Abstract
AIM
To investigate the clinical features of adult patients with ocular toxocariasis (OT) in north China and to diagnose adults OT patients in early stage.
METHODS
Clinical data of 24 adults with OT were retrospectively analyzed. Slit lamp photographs and fundus photographs and other imaging examinations of all the patients were reviewed. A questionnaire concerning the pet ownership and place of residence was completed to investigate the possible infection origin. Descriptive statistical analyses were performed on the demographic data, clinical features, funduscopic findings and ELISA results.
RESULTS
Among the 24 patients diagnosed with OT by Toxocara IgG antibody in intraocular fluid, 16 (66.7%) eyes were right eye. The onset age of 12 eyes (50.0%) was between 30 and 40 years old, and 21 (87.5%) eyes were of peripheral granuloma type. The most common sign was vitreous opacity. Granulomas were detected in all the eyes, and 20 (83.3%) patients resided in rural area. In 4 patients, the concentration of anti-Toxocara antibody both in anterior humor and in vitreous humor were detected, and the results showed the concentration in vitreous humor was much higher than aqueous humor.
CONCLUSION
Our study analyzes the clinical manifestation of OT in adults, which may have been under-recognized before. Eye side, residence, and detection of granuloma may help us in diagnosis of OT in patients with monocular vitreous opacity. For adult patients with presumed OT, negative results of anti-Toxocara antibody in anterior humor cannot rule out the possibility of OT, further detection of vitreous humor is suggested for final diagnosis.
Keywords: ocular toxocariasis, ELISA, intraocular fluids, granuloma, adults
INTRODUCTION
Toxocariasis is one of the most common zoonotic infections caused by larvae of Toxocara canis (T. canis) or Toxocara cati. Ocular toxocariasis (OT) is often caused by infection of T. canis larva that migrates into the eye[1]. T. canis larvae have a high affinity for brain tissue and eyes[2]. Human beings generally become infected through ingestion of embryonated eggs from contaminated sources such as soil or improperly cooked paratenic hosts. In addition, pet owners can sometimes be accidentally infected by their dogs or cats[3]–[4].
OT is underestimated severely in clinical works. One study in a Korean tertiary hospital about patients with uveitis of unknown etiology found that 29.8% were diagnosed with OT; among those patients with intermediate and posterior uveitis, the prevalence rates of OT were 47.1%, and 44.8% respectively[5]–[6]. Some reports of OT case series have addressed clinical features in children in China, these young patients were under 14 years of age[7].
However, little is known about the clinical features of OT in adult patients, particularly in China. OT is mainly diagnosed by immunological and imaging methods[8]–[11]. Enzyme-linked immunosorbent assay (ELISA) is relatively sensitive and specific to detect the antibody of T. canis in the serum or intraocular fluid[8],[12]. Hereby, we reported clinical features of 17 adult patients with OT in this study, in whom an intraocular Toxocara infection was confirmed with ELISA testing for IgG antibody to the Toxocara larva crude antigen in intraocular fluid.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Institutional Ethics Committee of the Beijing Chaoyang Hospital Affiliated to the Capital Medical University and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients.
Patients
The clinical data of 24 adult patients (≥18 years old) who were diagnosed as OT in Department of Ophthalmology, Beijing Chaoyang Hospital Affiliated to Capital Medical University from November 2016 to August 2021 were retrospectively reviewed. A standardized face-to-face interview was conducted by a trained interviewer (medical doctor) to collect data concerning residence and contact with animals and soil. Aqueous humor (AH) or vitreous humor of these patients was tested positive for Toxocara antibody.
To calculate the normal range of Toxocara antibody, the intraocular fluids of 16 patients with confirmed diagnoses including macular hole, epimacular membrane, and proliferative diabetic retinopathy were used.
A clinical diagnosis of OT was made based on 1) unilaterally involved; 2) clinical features of presumed OT, including the presence of a peripheral granuloma (focal, white peripheral nodule with pigmentary scarring or traction retinal detachment), posterior pole granuloma (focal, white nodule with or without posterior pole variable pigmentation), or vitritis with unknown cause, generally without inflammatory signs in the anterior chamber; 3) positive Toxocara antibody in the intraocular fluids; 4) exclusion of other intraocular granulomatous diseases.
