GIB treatment. The most common presentation in GIB patients is melena (n = 117, 47.5%). Peptic, esophageal, and rectal ulcers were the most common endoscopic findings in upper (48.4%) and lower (36.4%) endoscopies. The GIB cohort had worse outcomes and higher incidence of hypertension (61.1%), liver disease (11.2%), and cancer (13.6%) than the non-GIB cohort. Death was strongly associated with hypertension (P < .001, r = 0.814), hematochezia (P < .001, r = 0.646), and esophagogastroduodenoscopy (P < .001, r = 0.591) in COVID-19 patients with GIB.
Conclusion
Overall, the incidence of GIB in COVID-19 patients is similar to that estimated in the overall population, with melena being the most common presentation. The common endoscopic findings in GIB COVID-19 patients were ulcers, esophagitis, gastritis, and colitis. Patients with GIB were more prone to death than non-GIB COVID-19 patients.
Keywords: Coronavirus Disease-19, Gastrointestinal Bleeding, Endoscopy, Melena, Hematemesis
Introduction
The SARS-CoV-2 virus has infected over 404 million people worldwide; many of them developed the coronavirus disease-2019 (COVID-19).1 SARS-CoV-2 associates with a wide variety of manifestations, partly because there are several variants in the world due to mutations but also because of geographic and population’s specificities and differences. The most common COVID-19 symptoms are fever, cough, dyspnea, sore throat, rhinorrhea, anosmia, dysgeusia, fatigue, and myalgia.2 However, gastrointestinal (GI) symptoms (nausea, diarrhea, vomiting, abdominal pain, and anorexia) are also commonly reported.2,3 A specific GI manifestation that has been identified in 3%–12% of COVID-19 patients is GI bleeding (GIB).4, 5, 6
Coagulopathy is a major concern in COVID-19 patients.7 Endothelial dysfunction and hypercoagulability are likely associated with a strong immune response of the host toward the virus, which generates a cytokine storm and subsequent complications.7 Therefore, anticoagulants and thromboprophylaxis are frequently used in the treatment of COVID-19. Coagulopathies, adoption of anticoagulant therapies, and other viral effects make COVID-19 patients more prone to develop GIB.6 In particular, the virus is thought to cause both direct and indirect damage, via a “double-hit” mechanism.8 Direct damage refers to the GI mucosal injury with consequent immune response and inflammation, and indirect damage is the subsequent hypoxic stress that stems from coagulopathy.9 Little is known about the underlying conditions that increase the risk of developing GIB.
In normal circumstances, it is recommended that patients developing upper GIB (UGIB) undergo endoscopy within 24 hours from presentation.9 However, the COVID-19 pandemic has led to several changes in the allocation of resources and performance of such procedures, both to free health care providers to deal with patients infected by the virus and to limit the risk of infection in health care facilities. A study from Northern Italy reported that most of the endoscopic procedures that were supposed to take place in March 2020 were either postponed or canceled.10 Another study from central Italy reported similar findings, with the number of patients admitted for urgent upper endoscopy in March–May 2020 dropping 50% from the same period before lockdown.11 Finally, a nation-wide Italian study showed a statistically significant decrease in upper and lower endoscopies during the COVID-19 pandemic, mainly due to patients avoiding exposure to the virus in hospital settings.12 Consequently, post-UGIB endoscopy survival was shown to be reduced during the COVID-19 pandemic.13
In this review, we analyze and discuss the results of studies including mainly patients from Western countries that report GIB and endoscopic findings in COVID-19 patients. We report the current consensus and available information on different types of treatments and investigate the differences in underlying conditions and outcomes between COVID-19 patients with and without GIB.
Methods
Search Strategy and Selection Criteria
We conducted a systematic literature search of published articles using PubMed and Google Scholar databases from June 20, 2020, to July 10, 2021. We used the following search words: COVID-19, COVID-19 GI bleeding, hematemesis, hematochezia, melena COVID-19 gastrointestinal bleeding, COVID-19 endoscopic findings, COVID-19 endoscopy, COVID-19 clinical characteristics, COVID-19 comorbidities, COVID-19 underlying conditions, COVID-19 outcome, COVID-19 Italy, and COVID-19 Unites States. The protocol of this systematic review and analysis for COVID-19 patients’ data is in accordance with the PRISMA-P (Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols) guidelines.14
Selection and Identification of Relevant Literature
Using the listed inclusion and exclusion criteria, we first sorted the GIB and endoscopy COVID-19 studies by the title and abstract; then, we compiled the papers by relevance and conducted a new selection process by a thorough review of the data (Figure 1). We incorporated studies that reported GIB and/or endoscopic findings in COVID-19 patients. Then, we selected articles on non-GIB COVID-19 patients from Italy and the United States for comparison. From the selected papers, tables were generated for each data set on Microsoft Excel. These tables included the following information for each study (when available): general information about the study (year, location, hospital or city, state and country, publication date), confirmed cases, GIB (general, upper, lower), indication to gastroscopy/GIB manifestation (melena, hematochezia, anemia, hematemesis, etc.), number and type of endoscopies, main upper and lower endoscopy findings, COVID-19 treatments, GIB treatments, outcome (rebleeding, hospitalizations, intensive care unit [ICU] transfers, deaths), respiratory support (supplemental oxygen, noninvasive ventilation/continous positive airway pressure, intubation), general signs and symptoms, GI symptoms, and comorbidities. We compared clinical manifestations, comorbidities, treatment, and outcomes between COVID-19 patients with GIB vs non-GIB COVID-19 patients.
Figure 1.
PRISMA flow diagram.
Inclusion Criteria
The following inclusion criteria were adopted to validate article selection: any study including patients with confirmed diagnosis of COVID-19 through reverse transcriptase -polymerase chain reaction (RT-PCR) test positive) with specified GIB or endoscopic findings; any study with 5 or more patients; any study with all or the majority of patients from Western countries; and no distinction with regard to sex, age, severity of disease, inpatient or outpatient management, data collection date, treatment, and outcome. Confirmed diagnosis of COVID-19 (RT-PCR) patients without GIB is included for comparison with GIB patients.
Exclusion Criteria
The following exclusion criteria were adopted to filter out incomplete data: studies where cases were not confirmed by PCR, studies with fewer than 5 patients, studies which did not distinguish between COVID-19 patients with GIB and COVID-19 patients without GIB, systematic reviews, and meta-analyses. Studies from Eastern countries were also excluded to have more homogeneous data.
Statistical Analysis
The collected data were used to calculate the most common endoscopic findings in the included COVID-19 patients’ studies. Different GIB presentations, common symptoms, comorbidities, treatment strategies, respiratory support, and outcomes were combined and analyzed by weighted analysis methods where applicable. Variables reported in only one study were excluded from statistical analysis. Correlation coefficients were calculated together with regression analysis to establish associations between comorbidities and mortality. The effect of symptoms was reported using weighted analysis, where weights were related to the size of the reported study. Differences in comorbidities and outcomes between COVID-19 patients with and without GIB were computed. SPSS (SPSS Inc, Chicago, IL) was used for this analysis.
