Abstract
AIM
To evaluate the long-term anatomical and visual outcomes of drusenoid pigment epithelial detachment (D-PED) in intermediate age-related macular degeneration (AMD) eyes treated with 577 nm yellow subthreshold micropulse laser (SML).
METHODS
In this retrospective study, 21 eyes of 16 patients with D-PED in intermediate AMD were consecutively included and assessed. All the eyes were treated with 577 nm SML in several sessions according to D-PED growth status. The logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) were assessed at the initial visit and after treatment. Spectral-domain optical coherence tomography (SD-OCT) was performed to evaluate the D-PED lifecycle by volumetric calculations. Regression analysis was used to determine the breakpoint, growth, and collapse rate of the D-PED lesions. The progression to advanced AMD was also documented.
RESULTS
All the eyes were treated with SML for 2.9±1.0 sessions. The mean follow-up period was 25.3±12.6mo. The BCVA was stable from the baseline to final visit. All the eyes were categorized into two groups according to the anatomical changes of the D-PED lesion: the collapse group (n=6, 28.6%) and non-collapse group (n=15, 71.4%). The change in logMAR BCVA did not differ significantly between the collapse group 0.00 (-0.31, 0.85) and non-collapse group 0.00 (0.00, 0.00; P=1). Regression analysis showed that the growth rate was significantly higher in the collapse group (0.090±0.095 mm3/mo) than in the non-collapse group (0.025±0.035 mm3/mo; P<0.001). One eye (4.8%) developed macular neovascularization at 11mo after SML treatment in the non-collapse group. Three eyes (14.3%) developed geographic atrophy (GA) in the collapse group.
CONCLUSION
Compared to the natural course of D-PED reported by previous studies, our results preliminarily show that SML can alleviate visual loss and possibility of progression to advanced AMD in eyes with D-PED in intermediate AMD. A controlled clinical trial needs to further verify the benefit of the intervention.
Keywords: age-related macular degeneration, drusenoid pigment detachment, optical coherence tomography, retinal pigment epithelium, subthreshold micropulse laser
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of severe vision loss in the elderly in the global society[1]–[2]. Clinically, AMD is classified as early-stage (medium-sized drusen and retinal pigmentary changes) to late-stage (neovascular and atrophic AMD)[2]. Intermediate AMD was defined as large drusen >125 µm and/or any AMD pigmentary abnormalities. According to Age-Related Eye Diseases Study (AREDS) severity scale, large drusen are an important risk of AMD[3]. Patients with a large drusen size and extensive drusen area are more likely to progress to advanced AMD[4]–[6].
Drusenoid pigment epithelial detachment (D-PED) is defined as a well-defined, pale yellow or white, large mound consisting of many large drusen or confluent drusen which is at least 350 µm in the narrowest diameter and appears elevated on stereoscopic fundus photographs in the AREDS study[4],[7]. D-PED is characterized by the accumulation of extracellular lipid-rich deposits between the retinal pigment epithelium (RPE) and Bruch's membrane (BrM) and is associated with an increased risk of progression to advanced AMD[7]–[9]. Although the precise mechanism underlying D-PED is unknown, RPE dysfunction was demonstrated to play a key role in the progression of D-PED[10]–[11]. Long separation of RPE from the underlying BrM/choriocapillaris complex results in a decline in RPE function and the death of photoreceptors over time.
As mentioned in AREDS Report No.28, advanced AMD developed within 5y in 42% of D-PED eyes [19% central geographic atrophy (GA) and 23% neovascular AMD] without advanced AMD at baseline, as a results of which 40% of the eyes lost three lines of BCVA (equivalent to 0.3 logMAR)[3]. The D-PED lifecycle typically shows an initial slow growth phase and then followed by a rapid collapse phase, accompanied by RPE layer disruption and anterior migration[12]. The connecting point between the growth phase and the collapse phase was defined as “breakpoint” of the lifecycle curve. Volumetric calculation has revealed that the lifecycle of D-PED is asymmetric; the collapse rate (0.199 mm3/mo) is significantly higher than the growth rate (0.022 mm3/mo). The appearance of intraretinal hyperreflective foci and acquired vitelliform lesions (AVLs) in optical coherence tomography (OCT), represented by anterior migration of RPE and disintegration of the RPE layer, precedes the breakpoint of D-PED[5].
Except to lifestyle changes and the use of vitamin supplements, there were limited treatment options available for intermediate AMD[13]. Recently, high-density/low-intensity subthreshold micropulse laser (SML) treatment has been studied in a number of retinal diseases[14]–[19]. An observational retrospective cohort study has shown that high-risk dry AMD eyes of AREDS category 2 or greater treated with 810 nm SML have a very low incidence of choroidal neovascularization (CNV)[16]. SML delivers energy via multiple, repetitive, short pulses within an envelope whose width is typically 0.1-0.5s[20]. Compared with continuous-wave laser, the time between laser bursts (referred to as the duty cycle) is long enough to target RPE and help reduce the spread of heat from the light-absorbing RPE and choroid[2]. More importantly, SML was proved to improve retinal and visual function in eyes with dry AMD-related photoreceptor degeneration[21]. The incorporation of micropulse laser technology with a 577 nm yellow laser system, which facilitates the titration of the threshold coagulation power, provides a shorter envelope (20ms), and is more suitable for the treatment of the macular disorders.
