Abstract
AIM
To confirm the changes in proteins related with hypoxia-induced retinal cell death and to assess the effects of resveratrol (Res).
METHODS
The therapeutic effect of Res was verified using an ischemic/reperfusion (I/R) model in vivo and a hypoxia modelin retinal ganglion cells (RGCs) in vitro. Death of RGCs were confirmed by TUNEL assay. Protein expression was confirmed by Western blotting and immunohistochemistry. In addition, flow cytometric analysis was used to confirm the response in the cell unit to obtain more accurate data.
RESULTS
ErbB2 expression and apoptosis in the ganglion cell layer (GCL) increased after I/R injury. Treatment of Res rescued I/R-induced ganglion cell death, downregulated apoptosis and ErbB2 protein expression in the retina. In subsequent in vitro models, Res affects apoptosis by regulating the phosphorylation and expression of mouse double minute 2 homolog (MDM2), along with those of ErbB2. These results suggest that Res reverses GCL-specific apoptosis via downregulation of ErbB2 in ischemic injury.
CONCLUSION
In light of Res favorable properties, it should be evaluated in the treatment of RGC death and related retinal disease characterized by ErbB2 and MDM2 expression. Therefore, Res is appropriate therapeutic agent for treating ischemic injury-related eye diseases by targeting the expression of ErbB2 and MDM2.
Keywords: ischemia/reperfusion injury, hypoxia, retinal ganglion cell, resveratrol, ErbB2
INTRODUCTION
Retinal disease ultimately results in blindness, so it is important for patients to receive treatment at an early stage. Diabetic retinopathy, a complication of diabetes, is often caused by disorders of blood flow due to diabetes. Macular degeneration is caused by inflammation or capillary perfusion disorders. In the case of glaucoma, intraocular pressure (IOP) is generally the main cause of ischemic injury[1]–[3]. Ischemic injury is one of the major causes of eye diseases[4]. Ischemic injury primarily induces hypoxia. Some studies have shown that hypoxia exposes retinal cells to a wide range of abnormal conditions such as inflammation, oxidative stress, and endoplasmic reticulum stress[5]–[6]. Therefore, hypoxia plays a consequential role in the pathology of many major eye diseases[7].
As the population ages and increasingly irregular eating habits, the number of people with hypertension and diabetes increase. These diseases were accompanied with impaired blood flow to the eye, thus resulting in an increased prevalence of retinal disease[8]. In recent decades, research has shown that various natural products have a beneficial effect on the eyes. Resveratrol (Res), an antioxidant derived from grapes, has been shown to prevent or attemper the effects of eye diseases by diet[9]–[11] and is effective in hypoxic conditions causing reactive oxygen species (ROS)[12]. Res protects the pathologic symptoms of ocular diseases by regulating transcription-related proteins[13]–[14]. Various signaling proteins are expressed by hypoxic stimulation[15]. Therefore, Res modulates inflammation, neovascularization, and apoptosis through these wide ranges of protective effect[16]. Based on these results, the present study aimed to suppress retinal cell death using Res.
In the diseased retina, regulator protein of ROS or cytotoxicity, such as receptor tyrosine protein kinase erbB-2 (ErbB2) and mouse double minute 2 homolog (MDM2), that regulate the transcription of genes, change expression after ischemia. ErbB2 (HER2/neu) is a member of the ErbB family of tyrosine kinase receptors that activate several pathways, including PI3K-Akt and RAS-MAPK[17]. These pathways regulate many cell functions including proliferation, survival, and cell death[18]. One of the most important cellular functions associated with neuronal cell death is apoptosis. As upstream regulators, both ErbB2 and MDM2 are major targets for controlling apoptosis[19]. The present study aimed to confirm the changes in upstream proteins related with hypoxia-induced retinal cell death and assess the effects of Res for early-stage treatment of retinal disease.
MATERIALS AND METHODS
Ethical Approval
All experimental processes were performed in correspondence with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Guidelines of the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU-170804-M0036) were applied to each mice strictly. The 8-week-old and weighing 20-25 g male C57BL/6J mice (Central Lab. Animal Inc., Seoul, Korea) were used in experiment (n=16). All mice were provided a standard rodent diet and arbitrary water supply. Under controlled lighting conditions (12h light/12h dark cycles) were provided to all mouse.