Results
UGIB Occurred More Often Than Lower GIB
The studies reporting GIB included a total of 808 patients (Table 1). Six of these studies reported the incidence of patients with GIB in their overall COVID-19 population which amounts to 0.06%. Within the selected 808 patients with endoscopic findings from the 12 studies, 92.7% displayed GIB. Not all studies specified the type or presence of GIB. Among 633 patients, the bleeding was localized in the upper GI tract in 66.0% (418) of patients and in the lower GI tract in 24.8% (157). The remaining 9.2% (58) had unspecified GIB location.
Table 1.
Demographics and Clinical Characteristics of Patients With COVID-19 Reporting GI Bleeding
| Study (n = 808) | González González et al15 | Kuftinec et al16 | Martin et al6 | Massironi et al17 | Mauro et al18 | Melazzini et al19 | Rustgi et al20 | Trindade et al5 | Vanella et al21 | Holzwanger et al22 | Ierardi et al23 | Cavaliere et al24 | Total % (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total # of patients | 83 | 24 | 41 | 38 | 23 | 5 | 146 | 314 | 106 | 11 | 11 | 6 | 100 (808) |
| Mean age | 77 | 65.2 | 68.7 | 69 | 75 | 73 | 67.4 | 70 | 68 | 64 | 65 | 67.8 | 69.7 |
| Male | 61.4 (51) | 83.3 (20) | 66 (27) | 73.7 (28) | 78.3 (18) | 80 (4) | 53.4 (78) | 59.6 (187) | 70.8 (75) | 55 (6) | 63.6 (7) | 50 (3) | 62.4 (504) |
| Female | 38.6 (32) | 16.7 (4) | 34 (14) | 26.3 (10) | 21.7 (5) | 20 (1) | 46.6 (68) | 40.5 (127) | 29.2 (31) | 45 (5) | 36.4 (4) | 50 (3) | 37.6 (304) |
| GI bleed | 100 (83) | 54.2 (13) | 100 (41) | 86.8 (33) | 100 (23) | 100 (5) | 100 (146) | 100 (314) | 59.4 (63) | 100 (11) | 100 (11) | 100 (6) | 92.7 (749) |
| UGIB | 100 (83) | - | 75.6 (31) | 39.5 (15) | 100 (23) | - | - | 68.2 (214) | 38.7 (41) | - | 45.5 (5) | 100 (6) | 66 (418) |
| LGIB | - | - | 24.4 (10) | 21.1 (8) | - | - | - | 31.9 (100) | 20.8 (22) | 100 (11) | 54.5 (6) | - | 24.8 (157) |
| Reason for endoscopy/GI bleeding manifestation (n = 382) | |||||||||||||
| Melena | 68.7 (57) | - | 48.8 (20) | 34.2 (13) | 52 (12) | 100 (5) | 35.3 (6) | - | - | - | - | 66.7 (4) | 47.5 (117) |
| Hematochezia | - | - | 26.8 (11) | 18.4 (7) | - | - | 41.2 (7) | - | - | 100 (11) | - | - | 37.8 (36) |
| Anemia | - | 4.2 (1) | - | 34.2 (13) | - | 100 (5) | 17.6 (3) | - | - | - | - | - | 21 (22) |
| Hematemesis | 32.5 (27) | - | 4.9 (2) | 7.9 (3) | 22 (5) | - | 11.8 (2) | - | - | - | - | 33.3 (2) | 16.7 (41) |
| Coffee-ground emesis | - | - | 4.9 (2) | - | 13 (3) | - | - | - | - | - | - | - | 7.8 (5) |
| Diarrhea | - | 4.2 (1) | - | 10.5 (4) | - | - | - | - | - | - | - | - | 8.1 (5) |
| Endoscopy (n = 298) | |||||||||||||
| EGD | 100 (39) | 75 (20) | 66.7 (10) | 63.2 (28) | 100 (18) | - | 82.4 (14) | 85 (17) | 67 (76) | - | - | - | 81.7 (222) |
| Colonoscopy | - | 29.2 (8) | 6.7 (1) | 52.6 (23) | - | - | 35.3 (6) | 10 (2) | 25.5 (29) | - | - | - | 20.9 (69) |
| Enteroscopy | - | - | - | - | - | - | 5.9 (1) | - | 0.9 (1) | - | - | - | 3.8 (2) |
| Sigmoidoscopy | - | - | 26.7 (4) | - | - | - | 11.8 (2) | 5 (1) | - | - | - | - | 8.7 (7) |
| ERCP | - | 8.3 (2) | - | - | - | - | - | - | 9.4 (11) | - | - | - | 9.2 (13) |
| Main UGI findings (n = 241) | |||||||||||||
| Peptic ulcer | 46.2 (18) | 55.6 (10) | 80 (16) | 20.8 (5) | 44 (8) | 75 (3) | 28.5 (4) | 64.7 (11) | 25.3 (22) | - | - | - | 48.4 (97) |
| Esophagitis/esophageal ulcer | 28.9 (11) | - | 30 (6) | 20.8 (5) | - | - | 14.3 (2) | 5.8 (1) | 42.5 (37) | - | - | - | 17.6 (62) |
| Esophageal varices | 2.6 (1) | 5.6 (1) | - | - | - | - | 7.1 (1) | - | - | - | - | - | 5.5 (3) |
| Erosive gastritis or hemorrhagic gastritis | - | 5.6 (1) | - | 16.6 (4) | 22 (4) | - | 28.6 (4) | 11.7 (2) | 16.1 (14) | - | - | - | 16.6 (29) |
| Mallory-Weiss tear | - | - | - | 4.1 (1) | 11 (2) | - | 7.1 (1) | 5.8 (1) | - | - | - | - | 6.3 (5) |
| Dieulafoy lesions | - | - | - | - | 11 (2) | - | - | - | 1.1 (1) | - | - | - | 2.9 (3) |
| Gastropathy/duodenopathy | - | - | - | - | - | 25 (1) | - | - | 9.2 (8) | - | - | - | 9.9 (9) |
| Normal | - | 5.6 (1) | - | 25 (6) | 6 (1) | - | - | - | 29.9 (26) | - | - | - | 23 (34) |
| Main LGI findings (n = 71) | |||||||||||||
| Rectal ulcer | - | 14.3 (1) | 60 (3) | - | - | - | 33.3 (3) | - | - | - | - | - | 36.4 (7) |
| Colitis—hemorrhagic/lymphocytic/microscopic | - | - | 20 (1) | 20 (4) | - | - | - | 33.3 (1) | - | - | - | - | 30.7 (6) |
| Hemorrhoids | - | 42.9 (3) | - | - | - | - | 11.1 (1) | - | - | - | - | - | 15.6 (4) |
| Diverticular bleeding/diverticulosis | - | 8.6 (1) | 20 (1) | 25 (5) | - | - | 11.1 (1) | 33.3 (1) | - | - | - | - | 25 (9) |
| Colon ischemia | - | - | - | 20 (4) | - | - | - | - | 33.3 (9) | - | - | - | 29.8 (13) |
| Blood without source/LGIB without other abnormalities | - | 14.3 (1) | - | - | - | - | - | - | 11.1 (3) | - | - | - | 11.7 (4) |