To our knowledge, few study has investigated the effect of 577 nm yellow SML in patients with dry AMD. In this study, we aimed to assess the anatomical and visual outcomes in intermediate AMD patients with D-PED who were treated with 577 nm SML and to determine safety profile of SML and its effect on the D-PED lifecycle.
SUBJECTS AND METHODS
Ethical Approval
This retrospective cohort study was conducted under the institutional review board guidelines at the General Hospital of Central Theater Command in accordance with the tenets of Declaration of Helsinki. Informed consent was obtained from the patients.
Enrollment of Subjects
This study was conducted between June 1, 2016 and December 29, 2020. The eligibility criteria were as follows: 1) Patients aged over 50y who were diagnosed with intermediate AMD and the presence of large D-PED lesions >350 µm[4]; 2) Eyes were followed up for over 6mo. The exclusion criteria were as follows: 1) the presence of significant GA or macular neovascularization (MNV) at baseline; 2) the presence of other eye diseases at baseline that could reduce visual acuity (excluding mild cataract), such as retinal vascular disorders or macular dystrophies; 3) prior ocular therapies at initial visit, such as laser photocoagulation or intravitreal therapy, macular photocoagulation, photodynamic therapy, or anti-vascular endothelial growth factor (VEGF) therapy.
Subthreshold Micropulse Laser Treatment
All the eyes were treated with 577 nm SML after enrollments. All laser treatments were performed by an experienced ophthalmologist (Song YP). After pupillary dilation, topical benoxinate was applied to the cornea. A Volk Area Centralis contact lens (Volk Optical, Mentor, OH, USA) was placed on the cornea with a viscoelastic solution. Before SML treatment, a test burn was performed outside the vascular arcade to determine the threshold power for each eye. The threshold power was determined using a 200 µm spot with a 200ms exposure duration and titrated from 50 mW in the continuous wave emission mode until a light grey white burn was barely visible for each individual. Then, the laser was changed to a micropulse emission mode with a 5% duty cycle, and the resulting power was four-to-eight fold higher than the threshold power with the same exposure duration (IQ577, Oculight SLx; Iridex, Corp, Mountain View, CA, USA). Multipoint scanning laser was delivered to the whole D-PED area with no space between laser spots, including the foveal center, to provide as tight coverage as possible.
Patients was followed at 1-2mo interval. SML treatment was repeated if there was no obvious D-PED collapse over 3mo prior to the former SML treatment. The SML treatment intervals was over 3mo. The power, number of spots, and number of SML treatment sessions were collected.
Demographic and Eye Examinations
Data regarding baseline characteristics, such as age, sex, visual acuity, and follow-up periods, were collected. The best-corrected visual acuity (BCVA) was assessed at the initial visit and after treatment and were converted to logarithm of the minimum angle of resolution (logMAR) values for statistical analyses.
OCT for Morphological Changes and Measurement of D-PED
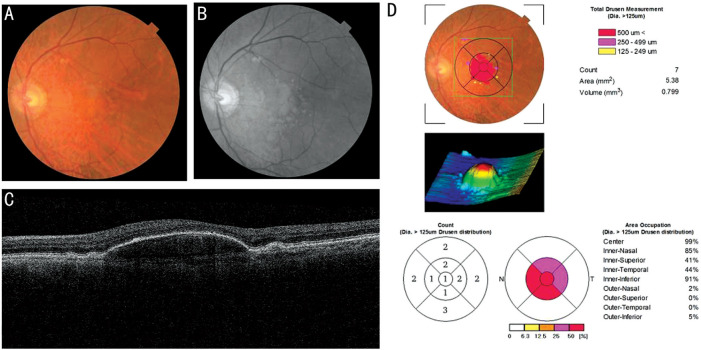
Anatomic characteristics were collected by a high-speed spectral-domain optical coherence tomography (SD-OCT) device (3D-OCT 2000 MARK 2, Topcon Corporation, Tokyo, Japan). The drusen area and volume of the D-PED lesions at every 3mo were measured by built-in algorithms for the 3D macular 512×128 scans (6×6 mm2) result using “Drusen Analysis Mode” by the reviewer software Topcon IMAGEnet 2000 (Figure 1). The height of D-PED was measured manually from the RPE to BrM at its greatest height. Two independent examiners (Huang Z and Deng KY) analyzed the OCT images. Any disagreement was settled by discussion between the two examiners.
Figure 1. Spectral-domain optical coherence tomography of typical drusenoid pigment detachment (D-PED) eye.