Resveratrol Administration
Resveratrol (Res; Tocris, Ellisville, MO, USA) was dissolved in saline. Mice were randomly divided into two groups and intraperitoneally injected with saline or Res (20 mg/kg). Injection was applied from two days before ischemic/reperfusion (I/R) injury and continuing until the sacrifice once per day (Figure 1A). The dose of Res was followed to similar studies[9],[11].
Figure 1. Schematic diagrams of the resveratrol (Res) treatment and retinal I/R injury.
A: Schematic diagram for in vivo; B: Schematic diagram for in vitro.
Retinal Ischemic/Reperfusion Model
Mice were anesthetized with 10 mL/kg 2.5% 2,2,2-tribromoethanol (Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection as used in a previous study[20]. Anterior chamber was cannulated with a 30-gauge needle to increase the IOP to 60 mm Hg. Saline was injected to the right eye; the high IOP was kept for 60min. The opposite eye considered as a control. The IOP was measured by a tonometer (TonoLab, Raleigh, NC, USA). After 60min, the needle was removed.
TUNEL Assay
The collected eyes were fixed in 4% paraformaldehyde (Sigma-Aldrich) for 1h. After eliminating the anterior segment of the eye, the eye cups were fixed for 1h additionally at 4°C. For the cryo-protection, the eyes were incubated in 30% sucrose at 4°C overnight. Next, the eyes were embedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA). The cryo-blocks were processed to frozen sectioning. The eyeballs were sectioned perpendicular to the iris including the optic nerve by a cryostat (5-µm thick; Leica, Wetzlar, Germany). The terminal deoxynucleotidyl transferase-mediated biotinylated uridine triphosphate nick-end labeling (TUNEL) assay was applied with 4′,6-diamidino-2-phenylindole (DAPI) staining, following to manufacturer's instructions (In Situ Cell Death Detection; Roche Molecular Biochemicals, Penzberg, Germany). After staining, retinal sections were mounted in fluorescence mounting medium (ibidi GmbH, Gräfelfing, Germany).
Immunohistochemistry
Immunohistochemical staining was performed following the protocol of the 3,3′-diaminobenzidine (DAB) Peroxidase (horseradish peroxidase) Substrate Kit (Vector Labs, Burlingame, CA, USA). Immunoreactive scoring was performed by comparing right eye samples with the left eye as the control.
Reagents
High-glucose Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). Res was purchased from Tocris, and dimethyl sulfoxide (DMSO) was purchased from Amresco (Solon, OH, USA). Antibodies specific for ErbB2 (1:1000, MA5-13105), goat anti-rabbit (31460) and goat anti-mouse (31430) immunoglobulin G secondary antibodies (1:10 000) were purchased from Thermo Fisher Scientific (Waltham, MA, USA); MDM2, from Abcam (1:1000, ab3110, Cambridge, UK); phospho-MDM2, from Cell Signaling Technology (1:1000, #3521, Danvers, MA, USA); and anti-β-actin, from Pierce (1:10 000, St. Louis, MO, USA). Res was dissolved in DMSO.
Retinal Ganglion Cell Line Culture and Resveratrol Treatment
Cells from the retinal ganglion cell (RGC) line RGC-5, which was used in a previous study[21], were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in an atmosphere with 5% CO2 at 70% confluence. For hypoxic experiments, RGC-5 cells were cultured in DMEM in the presence of designated concentrations of CoCl2, 1% O2, and/or Res (1-50 µmol/L). Cells were grown as a monolayer (Figure 1B).
Western Blotting
The concentration of total protein was confirmed by bicinchoninic acid protein (BCA) assay kit (ThermoFisher Scientific). Same volume of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer was added to the sample including 15-20 µg protein. Equal amount of protein (15-20 µg) was loaded by SDS-PAGE on 15%-20% polyacrylamide gels. Then, the proteins were transferred to nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). The semi-dry transfer apparatus (Bio-Rad Laboratories, Hercules, CA, USA) was used for transfer in 15 V during 25min. Both membrane and gel were submerged in transfer buffer (pH 8.3; 20% methanol, 192 mmol/L glycine and 25 mmol/L Tris). The membrane was incubated with 5% skim milk with 0.1% Tris buffered saline with Tween 20 (TBST) for blocking. After blocking, the membrane was treated with antibodies (anti-ErbB2, anti-p-MDM2, anti-MDM2, or anti-β-actin) for overnight at 4°C. TBST and incubated in TBST was using for washing. Goat anti-rabbit (ThermoFisher Scientific) and goat anti-mouse (ThermoFisher Scientific) immunoglobulin G secondary antibodies were treated for 50min at room temperature. Immunoreactivity was reacted with enhanced chemiluminescence (Advansta, Menlo Park, CA, USA). LAS 3000 instrument (Fujifilm, Tokyo, Japan) was used for detection. Intensity of bands were measured by Multi Gauge 3.0 software (Fujifilm).