| Normal | - | 14.3 (1) | - | 30 (6) | - | - | - | - | 18.5 (5) | - | - | - | 20.5 (12) |
| COVID-19 treatments (n = 719) | |||||||||||||
| Anticoagulants/thromboprophylaxis | - | - | 70.7 (29) | 76 (29) | 78 (18) | 100 (5) | 43.2 (63) | 61.9 (194) | 54.8 (58) | 73 (8) | 90 (10) | - | 59.6 (414) |
| Steroids | - | - | - | 35.9 (14) | - | - | 33.6 (49) | 54.3 (170) | 28 (30) | - | - | - | 43.5 (263) |
| Remdesivir | - | 12.5 (3) | - | 11 (4) | - | - | - | - | - | - | - | - | 11.6 (7) |
| Tocilizumab | - | 16.7 (4) | - | 8 (3) | - | - | - | - | - | - | - | - | 11.4 (7) |
| Hydroxychloroquine | - | 66.7 (16) | - | 47 (18) | - | - | 58.9 (86) | - | 41.4 (44) | - | - | - | 52.1 (164) |
| Azithromycin or other antibiotics | - | - | - | - | - | - | 58.2 (85) | - | 89.1 (94) | - | - | - | 71.2 (179) |
| Ritonavir/lopinavir/antivirals | - | - | - | 26 (10) | - | - | - | - | 45.9 (49) | - | - | - | 40.6 (59) |
| Antiplatelet | - | - | 48.8 (20) | - | 30 (7) | - | - | 43.8 (138) | - | 36 (4) | - | - | 43.3 (168) |
| GI bleeding treatments (n = 664) | |||||||||||||
| Endoscopic interventions | 14.5 (12) | 41.7 (10) | 17.1 (7) | - | 26.1 (6) | 20 (1) | 4.1 (6) | - | - | - | - | - | 6.3 (42) |
| Medical only | 85.5 (71) | 58.3 (14) | 82.9 (34) | - | 73.9 (17) | 80 (4) | 95.9 (140) | 100 (314) | - | 100 (11) | 100 (11) | 100 (6) | 93.7 (622) |
| Interventional radiology | - | - | - | - | 8.7 (2) | - | 2.1 (3) | - | - | 9 (1) | 100 (11) | - | 8.9 (17) |
| Transfusions | - | - | 73.2 (30) | - | - | - | 69.2 (101) | 45.9 (144) | - | 64 (7) | - | 66.7 (4) | 55.2 (286) |
| Proton pump inhibitor | - | - | 90.2 (37) | - | 95.7 (22) | - | - | 37.6 (118) | - | - | - | 100 (6) | 47.7 (183) |
| H2 receptor blocker | - | - | 14.6 (6) | - | - | - | - | 15.2 (48) | - | - | - | - | 15.2 (54) |
| Vasopressor support | - | 66.7 (16) | - | - | 4.3 (1) | - | - | - | - | 82 (9) | - | - | 44.9 (26) |
| Outcome (n = 808) | |||||||||||||
| Rebleeding | - | - | 12.2 (5) | - | 17 (4) | 20 (1) | 1.4 (2) | - | - | 9 (1) | 9 (1) | - | 5.9 (14) |
| Hospitalized | 86.7 (72) | 100 (24) | 100 (41) | 97 (37) | 100 (23) | - | - | 100 (314) | 67 (71) | 100 (11) | - | - | 92.6 (593) |
| ICU transfer | 10.8 (9) | 100 (24) | 46.3 (19) | 21 (8) | - | - | 44.5 (65) | 45.7 (144) | 33 (35) | 90.9 (10) | - | - | 39.9 (313) |
| Death | 26.5 (22) | 29.2 (7) | 24.4 (10) | 13 (5) | 22 (5) | 20 (1) | 35.6 (52) | - | 21.7 (23) | 3 (0) | - | - | 25.4 (125) |
| Respiratory support (n = 249) | |||||||||||||
| Supplemental oxygen (NC/VM/NRB) | - | - | 34 (14) | 28.9 (11) | 47.8 (11) | - | - | - | - | - | - | 83.3 (5) | 38.3 (41) |
| Non-invasive ventilation/CPAP | - | 83.3 (20) | 34 (14) | 23.7 (9) | 35 (8) | - | - | - | - | - | - | - | 40.5 (51) |
| Intubated (invasive ventilation) | - | - | 46.3 (19) | 23.7 (9) | - | - | - | - | 33 (35) | 73 (8) | - | 16.7 (1) | 35.6 (72) |
| Signs and symptoms (n = 409) | |||||||||||||
| Fever | 36.1 (30) | - | 61 (25) | 73.7 (28) | - | 100 (5) | - | - | - | - | - | 100 (6) | 54.3 (94) |
| Shortness of breath | 39.8 (33) | - | 54 (22) | 70.3 (27) | - | 100 (5) | - | - | 81.1 (73) | - | - | 100 (6) | 64.1 (179) |
| Cough | 30.1 (25) | - | 64 (26) | 42.1 (16) | - | 100 (5) | - | - | - | - | - | - | 43.2 (72) |
| Loss of taste (ageusia) | 2.4 (2) | - | 5 (2) | - | - | - | - | - | - | - | - | - | 2.5 (4) |
| Loss of smell (anosmia) | 2.4 (2) | - | - | - | - | - | - | - | - | - | - | - | 1.6 (2) |
| Abdominal pain | 30.1 (25) | - | 2 (1) | - | - | - | - | - | 27.8 (25) | - | - | - | 24 (55) |
| Nausea | - | - | - | 12.5 (5) | - | - | 28.1 (41) | - | 17.8 (16) | - | - | - | 22.3 (65) |
| Vomiting | 37.3 (31) | - | - | 15.2 (6) | - | - | 21.2 (31) | - | 14.4 (13) | - | - | - | 22.2 (83) |
| Diarrhea | 27.7 (23) | - | 27 (11) | 34.2 (13) | - | - | 30.1 (44) | - | 15.6 (14) | - | - | - | 26 (108) |
| Anorexia | - | - | 17 (7) | - | - | - | - | - | 11.1 (10) | - | - | - | 12.7 (19) |
| Comorbidities/risk factors (n = 766) | |||||||||||||
| Respiratory disease | - | - | 12 (5) | - | - | - | - | - | 10.5 (11) | - | - | - | 10.9 (16) |
| Hypertension | 68.7 (57) | - | 66 (27) | 48.6 (18) | 70 (16) | 80 (4) | - | 62 (195) | 52.4 (55) | - | - | - | 61.1 (373) |
| Cardiac disease | - | - | 24 (10) | 42.1 (16) | 39 (9) | - | - | - | 16.2 (17) | - | - | - | 25 (52) |
| Metabolic, diabetes | 34.9 (29) | - | 37 (15) | 31.6 (12) | 48 (11) | - | - | 35.6 (112) | 21.9 (23) | - | - | - | 33.4 (202) |
| Obesity | 13.3 (11) | - | - | 23.5 (9) | 9 (2) | - | - | - | 11.4 (12) | - | - | - | 13.7 (34) |
| Smoking | 8.4 (7) | - | - | 28.1 (11) | - | - | 27 (39) | - | - | - | - | - | 21.4 (57) |
| H/O IBD | - | - | 5 (2) | 4 (2) | - | - | - | 0.6 (2) | - | - | - | - | 1.4 (6) |
| H/O liver disease | 12 (10) | - | 5 (2) | 16.2 (6) | - | - | - | - | - | - | - | - | 11.2 (18) |