A: Fundus photography shows a large D-PED in the eye; B: Red-free photography of D-PED; C: B-scan of D-PED; D: Drusen analysis report by Topcon IMAGEnet 2000 shows the 3D image of D-PED and drusen area and volume.
The presence of D-PED collapse, defined as fading of the drusenoid material associated with flattening of RPE. According to the anatomical outcome of the D-PED lesions, the eyes were divided into two groups: the collapse group and non-collapse group. The presence of hyperreflective foci, presence of AVLs, and disruption of RPE were also evaluated at baseline and at the final visit through OCT and fundus findings.
Fluorescein Angiography or OCT Angiography
Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA; Heidelberg Engineering, Dossenheim, Germany) were performed at baseline to exclude neovascular AMD and repeated when MNV was suspected during the follow-up periods. While FFA and ICGA is not allowed, OCT angiography was carried out. Once new MNV identified, eyes were managed with intravitreal anti-VEGF inhibitors in a regular manner. The progression to advanced AMD, is defined as the presence of GA or MNV on the basis of fundus photographs, OCT, FFA and ICGA findings.
Statistical Analysis
Statistical analyses were performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Frequencies, means, and medians were calculated to summarize the data. Continuous variables that obeyed a normal distribution were calculated using two-dependent t-test or two-paired t-test and are presented as mean±SD. Continuous variables that did not obey a normal distribution were calculated using the Mann-Whitney U test or Wilcoxon matched pairs signed rank sum test and are presented as the median (P25, P75). Categorical variables were calculated using the Chi-square test or McNemar test for correlated proportions and are presented as frequencies.
Nonlinear regression analysis was used to evaluate any significant slope change in the curves depicting the PED volume as a function of time in the collapse group[5]. The “breakpoints” were defined as the point at which the slope changed significantly between periods of growth and collapse. The growth and collapse rates were identified in all collapse cases. In the non-collapse group, linear regression analysis was performed to calculate the growth rate of the D-PED lesions.
Spearman's correlation coefficient for ranked data was used to determine the correlations among the change in BCVA, collapse of D-PED lesions, baseline characteristics, PED morphometry, SML treatment parameters, and incidence of MNV/GA. A two-sided P<0.05 was considered statistically significant.
RESULTS
Demographics
Nineteen AMD patients (24 eyes) with D-PED were recruited in this study. All eyes were graded as intermediate AMD at baseline. Three patients (3 eyes) were lost to follow-up in less than 6mo due to changes in medical insurance, relocation, or loss of contact. Thus, 16 patients (11 males and 5 females, 21 eyes) fulfilled the criterions were included in the statistical analyses. The baseline demographics of these patients are presented in Table 1. The average age of the patients was 72.8±10.4y (range, 54-90y). The mean follow-up period was 25.3±12.6mo (range, 8-39mo).
Table 1. Baseline characteristics of individuals with drusenoid pigment epithelial detachment of intermediate age-related macular degeneration.
| Patients' No. | Age | Sex | Eye | AREDS scale | Baseline logMAR BCVA | Follow-up (mo) | Number of follow-ups | Number of SML treatments | Incidence of GA or MNV |
| 1 | 76 | M | OD | 4 | 1.00 | 39 | 19 | 3 | - |
| OS | 4 | 0.82 | 36 | 18 | 4 | - | |||
| 2 | 58 | F | OD | 4 | 0.60 | 37 | 19 | 4 | GA |
| OS | 4 | 0.52 | 37 | 37 | 2 | GA | |||
| 3 | 84 | M | OS | 2 | 0.30 | 10 | 6 | 3 | - |
| 4 | 90 | M | OS | 3 | 0.60 | 18 | 12 | 1 | MNV |
| 5 | 79 | M | OD | 4 | 0.30 | 9 | 4 | 3 | - |
| OS | 4 | 0.30 | 9 | 4 | 3 | - | |||
| 6 | 54 | F | OD | 2 | 0.22 | 28 | 8 | 3 | - |
| 7 | 78 | M | OS | 3 | 0.52 | 39 | 7 | 1 | - |
| 8 | 68 | M | OS | 2 | 0.40 | 29 | 15 | 4 | - |
| 9 | 73 | M | OS | 2 | 0.36 | 13 | 6 | 3 | - |
| 10 | 70 | F | OS | 4 | 0.30 | 39 | 11 | 2 | - |
| 11 | 84 | M | OD | 3 | 0.52 | 24 | 2 | 2 | - |
| 12 | 67 | M | OS | 2 | 0.22 | 39 | 7 | 4 | - |
| 13 | 56 | M | OD | 2 | 1.00 | 8 | 6 | 3 | - |
| 14 | 70 | M | OD | 3 | 0.70 | 12 | 5 | 3 | GA |
| OS | 3 | 0.30 | 12 | 5 | 4 | - | |||
| 15 | 79 | F | OD | 3 | 0.40 | 18 | 8 | 4 | - |
| OS | 3 | 0.60 | 18 | 8 | 4 | - | |||
| 16 | 78 | F | OD | 2 | 0.22 | 37 | 3 | 1 | - |
AREDS: Age-Related Eye Diseases Study; logMAR: Logarithm of the minimum angle of resolution; SML: Subthreshold diode micropulse laser; BCVA: Best-corrected visual acuity; M: Male; F: Female; GA: Geographic atrophy; MNV: Macular neovascularization.
