Abstract
AIM
To compare the changes in the objective visual quality of patients with low and moderate myopia postoperatively after transepithelial photorefractive keratectomy using the smart pulse technology (SMART) and femtosecond laser in situ keratomileusis (FS-LASIK).
METHODS
Corneal higher-order aberrations (HOAs), horizontal coma, vertical coma and spherical aberration were measured using Pentacam, and cutoff for modulation transfer function (MTF cutoff), objective scatter index (OSI) and Strehl ratio (SR) was measured using an optical quality analysis system (OQAS-II), before and after operation at 1, 3, and 6mo, and data were analyzed by repeated measurement two-way analysis of variance.
RESULTS
The difference in uncorrected distance visual acuity between SMART and FS-LASIK was statistically significant only 1wk postoperatively. Approximately 86.36% and 80.69% of patients with spherical equivalent (SE) in ±0.50 D were observed in the SMART and FS-LASIK groups, respectively. No significant difference was observed in SE between the two groups (P=0.509). The HOAs increased postoperatively compared with those before surgery in both groups (P<0.05). No significant difference in HOA, corneal horizontal coma, spherical aberration, ΔHOA, Δhorizontal coma, and Δspherical aberration were observed between the two group (P>0.05). Corneal vertical coma and Δcorneal vertical coma in the FS-LASIK group were higher than those in the SMART group (P<0.05). The OSI of both groups at 1mo after surgery was higher than that before surgery (P<0.05). At 3 and 6mo postoperatively, the OSI in the FS-LASIK group was slightly higher than that in the SMART group (P=0.040 and 0.047, respectively). At 6mo after surgery, the MTF cutoff was statistically significant different between the two groups (P=0.026). No significant difference in SR between the FS-LASIK and SMART groups was observed at 1, 3, and 6mo postoperatively (P>0.05).
CONCLUSION
The HOAs increase and visual quality is delayed in both groups postoperatively, and the long-term objective visual quality after SMART is slightly better than that after FS-LASIK.
Keywords: myopia, FS-LASIK, higher-order aberrations, visual quality, smart pulse technology
INTRODUCTION
With the rapid development of equipment and technology and continuation in upgradement in corneal refractive surgery, the safety and effectiveness of surgery have been significantly improved, and satisfactory clinical results have been achieved[1]–[2]. The therapeutic effect of corneal refractive surgery is evaluated not only to meet the recovery of vision, but also to pursue the improvement of visual quality. It can effectively avoid the interference and decline of postoperative visual quality, improve the comfort and satisfaction of patients, and improve their quality of life. Although the postoperative uncorrected visual acuity reached 1.0, some patients still complained of symptoms related to visual quality deterioration, such as glare, ghost, poor night vision and so on[3]–[5]. Several studies[6]–[8] have shown that the postoperative visual quality of patients who underwent femtosecond laser in situ keratomileusis (FS-LASIK) is better than those who underwent LASIK with mechanical microkeratome. Moreover, the application of smart pulse technology has been reported to effectively reduce the introduction of transepithelial photorefractive keratectomy (Trans-PRK) induced aberrations and obtain better visual quality. However, relatively few comparative studies on transepithelial photorefractive keratectomy using the smart pulse technology (SMART) and FS-LASIK are available. Jiang et al[9] compared the changes in contrast sensitivity after Trans-PRK and FS-LASIK, and found that the difference between the two groups was not significant 3mo after surgery. In this study, the effects of SMART and FS-LASIK on the objective visual quality were compared and analyzed.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Ethics Committee of Tianjin Medical University Eye Hospital (ethical approval No.2019KY-17). All the patients signed the informed consent of the procedure and study. The study has been registered in the Chinese Clinical Trial Registry with the registration number ChiCTR1900027341.
Study Design
A prospective, non-randomized, controlled study was conducted. A total of 76 patients (152 eyes) who underwent corneal refractive surgery at the Tianjin Medical University Eye Hospital between May and September in 2019 were enrolled. Doctors recommended suitable surgical methods for patients according to their eye parameters, such as spherical equivalent (SE) and central corneal thickness (CCT). The inclusion criteria were as follows: the age was over 18 years old, a relatively stable spherical diopter for at least 2y, cessation of soft contact lens use for more than 1wk, use of rigid contact lens for more than 4wk, no keratoconus tendency, and no active ocular disease or systemic disease. Thirty-two patients (64 eyes) received SMART, and 44 patients (88 eyes) received FS-LASIK. General data are shown in Table 1, and no significant difference in the parameters was observed between the two groups (P>0.05) except for CCT (P<0.001).