Reactive Oxygen Species Detection
Cells were plated on a 60-mm dish and treated with different concentrations of CoCl2 and/or Res for 24h. After treatment, cells were incubated with 10 µL 5-(and 6)-Carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Thermo Fisher Scientific). Reactive oxygen species (ROS) production was detected using an Attune NxT flow cytometer (Thermo Fisher Scientific).
Cell Cycle Analysis
Cells were plated on 100-mm dishes at a concentration of 5×105 cells per dish. Cells were treated with CoCl2 and/or Resfor 24h. After treatment, cells were fixed in 70% ethanol at 4°C for 30min and stained with propidium iodide (PI)-RNase staining solution (Invitrogen) at room temperature for 30min. The cell cycle stages were analyzed using the Attune NxT flow cytometer (Thermo Fisher Scientific).
Annexin/PI Stain Analysis
Cells were plated on 100-mm dishes at a concentration of 5×105 cells per dish for 12h and treated with CoCl2 and/or Resfor 24h. Cells were suspended in the binding buffer and stained with annexin V-fluorescein isothiocyanate (FITC)/PI solution (Invitrogen). The number of apoptotic cells was analyzed using the Attune NxT flow cytometer (Thermo Fisher Scientific).
Statistical Analysis
Data are presented as the means±standard error of the mean (SEM). One-way analysis of variance was performed using Dunnett's post-test (Prism 5; GraphPad Software, La Jolla, CA, USA). A P value less than 0.05 was regarded to indicate a significant difference in statistical.
RESULTS
Resveratrol Suppresses I/R Injury–Induced Retinal Cell Death
To evaluate the effect of Res on retinal cell death after I/R injury, TUNEL staining was performed. C57BL/6J mice were injected by Res (20 mg/kg) for 2 consecutive days before I/R injury and 3 consecutive days after I/R injury (Figure 1A). Four groups of retina samples were analyzed: control (CTL) and I/R retinas from non-Res-treated mice; I/R+Res and Res retinas from Res-treated mice. Collected retinas were confirmed with TUNEL and DAPI (Figure 2A). The I/R group showed significantly more TUNEL-positive cells, especially in the ganglion cell layer (GCL; 6.5±0.64 fold, P<0.001) than the CTL group. By contrast, the I/R+Res group showed significantly less TUNEL-positive cells than the I/R group (3.33±0.33 fold; P<0.001; Figure 2B). These results confirm that Res suppressed retinal cell death by I/R injury in the GCL.
Figure 2. Effects of Res on I/R injury-induced TUNEL-positive cells in the GCL.

A: TUNEL and API staining of retinal cross-sections by group; B: Number of TUNEL-positive cells in the GCL (white arrows) were counted using Image J software (top of the GCL-bottom of the INL). Data are represented as the means±SEM and were analyzed using one-way ANOVA analysis of variance with Dunnett's post-test (n=3-4 for each group). Scale bar: 50 µm. aP<0.001 vs control, bP<0.001 vs I/R.
Res Suppresses I/R Injury–Induced ErbB2 Expression
To confirm the expression of ErbB2, C57BL/6J mice were injected with Res (20 mg/kg) for 2 consecutive days before I/R injury and 2 consecutive days after, retinas were collected (Figure 1A). Then immunohistochemistry was performed (Figure 3A). Expression of ErbB2 increased in I/R group (1.66±0.20 fold; P<0.05). However, these increases were reduced in I/R+Res group (0.94±0.09 fold; P<0.01; Figure 3B) and same as the results of TUNEL assay in the GCL. These results demonstrated that Res effectively inhibited I/R injury-induced ErbB2 expression in the GCL.
Figure 3. Effect of Res on I/R injury-induced ErbB2 expression in the retina.