Subthreshold Micropulse Laser Treatment
The number of SML treatments for the D-PED lesions was 2.9±1.0 (range, 1-4). The average laser power was 442.9±98.7 mW (range, 280-600 mW) and the total spots used each time were 1778.8±1086.9, respectively. After SML treatment, the D-PED lesions showed two different patterns: collapse (28.6%, n=6) and non-collapse (71.4%, n=15). Typical cases of these two groups were shown in Figures 2 and 3 separately.
Figure 2. D-PED volumetric changes after treated with 577 nm subthreshold micropulse laser in the collapse group.
A: Illustrative plots of the D-PED volume of a 68-year-old patient. Nonlinear regression analysis determined the breakpoint at 21mo where the D-PED volumetric slope changed significantly between periods of growth and collapse. The growth rate was 0.013 mm3/mo whereas the collapse rate was 0.076 mm3/mo. B: SD-OCT images showed the change of D-PED lesion against time. The eye received 3 times SML treatment at baseline, 5, and 13mo separately.
Figure 3. D-PED volumetric changes after treated with 577nm subthreshold micropulse laser in the non-collapse group.
A: Illustrative plots of the D-PED volume of a 67-year-old patient. Linear regression analysis the growth rate was 0.012 mm3/mo. The eye received 4 times SML treatment at baseline, 4, 8, and 12mo separately. B: SD-OCT images showed the change of D-PED lesion against time.
Change in Best-Corrected Visual Acuity
At the final visit, the mean logMAR BCVA was 0.55±0.36 (Snellen equivalent 20/63, range, 0.22-1.4) compared to 0.49±0.24 (Snellen equivalent 20/63, range, 0.22-1) at baseline as shown in Table 2. The BCVA was stable from the baseline compared to final visit (Z=-1.572, P=0.116, Wilcoxon signed ranks test). The change in logMAR BCVA did not differ significantly between the collapse group 0.00 (-0.31, 0.85) and non-collapse group 0.00 (0.00, 0.00; Z=1.000, P=1.000, Mann-Whitney U test).
Table 2. The change of logMAR best-corrected visual acuity in eyes of drusenoid pigment detachment after 577 nm subthreshold diode micropulse laser treatment.
| Parameters | D-PED Relapse |
||
| Yes (n=6) | No (n=15) | P | |
| Baseline BCVA, mean±SD | 0.56±0.19 | 0.46±0.26 | 0.404a |
| Final BCVA, mean±SD | 0.66±0.44 | 0.50±0.34 | 0.388a |
| BCVA change, M (P25, P75) | 0.00 (-0.31, 0.85) | 0.00 (0.00, 0.00) | 1.000c |
| Change of logMAR BCVA, n (%) | 0.793d | ||
| Increase≥0.1 | 0 | 1 (16.7) | |
| Stable<0.1 | 13 (86.7) | 3 (50.0) | |
| Decrease≥0.1 | 2 (13.3) | 2 (33.3) | |
logMAR: Logarithm of the minimum angle of resolution; BCVA: Best corrected visual acuity; D-PED; Drusenoid pigment detachment. aIndependent-samples t-test; cMann-Whitney U test; dKruskal Wallis test.
Morphological Characteristics
The baseline and final morphological characteristics are shown in Table 3. The height, area, and volume of the D-PED lesions decreased after SML treatment at the final visit compared with the baseline values; however, the difference was not statistically significant (Z=0.504, 0.504 and 0.901, P=0.614, 0.614, and 0.357, respectively, Wilcoxon matched pairs signed rank sum test). The presence of intraretinal hyperreflective foci, presence of AVLs, and disruption of RPE increased (n=11, 10 and 4, P=0.250, 0.625 and 0.250, respectively, McNemar test) with no significant difference. However, as shown in Table 4, the height, area, and volume of the D-PED lesions clearly decreased in the collapse group and slightly increased in the non-collapse group.
Table 3. Overview of baseline and final morphological characteristics of drusenoid pigment detachment after 577 nm subthreshold diode micropulse laser treatment.
| Parameters | Baseline | Final visit | P |
| D-PED height (µm), mean±SD | 366.7±299.6 | 254.9±286.7 | 0.614a |
| D-PED area (mm2), mean±SD | 7.39±6.09 | 5.72±5.35 | 0.614a |
| D-PED volume (mm3), mean±SD | 2.11±2.83 | 1.02±2.01 | 0.357a |
| Presence of intraretinal hyperreflective foci, n (%) | 8 (38.1) | 11 (52.4) | 0.250b |
| Presence of acquired vitelliform lesions, n (%) | 8 (38.1) | 10 (47.6) | 0.625b |
| Disruption of retinal pigment epithelium, n (%) | 1 (4.8) | 4 (19.0) | 0.250b |
| Presence of subretinal fluid, n (%) | 7 (33.3) | 4 (17.4) | 0.250b |
D-PED: Drusenoid pigment epithelium detachment; aWilcoxon matched samples signed rank sum test; bMcNemar test.