Table 1. Preoperative demographics of the eyes undergoing FS-LASIK and SMART.
| Demographics | FS-LASIK (n=88) | SMART (n=64) | t/χ2 | P |
| Age (y) | 25.32±5.99 | 26.59±6.97 | -0.855 | 0.395 |
| Sex (male/female) | 23/21 | 15/17 | 0.216 | 0.642 |
| Spherical (D) | -3.87±1.05 | -3.92±1.02 | 0.393 | 0.574 |
| Cylindrical (D) | -0.68±0.49 | -0.85±0.44 | -1.889 | 0.061 |
| Spherical equivalent (D) | -4.22±1.09 | -4.36±1.00 | 0.511 | 0.611 |
| CCT (µm) | 555.05±27.96 | 527.84±27.24 | 5.105 | <0.001 |
| K1 (D) | 42.98±1.32 | 42.89±1.09 | 0.481 | 0.631 |
| K2 (D) | 44.04±1.34 | 43.93±1.20 | 0.580 | 0.556 |
FS-LASIK: Femtosecond laser in situ keratomileusis; SMART: Transepithelial photorefractive keratectomy using the smart pulse technology; D: Diopters; CCT: Central corneal thickness; K1: Cornea flat meridian curvature; K2: Cornea steep meridian curvature.
mean±SD/ratio
Preoperative and Postoperative Assessment
All patients underwent a detailed ophthalmological examination before surgery. Corneal aberrations were measured using a three-dimensional anterior segment analysis system (Pentacam 70700, Oculus, Germany). The measurements were performed in a dark room. The patient was asked to sit and blink, then focus automatically. Interference caused by poor quality of the tear film and eyelid occlusion was avoided. The images that quality specification (QS) shows “OK” and images with corneal exposure greater than 9 mm were accepted.
Optical quality analysis system (OQAS-II, Vision Metrics, Spain) was used to measure the cutoff for the modulation transfer function (MTF cutoff), objective scatter index (OSI), and Strehl ratio (SR). Before the measurements, the room illumination was kept low and the pupil diameter was more than 4.0 mm in all eyes during testing. The patient's head position was adjusted. The patients' manifested refractive error was fed into the system and was corrected fully during these measurements. Objective refraction was first performed. Based on objective refraction, the MTF cutoff, OSI, and SR were assessed.
Each examination was performed by the same person, and each eye was measured three times. Visual acuity, objective refraction (sphere, cylinder, and SE), corneal aberrations, MTF cutoff, OSI, and SR were followed-up before surgery and at 1, 3 and 6mo postoperatively.
Surgical Procedure
Before the SMART surgery, the conjunctival sac was washed with balanced solution, disinfected with iodophor, covered with disposable sterile towel, anesthetized with 0.4% oxybuprocaine hydrochloride eye drops, and the cornea was completely exposed with an eyelid opener. A Schwind Amaris 1050RS excimer laser system was used. The laser spot was 0.54 mm in diameter. A seven-dimensional eye-tracking system and smart pulse technology were used to complete the operation. After cutting, cold balanced liquid was used for washing, and corneal bandage lens was worn postoperatively.
The eyes of FS-LASIK were anesthetized with 0.4% oxybuprocaine hydrochloride eye drops, and the eyelid opener was used to open the eyelids. After the corneal surface was smooth, a negative pressure suction ring was placed in the center of the cornea. When the corneoscleral edge and the center of the negative pressure suction ring basically coincided, the eyeball was fixed with negative pressure. The corneal stromal flap was made using the IntraLase FS laser equipment (USA). The diameter of the flap was 8.5 mm, and the hinge was located above. After all the bubbles under the corneal flap were absorbed, the corneal flap was separated using a splitter, and the stromal bed was cut with a Schwind Amaris 1050RS excimer laser system. After cutting, the corneal flap was restored by washing with balanced salt solution.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics software (version 23; IBM Corporation, Armonk, NY, USA). The Kolmogorov-Smirnov test was used to test for normality and data are expressed as mean values±standard deviation. The Levene's test found that the data between the two groups met the homogeneity of variance. Higher-order aberrations (HOAs), horizontal coma, vertical coma and spherical aberration were analyzed by root mean square. ΔHOA, Δvertical coma, Δhorizontal coma and Δspherical aberration were used to represent after 6mo with preoperative difference. The independent sample t-test was used to compare the general data between the two groups. Before and after operation, the corneal HOA, horizontal coma, vertical coma, spherical aberration, MTF cutoff, OSI, and SR were compared by repeated measurement two-factor (ANOVA), and pairwise comparisons between groups and within groups were performed using the least significant difference (LSD) t-test. Statistical significance was set at P<0.05.