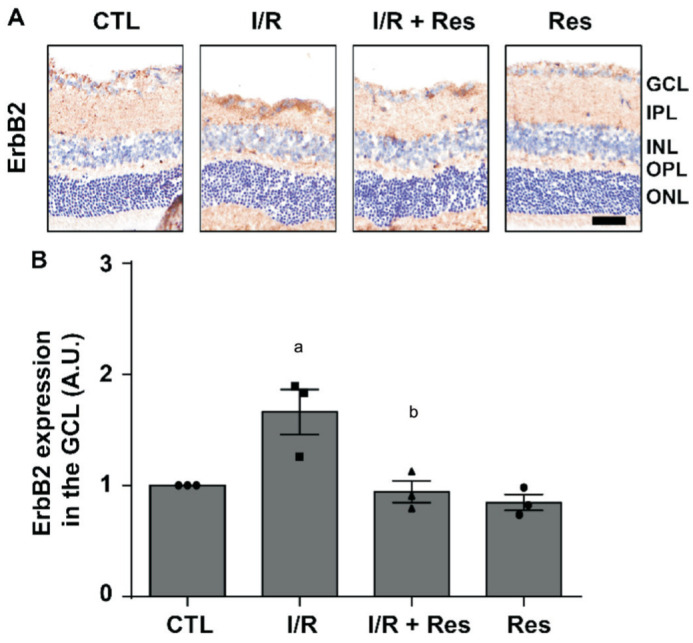
A: Retinal ErbB2 expression was detected by immunohistochemistry (hematoxylin counterstain). Scale bar represents 50 µm; B: ErbB2 protein expression decided using Image J software and displayed as the change value of each group relative to the control by an arbitrary unit. Data are indicated as the means±SEM and were tested using one-way analysis of variance with Dunnett's post-test (n=3 for each group). aP<0.05 vs control, bP<0.01 vs I/R.
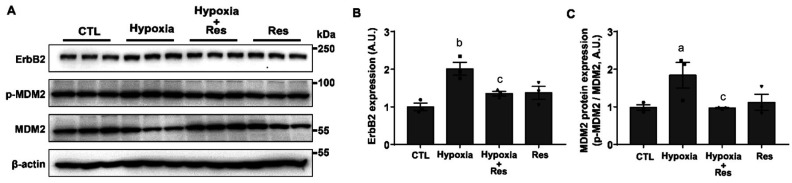
Res Affects the Expression of Cell Death-Related Upstream Regulator in Hypoxia
To examine hypoxia-induced changes in protein expression, RGC-5 cells were incubated with 1% O2 and Res (10 µmol/L) for 6h. In addition, cells were treated with Res for 2h before being subjected to hypoxia. Levels of ErbB2 and MDM2 protein expression and phosphorylated MDM2 levels were determined by Western blotting (Figure 4A). The expression of ErbB2 was increased by hypoxia (2.01±0.17 fold, P<0.01) and reduced by Res treatment (1.35±0.06 fold, P<0.05; Figure 4B). The phosphorylation of MDM2 was suppressed significantly by Res treatment in hypoxic conditions. In contrast, total MDM2 was increased by Res treatment. The ratio of phosphorylated/total MDM2 protein was increased by hypoxia (1.87±0.34 fold, P<0.05) and was reduced by Res treatment (0.98±0.01 fold, P<0.05; Figure 4C). Hypoxia increased the expression of ErbB2 and the phosphorylation of MDM2. However, the protein expression of MDM2 was downregulated under 1% O2 hypoxic conditions. Taken together, our results suggest that Res affected apoptosis by regulating upstream regulators.
Figure 4. Effect of Res on expression of cell death-related upstream regulator.
A: RGC-5 cells were incubated in 1% O2 hypoxic condition (hypoxia), pretreated with Res (10 µmol/L) for 2h and then co-treated with 1% O2 and Res (10 µmol/L) for 6h (hypoxia+Res) or treated with Res (10 µmol/L) for 8h (Res). Western blots showing the protein expression of ErbB2, p-MDM2, and MDM2 in RGC-5 cells. B: ErbB2 protein expression levels. C: The p-MDM2/MDM2 protein expression ratio performed using Image J software and displayed as the change value of each group relative to the control (CTL) by an arbitrary unit. Data are indicated as the means±SEM and were analyzed using one-way analysis of variance with Dunnett's post-test (n=3). aP<0.05, bP<0.01 vs control, cP<0.05 vs hypoxia.