Eye Examination and Laboratory Test
A slit-lamp examination with a noncontact lens and binocular indirect ophthalmoscope were conducted to thoroughly examine the eyes of patients. Depending on the condition of these patients, B-scan ultrasonography (ODM-1000A/P, Tianjin Maida Medical Technology Co., Ltd., China) and fundus fluorescein angiography (HRA2; Heidelberg Engineering GmbH, Dossenheim, Germany) were tested when necessarily.
ELISA was used for quantitatively determining IgG antibodies against T. canis in intraocular fluid (RE-58721; IBL International, GmbH, Hamburg, Germany). Samples with a value >3 U were considered positive for OT. The ELISA test was performed on undiluted anterior humor or vitreous sample (obtained during vitreous surgery) in patients who were treated with vitreoretinal surgery.
Statistical Analysis
The data were analyzed using SPSS 19.0 software. Descriptive statistical analyses were performed on the demographic data, clinical features, funduscopic findings and ELISA results. The data that were distributed normally were presented as the mean±standard deviation (SD). All P values were 2-sided and were considered statistically significant when the values were less than 0.05.
RESULTS
Measurement Outcome of Toxocara IgG
The Toxocara IgG concentration in the intraocular fluids from non-OT patients (including macular hole, epimacular membrane, and proliferative diabetic retinopathy) varied from 0.800 U/mL to 1.640 U/mL (95% confidence interval: 1.204 U/mL to 1.398 U/mL). Among them, 3 samples were AH and the others were vitreous. Mean±2SD was 0.907 U/mL to 1.695 U/mL. After discussion between statistician and the authors, hereby, the consensus was accessed, i.e., if the concentration of the Toxocara IgG is over 3.0 U/mL, it was regarded as positive.
Patient Demographics
Patient demographics was presented in Table 1. There were 11 male and 13 female patients. All cases were unilaterally involved, and more patients were infected in the right eye (66.7%). Mean age of presentation was 33.5±10.42 years old and 50.0% was between 30 and 40 years old. Most (83.3%) of the patients lived in rural areas.
Table 1. Demographic characteristics of adults with ocular toxocariasis.
| Characteristics | n (%) |
| Age, range (y) | 18-58 |
| Gender | |
| Male | 11 (45.8) |
| Female | 13 (54.2) |
| Ocular side | |
| Right | 16 (66.7) |
| Left | 8 (33.3) |
| Residence | |
| Rural area | 20 (83.3) |
| Urban area | 4 (16.7) |
| Pet exposure | 13 (54.2) |
Clinical Characteristics
Table 2 summarizes the clinical characteristics of the patients who visited the hospital for the first time. The visual acuity (VA) at baseline varied between 20/20 and light perception (LP); 17 cases (70.8%) had a VA of 20/100 or better, and only 7 cases (29.2%) had a VA of less than 20/200. Blurring of vision were the most common complaints of these patients at the first visit. Before they were referred to our hospital, only 2 cases (8.3%) were diagnosed with OT, with a granuloma which could be easily observed during fundoscopy (Figure 1). Two cases were presumed to be OT with the concentrations of anti-T. canis IgG antibody of 2.44 U and 2.5 U in AH, which were negative results according to our standard. However, the following tests of vitreous fluid showed a much higher concentration of 39.41 U and confirmed the diagnosis. In addition, 83.8% of the patients were diagnosed as other diseases, such as uveitis and retinal detachment (Table 2).