RESULTS
Visual Acuity
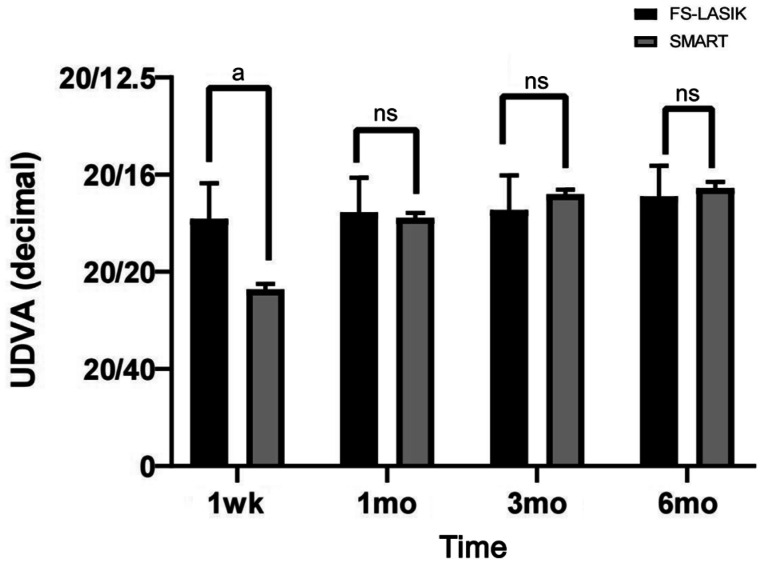
Uncorrected distance visual acuity (UDVA; decimal) was significantly different between the SMART and FS-LASIK groups at 1wk postoperatively (t=11.125, P<0.001), but there was no significant difference in the UDVA between the two groups at 1, 3, and 6mo postoperatively (t=-0.990, -0.769, and -0.961, respectively, P>0.05; Figure 1). The visual acuity recovery of the patients in the SMART group was slightly slower than that in the FS-LASIK group. At 1mo postoperatively, the visual acuity of the two groups recovered to the expected preoperative vision.
Figure 1. Visual acuity recovery after the operation between the FS-LASIK and SMART groups.
ns: Not significant. aP<0.001 indicates a significant difference.
Refractive Results and Accuracy
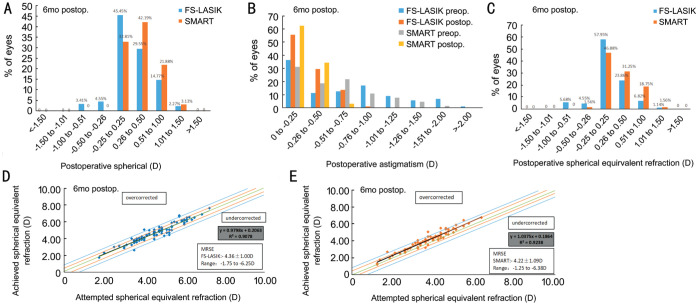
In the FS-LASIK and SMART groups, 79.55% and 75% of the patients had the spherical diopter of ±0.50 D, respectively, and the difference was not statistically significant (P=0.286; Figure 2A). Astigmatism in the FS-LASIK and SMART groups were -0.68±0.49 and -0.85±0.44 respectively, before the surgery, and -0.32±0.20 and -0.27±0.16, respectively, at 6mo postoperatively, and no significant difference was observed (P=0.111; Figure 2B). No significant difference was noted in SE between the FS-LASIK and SMART groups at 6mo postoperatively (P=0.509). The patients with SE in ±0.50 D were 86.36% and 80.69% respectively (Figure 2C). In addition, Figure 2D and 2E shows the correlation between the attempted SE refraction and achieved SE refraction in the two groups.
Figure 2. Comparison between the FS-LASIK and SMART groups at 6mo.
A: Postoperative spherical; B: Preoperative and postoperative refractive astigmatism; C: SE refractive accuracy at 6mo; D: SE attempted versus achieved in FS-LASIK group; E: SE attempted versus achieved in SMART group.