| H/O GERD/PUD | 5 (4) | - | - | - | - | - | - | 2.9 (9) | - | - | - | - | 3.3 (13) |
| Chronic kidney disease | 24.1 (20) | - | 22 (9) | - | 17 (4) | - | - | 8.9 (28) | 14.3 (15) | - | - | - | 13.4 (76) |
| Cancer | - | - | 27 (11) | - | 13 (3) | - | - | - | 8.6 (9) | - | - | - | 13.6 (23) |
| Prior anticoagulant therapy | - | - | 39 (16) | - | - | - | 43.2 (63) | - | - | - | - | - | 42.3 (79) |
| Prior steroid therapy | - | - | 15 (6) | - | - | - | 33.6 (49) | - | - | - | - | - | 29.5 (55) |
| Prior proton pump inhibitors< | - | - | - | - | - | - | 63.7 (93) | 37.6 (118) | - | - | - | - | 45.9 (211) |
| Cirrhosis | - | - | - | - | 9 (2) | - | - | - | 8.6 (9) | - | - | - | 8 (11) |
| Prior GI bleed | - | - | - | - | - | - | - | 2.9 (9) | - | 9 (1) | - | - | 3.1 (10) |
ERCP, endoscopic retrograde cholangiopancreatography; GERD, gastro-esophageal reflux disease; H/O, history of; IBD, Inflammtory bowel disease; LGI, lower gastrointestinal; LGIB, lower gastrointestinal bleeding; NC, nasal cannula; NRB, non rebreather mask; PUD, peptic ulcer disease; VM, venturi mask.
From the 808 patients in all the studies reporting GIB, the mean age was 69.7 years compared to 62.3 in the control group (Tables 1 and 2). The control population consists of a large general COVID-19 cohort, of which the characteristics are presented in Table 2 (non-GIB cohort). In the GIB cohort, 62.4% were males and 37.6% were females, while the distribution in the control cohort was 52.6% and 47.4%, respectively. In the GIB cohort, men are older than in the control group (P < .001).
Table 2.
Demographics and Clinical Characteristics of Patients With COVID-19 Not Reporting GI Bleeding
| Study | Balla et al25 | Incerti et al26 | Schettino et al27 | Colaneri et al28 | Aghemo et al29 | Vena et al30 | Total % (N) |
|---|---|---|---|---|---|---|---|
| Total # of patients | 217 | 17,086 | 190 | 44 | 325 | 317 | 100 (18,179) |
| Mean age | 63.13 (137) | 62 (10,593) | 67 (127) | 67.5 (30) | 67 (218) | 71 (225) | 62.3 |
| Male | 47 (102) | 51.9 (8868) | 66.84 (127) | 63.64 (28) | 68.62 (223) | 67.19 (213) | 52.6 (9561) |
| Female | 53 (115) | 48.1 (8218) | 33.16 (63) | 36.36 (16) | 31.38 (102) | 32.81 (104) | 47.4 (8618) |
| Respiratory support | |||||||
| Supplemental oxygen (NC/VM/NRB) | - | - | 80.95 (154) | 38.64 (17) | 45.99 (149) | - | 57.3 (320) |
| Noninvasive ventilation/CPAP | - | - | 40 (76) | - | 7.69 (25) | 35.02 (111) | 25.5 (212) |
| Intubated (invasive ventilation) | - | - | 11.05 (21) | - | 34.59 (112) | 18.93 (60) | 23.2 (193) |
| Severity/hospitalizations/outcome | |||||||
| Hospitalized | 100 (217) | 100 (17,086) | 88.57 (168) | 100 (44) | 100 (325) | 86.75 (275) | 99.6 (18,115) |
| ICU transfer | - | - | 13.21 (25) | 6.82 (3) | 17.85 (58) | 20.5 (65) | 17.2 (151) |
| Death | 10.6 (23) | 15.8 (2700) | 21.58 (41) | 4.5 (2) | 21.85 (71) | 43.64 (138) | 16.4 (2975) |
| Comorbidities/risk factors/medical history | |||||||
| Respiratory disease | 39.17 (85) | 17 (2905) | - | 4.55 (2) | 10.15 (33) | 5.68 (18) | 16.9 (3043) |
| Hypertension | 70.97 (154) | 58.6 (10,012) | 58.95 (112) | 34.09 (15) | 51.38 (167) | 47 (149) | 58.4 (10,609) |
| Cardiac disease | 41.94 (91) | 26.6 (4545) | 19.47 (37) | 25 (11) | 17.85 (58) | - | 26.5 (4742) |
| Metabolic, diabetes | 43.32 (94) | 33.8 (5775) | 21.58 (41) | 15.91 (7) | 22.46 (73) | 15.46 (49) | 33.2 (6039) |
| Obesity | 57.14 (124) | - | 18.52 (35) | 8.5 (4) | 25.1 (82) | - | 31.5 (244) |
| Smoking | 38.25 (83) | - | - | - | 87.23 (283) | - | 67.6 (366) |
| A fib/arrhythmia | - | - | 6.32 (12) | - | 10.46 (34) | - | 8.9 (46) |
| H/O liver disease | 7.3 (16) | - | - | 4.55 (2) | - | - | 6.8 (18) |
| Chronic kidney disease | - | 20.7 (3537) | 3.16 (6) | 2.27 (1) | 8.95 (29) | 6.94 (22) | 20 (3595) |
| Cancer | 8.3 (18) | 12.3 (2102) | 3.16 (6) | - | - | 3.47 (11) | 12 (2137) |
| Stroke | - | 10.5 (1794) | 6.84 (13) | - | - | - | 10.5 (1807) |
| Oncologic | - | - | - | 13.64 (6) | - | 3.79 (12) | 5 (18) |
| Other neurological disease and mental disorder | - | 10.2 (1743) | 6.32 (12) | - | - | 8.83 (28) | 10.1 (1783) |
GIB Was Strongly Correlated With GI Symptoms, Diabetes, Cancer, Hypertension, and Heart Disease But Not With Anticoagulants
Several symptoms, comorbidities, and treatments were associated with GIB. Positive associations were found with diarrhea (P < .001, r = 0.884), loss of taste (P < .001, r = 0.791), nausea (P < .001, r = 0.734), and vomiting (P < .001, r = 0.651). Negative associations with shortness of breath (P < .001, r = −0.770) and fever (P < .001, r = −0.524) were noted. As for comorbidities, positive correlations were found between GIB and diabetes (P < .001, r = 0.915), cancer (P < .001, r = 0.844), hypertension (P < .001, r = 0.754), and cardiac disease (P < .001, r = 0.714).