Table 2. Clinical characteristics of patients at their first visit to the hospital.
| Clinical characteristics | n (%) |
| Visual acuity | |
| >20/100 | 17 (70.8) |
| >20/200 | 19 (79.2) |
| Finger counting | 3 (12.5) |
| Hand moving | 3 (12.5) |
| Light perception | 1 (4.2) |
| Intraocular pressure, mm Hg | |
| 11-21 | 23 (95.8) |
| >21 | 1 (4.2) |
| Symptom | |
| Blurred vision | 24 (100) |
| Red eye | 3 (12.5) |
| Diagnosis at first visit | |
| Uveitis | 18 (75.0) |
| Retinal detachment | 2 (8.3) |
| Suspected ocular toxocariasis | 2 (8.3) |
| Ocular toxocariasis | 2 (8.3) |
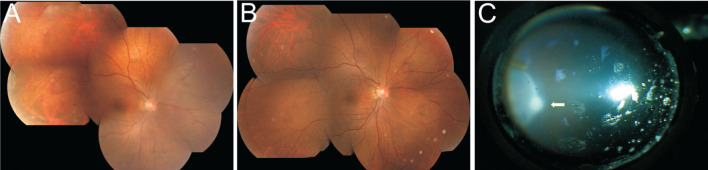
Figure 1. Fundus photo of a 30-year-old female.
A: A granuloma (arrow) can be easily observed before surgery; B: One year after vitrectomy and laser photocoagulation, scar formed with pigment surrounding.
Ocular Signs
Table 3 presents the clinical signs of these adults OT. The most common sign at presentation was vitreous opacity (Figure 2). Granulomas were found at the first visit in 13 eyes (Figure 3) and became visible in 10 eyes after vitreous opacities had been cleared by vitrectomy or pressing peripheral retina during vitrectomy (Figure 4). Toxocara granulomas were classified as posterior pole (3 eyes, 12.5%), peripheral (21 eyes, 87.5%). Temporal granulomas were detected in 50.0% of the patients and nasal granulomas were detected in 50.0% cases. Anterior segment inflammation was observed in 11 eyes (45.8%), including keratic precipitates and floating cells.
Table 3. Clinical characteristics of patients with ocular toxocariasis.
| Ocular signs | n | Peripheral granuloma | Posterior pole granuloma | Endophthalmitis | Atypical |
| Anterior segment inflammation | 11 | 8 | 1 | 1 | 1 |
| Cataract | 3 | 2 | 0 | 0 | 1 |
| Vitritis | 23 | 19 | 2 | 1 | 1 |
| Mild | 3 | 3 | 0 | 0 | 0 |
| Moderate | 18 | 16 | 2 | 0 | 0 |
| Severe | 2 | 0 | 0 | 1 | 1 |
| Retinal detachment | 2 | 1 | 0 | 0 | 1 |
Figure 2. Vitreous opacity (arrow) in the right eye (A) and normal anterior vitreous image in the left eye (B) of a 47-year-old male.
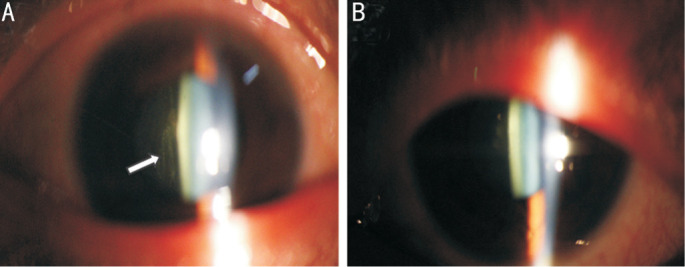
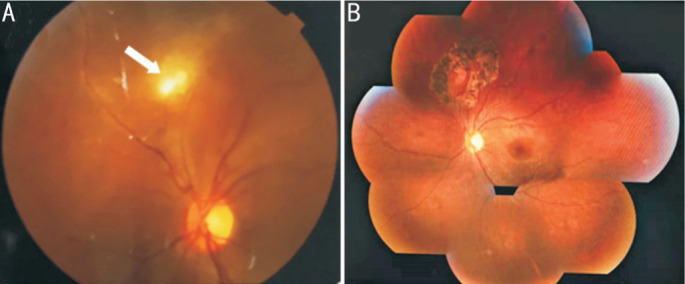
Figure 3. Fundus photo of a 30-year-old female with a mid-periphery granuloma (black arrowhead) and vitritis.