Changes of Corneal Aberrations in the Two Groups
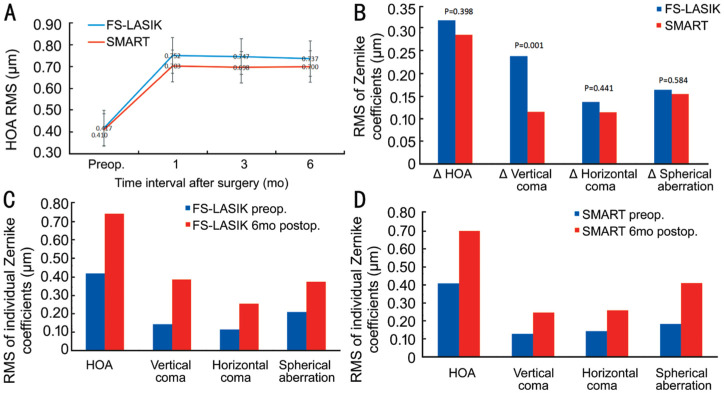
The HOAs increased at 1, 3, and 6mo postoperatively in both the FS-LASIK and SMART groups (Figure 3A). At 6mo, the corneal HOA, vertical coma, horizontal coma, and spherical aberration in the FS-LASIK and SMART groups were significantly higher than those before surgery (P<0.05; Figure 3C, 3D). There was no significant difference in the parameters of HOAs between 6 and 1mo postoperatively (P>0.05; Figure 3A). With the extension of time, the parameters of HOAs in the two groups did not change significantly.
Figure 3. HOAs in the FS-LASIK and SMART groups.
A: Preoperative and postoperative mean root mean square of HOA; B: Absolute changes in HOAs value in each individual Zernike coefficient; C: Mean of individual Zernike coefficient in the FS-LASIK group; D: Mean of individual Zernike coefficient in SMART group.
Comparison of Corneal Aberrations between Two Groups
No significant difference in preoperative corneal HOA, vertical coma, horizontal coma and spherical aberration between the FS-LASIK and SMART groups was noted (P>0.05). Regarding corneal HOA, horizontal coma and spherical aberration, no significant difference was observed between the two groups (P>0.05) at 1, 3, and 6mo postoperatively. The corneal vertical coma in the FS-LASIK group was slightly higher than that in the SMART group (P<0.001; Table 2). No significant difference was noted in ΔHOA, Δhorizontal coma and Δspherical aberration between the FS-LASIK and SMART groups (P>0.05). The Δcorneal vertical coma in the FS-LASIK group was higher than that in the SMART group (P=0.001; Figure 3B).
Table 2. Results of HOA parameters between the two groups.
| Groups | Preop. | 1mo | 3mo | 6mo | P (preop. vs 6mo) |
| HOA | |||||
| FS-LASIK | 0.42±0.07 | 0.75±0.19 | 0.75±0.24 | 0.74±0.24 | <0.001 |
| SMART | 0.41±0.09 | 0.70±0.17 | 0.70±0.20 | 0.70±0.19 | <0.001 |
| P | 0.575 | 0.125 | 0.194 | 0.300 | |
| Vertical coma | |||||
| FS-LASIK | 0.14±0.10 | 0.39±0.23 | 0.41±0.27 | 0.38±0.27 | <0.001 |
| SMART | 0.13±0.11 | 0.27±0.16 | 0.24±0.15 | 0.25±0.16 | 0.010 |
| P | 0.525 | <0.001 | <0.001 | <0.001 | |
| Horizontal coma | |||||
| FS-LASIK | 0.11±0.10 | 0.22±0.17 | 0.23±0.17 | 0.25±0.16 | <0.001 |
| SMART | 0.12±0.09 | 0.24±0.16 | 0.27±0.19 | 0.26±0.19 | 0.035 |
| P | 0.712 | 0.496 | 0.253 | 0.801 | |
| Spherical aberration | |||||
| FS-LASIK | 0.21±0.06 | 0.38±0.11 | 0.38±0.11 | 0.37±0.11 | <0.001 |
| SMART | 0.19±0.07 | 0.38±0.13 | 0.40±0.15 | 0.41±0.15 | <0.001 |
| P | 0.052 | 0.892 | 0.281 | 0.097 |
HOA: Higher-order aberration; FS-LASIK: Femtosecond laser in situ keratomileusis; SMART: Transepithelial photorefractive keratectomy using the smart pulse technology. HOA: F (group)=0.360, P=0.551; F (time)=160.711, P<0.001. Vertical coma: F (group)=11.32, P=0.001; F (time)=58.322, P<0.001. Horizontal coma: F (group)=1.043, P=0.312; F (time)=35.994, P<0.001. Spherical aberration: F (group)=0.245, P=0.622; F (time)=194.990, P<0.001.