Melena Was the Most Common Form of Reported GIB Manifestation
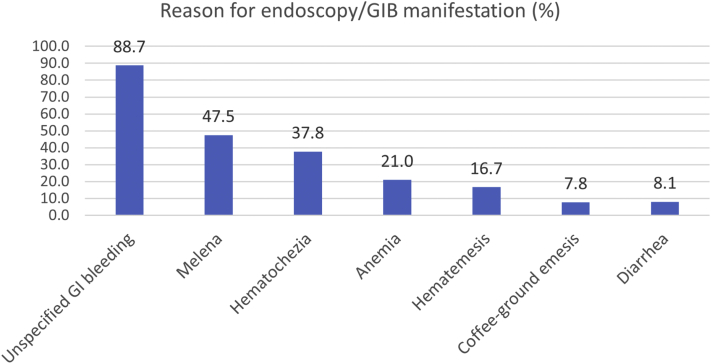
Endoscopic procedures were performed for varied reasons (Table 1). Only 9 out of the 12 studies reporting GIB also reported information regarding endoscopies. Most procedures were carried out due to GIB. The known GIB manifestations and reasons for endoscopy were melena (47.5%), hematochezia (37.8%), anemia (21.0%), hematemesis (16.7%), diarrhea (8.1%), and coffee-ground emesis (7.8%) (Figure 2). Performed endoscopic procedures were esophagogastroduodenoscopy (EGD) (81.7%), colonoscopy (20.9%), endoscopic retrograde cholangiopancreatography (9.2%), sigmoidoscopy (8.7%), and enteroscopy (3.8%).
Figure 2.
Clinical manifestations/reason for endoscopy in the GIB patients with COVID-19.
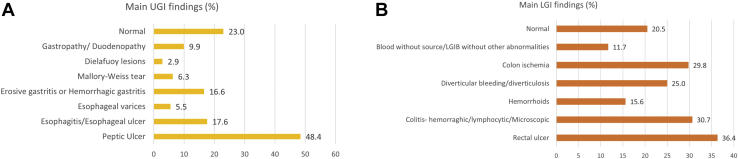
Ulcer Was the Main Finding in Both Upper and Lower Endoscopies
The upper and lower endoscopies revealed a wide spectrum of findings (Table 1). The upper endoscopy findings were the following: peptic ulcer (48.4%), esophagitis or esophageal ulcer (17.6%), erosive or hemorrhagic gastritis (16.6%), gastropathy or duodenopathy (9.9%), Mallory-Weiss tears (6.3%), esophageal varices (5.5%), and Dieulafoy lesions (2.9%), while 23% of upper endoscopies had no abnormal findings (Figure 3A). As for the lower endoscopies, the most reported findings were rectal ulcer (36.4%), hemorrhagic, lymphocytic, or microscopic colitis (30.7%), colon ischemia (29.8%), diverticular bleeding or diverticulosis (25.0%), hemorrhoids (15.6%), and blood without source (11.7%; Figure 3B). In 20.5% of cases, no abnormal findings were identified.
Figure 3.
Endoscopic gastrointestinal findings in the GIB patients. (A) Upper gastrointestinal findings in the GIB patients. (B) Lower gastrointestinal (LGI) findings in the GIB patients.
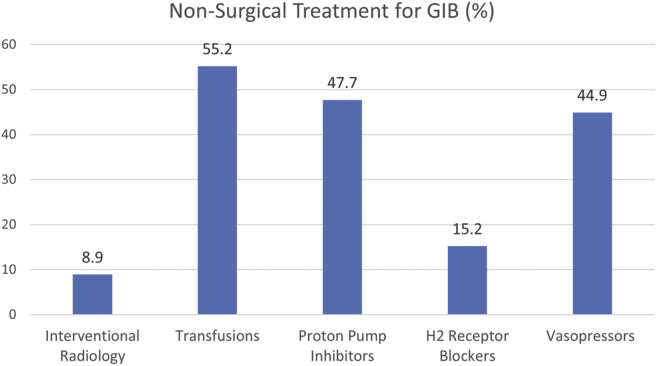
Endoscopic Treatment Was Rarely Necessary to Treat GIB
In the cohort of 808 GIB patients, endoscopic treatment (in the form of cautery, clips, and others) was only performed in 6% of the cases (Table 1). In the remaining 94%, treatment for bleeding was either medical only or not performed. The most frequent nonendoscopic treatments for GIB were the following: transfusions (55.2%), proton pump inhibitor (47.7%), vasopressor support (44.9%), H2 receptor blockers (15.2%), and interventional radiology (8.9%; Figure 4). Different kinds of COVID-19 treatments were also reported as follows: azithromycin or other antibiotics (71.2%), anticoagulants, low-molecular-weight heparin, or thromboprophylaxis (59.6%), hydroxychloroquine (52.1%), steroids (43.5%), antiplatelets (43.3%), remdesivir (11.6%), and tocilizumab (11.4%; Figure A1).
Figure 4.
Nonsurgical treatments for gastrointestinal bleeding in patients with COVID-19.
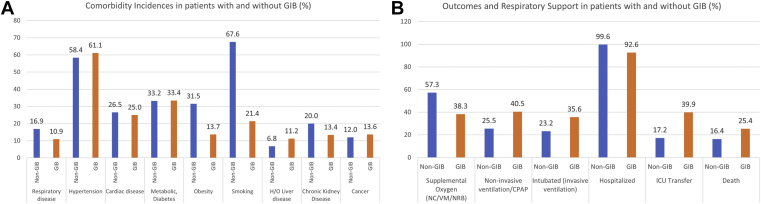
The GIB Cohort Had Higher Incidence of Hypertension, Liver Disease, and Cancer
The GIB cohort and the control cohort had different incidences of comorbidities (Figure 5A), most of which were significant. The GIB cohort had a higher incidence of hypertension (61.1% vs 58.4%, P < .001), liver disease (11.2% vs 6.8%, P < .001), and cancer (7.7% vs 1.5%, P = .006). The control group, on the other hand, had higher prevalence of respiratory disease (16.9% vs 10.9%, P < .001), obesity (31.5% vs 13.7%, P < .001), smoking (67.6% vs 21.4%, P < .001), and chronic kidney disease (20.0% vs 13.4%, P < .001).
Figure 5.
Comparing comorbidities, outcomes, and respiratory support between GIB and non-GIB COVID-19 patients. (A) Differences in comorbidities among the GIB and non-GIB patients. (B) Differences in respiratory support and outcomes among GIB and non-GIB COVID-19 patients.