Table 4. The change of drusenoid pigment detachment lesion after 577 nm subthreshold diode micropulse laser treatment.
| Parameters | Collapse of D-PED lesion |
||
| Yes (n=6) | No (n=15) | P a | |
| Change of D-PED height (µm) | -553.2±287.5 | 64.8±148.7 | <0.001 |
| Change of D-PED area (mm2) | -8.02±5.07 | 0.87±1.85 | <0.001 |
| Change of D-PED volume (mm3) | -4.30±2.84 | 0.19±0.44 | <0.001 |
a2-independent samples t-test; D-PED: Drusenoid pigment epithelium detachment.
Illustrative plots of the D-PED volume against time in the collapse and non-collapse groups are shown in Figures 2A and 3A, respectively. In the collapse group, the dynamic change in D-PED volume suggested that the D-PED lesions gradually increased and then suddenly decreased (Figure 2). The average breakpoint of the D-PED lesions was 12.3±9.5mo (range, 1-21mo) and the duration of lesion collapse was 6.5±4.6mo in the collapse group.
In the non-collapse group, the dynamic changes in D-PED indicate a slight increase in the volume with time (Figure 3). The growth rate of the D-PED lesions was significantly higher in the collapse group (0.090±0.095 mm3/mo) than in the non-collapse group (0.025±0.035 mm3/mo; t=-2.231, P<0.001, independent t-test). The collapse rate of D-PED lesions was 0.718±0.729 mm3/mo in the collapse group.
Correlations Among Change in BCVA, Collapse of D-PED Lesions, Baseline Characteristics, PED Morphometry, SML Treatment Parameters
The correlations among the change in BCVA, collapse of D-PED lesions, baseline characteristics, PED morphometry, and SML treatment parameters are shown in Table 5 (Spearman's correlation test).
Table 5. The correlations of change of BCVA, collapse of D-PED lesion, baseline characterizes, morphometry and SML treatment parameters.
| Parameters | Collapse of D-PED lesion |
Change of logMAR BCVA |
||
| r c | P | r c | P | |
| Relapse of D-PED lesion | - | - | -0.087 | 0.707 |
| Change of logMAR BCVA | 0.087 | 0.707 | - | - |
| Baseline logMAR BCVA | 0.290 | 0.202 | 0.533a | 0.013 |
| Age | -0.447a | 0.042 | 0.254 | 0.267 |
| Height of D-PED lesion | 0.697b | 0.000 | 0.182 | 0.429 |
| Area of D-PED lesion | 0.487a | 0.025 | 0.254 | 0.267 |
| Volume of D-PED lesion | 0.574b | 0.006 | 0.238 | 0.300 |
| Growth rate of D-PED lesion volume | 0.491 | 0.089 | 0.282 | 0.350 |
| Collapse of D-PED lesion volume | - | - | 0.696 | 0.125 |
| Presence of intraretinal hyperreflective foci | 0.372 | 0.097 | 0.447a | 0.042 |
| Presence of acquired vitelliform lesions | 0.589b | 0.005 | 0.061 | 0.793 |
| Disruption of retinal pigment epithelium | 0.354 | 0.116 | 0.324 | 0.152 |
| Presence of subretinal fluid | 0.533a | 0.013 | 0.480a | 0.028 |
| Number of SML laser treatment | 0.226 | 0.325 | -0.126 | 0.126 |
| Energy of laser spot | 0.299 | 0.189 | 0.091 | 0.694 |
| Total number of SML laser spot | 0.244 | 0.287 | 0.002 | 0.994 |
| Incidence of MNV/GA | 0.499a | 0.021 | 0.351 | 0.118 |
D-PED: Drusenoid pigment epithelium detachment; SML: Subthreshold diode micropulse laser; MNV: Macular neovascularization; GA: Geographic atrophy. aP<0.05; bP<0.01; cSpearman correlation coefficient.
The height, area, and volume of the D-PED lesions; presence of AVLs; presence of subretinal fluid; and incidence of MNV/GA were positively correlated with the collapse of D-PED (r=0.697, P<0.001; r=0.487, P=0.025; r=0.574, P=0.006; r=0.589, P=0.005; r=0.533, P=0.013; and r=0.499, P=0.021, respectively). Age was inversely correlated with the collapse of D-PED (r=−0.447, P=0.042).
The baseline logMAR BCVA, presence of intraretinal hyperreflective foci, and presence of subretinal fluid were positively correlated with the change in logMAR BCVA (r=0.533, P=0.013; r=0.447, P=0.042; and r=0.480, P=0.028, respectively).