Changes of Visual Quality in the Two Groups
At 1, 3, and 6mo postoperatively, the OSI increased in both groups compared to that preoperatively. There were statistically significant differences between 1mo post-operative and pre-operative in both the groups (P<0.05). At 3 and 6mo after surgery, no significant difference was noted in the OSI compared with that before surgery (P>0.05). The MTF cutoff in the two groups at 1 and 3mo post surgery showed no significant change compared with that before surgery (P>0.05). No significant difference in the SR was observed in both groups at 1, 3 and 6mo after surgery (P>0.05; Table 3).
Table 3. Results of the visual quality parameters between the two groups.
| Groups | Preop. | 1mo | 3mo | 6mo | P (preop. vs 6mo) |
| OSI | |||||
| FS-LASIK | 0.79±0.32 | 0.90±0.32a | 0.81±0.28 | 0.81±0.30 | 0.900 |
| SMART | 0.71±0.20 | 0.81±0.27a | 0.71±0.29 | 0.72±0.28 | 0.966 |
| P | 0.057 | 0.064 | 0.040 | 0.047 | |
| MTF cutoff | |||||
| FS-LASIK | 40.68±6.67 | 39.80±5.55 | 40.79±6.50 | 39.44±6.63 | 0.809 |
| SMART | 41.36±6.17 | 40.84±6.81 | 41.60±5.42 | 41.10±5.46 | 0.687 |
| P | 0.419 | 0.314 | 0.427 | 0.026 | |
| SR | |||||
| FS-LASIK | 0.24±0.06 | 0.24±0.06 | 0.25±0.06 | 0.24±0.06 | 0.682 |
| SMART | 0.26±0.05 | 0.24±0.06 | 0.25±0.05 | 0.25±0.05 | 0.387 |
| P | 0.065 | 0.626 | 0.818 | 0.187 |
OSI: Objective scatter index; MTF: Modulation transfer function; SR: Strehl ratio; FS-LASIK: Femtosecond laser in situ keratomileusis; SMART: Transepithelial photorefractive keratectomy using the smart pulse technology. OSI: F (group)=3.976, P=0.039; F (time)=9.896, P<0.001. MTF: F (group)=0.276, P=0.045; F (time)=1.641, P=0.182. SR: F (group)=0.059, P=0.810; F (time)=1.608, P=0.190. aStatistically significant compared with preoperative values.
Comparison of Visual Quality Between the Two Groups
Preoperative OSI, MTF cutoff and SR were not significantly different between the FS-LASIK and SMART groups (P>0.05). There was no significant difference in OSI between the two groups at 1mo postoperatively (P>0.05), however, a significant difference was found between the two groups at 3 and 6mo after surgery (P=0.040 and 0.047 respectively). No significant difference in MTF cutoff was noted between the two groups at 1 and 3mo postoperatively (P>0.05). At 6mo, the MTF cutoff in the SMART group was slightly higher than that in the FS-LASIK group (P=0.026). At 1, 3, and 6mo postoperatively, no significant difference in the SR was observed between the FS-LASIK and SMART groups (P>0.05; Table 3).
DISCUSSION
At present, the visual quality after refractive surgery has become another focus of doctors. Common visual quality evaluation methods include subjective and objective visual quality evaluations. In contrast, the former has a certain degree of subjectivity, which is related to the cognitive understanding ability and degree of cooperation of the patients, and the evaluation accuracy and repeatability error are relatively large. Objective visual quality assessment reduces the error caused by patient cooperation, and has a certain degree of operability and repeatability, and has a wide range of clinical application value. In the current study, it was found that both FS-LASIK and SMART surgeries have good efficacy and safety in the correction of low to moderate myopia and astigmatism, but the postoperative objective visual quality of both groups decreased compared with the preoperative level, while the HOA increased.
As is known to all, improving the UDVA is the primary goal of corneal refractive surgery. Previous studies have shown that both FS-LASIK and SMART surgery are safe and effective, and visual acuity can be significantly improved after surgery[10]–[11]. Luger et al[12] observed the diopter and visual acuity of Trans-PRK (196 eyes) and FS-LASIK (196 eyes) for one year. It was found that there was no significant difference in diopter and visual acuity 1y postoperatively between the two groups, but the recovery time of Trans-PRK group was longer than that of FS-LASIK group. Our study shows that the visual acuity recovery after SMART surgery is slower than that of FS-LASIK, but there is no significant difference in the visual acuity recovery and diopter correction between the two surgical methods in the long term. This finding is consistent with previous studies.
