Abstract
Colibactin is a chemically unstable small-molecule genotoxin that is produced by several different bacteria, including members of the human gut microbiome1,2. Although the biological activity of colibactin has been extensively investigated in mammalian systems3, little is known about its effects on other microorganisms. Here we show that colibactin targets bacteria that contain prophages, and induces lytic development through the bacterial SOS response. DNA, added exogenously, protects bacteria from colibactin, as does expressing a colibactin resistance protein (ClbS) in non-colibactin-producing cells. The prophage-inducing effects that we observe apply broadly across different phage–bacteria systems and in complex communities. Finally, we identify bacteria that have colibactin resistance genes but lack colibactin biosynthetic genes. Many of these bacteria are infected with predicted prophages, and we show that the expression of their ClbS homologues provides immunity from colibactin-triggered induction. Our study reveals a mechanism by which colibactin production could affect microbiomes and highlights a role for microbial natural products in influencing population-level events such as phage outbreaks.
Subject terms: Natural products, Phage biology
The bacterial genotoxin colibactin triggers prophage-mediated lysis of neighbouring bacteria, a finding that provides insight into the dynamics of microbial communities and relationships between bacterial metabolite production and phage behaviour.
Main
Microbial communities, including the human microbiome, are rich sources of bioactive natural products. However, the biological roles of natural products in these habitats are typically poorly understood. A bacterial natural product of particular relevance to human health is colibactin, a chemically reactive small-molecule genotoxin produced by gut bacteria that have a 54-kb hybrid nonribosomal peptide synthetase-polyketide synthase (NRPS-PKS) biosynthetic gene cluster known as the pks island (Fig. 1a). This gene cluster is predominantly found in human-associated strains of Escherichia coli that belong to phylogenetic group B2, but is also present in other human gut Enterobacteriaceae, as well as bacteria from the honey bee gut, a marine sponge and an olive tree knot4–6. Mechanistic studies have revealed that colibactin induces inter-strand DNA cross-links in vitro, causes cell-cycle arrest in eukaryotic cell culture and affects tumour formation in mouse models of colorectal cancer. Colibactin–DNA adducts have been detected in mammalian cells and in mice7, and studies have identified colibactin-associated mutational signatures in cancer genomes, predominantly from colorectal cancer8,9. Despite its important biological activity, colibactin has eluded traditional isolation and structural elucidation. Information regarding its chemical structure has largely been derived from bioinformatic analyses and biochemical characterization10–13. These studies suggest that colibactin has a pseudodimeric structure, with a reactive cyclopropane warhead at each end that accounts for its characteristic DNA-alkylating ability7,14,15 (Fig. 1a).
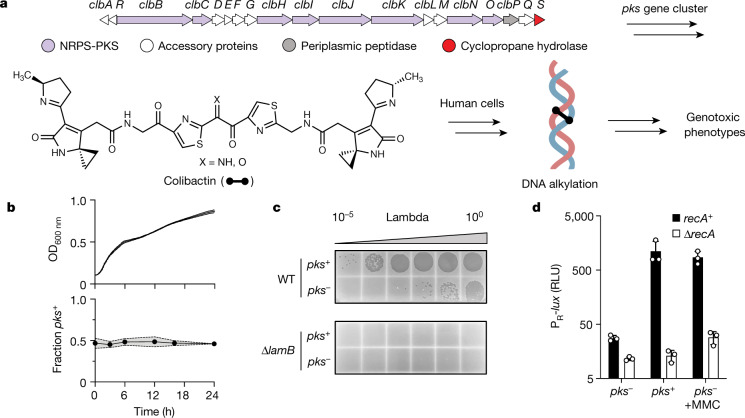
Fig. 1. Colibactin production specifically affects prophage-carrying bacteria.