Incidence of CNV/GA
One eye (4.8%) developed MNV at 11mo after SML treatment in the non-collapse group and received rescue anti-VEGF injections. In the collapse group, 3 eyes (14.3%) developed GA after the collapse of PED at 6, 12, and 21mo after SML treatment separately. No eyes in the non-collapse group developed GA during the study.
DISCUSSION
Although a number of large multicenter randomized clinical trials have been conducted in the past decades to assess whether prophylactic continuous laser treatment is beneficial for treating high-risk AMD, the results were disappointing due to the higher incidence of CNV compared with the observation[22]–[23]. Recently, the world-first 36mo multicenter double-masked clinical trial, Laser Intervention in Early Stages of Age-Related Macular Degeneration (LEAD), revealed that subthreshold nanosecond laser could slow progression for intermediate AMD except with reticular pseudodrusen[24]. A 12mo prospective study shows primary results that D-PED treated with 532 nm Nd:YAG laser at a very short pulse duration and prophylactic intravitreal VEGF injections improved vision acuity and D-PED regressed without developing center involving GA[25]. In this study, we firstly evaluated the effect of 577 nm SML in intermediate AMD with D-PED case series.
The exact mechanism of 577 nm SML is unknown. But compared with continuous-wave laser, 577 nm SML with a short envelope and duty cycle can target RPE and reduce the spread of heat from light-absorbing RPE and the choroid, thereby minimizing the thermal effect on neural retinal and deeper structures[26]–[27]. For the treatment of D-PED, SML can reduce direct thermal damage to the retina compared with traditional laser. SML is believed to be photo stimulation by upregulation of heat-shock protein (HSP) 70 expression to restore retinal fluid extravasation[18] and aquaporin (AQP) 3 by 100-fold to drain subretinal fluid[28]. The recruitment of mononuclear cells to RPE accompanied by the upregulation of inflammatory cytokine and HSP genes may mediate the effect of SML[29]. Clinically, BrM thickness and AMD-like RPE alterations can be reduced by SML[30]. Few study has reported anti-VEGF therapy for D-PED but it just for the prevent complications of MNV during the laser treatment session.
In our study, large D-PED lesions were included, with an average volume of 2.11±2.83 mm3 and average height of 366.7±299.6 µm. Two years after SML treatment, BCVA was stable compared with baseline. Balaratnasingam et al[5] reported that in a follow-up period of 4.1y, the mean BCVA of large D-PED lesions (0.22 logMAR) significantly decreased at the final visit (0.8 logMAR). Alexandre de Amorim Garcia Filho et al[31] also reported that half of D-PED eyes progressed to GA or CNV and had a worse BCVA in 18.5mo without intervention. BCVA was stable in the study, suggesting that SML treatment can alleviate vision loss compared with natural cause reported as the above-mentioned studies.
In the natural course, drusen volumes were increased by a cubic function and then spontaneous decreased, following of progression to advanced AMD[32]. In our study, 6 of 21 eyes (28.6%) showed a collapse of D-PED lesion at the final visit after SML treatment. The collapse group showed a faster growth rate of D-PED lesions (0.090 mm3/mo) than the non-collapse group (0.025 mm3/mo) and that during the natural course (0.022 mm3/mo)[5]. The collapse rate in the collapse group (0.718 mm3/mo) was also higher than that during the natural course (0.199 mm3/mo)[5]. The incidence of MNV (4.8%) and GA (14.3%) in our study was also lower than that during the natural course of D-PED[4]. So we infer that 577 nm SML accelerates the lifecycle of part of D-PED lesions, resulting faster growth and collapse rate of the lesion without obvious BCVA damage, by avoiding long separation of RPE from the underlying BrM/choriocapillaris complex and alleviating RPE damage.
Our findings revealed that the height, area, and volume of the D-PED lesions were positively correlated with the collapse of D-PED, which suggests that larger D-PED lesions are more likely to collapse after SML treatment. The presence of AVLs and the presence of subretinal fluid were positively correlated with the collapse of D-PED, suggesting that AVLs and subretinal fluid predict the collapse of D-PED, which was consistent with former studies[5],[33]. Moreover, the incidence of MNV/GA was more frequent after the collapse of D-PED. Tvenning et al[34] reported that the subfoveal location of D-PEDs and the presence of AQLs is associated with a reduction in BCVA, while in our study a lower baseline BCVA, the presence of intraretinal hyperreflective foci, and the presence of subretinal fluid predicted a decrease in BCVA after SML treatment.
In this study, the average height of D-PED lesions was 367 µm, which suggested that the D-PED lesions were large; this facilitated the precise evaluation of the lesions. However, the lack of a control group, small sample size and retrospective nature of this study were the limitations of this study. Sufficient baseline data, such as blood glucose levels, blood pressure, lipid levels, obesity, ocular factors, corneal lipid levels, annular ocular axis, cataract, and choroid thickness, were not recorded completely in this study. A longer follow-up period is also needed regarding to the slow progression of the D-PED lesions. The small-size, retrospective feature and no control group also restrict the analysis of the risk factor of the intervention.