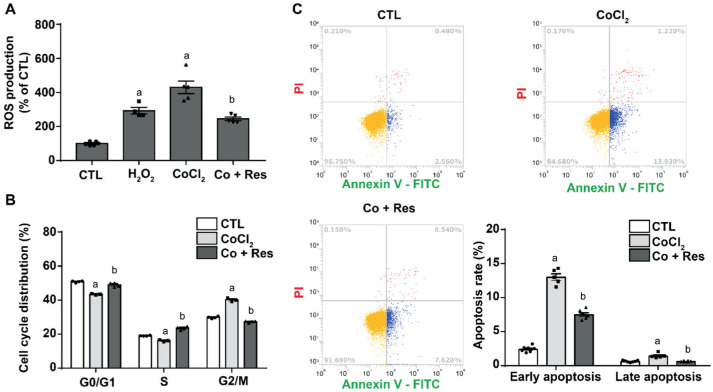
Resveratrol Suppresses ROS Production, Cell Cycle Arrest, and Apoptosis
To identify the effect of Res on the death of RGCs, RGC-5 cells were treated with CoCl2 (200 µmol/L) for 24h, and Res (10 µmol/L) was pretreated for 2h. In addition, Res was treated together with CoCl2 for 24h. Then, to verify the effect of Res in hypoxia, the ROS production, cell cycle arrest, and apoptosis rates were measured using flow cytometry. Res reduced both CoCl2-induced ROS production and cell cycle arrest, as well as both early and late apoptosis (P<0.001; Figure 5). These results indicate that Res protects RGC-5 cells from hypoxia-induced RGC death.
Figure 5. Effects of Res on CoCl2-induced ROS production, cell cycle arrest and CoCl2-induced apoptosis.
A: Cells were pretreated with Res (10 µmol/L) for 2h and then co-treated with CoCl2 (200 µmol/L) and Res for 24h. ROS production measured by carboxy-H2DCFDA staining. B: Cell cycle arrest measured by propidium iodide staining. C: Apoptosis measured by annexin V-FITC/PI staining. Data are represented as the means±SEM and were analyzed by one-way analysis of variance with Dunnett's post-test (n=4-6 for each group). aP<0.001 vs control, bP<0.001 vs CoCl2.
DISCUSSION
The major medical cure for ischemic disease of retina is anti-vascular endothelial growth factor agents[22]. Although these agents have significant anti-neovascular and anti-inflammatory effects, they also have limitations[23]. Recently, researchers have begun to focus on the identification of natural compounds that can overcome the limitations of such therapies[24]. Natural compounds are derived from foods in usual and are known to exert a wide range of effects against various diseases[25]. Res is a compound from nature that has been revealed to alleviate the symptoms of various eye diseases[26]–[27]. However, further research is still required to identify the mechanism of Res's beneficial effects on ischemic injury. Based on these previous results, we aimed to identify an upstream regulator of cell death that is affected by Res. Therefore, the purpose of the present study was to explain such a mechanism, at least in partial, by using well-established mouse I/R injury models and hypoxia models in cells, and to investigate the protective effects of Res for cell death in retina.
First, apoptosis that occurred in the tissue was confirmed using the TUNEL assay. Through the TUNEL assay, it is possible to confirm the locational relationship between apoptosis and RGC histologically[28]. As a result, the increase in apoptosis was a GCL-specific change. These results demonstrate that the morphological changes caused by I/R injury are due to apoptosis in the GCL and that these effects are alleviated by Res. In fact, the increase in TUNEL-positive cells in the GCL was also reduced by Res treatment (Figure 2). Caspase, which controls apoptosis, is regulated by many upstream proteins[29]. In this study, we selected the upstream protein, ErbB2, and monitored changes in its expression. As in previous results, the expression levels of ErbB2 were increased by I/R injury and downregulated by Res. In particular, the expression of ErbB2 was evident in RGCs, suggesting that ErbB2 may play a major role in apoptosis in the GCL.
Next, to further confirm the mechanism at the tissue level, the change in protein expression caused by hypoxia was examined in RGC-5 cells. As with the experiments in the I/R injury model, cells were pretreated with Res and then subjected to hypoxic conditions to verify the preventive effect of Res. The increased protein expression of ErbB2 under the hypoxia conditions suggests that hypoxia may induce changes in ErbB2 expression. In the case of MDM2, hypoxia increased MDM2 phosphorylation but decreased MDM2 protein expression.