Previous studies have shown that coma and spherical aberration are the main HOAs after corneal refractive surgery[13]–[14]. Therefore, corneal HOA, third-order coma and fourth-order spherical aberration were selected as observation indices in this study. Adib-Moghaddam et al[15] reported that corneal HOAs increased 18mo after Trans-PRK. Wang et al[16] observed the changes of corneal HOAs after FS-LASIK, and found that the corneal HOA, corneal coma and corneal spherical aberration increased 12mo after FS-LASIK. Hashemi et al[17] reported that FS-LASIK can induce more HOAs than that in PRK, mainly coma. The results showed that there was no significant difference in corneal HOA, corneal horizontal coma and corneal spherical aberration between the two groups at 6mo postoperatively. The vertical coma of FS-LASIK was slightly higher than that of SMART and the corneal aberrations of FS-LASIK were significantly increased compared to those before surgery. It is concluded that these two operations inevitably induce HOAs. Compared with FS-LASIK, the corneal coma introduced by SMART surgery is smaller. Previous studies have shown that the coma size is related to the degree of decentration[18]. This study indicates that FS-LASIK requires a certain thickness of the corneal flap, and the process of creating a corneal flap and its healing process can increase the HOAs. It has been suggested that the coma caused by the hinge of the corneal flap is consistent with the direction of the hinge, which may be related to the wound healing reaction along the edge and hinge direction of the corneal flap. The retraction and tension along the hinge axis caused by the hydration of the corneal flap leads to an increase in the asymmetry of the corneal flap relative to the hinge axis, resulting in a change in coma. The center of the surgical optical area is closer to the patients' visual axis and the cutting eccentricity is smaller, thus reducing the introduction of surgical coma. Some studies have shown that the formation of spherical aberration may be related to the changes in corneal anterior surface morphology, which is mainly related to the cutting amount[19]. Therefore, no significant difference was observed between FS-LASIK and SMART.
In the optical system of the eye, in addition to aberration, scattering and diffraction are also factors that cause the degradation of visual quality. Using the objective optical quality analysis system, OQAS II, based on the principle of dual-channel retinal imaging, the influence of higher-order aberration and scattering on visual quality was recorded and analyzed. Several studies have demonstrated that it has good accuracy and repeatability[20]–[21]. Scattering refers to the phenomenon in which some light rays deviate from the original direction when passing through an inhomogeneous medium, which is mainly affected by the transparency and surface regularity of refractive media such as the cornea and lens. Previous studies have shown that the visual quality after corneal refractive surgery decreases or the change is not obvious[22]–[23]. Ondategui et al[24] used OQAS to evaluate the visual parameters of PRK (34 eyes) and LASIK (55 eyes) before and 3mo after surgery. The results showed that the MTF cutoff and SR were lower than those before operation, while OSI was higher than that before operation, indicating that the two surgical methods would lead to early visual quality decline.
This study revealed that there was no significant difference in the MTF cutoff and SR between FS-LASIK and SMART. The MTF cutoff and OSI of FS-LASIK were higher than those of SMART at 6mo postoperatively. Postoperative OSI was increased compared with that before surgery in both groups, and the OSI in the FS-LASIK group was higher than that in the SMART group at 6mo. It is concluded that both FS-LASIK and SMART can increase the postoperative scattering, and the long-term objective visual quality of SMART is better than that of FS-LASIK. When femtosecond laser cuts and separates corneal tissue, the regularity of its surface will affect the optical imaging. For example, the discontinuous tissue connection between bubbles and the depression produced by the melting part of bubbles may cause the corneal regularity change on the stromal surface, which then affects the visual quality[25]–[26]. Second, FS-LASIK cuts off a large number of nerve fibers when making a corneal flap, and only the nerve fibers in the hinge can be reserved, which makes the density of nerve fibers significantly decrease in the early postoperative period of FS-LASIK and also affects the speed of nerve fiber recovery. Compared with FS-LASIK, the damage depth of SMART to corneal nerve fibers is shallower and lighter. It has been reported that the nerve fibers after the FS-LASIK operation are still in the stage of growth and recovery in about 1y and are significantly lower than that preoperative[27]–[28]. Erie et al[29] found that PRK nerve fiber density can be restored to the preoperative level in 2y, but LASIK still cannot restore the preoperative density in the fifth year postoperatively. This can also explain why the long-term objective visual quality after SMART surgery is slightly better than that of FS-LASIK.