Overall, our primary findings suggest that 577 nm SML can alleviate visual loss and possibility of progression to advanced AMD in eyes with D-PED in intermediate AMD. Larger D-PED lesions are more likely to collapse after SML treatment. A controlled clinical trial needs to further verify the benefit of the intervention.
Acknowledgments
Conflicts of Interest: Huang Z, None; Deng KY, None; Deng YM, None; Hui YN, None; Song YP, None.
REFERENCES
- 1.Mao FF, Yang XH, Yang K, Cao XS, Cao K, Hao J, Zhang Y, Wang NL. Six-year incidence and risk factors for age-related macular degeneration in a rural Chinese population: the Handan eye study. Invest Ophthalmol Vis Sci. 2019;60(15):4966. doi: 10.1167/iovs.19-27325. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392(10153):1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 3.Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BEK, Klein R, Ferris FL, Bressler SB, Milton RC, Group AREDS The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cukras C, Agrón E, Klein ML, Ferris FL, III, Chew EY, Gensler G, Wong WT. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: age-related eye disease study report no. 28. Ophthalmology. 2010;117(3):489–499. doi: 10.1016/j.ophtha.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balaratnasingam C, Yannuzzi LA, Curcio CA, Morgan WH, Querques G, Capuano V, Souied E, Jung J, Freund KB. Associations between retinal pigment epithelium and drusen volume changes during the lifecycle of large drusenoid pigment epithelial detachments. Invest Ophthalmol Vis Sci. 2016;57(13):5479–5489. doi: 10.1167/iovs.16-19816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitnilska V, Kersten E, Altay L, Schick T, Enders P, de Jong EK, Langmann T, Hoyng CB, den Hollander AI, Fauser S. Major predictive factors for progression of early to late age-related macular degeneration. Ophthalmologica. 2020;243(6):444–452. doi: 10.1159/000507196. [DOI] [PubMed] [Google Scholar]
- 7.Yu JJ, Agrón E, Clemons TE, Domalpally A, van Asten F, Keenan TD, Cukras C, Chew EY, Research Group AREDS2 Natural history of drusenoid pigment epithelial detachment associated with age-related macular degeneration: age-related eye disease study 2 report no. 17. Ophthalmology. 2019;126(2):261–273. doi: 10.1016/j.ophtha.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roquet W, Roudot-Thoraval F, Coscas G, Soubrane G. Clinical features of drusenoid pigment epithelial detachment in age related macular degeneration. Br J Ophthalmol. 2004;88(5):638–642. doi: 10.1136/bjo.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lains I, Pundlik SJ, Nigalye A, Katz R, Luo G, Kim IK, Vavvas DG, Miller JW, Miller JB, Husain D. Baseline predictors associated with 3-year changes in dark adaptation in age-related macular degeneration. Retina. 2021;41(10):2098–2105. doi: 10.1097/IAE.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaratnasingam C, Messinger JD, Sloan KR, Yannuzzi LA, Freund KB, Curcio CA. Histologic and optical coherence tomographic correlates in drusenoid pigment epithelium detachment in age-related macular degeneration. Ophthalmology. 2017;124(5):644–656. doi: 10.1016/j.ophtha.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilely A, Au A, Freund KB, et al. Non-neovascular age-related macular degeneration with subretinal fluid. Br J Ophthalmol. 2021;105(10):1415–1420. doi: 10.1136/bjophthalmol-2020-317326. [DOI] [PubMed] [Google Scholar]
- 12.Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(6):BIO211–BIO226. doi: 10.1167/iovs.17-21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study 2 (AREDS2) Research Group. Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/Zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89(1):74–80. doi: 10.1136/bjo.2004.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koss MJ, Beger I, Koch FH. Subthreshold diode laser micropulse photocoagulation versus intravitreal injections of bevacizumab in the treatment of central serous chorioretinopathy. Eye (Lond) 2012;26(2):307–314. doi: 10.1038/eye.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrull JK, Sinclair SH, Elmann S, Glaser BM. Low incidence of choroidal neovascularization following subthreshold diode micropulse laser (SDM) in high-risk AMD. PLoS One. 2018;13(8):e0202097. doi: 10.1371/journal.pone.0202097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arsan A, Kanar HS, Sonmez A. Visual outcomes and anatomic changes after sub-threshold micropulse yellow laser (577-nm) treatment for chronic central serous chorioretinopathy: long-term follow-up. Eye (Lond) 2018;32(4):726–733. doi: 10.1038/eye.2017.