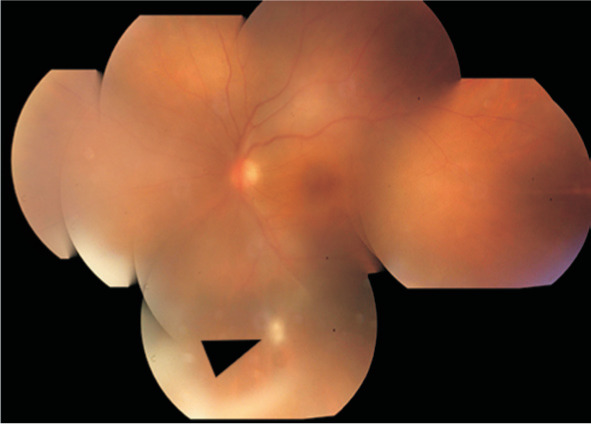
Figure 4. Fundus photos of a 43-year-old female.
A: Vitreous opacity in right eye and no granuloma; B: One month after vitrectomy; C: A periphery granuloma was observed with sclera press during surgery.
Imaging Characteristics
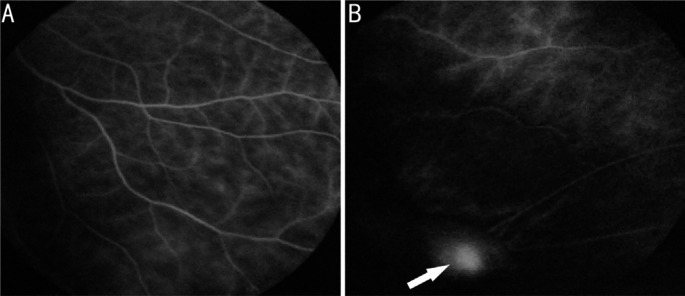
Fundus fluorescence angiography (FFA) was examined in 5 patients. Capillary fluorescence leakage and staining of the granuloma were observed in the affected eyes (Figure 5). There was no abnormal fluorescence in the fellow eyes.
Figure 5. FFA of a 36-year-old female with a granuloma in right eye.
A: Capillary fluorescence leakage; B: Staining of granuloma (arrow).
DISCUSSION
OT is an important cause of reduced VA, mostly in children, and the involvements of adults are often overlooked. In this study, the clinical manifestation of 24 OT patients with mean age of 33.5 years old was reported.
Direct contact with a puppy or kitten, 54.2% of adult OT patients in our study, was in approximately 20% of Korean adult OT patients[13], and in 0 of European adult patients. However, this does not exclude the possibility of ingesting food that has been soiled by dog or cat feces. This suggests that OT diagnosis should not be dismissed in case of absence of close or direct contact with pets[14]. However, in a previous study in China about OT in children, 91% of OT patients had contact with pets[7].
In our study, male/female ratio was 1.4/1. Previously, male predominance has been reported in Japanese[15] (male/female ratio=2.5/1) and Korean[16] (4/1) populations. It suggested that Japanese and Korean men may have a toxocariasis-related behavior, for example, the ingestion of raw cow liver[15]–[18]. Such a dietary habit was not common to Chinese men, suggesting that there was some difference in pathogenesis of adults OT between South Korea, Japan and north China.
Most patients (83.3%) resided in rural areas. In rural areas of China, dogs and cats are not generally fed in a pen, and their excrements are not disposed properly. These patients live in areas where there is more opportunity to contact eggs of worms in the soil. This study confirmed that the infection source may differ based on geographic and behavioral differences[3].
Demographic analyses revealed that OT predominantly occurred in right eye (2/1), which has not been analyzed previously. Several factors possibly contributed to it. First, OT is caused by the migration of Toxocara larvae from the circulatory system into the posterior segment of the eye. Brachiocephalic trunk is the first branch of arch of aorta, then divided into right common carotid artery and right subclavian artery; left common carotid artery is the second branch of arch of aorta; It is a separated branch, which means the size of the opening of left common carotid artery is smaller than that of brachiocephalic trunk. Second, the direction of circulation is along with the angle of right common carotid artery but is against the angle of left common carotid artery[19]. All of these factors make it much easier for the larvae to goes into the right common carotid artery than into the left one.