Death, ICU Transfer, Ventilation, and Intubation Were More Common in the GIB Cohort
Death had an incidence of 25.4% in the GIB cohort, vs 16.4% in the control group (P < .001) (Tables 1 and 2). It is worth noting that most patients in both cohorts were hospitalized (92.6% in the GIB cohort and 99.6% in the control cohort). ICU transfer, however, was much higher in the GIB cohort, with an incidence of 39.9% vs 17.2% in the control group. Respiratory support rates were also different, with supplemental oxygen being more common in the control group (57.3% vs 38.3% in GIB) as opposed to noninvasive ventilation (40.5% in the GIB cohort vs 25.5% in the controls) and intubation (35.6% vs 23.2%) (Figure 5B). There was also a 5.9% incidence of rebleeding in the GIB cohort. All these differences in outcomes were significant (P < .001).
Death Was Positively Associated With Hypertension, EGD, and Hematochezia in GIB Patients
Several associations with death were found in the GIB cohort. Among all the comorbidities, death was strongly associated with hypertension (P < .001, r = 0.814). Other strong positive correlations with death were EGD (P < .001, r = 0.591) and hematochezia (P < .001, r = 0.646).
Discussion
GIB has been identified in some COVID-19 patients as a GI manifestation. In this systematic review, we analyzed the findings of 12 studies which reported GIB and endoscopic findings in COVID-19 patients. These studies included 808 patients. The data retrieved from the GIB cohort were compared with a control group consisting of 18,179 COVID-19 mostly hospitalized patients. GIB was found to have an incidence of about 0.06% in COVID-19 patients. This rate was calculated from the studies from which we extracted and constructed our GIB cohort. This rate is slightly greater than the incidence in the overall population, which is estimated around 0.05% (0.06% for UGIB and 0.03% for lower GIB ).31 In our cohort, as in the overall population, bleeding in the upper GI tract occurred more often than that in the lower GI tract. Because not all the analyzed studies provided a specific subdivision of upper and lower GIB, we could not calculate an exact ratio, but from the available data, it is approximately 7:3. Additionally, males were found to be more susceptible to GIB than females. This is also confirmed by the studies, which state that both UGIB and lower GIB are more common in men than those in women.31,32 As such, it seems unlikely that COVID-19 affects GIB or its upper or lower distribution since the profile in the general population fits the one in the GIB COVID-19 cohort. It is worth noting, however, that COVID-19 associates with coagulopathies and veinous thromboembolism and that many patients receive anticoagulants as part of their treatment regimen. While anticoagulants’ use is known to associate with bleeding disorders, including in the GI tract, such an association was not confirmed in our cohort. Consequently, neither COVID-19 nor anticoagulants’ use can be suspected to be causative of GIB in the analyzed cohort.
Since UGIB was more common than lower GIB, it follows that the most common type of endoscopy was EGD and the most common bleeding presentation was melena, which mainly occurs when the bleeding lesions are located above the ligament of Treitz.31,33 Hematemesis, which was the fourth most common presentation in the papers included in the study, confirms that the bleeding is mostly located in the upper GI tract and that the hemorrhage is large, often leading to the loss of large amounts of blood and ultimately to anemia.33 Altogether, melena and hematemesis usually derive from peptic ulcers, esophagitis, varices, Mallory-Weiss tears, and other upper GI etiologies. Finally, hematochezia, which was the second most common presentation, indicates lower GIB.
Endoscopic findings revealed that ulcers were the most common lesions in both upper and lower GIB patients, followed by varied inflammations such as esophagitis, gastritis, and colitis. It is unclear whether the GI damage present in these patients is caused or exacerbated by SARS-CoV-2. At least from the inflammatory conditions, the viral infection might have been a factor, if not the trigger. Since we do not have access to these patients’ medical records prior to infection, it is difficult to rule whether the co-occurrence is causative, cumulative, or coincidental.
In patients where the association might be judged as causative, the virus might lead to GIB through direct mucosal damage followed by a strong immune response and indirect consequences of viral-induced hypoxia and coagulopathy.8 Indeed, SARS-CoV-2 can bind to the angiotensin-converting enzyme 2 (ACE2) receptors present on cells as the main route of access.34 These receptors are abundant on the epithelial cells lining not only the lungs, but also the GI tract. By binding to these receptors, the virus reduces their action, increasing the concentration of angiotensin II, which is a vasoconstrictor. This leads to thrombosis, oxidative stress, and inflammation.34 Disturbances in the capillaries can also cause hypoxia, which triggers cytokines release, creating a positive feedback loop that leads to more capillary dysfunction and more severe hypoxia.34 As for the mucosal damage and immune response, we know that SARS-CoV-2 activates T cell lymphocytes, which induce an abnormal release of proinflammatory cytokines such as interleukin-1, interleukin-6, and interferon-gamma.35 This immune response can lead to organ damage and could be associated with some of the aforementioned endoscopic findings. For instance, IL-6 and tumor necrosis factor- alpha (directly correlated with the severity of COVID-19) have been shown to contribute to the onset and maintenance of ulcers in later stages of tissue inflammation.36 A similar mechanism could be taking place in the GI tract to cause peptic ulcers.
Steroids’ use was positively associated with GIB, a finding consistent with the previous study by Narum et al.37 Interestingly, among all the patients who exhibited GIB and/or underwent endoscopy, only 6% required an endoscopic treatment. This means that 94% of the patients fully recovered with medical treatment or no treatment at all. The medical treatments mostly consisted of blood transfusions, proton pump inhibitors, and vasopressor support, which are used routinely to deal with blood loss and to reduce stomach acid production, whether or not a COVID-19 background is present.38
Overall, the GIB cohort exhibited worse outcomes than the control group. This is reflected by the higher incidence of ventilation, intubations, ICU transfers, and deaths. Furthermore, our analysis showed a direct correlation between death and both EGD (P = .000, r = 0.591) and hematochezia (P = .0001, r = 0.646). It was reported that patients undergoing endoscopy secondary to UGIB in the COVID-19 era (regardless of whether or not they are infected) were more likely to die than before the pandemic.13 These results were attributed to 2 main factors: patients’ avoidance of hospitals to reduce exposure to the virus during the pandemic and hospital staff and resource relocation to respond to more severe COVID-19 cases, thus leading to a reduction and delay in endoscopic procedures, leading to the exacerbation of patients’ conditions.13
The reduced survival in GIB patients could also be linked to comorbidities. According to our analysis, in the GIB cohort, death was positively associated with hypertension. Hypertension was found to be directly correlated with GIB and more prevalent in the GIB cohort than that in the control group. According to a recent meta-analysis, high blood pressure was independently associated with increased mortality in COVID-19 patients.39 A specific mechanism connecting hypertension with COVID-19–related death was not identified. Of note, many antihypertensive drugs can increase the expression of ACE2, thus increasing the number of entry points for the virus. By contrast, other researchers proposed a diametrically opposite reasoning, stating that hypertensive patients might have reduced ACE2 expression, which would lead to a higher concentration of angiotensin upon binding with SARS-CoV-2, and consequent COVID-19 development.39
Other variables that were associated with GIB or had a higher incidence in the GIB cohort were GI symptoms (diarrhea, nausea, vomiting), loss of taste, diabetes, liver disease, cancer, and heart disease. Diabetes has been associated with an increased incidence of GIB in diabetic ketoacidosis patients.40 Patients with liver cirrhosis are known to be at a higher risk of GIB from different lesions, including gastroesophageal varices, which were identified in this analysis.41 Same goes for cancer patients, who are often subject to GIB.42 Of note, the studies included in the analysis did not specify the types of cancer detected in their cohorts. Finally, heart disease is normally considered a risk factor for GIB because these patients take anticoagulant or antiplatelet medications.43,44 However, in our data analysis, no correlation between the use of anticoagulants/antiplatelets and GIB was found.