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavinsky D, Wang J, Huie P, Dalal R, Lee SJ, Lee DY, Palanker D. Nondamaging retinal laser therapy: rationale and applications to the macula. Invest Ophthalmol Vis Sci. 2016;57(6):2488. doi: 10.1167/iovs.15-18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terashima H, Hasebe H, Okamoto F, Matsuoka N, Sato Y, Fukuchi T. Combination therapy of intravitreal ranibizumab and subthreshold micropulse photocoagulation for macular edema secondary to branch retinal vein occlusion: 6-month result. Retina. 2019;39(7):1377–1384. doi: 10.1097/IAE.0000000000002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorin G. Subthreshold and micropulse diode laser photocoagulation. Semin Ophthalmol. 2003;18(3):147–153. doi: 10.1076/soph.18.3.147.29812. [DOI] [PubMed] [Google Scholar]
- 21.Luttrull JK, Margolis BWL. Functionally guided retinal protective therapy for dry age-related macular and inherited retinal degenerations: a pilot study. Invest Ophthalmol Vis Sci. 2016;57(1):265–275. doi: 10.1167/iovs.15-18163. [DOI] [PubMed] [Google Scholar]
- 22.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006;113(11):1974–1986. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Owens SL, Bunce C, Brannon AJ, Wormald R, Bird AC. Prophylactic laser treatment appears to promote choroidal neovascularisation in high-risk ARM: results of an interim analysis. Eye (Lond) 2003;17(5):623–627. doi: 10.1038/sj.eye.6700442. [DOI] [PubMed] [Google Scholar]
- 24.Guymer RH, Wu ZC, Hodgson LAB, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126(6):829–838. doi: 10.1016/j.ophtha.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Ryoo NK, Park KH. Laser and anti-vascular endothelial growth factor treatment for drusenoid pigment epithelial detachment in age-related macular degeneration. Sci Rep. 2020;10(1):14370. doi: 10.1038/s41598-020-71401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Quan Y, Dalal R, Palanker D. Comparison of continuous-wave and micropulse modulation in retinal laser therapy. Invest Ophthalmol Vis Sci. 2017;58(11):4722–4732. doi: 10.1167/iovs.17-21610. [DOI] [PubMed] [Google Scholar]
- 27.Kim HD, Han JW, Ohn YH, Brinkmann R, Park TK. Functional evaluation using multifocal electroretinogram after selective retina therapy with a microsecond-pulsed laser. Invest Ophthalmol Vis Sci. 2014;56(1):122–131. doi: 10.1167/iovs.14-15132. [DOI] [PubMed] [Google Scholar]
- 28.Hirabayashi K, Kakihara S, Tanaka M, Shindo T, Murata T. Investigation of the therapeutic mechanism of subthreshold micropulse laser irradiation in retina. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):1039–1047. doi: 10.1007/s00417-020-04638-3. [DOI] [PubMed] [Google Scholar]
- 29.Caballero S, Kent DL, Sengupta N, Calzi SL, Shaw L, Beli E, Moldovan L, Dominguez JM, 2nd, Moorthy RS, Grant MB. Bone marrow-derived cell recruitment to the neurosensory retina and retinal pigment epithelial cell layer following subthreshold retinal phototherapy. Invest Ophthalmol Vis Sci. 2017;58(12):5164–5176. doi: 10.1167/iovs.16-20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tode J, Richert E, Koinzer S, Klettner A, von der Burchard C, Brinkmann R, Lucius R, Roider J. Selective retina therapy reduces bruch's membrane thickness and retinal pigment epithelium pathology in age-related macular degeneration mouse models. Trans Vis Sci Tech. 2019;8(6):11. doi: 10.1167/tvst.8.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexandre de Amorim Garcia Filho C, Yehoshua Z, Gregori G, Farah ME, Feuer W, Rosenfeld PJ. Spectral-domain optical coherence tomography imaging of drusenoid pigment epithelial detachments. Retina. 2013;33(8):1558–1566. doi: 10.1097/IAE.0b013e318285cbd2. [DOI] [PubMed] [Google Scholar]
- 32.Schlanitz FG, Baumann B, Kundi M, Sacu S, Baratsits M, Scheschy U, Shahlaee A, Mittermüller TJ, Montuoro A, Roberts P, Pircher M, Hitzenberger CK, Schmidt-Erfurth U. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. 2017;101(2):198–203. doi: 10.1136/bjophthalmol-2016-308422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim M, Lee YJ, Park W, Park YG, Park YH. Optical coherence tomography biomarkers for reduction of drusenoid pigment epithelium detachment. Retina. 2021;41(2):402–408. doi: 10.1097/IAE.0000000000002844. [DOI] [PubMed] [Google Scholar]
- 34.Tvenning AO, Krohn J, Forsaa V, Malmin A, Hedels C, Austeng D. Drusenoid pigment epithelial detachment volume is associated with a decrease in best-corrected visual acuity and central retinal thickness: the Norwegian Pigment Epithelial Detachment Study (NORPED) report No.1. Acta Ophthalmol. 2020;98(7):701–708. doi: 10.1111/aos.14423. [DOI] [PubMed] [Google Scholar]