In adult OT patients, VA was better than 20/100 in 70.8% and better than 20/200 in 79.2% of the patients. This VA score is much better than reported children OT patients in China. In previous study, 36 pediatric cases (84%) had a VA of 20/200 or worse at baseline[7]. Children can't express themselves clearly, and it will be a long time after the attack before their parents found the abnormality of the eyes because of strabismus or other signs[20]. Adult patients can detect VA decrease in time and present in hospital in the early stage.
In this study, granuloma and vitreous opacities were common comorbidities of OT. Causes of granulomatous uveitis in the developed world constitute sarcoidosis (0.5%-18.1%), Vogt-Koyanagi-Haradasyndrome (0.4%-10.3%), and sympathetic ophthalmia (0.2%-2.1%)[21]. However, the most common non-infective uveitis with retinal granuloma is bilateral involvement. In our study, most of granulomas (87.5%) located in the periphery, which suggests that careful examination including fundus examination and various imaging examinations for granuloma is necessary in patients with monocular vitreous opacities and slight or no anterior segment inflammation. These results were consistent with the usual clinical features of adult OT in South Korea and Europe[13]–[14]. There was no significant difference between temporal or nasal side when it came to the localization of granuloma. Vitreous opacities were the most common cause of vision loss in our OT patients. There was one patient presented as retinal detachment and white cataract, accompanied with choroidal detachment and anterior segment inflammation, which implied that in adults OT patients, retinal detachment may possibly develop without treatment, and the natural prognosis may be bad. However, in children, the most common signs of ocular toxocariasis at the first visit were tractional retinal detachment and the development of vitreous strands[7].
OT can be definitively diagnosed with a biopsy for direct confirmation of Toxocara infection. But it is risky and not reasonable to obtain a biopsy specimen by retinotomy in an eye with only vitreous opacity. Clinically it is mainly diagnosed by typical clinical manifestation. But in our study, most of granulomas (87.5%) located in the periphery retina, and it is difficult to diagnose OT with atypical manifestation. The atypical subtype, which showed advanced stages of retinal and choroidal detachment, was difficult to be diagnosed. Scleral indentation during surgery could detect doubtful peripheral granuloma, which reminded the possible etiology of retinal, and detachment might be OT, and final diagnosis was confirmed by the positive results of the anti-T. canis IgG test of vitreous humor. ELISA test of anti-T. canis larva IgG is currently used for diagnosis of OT[22]. It has been reported that the diagnostic value of the serum toxocariasis ELISA test in OT patients with typical clinical manifestation is high in terms of sensitivity and specificity[23], but A negative serology does not rule out a diagnosis of ocular toxocariasis[24], in cases of OT without the visceral toxocariasis and without breakdown of the blood-AH barrier, there will be no leakage of IgG from intraocular fluids into serum, which leads to a low or undetectable specific antibodies in the serum[2],[25]. Sero-epidemiological surveys have demonstrated that some healthy subjects also have specific anti-Toxocara antibodies in their serum, corresponding to past, self-cured systemic infection[26]. Anti-Toxocara IgG level in intraocular fluids will be more precise and specific for the diagnosis of OT. AH is much easier to be collected than the vitreous humor, but in our study, the concentration gradient between the vitreous humor and AH was found, which is similar with other previous study[27]. The following reasons could be associated with it. First, the vitreous humor is closer to the lesion; second, intact anterior limiting membrane can prevent the distribution of IgG from vitreous body to AH. It suggested that vitreous humor is of great value in susceptible OT cases with a low anti-Toxocara IgG level in AH.
There are limitations in this study. First, it is a retrospective study. Goldman-Witmer coefficient is a very important result for diagnosis of OT, while not all patients underwent this examination. Second, there might have been a selection bias because patients were recommended to the uveitis center in our hospital from other clinics. Third, the number of patients is relatively small, so more cases are still needed to further investigate into this disease to illustrate it in our clinical practice. Despite of all above limitations, to our knowledge, this is the first report about OT in adults, particularly in Chinese.
In conclusion, OT is an etiological factor of infectious uveitis, but its adults' involvement seems to be neglected and underestimated[3],[28]. Early diagnosis of OT is not easy in adult patients without typical manifestation in early stage but is important for a good visual prognosis[29]–[30]. It is necessary to routinely differentiate ocular toxocariasis from patients with monocular intermediate or posterior uveitis with vitreous opacity and unknown etiology.