The phosphorylation of MDM2 is regulated by Akt (protein kinase B). In general, MDM2 phosphorylation by Akt is known to activate MDM2[30]. However, recent studies have shown that phosphorylation by Akt inhibits the stability of MDM2, causing its degradation[31]. In addition, Akt is a representative protein regulated by ErbB2. Therefore, the changes in ErbB2 and MDM2 protein expression by hypoxia suggests that they may be closely related to the regulation of apoptosis in RGCs. All of these changes were reversed by Res. Therefore, Res involved in the regulation of upstream proteins, and mitigation of apoptosis. To our knowledge, this is the first study to describe changes in the expression of ErbB2 and MDM2 induced by hypoxia and the protective effect of Res in retinal cells.
Finally, the effects of Res on cell death in RGCs were examined in detail. For ease of experimental design, the model was reestablished using the hypoxia-inducing compound CoCl2[32]. The optimal concentration of the compound was confirmed using a cell viability assay. Res does not have a detrimental effect on cell viability at low concentrations (below 10 µmol/L). However, the most effective Res concentration that restored viability lowered by hypoxia was 10 µmol/L (Data was not shown). Res not only inhibited ROS production but also significantly restored G2/M arrest induced by hypoxia (Figure 5). G2/M arrest is known to result in apoptosis[33]–[34]. Indeed, Res inhibited both early- and late-stage hypoxia-induced apoptosis. These results suggest that Res reverses GCL-specific apoptosis via downregulation of ErbB2 in ischemic injury. In conclusion, our data identified that Res is appropriate as a remedy for healing hypoxia-related eye diseases by aim to the regulation of ErbB2 and MDM2.
Acknowledgments
Foundation: Supported by the Biomedical Research Institute Fund (GNUHBRIF-2017-0003) from Gyeongsang National University Hospital.
Conflicts of Interest: Seong H, None; Jeong JY, None; Ryu J, None; Park J, None; Han YS, None; Cho HK, None; Kim SJ, None; Park JM, None; Kang SS, None; Seo SW, None.
REFERENCES
- 1.Miller JW, le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120(1):106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 2.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasiak J, Petrovski G, Veréb Z, Facskó A, Kaarniranta K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed Res Int. 2014;2014:768026. doi: 10.1155/2014/768026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhard J, Renner M, Wiemann S, Shakoor DA, Stute G, Dick HB, Faissner A, Joachim SC. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci Rep. 2017;7:43470. doi: 10.1038/srep43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ola MS, Al-Dosari D, Alhomida AS. Role of oxidative stress in diabetic retinopathy and the beneficial effects of flavonoids. Curr Pharm Des. 2018;24(19):2180–2187. doi: 10.2174/1381612824666180515151043. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SX, Sanders E, Wang JJ. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4(1-2):51–61. doi: 10.1007/s12177-011-9075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MH, Hsiao G, Al-Shabrawey M. Eicosanoids and oxidative stress in diabetic retinopathy. Antioxidants (Basel) 2020;9(6):E520. doi: 10.3390/antiox9060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashemi H, Khabazkhoob M, Nabovati P, Ostadimoghaddam H, Shafaee S, Doostdar A, Yekta A. The prevalence of age-related eye disease in an elderly population. Ophthalmic Epidemiol. 2017;24(4):222–228. doi: 10.1080/09286586.2016.1270335. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Dong XG, Liu ZX, et al. Resveratrol suppresses rotenone-induced neurotoxicity through activation of SIRT1/Akt1 signaling pathway. Anat Rec (Hoboken) 2018;301(6):1115–1125. doi: 10.1002/ar.23781. [DOI] [PubMed] [Google Scholar]
- 10.Josifovska N, Albert R, Nagymihály R, Lytvynchuk L, Moe MC, Kaarniranta K, Veréb ZJ, Petrovski G. Resveratrol as inducer of autophagy, pro-survival, and anti-inflammatory stimuli in cultured human RPE cells. Int J Mol Sci. 2020;21(3):E813. doi: 10.3390/ijms21030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35(Pt 5):1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 12.Oh WY, Shahidi F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018;261:267–273. doi: 10.1016/j.foodchem.2018.03.085. [DOI] [PubMed] [Google Scholar]