The main limitation of this study is certainly the heterogeneity of the data reported in the included studies. Indeed, all collected data were in an aggregated format. This means that calculations reporting associations between variables, such as correlations with GIB and death, have a more potential statistical error than would be yielded by an analysis of deidentified single patients’ data. Moreover, the studies were published by different authors, who differently decided (1) what data to collect, (2) how to collect them, and (3) what information to share. Some papers did not report the reason for the endoscopy. Not every research article (except Massaroni et al) mentioned the reason for the endoscopy. Massironi et al17 evaluated GIB for the COVID-19–positive patients including all patients who were managed in 7 hospitals in Lombardy, Northern Italy, and underwent endoscopic examination between February 1 and April 20, 2020: among these patients, GIB was detected in 20/24 EGDs and 20/20 colonoscopies. Reasons for EGD were melena in 10 (42%), anemia in 8 (33.4%), and hematemesis in 2 (8.3%) cases. Reasons for colonoscopies were bloody diarrhea in 5 (25%), hematochezia in 8 (40%), melena with negative EGD in 4 (20%), and anemia in 3 (15%) cases. The main findings at EGD consisted of esophagitis in 5 cases (20.8%) (2 Los Angeles grade B, 2 Los Angeles grade C, and 1 Los Angeles grade D), bulbar ulcer in 5 cases (20.8%) (2 Forrest II a, 2 Forrest II c, and 1 Forrest I b), erosive gastritis in 4 cases (16.6%), neoplasm in 2 cases (8.3%), and Mallory Weiss in 1 case (4.1%). The main findings at colonoscopy were segmental colitis associated with diverticulosis in 5 cases (25%), ischemic colitis in 4 cases (20%), diffuse hemorrhagic colitis with a mild edematous mucosa and multiple red mucosal spots in 1 case, and neoplasm in 1 case. For other studies, it was difficult to estimate what percent of suspected GIB patients have endoscopically confirmed GIB. Furthermore, for this reason, the analysis of several comorbidities, treatments, and symptoms was made impossible by the fact that not all authors reported the same variables.
GIB in patients with SARS-CoV-2 poses unique challenges, particularly for endoscopists and other health care staff, because of the potential for aerosol spread. Not every GIB may have been reported at the pandemic's peak. Due to the risk of transmission of the virus, shortage of medical facilities, avoidance of preventive care, and overwhelming burden on the health care workers, there was a significant delay in patient care. All these factors may have cumulatively underestimated the presence of these bleeds and significantly delayed patients’ care. It was difficult to judge the nature of GI symptoms. We could not evaluate the severity and duration of GI symptoms from the currently available data. Additionally, some GI symptoms might not be caused by SARS-CoV-2 infection.
Prospective studies that use patients’ medical records prior to COVID-19 and follow their symptoms and prognosis after infection are needed to establish the potential role of COVID-19 in the onset of GIB and whether such an infection would speed up GIB occurrence in patients with predisposing conditions or worsen the outcome when GIB is preceding the infection. Studies describing the percent of patients who had been consulted for GIB or those who had suspected GIB but could not get an upper endoscopy along with the number of days health care was delayed in patients with suspected GIB are required. The role of anticoagulants needs also further investigation as it is part of COVID-19 patients’ treatment and known to cause GIB.
In conclusion, we found that the incidence of GIB in COVID-19 patients is similar to that estimated in the overall population, with men being more susceptible than women and melena being the most common presentation. Nonetheless, patients with GIB were more likely to die than non-GIB COVID-19 control patients. Common endoscopic findings in GIB COVID-19 patients were ulcers, esophagitis, gastritis, and colitis. GIB was not found to be associated with anticoagulants’ use. There was a higher incidence and association of GI symptoms, diabetes, heart disease, hypertension, liver disease, and cancer in the GIB cohort. The latter 3 were positively associated with death in the GIB cohort.
Acknowledgments:
The authors appreciate the work and invaluable and enthusiastic support of all health care providers in this COVID-19 pandemic. They also would like to thank the funding agency.
Authors' Contributions:
Hassan Ashktorab and Hassan Brim contributed to study concept and design, analysis and interpretation of data, and drafting of the manuscript; Tiziano Russo and Lakshmi G. Chirumamilla contributed to acquisition of data and analysis and interpretation of data drafting of the manuscript; Gholamreza Oskrochi. contributed to interpretation of data and statistical analysis; Adeyinka O. Laiyemo and Giovanni Latella contributed to critical revision of the manuscript for important intellectual content.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: This project was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: They will be made available to other researchers upon request.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.02.021.
Supplementary Materials
References
- 1.WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/ Accessed Februray 12, 2022.