Acknowledgments
Authors' contributions: Hu XF: Collected, analyzed the data, and wrote the paper. Feng J, Kang H, Wang H, Liu XH: Collected and analyzed the dada. Tao Y: Conceived and designed the paper.
Foundations: Supported by National Natural Science Foundation of China (No.82070948); Scientific Research Program of Beijing Municipal Commission of Education (No.KM202010025020); Beijing Talent Project (No.2020027); Shunyi District “Beijing Science and Technology Achievements Transformation Coordination and Service Platform” Construction Fund (No.SYGX202010).
Conflicts of Interest: Hu XF, None; Feng J, None; Kang H, None; Wang H, None; Liu XH, None; Tao Y, None.
REFERENCES
- 1.Chen J, Liu Q, Liu GH, Zheng WB, Hong SJ, Sugiyama H, Zhu XQ, Elsheikha HM. Toxocariasis: a silent threat with a progressive public health impact. Infect Dis Poverty. 2018;7(1):59. doi: 10.1186/s40249-018-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alba-Hurtado F, Muñoz-Guzmán MA, Valdivia-Anda G, Tórtora JL, Ortega-Pierres MG. Toxocara Canis: larval migration dynamics, detection of antibody reactivity to larval excretory-secretory antigens and clinical findings during experimental infection of gerbils (Meriones unguiculatus) Exp Parasitol. 2009;122(1):1–5. doi: 10.1016/j.exppara.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104(1):3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 4.Fata A, Hosseini SM, Woo SJ, Zibaei M, Berenji F, Farash BRH, Moghaddas E. Frequency of toxocara antibodies in patients clinically suspected to ocular toxocariasis, northeast of Iran. Iran J Parasitol. 2021;16(2):305–311. doi: 10.18502/ijpa.v16i2.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae KW, Ahn SJ, Park KH, Woo SJ. Diagnostic value of the serum anti-Toxocara IgG titer for ocular toxocariasis in patients with uveitis at a tertiary hospital in Korea. Korean J Ophthalmol. 2016;30(4):258–264. doi: 10.3341/kjo.2016.30.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon JW, Lee SY, Jee D, Cho YK. Prognosis for ocular toxocariasis according to granuloma location. PLoS One. 2018;13(8):e0202904. doi: 10.1371/journal.pone.0202904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhang Q, Li J, Ji X, Xu Y, Zhao P. Clinical characteristics of pediatric patients with ocular toxocariasis in China. Ophthalmologica. 2016;235(2):97–105. doi: 10.1159/000443215. [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Sun L, Liu C, Li S, Zhang T, Luo X, Ding X. Diagnosis of ocular toxocariasis by serum and aqueous humor IgG ELISA. Transl Vis Sci Technol. 2021;10(8):33. doi: 10.1167/tvst.10.8.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zyoud SH. Global toxocariasis research trends from 1932 to 2015: a bibliometric analysis. Health Res Policy Syst. 2017;15(1):14. doi: 10.1186/s12961-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karska-Basta I, Kubicka-Trząska A, Chrząszcz M, Pociej-Marciak W, Romanowska-Dixon B. Toxocara optic disc granuloma: deep range imaging optical coherence tomography findings. Case Rep Ophthalmol. 2019;10(3):339–343. doi: 10.1159/000503139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Liu H, Li M, Fan K, Li S, Lei B. Multimodality image analysis in a cohort of patients with atypical juvenile ocular toxocariasis. J Ophthalmol. 2021;2021:4853531. doi: 10.1155/2021/4853531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arevalo JF, Espinoza JV, Arevalo FA. Ocular toxocariasis. J Pediatr Ophthalmol Strabismus. 2013;50(2):76–86. doi: 10.3928/01913913-20120821-01. [DOI] [PubMed] [Google Scholar]