In conclusion, both FS-LASIK and SMART increased postoperative HOA parameters, and FS-LASIK introduced a higher vertical coma than that in SMART. In the long term, the postoperative OSI and MTF cutoff in SMART were slightly better than that in FS-LASIK, indicating that postoperative visual quality of SMART was slightly better than FS-LASIK. It is expected to provide some reference for the selection of surgical methods. For example, in patients with higher coma before surgery, the postoperative visual quality of SMART surgery may be better than that of FS-LASIK.
At present, research is limited to the evaluation of postoperative objective visual quality, which needs to be further combined with subjective visual quality to evaluate the changes in postoperative visual quality more comprehensively.
Acknowledgments
We thank all of the participants recruited for this study.
Authors' contributions: Study concept and design (Wu Y, Huang Y, Wang SH, Yu AM, Zhao SZ, Wei RH, Zhang C, Yang RB); data collection (Wu Y, Wang SH, Wang GQ, Yu AM); analysis and interpretation of data (Wu Y, Huang Y, Wang SH); writing the manuscript (Wu Y); critical revision of the manuscript (Huang Y).
Foundation: Supported by Tianjin Clinical Key Discipline Project (No.TJLCZDXKM013).
Conflicts of Interest: Wu Y, None; Huang Y, None; Wang SH, None; Wang GQ, None; Yu AM, None; Zhao SZ, None; Wei RH, None; Yang RB, None; Zhang C, None.
REFERENCES
- 1.Řeháková T, Veliká V, Jirásková N. Correction of myopia and myopic astigmatism by femtosecond laser in situ keratomileusis. Cesk Slov Oftalmol. 2019;75(2):65–71. doi: 10.31348/2019/2/2. [DOI] [PubMed] [Google Scholar]
- 2.Xi L, Zhang C, He YL. Single-step transepithelial photorefractive keratectomy in the treatment of mild, moderate, and high myopia: six month results. BMC Ophthalmol. 2018;18(1):209. doi: 10.1186/s12886-018-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan-Paul NI, Li J, Miller JS, Florakis GJ. Night vision disturbances after corneal refractive surgery. Surv Ophthalmol. 2002;47(6):533–546. doi: 10.1016/s0039-6257(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 4.Hersh PS, Steinert RF, Brint SF. Photorefractive keratectomy versus laser in situ keratomileusis: comparison of optical side effects. Summit PRK-LASIK Study Group. Ophthalmology. 2000;107(5):925–933. doi: 10.1016/s0161-6420(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 5.Lorente-Velázquez A, Nieto-Bona A, Collar CV, Mesa AG. Straylight and contrast sensitivity after corneal refractive therapy. Optom Vis Sci. 2011;88(10):1245–1251. doi: 10.1097/OPX.0b013e3182271449. [DOI] [PubMed] [Google Scholar]
- 6.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Salomão MQ, Ambrósio R, Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. 2009;35(10):1756–1760. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang YQ, Qian J, Yuan YF, Xue K, Guo J, Wang XN. Tear film and lacrimal excretion changes after lacrimal gland tumor removal. Zhonghua Yan Ke Za Zhi. 2013;49(1):27–31. [PubMed] [Google Scholar]
- 9.Jiang JJ, Jhanji V, Sun LX, Li JY, Zhang RP. Comparison of visual quality after Femto-LASIK and TransPRK in patients with low and moderate myopia. Int Ophthalmol. 2020;40(6):1419–1428. doi: 10.1007/s10792-020-01308-5. [DOI] [PubMed] [Google Scholar]
- 10.Adib-Moghaddam S, Haydar AA, Razi-Khosroshahi M, Soleyman-Jahi S, Tefagh G, Grentzelos MA, Arba-Mosquera S, Kymionis GD. Predictors of visual acuity improvement and supernormal vision after refined single-step transepithelial photorefractive keratectomy. J Refract Surg. 2019;35(12):771–780. doi: 10.3928/1081597X-20191025-01. [DOI] [PubMed] [Google Scholar]