- 2.Singhavi H., Pai A., Mair M., et al. SARS-Cov2: a meta-analysis of symptom distribution by continent in 7310 adult COVID-19 infected patients. Virusdisease. 2021;32:400–409. doi: 10.1007/s13337-021-00699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groff A., Kavanaugh M., Ramgobin D., et al. Gastrointestinal manifestations of COVID-19: a review of what we know. Ochsner J. 2021;21:177–180. doi: 10.31486/toj.20.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal U., Anwar H., Siddiqui H.U., et al. Acute gastrointestinal bleeding in COVID-19 patients: a systematic review and meta-analysis. Clin Endosc. 2021;54:534. doi: 10.5946/ce.2021.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trindade A.J., Izard S., Coppa K., et al. Gastrointestinal bleeding in hospitalized COVID-19 patients: a propensity score matched cohort study. J Intern Med. 2021;289:887–894. doi: 10.1111/joim.13232. [DOI] [PubMed] [Google Scholar]
- 6.Martin T.A., Wan D.W., Hajifathalian K., et al. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case-control study. Am J Gastroenterol. 2020;115:1609–1616. doi: 10.14309/ajg.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattioli M., Benfaremo D., Mancini M., et al. Safety of intermediate dose of low molecular weight heparin in COVID-19 patients. J Thromb Thrombolysis. 2021;51:286–292. doi: 10.1007/s11239-020-02243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dioscoridi L., Giannetti A. A “double-hit” damage mechanism can explain self-limited GI bleeding in COVID-19 pneumonia. Gastrointest Endosc. 2021;93:1192. doi: 10.1016/j.gie.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau J.Y., Yu Y., Tang R.S., et al. Timing of endoscopy for acute upper gastrointestinal bleeding. N Engl J Med. 2020;382:1299–1308. doi: 10.1056/NEJMoa1912484. [DOI] [PubMed] [Google Scholar]
- 10.Repici A., Pace F., Gabbiadini R., et al. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy. Gastroenterology. 2020;159:363–366.e3. doi: 10.1053/j.gastro.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Ovidio V., Lucidi C., Bruno G., et al. A snapshot of urgent upper gastrointestinal endoscopy care during the Covid-19 outbreak in Italy. J Gastroenterol Hepatol. 2020;35:1839–1840. doi: 10.1111/jgh.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salerno R., Conti C.B., De Silvestri A., et al. The impact of covid-19 pandemic on urgent endoscopy in Italy: a nation-wide multicenter study. Scand J Gastroenterol. 2020;55:870–876. doi: 10.1080/00365521.2020.1782466. [DOI] [PubMed] [Google Scholar]
- 13.Tavabie O.D., Clough J.N., Blackwell J., et al. Reduced survival after upper gastrointestinal bleed endoscopy in the COVID-19 era is a secondary effect of the response to the global pandemic: a retrospective cohort study. Frontline Gastroenterol. 2021;12:279–287. doi: 10.1136/flgastro-2020-101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González González R., Jacob J., Miró Ò., et al. Incidence, clinical characteristics, risk factors, and outcomes of upper gastrointestinal bleeding in patients with COVID-19: results of the UMC-19-S12. J Clin Gastroenterol. 2022;56:e38–e46. doi: 10.1097/MCG.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 16.Kuftinec G., Elmunzer B.J., Amin S. The role of endoscopy and findings in COVID-19 patients, an early North American cohort. BMC Gastroenterol. 2021;21:1–7. doi: 10.1186/s12876-021-01796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massironi S., Viganò C., Dioscoridi L., et al. Endoscopic findings in patients infected with 2019 novel coronavirus in Lombardy, Italy. Clin Gastroenterol Hepatol. 2020;18:2375–2377. doi: 10.1016/j.cgh.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauro A., De Grazia F., Lenti M.V., et al. Upper gastrointestinal bleeding in COVID-19 inpatients: incidence and management in a multicenter experience from Northern Italy. Clin Res Hepatol Gastroenterol. 2021;45:101521. doi: 10.1016/j.clinre.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melazzini F., Lenti M.V., Mauro A., et al. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115:1139–1140. doi: 10.14309/ajg.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rustgi S.D., Yang J.Y., Luther S., et al. Anticoagulation does not increase risk of mortality or ICU admission in hospitalized COVID-19 patients with gastrointestinal bleeding: results from a New York Health System. Clin Res Hepatol Gastroenterol. 2021;45:101602. doi: 10.1016/j.clinre.2020.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanella G., Capurso G., Burti C., et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzwanger E.A., Bilal M., Stallwood C.G., et al. Acute lower gastrointestinal bleeding during the COVID-19 pandemic–less is more! Endoscopy. 2020;52:816–817. doi: 10.1055/a-1194-4864. [DOI] [PubMed] [Google Scholar]
- 23.Ierardi A.M., Del Giudice C., Coppola A., et al. Gastrointestinal hemorrhages in patients with COVID-19 managed with transarterial embolization. Am J Gastroenterol. 2021;116:838–840. doi: 10.14309/ajg.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavaliere K., Levine C., Wander P., et al. Management of upper GI bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc. 2020;92:454. doi: 10.1016/j.gie.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balla M., Merugu G., Nesheiwat Z., et al. Epidemiological and clinical characteristics of 217 COVID-19 patients in Northwest Ohio, United States. Cureus. 2021;13 doi: 10.7759/cureus.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Incerti D., Rizzo S., Li X., et al. Prognostic model to identify and quantify risk factors for mortality among hospitalised patients with COVID-19 in the USA. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-047121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schettino M., Pellegrini L., Picascia D., et al. Clinical characteristics of COVID-19 patients with gastrointestinal symptoms in Northern Italy: a single-center cohort study. Am J Gastroenterol. 2021;116:306–310. doi: 10.14309/ajg.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 28.Colaneri M., Sacchi P., Zuccaro V., et al. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Eurosurveillance. 2020;25:2000460. doi: 10.2807/1560-7917.ES.2020.25.16.2000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghemo A., Piovani D., Parigi T.L., et al. COVID-19 digestive system involvement and clinical outcomes in a large academic hospital in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18:2366–2368.e3. doi: 10.1016/j.cgh.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vena A., Giacobbe D.R., Di Biagio A., et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiGregorio A.M., Alvey H. StatPearls [internet] StatPearls Publishing; Treasure Island: 2021. Gastrointestinal bleeding. [Google Scholar]
- 32.Elghuel A. The characteristics of adults with upper gastrointestinal bleeding admitted to Tripoli Medical Center: a retrospective case-series analysis. Libyan J Med. 2011;6 doi: 10.3402/ljm.v6i0.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson I.D. In: Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Walker H.K., Hall W.D., Hurst J.W., editors. Butterworths; Boston: 1990. Hematemesis, melena, and hematochezia. Chapter 85. [PubMed] [Google Scholar]
- 34.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol Rep. 2021;9 doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Geng X., Tan Y., et al. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gefen A., Ousey K. COVID-19: pressure ulcers, pain and the cytokine storm. J Wound Care. 2020;29:540–542. doi: 10.12968/jowc.2020.29.10.540. [DOI] [PubMed] [Google Scholar]
- 37.Narum S., Westergren T., Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta-analysis. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gastrointestinal Bleeding. Accessed April 4, 2022. https://www.mayoclinic.org/diseases-conditions/gastrointestinal-bleeding/diagnosis-treatment/drc-20372732.
- 39.Du Y., Zhou N., Zha W., et al. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: a meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31:745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badipatla K.R., Jadhav P., Vaddigiri S., et al. Predictors of acute gastrointestinal bleeding in diabetic ketoacidosis: a retrospective observational study in minority population. Gastroenterol Rep. 2017;5:293–297. doi: 10.1093/gastro/gox006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odelowo O.O., Smoot D.T., Kim K. Upper gastrointestinal bleeding in patients with liver cirrhosis. J Natl Med Assoc. 2002;94:712. [PMC free article] [PubMed] [Google Scholar]
- 42.Yarris J.P., Warden C.R. Gastrointestinal bleeding in the cancer patient. Emerg Med Clin. 2009;27:363–379. doi: 10.1016/j.emc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Schrutka L., Seirer B., Duca F., et al. Patients with heart failure and preserved ejection fraction are at risk of gastrointestinal bleeding. J Clin Med. 2019;8:1240. doi: 10.3390/jcm8081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thanapirom K., Ridtitid W., Rerknimitr R., et al. Outcome of acute upper gastrointestinal bleeding in patients with coronary artery disease: a matched case–control study. Saudi J Gastroenterol. 2016;22:203. doi: 10.4103/1319-3767.182452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.