- 13.Ahn SJ, Woo SJ, Jin Y, Chang YS, Kim TW, Ahn J, Heo JW, Yu HG, Chung H, Park KH, Hong ST. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis. 2014;8(6):e2938. doi: 10.1371/journal.pntd.0002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despreaux R, Fardeau C, Touhami S, Brasnu E, Champion E, Paris L, Touitou V, Bodaghi B, Lehoang P. Ocular toxocariasis: clinical features and long-term visual outcomes in adult patients. Am J Ophthalmol. 2016;166:162–168. doi: 10.1016/j.ajo.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida M, Shirao Y, Asai H, Nagase H, Nakamura H, Okazawa T, Kondo K, Takayanagi TH, Fujita K, Akao N. A retrospective study of ocular toxocariasis in Japan: correlation with antibody prevalence and ophthalmological findings of patients with uveitis. J Helminthol. 1999;73(4):357–361. doi: 10.1017/s0022149x99000608. [DOI] [PubMed] [Google Scholar]
- 16.Park SP, Park I, Park HY, Lee SU, Huh S, Magnaval JF. Five cases of ocular toxocariasis confirmed by serology. Korean J Parasitol. 2000;38(4):267–273. doi: 10.3347/kjp.2000.38.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoi K, Goto H, Sakai J, Usui M. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm. 2003;11(4):269–275. doi: 10.1076/ocii.11.4.269.18266. [DOI] [PubMed] [Google Scholar]
- 18.Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008;46(3):139–143. doi: 10.3347/kjp.2008.46.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca C, Silva AM, Freire S, Proença R. Ocular toxocariasis: atypical clinical course. BMJ Case Rep. 2019;12(4):e228717. doi: 10.1136/bcr-2018-228717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodhall DM, Garcia AP, Shapiro CA, Wray SL, Shane AL, Mani CS, Stimpert KK, Fox LM, Montgomery SP. Assessment of U.S. pediatrician knowledge of toxocariasis. Am J Trop Med Hyg. 2017;97(4):1243–1246. doi: 10.4269/ajtmh.17-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, Androudi S. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26(1):2–16. doi: 10.1080/09273948.2016.1196713. [DOI] [PubMed] [Google Scholar]
- 22.Saki J, Eskandari E, Feghhi M. Study of toxoplasmosis and toxocariasis in patients suffering from ophthalmic disorders using serological and molecular methods. Int Ophthalmol. 2020;40(9):2151–2157. doi: 10.1007/s10792-020-01393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Y, Shen C, Huh S, Sohn WM, Choi MH, Hong ST. Serodiagnosis of toxocariasis by ELISA using crude antigen of Toxocara canis larvae. Korean J Parasitol. 2013;51(4):433–439. doi: 10.3347/kjp.2013.51.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inchauspe S, Echandi LV, Dodds EM. Diagnosis of ocular toxocariasis by detecting antibodies in the vitreous humor. Arch Soc Esp Oftalmol (Engl Ed) 2018;93(5):220–224. doi: 10.1016/j.oftal.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 25.de Visser L, Rothova A, de Boer JH, van Loon AM, Kerkhoff FT, Canninga-van Dijk MR, Weersink AY, de Groot-Mijnes JD. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol. 2008;145(2):369–374. doi: 10.1016/j.ajo.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39(1):1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZJ, Zhou M, Cao WJ, Ji J, Bi YW, Huang X, Xu GZ. Evaluation of the Goldmann-Witmer coefficient in the immunological diagnosis of ocular toxocariasis. Acta Trop. 2016;158:20–23. doi: 10.1016/j.actatropica.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Hotez PJ, Wilkins PP. Toxocariasis: America's most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl Trop Dis. 2009;3(3):e400. doi: 10.1371/journal.pntd.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Guo D, Xu G, Jiang R. Ocular toxocariasis: long-term follow-up and prognosis of patients following vitrectomy. Ocul Immunol Inflamm. 2020;28(3):517–523. doi: 10.1080/09273948.2019.1597897. [DOI] [PubMed] [Google Scholar]
- 30.Padhi TR, Das S, Sharma S, Rath S, Rath S, Tripathy D, Panda KG, Basu S, Besirli CG. Ocular parasitoses: a comprehensive review. Surv Ophthalmol. 2017;62(2):161–189. doi: 10.1016/j.survophthal.2016.09.005. [DOI] [PubMed] [Google Scholar]