- 11.Gershoni A, Mimouni M, Reitblat O, Livny E, Ehrlich R, Bahar I. Is performing femtosecond laser-assisted in situ keratomileusis on the day of the initial consultation visit safe, predictable and efficacious? Eye Contact Lens. 2020;46(3):182–189. doi: 10.1097/ICL.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 12.Luger MHA, Ewering T, Arba-Mosquera S. Myopia correction with transepithelial photorefractive keratectomy versus femtosecond-assisted laser in situ keratomileusis: one-year case-matched analysis. J Cataract Refract Surg. 2016;42(11):1579–1587. doi: 10.1016/j.jcrs.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Chen XQ, Wang Y, Zhang JM, Yang SN, Li XJ, Zhang L. Comparison of ocular higher-order aberrations after SMILE and wavefront-guided femtosecond LASIK for myopia. BMC Ophthalmol. 2017;17(1):42. doi: 10.1186/s12886-017-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JF, Feng QQ, Ding WZ, Peng YS, Long KL. Comparison of clinical results between trans-PRK and femtosecond LASIK for correction of high myopia. BMC Ophthalmol. 2020;20(1):243. doi: 10.1186/s12886-020-01515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adib-Moghaddam S, Soleyman-Jahi S, Salmanian B, Omidvari AH, Adili-Aghdam F, Noorizadeh F, Eslani M. Single-step transepithelial photorefractive keratectomy in myopia and astigmatism: 18-month follow-up. J Cataract Refract Surg. 2016;42(11):1570–1578. doi: 10.1016/j.jcrs.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Ren YL, Liang K, Jiang ZX, Tao LM. Changes of corneal high-order aberrations after femtosecond laser-assisted in situ keratomileusis. Medicine. 2018;97(18):e0618. doi: 10.1097/MD.0000000000010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashemi H, Ghaffari R, Miraftab M, Asgari S. Femtosecond laser-assisted LASIK versus PRK for high myopia: comparison of 18-month visual acuity and quality. Int Ophthalmol. 2017;37(4):995–1001. doi: 10.1007/s10792-016-0364-7. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001;42(6):1396–1403. [PubMed] [Google Scholar]
- 19.Marcos S, Barbero S, Llorente L, Merayo-Lloves J. Optical response to LASIK surgery for myopia from total and corneal aberration measurements. Invest Ophthalmol Vis Sci. 2001;42(13):3349–3356. [PubMed] [Google Scholar]
- 20.Kamiya K, Shimizu K, Igarashi A, Kobashi H. Effect of femtosecond laser setting on visual performance after small-incision lenticule extraction for myopia. Br J Ophthalmol. 2015;99(10):1381–1387. doi: 10.1136/bjophthalmol-2015-306717. [DOI] [PubMed] [Google Scholar]
- 21.Saad A, Saab M, Gatinel D. Repeatability of measurements with a double-pass system. J Cataract Refract Surg. 2010;36(1):28–33. doi: 10.1016/j.jcrs.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 22.Gertnere J, Solomatin I, Sekundo W. Refractive lenticule extraction (ReLEx flex) and wavefront-optimized Femto-LASIK: comparison of contrast sensitivity and high-order aberrations at 1 year. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1437–1442. doi: 10.1007/s00417-012-2220-4. [DOI] [PubMed] [Google Scholar]
- 23.Jung JW, Chung BH, Han SH, Kim EK, Seo KY, Kim TI. Comparison of measurements and clinical outcomes after wavefront-guided LASEK between iDesign and WaveScan. J Refract Surg. 2015;31(6):398–405. doi: 10.3928/1081597X-20150521-06. [DOI] [PubMed] [Google Scholar]
- 24.Ondategui JC, Vilaseca M, Arjona M, Montasell A, Cardona G, Güell JL, Pujol J. Optical quality after myopic photorefractive keratectomy and laser in situ keratomileusis: comparison using a double-pass system. J Cataract Refract Surg. 2012;38(1):16–27. doi: 10.1016/j.jcrs.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 25.Kunert KS, Blum M, Duncker GIW, Sietmann R, Heichel J. Surface quality of human corneal lenticules after femtosecond laser surgery for myopia comparing different laser parameters. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1417–1424. doi: 10.1007/s00417-010-1578-4. [DOI] [PubMed] [Google Scholar]
- 26.Lubatschowski H. Overview of commercially available femtosecond lasers in refractive surgery. J Refract Surg. 2008;24(1):S102–S107. doi: 10.3928/1081597X-20080101-18. [DOI] [PubMed] [Google Scholar]
- 27.Kanellopoulos AJ, Asimellis G. Three-dimensional LASIK flap thickness variability: topographic central, paracentral and peripheral assessment, in flaps created by a mechanical microkeratome (M2) and two different femtosecond lasers (FS60 and FS200) Clin Ophthalmol. 2013;7:675–683. doi: 10.2147/OPTH.S40762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after laser in situ keratomileusis: femtosecond laser vs mechanical microkeratome. Arch Ophthalmol. 2010;128(11):1413–1419. doi: 10.1001/archophthalmol.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140(6):1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]