- 13.Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50(7):3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 14.Bola C, Bartlett H, Eperjesi F. Resveratrol and the eye: activity and molecular mechanisms. Graefes Arch Clin Exp Ophthalmol. 2014;252(5):699–713. doi: 10.1007/s00417-014-2604-8. [DOI] [PubMed] [Google Scholar]
- 15.Banasiak KJ, Xia Y, Haddad GG. Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol. 2000;62(3):215–249. doi: 10.1016/s0301-0082(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 16.Lançon A, Frazzi R, Latruffe N. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules. 2016;21(3):304. doi: 10.3390/molecules21030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Saenz A, Dreyer C, Campbell MR, Steri V, Gulizia N, Moasser MM. HER2 amplification in tumors activates PI3K/Akt signaling independent of HER3. Cancer Res. 2018;78(13):3645–3658. doi: 10.1158/0008-5472.CAN-18-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant S, Qiao L, Dent P. Roles of ERBB family receptor tyrosine kinases, and downstream signaling pathways, in the control of cell growth and survival. Front Biosci. 2002;7:d376–d389. doi: 10.2741/grant. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Marchenko ND. ErbB2 inhibition by lapatinib promotes degradation of mutant p53 protein in cancer cells. Oncotarget. 2017;8(4):5823–5833. doi: 10.18632/oncotarget.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seong H, Ryu J, Yoo WS, Kim SJ, Han YS, Park JM, Kang SS, Seo SW. Resveratrol ameliorates retinal ischemia/reperfusion injury in C57BL/6J mice via downregulation of caspase-3. Curr Eye Res. 2017;42(12):1650–1658. doi: 10.1080/02713683.2017.1344713. [DOI] [PubMed] [Google Scholar]
- 21.Cho HK, Kim S, Lee EJ, Kee C. Neuroprotective effect of ginkgo biloba extract against hypoxic retinal ganglion cell degeneration in vitro and in vivo. J Med Food. 2019;22(8):771–778. doi: 10.1089/jmf.2018.4350. [DOI] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Aiello LP, Rosenfeld PJ. Anti-vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology. 2016;123(10S):S78–S88. doi: 10.1016/j.ophtha.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 23.Shah AR, Xi MQ, Abbey AM, Yonekawa Y, Faia LJ, Hassan TS, Ruby AJ, Wolfe JD. Short-term efficacy of intravitreal dexamethasone implant in vitrectomized eyes with recalcitrant diabetic macular edema and prior anti-VEGF therapy. J Ophthalmic Vis Res. 2016;11(2):183–187. doi: 10.4103/2008-322X.183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quaggin SE. Turning a blind eye to anti-VEGF toxicities. J Clin Invest. 2012;122(11):3849–3851. doi: 10.1172/JCI65509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman MU, Wali AF, Ahmad A, Shakeel S, Rasool S, Ali R, Rashid SM, Madkhali H, Ganaie MA, Khan R. Neuroprotective strategies for neurological disorders by natural products: an update. Curr Neuropharmacol. 2019;17(3):247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Amero KK, Kondkar AA, Chalam KV. Resveratrol and ophthalmic diseases. Nutrients. 2016;8(4):200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delmas D, Cornebise C, Courtaut F, Xiao J, Aires V. New highlights of resveratrol: a review of properties against ocular diseases. Int J Mol Sci. 2021;22(3):1295. doi: 10.3390/ijms22031295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SJ, Kim MJ, Choi MY, Kim YS, Yoo JM, Hong EK, Ju S, Choi WS. Aralia elata inhibits neurodegeneration by downregulating O-GlcNAcylation of NF-κB in diabetic mice. Int J Ophthalmol. 2017;10(8):1203–1211. doi: 10.18240/ijo.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JQ, Kurokawa M. Regulation of MDM2 stability after DNA damage. J Cell Physiol. 2015;230(10):2318–2327. doi: 10.1002/jcp.24994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stommel JM, Wahl GM. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 2004;23(7):1547–1556. doi: 10.1038/sj.emboj.7600145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang GL, Xu SQ, Peng LT, Li H, Zhao Y, Hu YF. The hypoxia-mimetic agent CoCl2 induces chemotherapy resistance in LOVO colorectal cancer cells. Mol Med Rep. 2016;13(3):2583–2589. doi: 10.3892/mmr.2016.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HJ, Liu WW, Zhou X, et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol Med Rep. 2017;16(2):2069–2074. doi: 10.3892/mmr.2017.6838. [DOI] [PubMed] [Google Scholar]
- 34.Liu XJ, Song MY, Gao ZL, Cai XK, Dixon W, Chen XF, Cao Y, Xiao H. Stereoisomers of astaxanthin inhibit human colon cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. J Agric Food Chem. 2016;64(41):7750–7759. doi: 10.1021/acs.jafc.6b03636. [DOI] [PubMed] [Google Scholar]