a, Biosynthetic organization and chemical structure of the genotoxic natural product colibactin. The proposed mode of action toward human cells as a DNA-damaging agent is shown. b, Growth and relative abundance of pks− and pks+ E. coli in co-culture. Top, total culture density (optical density at 600 nm; OD600 nm) of pks−lacZ – E. coli co-cultured with pks+lacZ + E. coli, at a starting ratio of 1:1. Bottom, the proportion of lacZ + versus lacZ − within the same co-culture (see Extended Data Fig. 1b for swapped markers). c, Plaque assay obtained from 24-h co-cultures between pks+ or pks− E. coli with E. coli harbouring phage lambda. Supernatants were spotted onto wild-type (WT) E. coli (top) and the lambda-resistant ∆lamB mutant (bottom). d, Relative light units (RLU) produced from a bioluminescent reporter encompassing the DNA-damage-inducible region of phage lambda that regulates lysis–lysogeny (PR-lux). Reporter output measured in recA+ (black) and ∆recA (white) E. coli co-cultured with pks+ or pks− E. coli in the absence or presence of MMC. RLU was calculated by dividing bioluminescence by OD600 nm. Data are mean ± s.d. with n = 3 biological replicates (b, d); or n = 3 biological replicates from which a single representative image is shown (c).
In contrast to its effects on eukaryotic organisms, the effect of colibactin on the surrounding microbial community remains largely unknown. Previous studies have indicated that colibactin production may cause broad shifts in the composition of the gut microbial community in mice and inhibit the growth of a subset of staphylococci16,17. However, exposure to colibactin did not affect the growth of the vast majority (97%) of bacterial species tested, and the mechanism that underlies these effects has remained elusive. We aimed to shed additional light on the activity of colibactin and its potential ecological roles in microbial communities by studying its effects on bacteria. To begin, we exposed a laboratory strain of non-colibactin-producing (pks−) E. coli (BW25113) to supernatants from overnight cultures of colibactin-producing E. coli (a heterologous expression strain called BAC-pks; hereafter pks+). Culture supernatants did not inhibit the growth of the laboratory E. coli strain (Extended Data Fig. 1a), in line with analogous reports in mammalian cells1. To test whether growth inhibition requires the presence of live colibactin-producing cells, we co-cultured pks+ E. coli with pks− E. coli carrying chromosomally distinguishable markers (lacZ) and monitored the growth of the two populations. When started at a 1:1 ratio, the proportion of pks+ E. coli did not change over the course of the experiment, and this outcome occurred irrespective of which strain carried the lacZ marker (Fig. 1b, Extended Data Fig. 1b). These results suggest that, under the conditions tested, colibactin production by one bacterium does not inhibit the growth of an isogenic, non-producing strain.
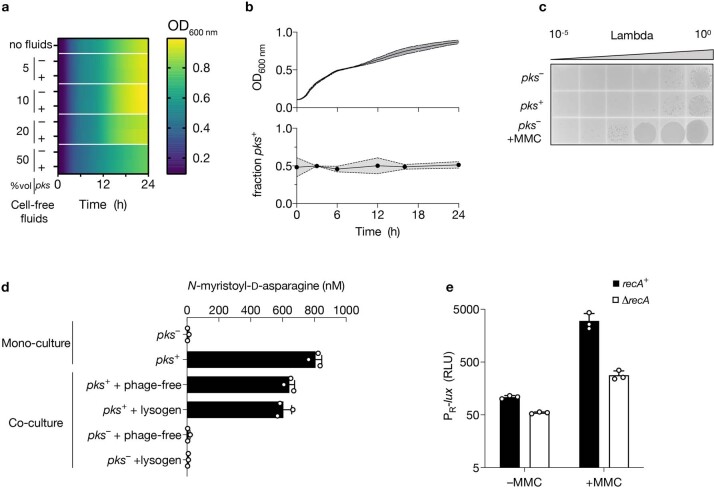
Extended Data Fig. 1. Colibactin production does not generally inhibit bacterial growth but induces DNA damage.
a, Growth of pks− E. coli grown in the presence of the indicated relative volume of cell-free fluids from overnight cultures of pks+ E. coli, pks− E. coli or without cell-free fluids added (top row). b, Growth and frequency of pks− and pks+ E. coli in co-culture as in Fig. 1b but with the pks and lacZ combination swapped. Upper, total culture density of pks− lacZ + E. coli co-cultured with pks+ lacZ − E. coli at a starting ratio of 1:1; lower, the proportion of lacZ + versus lacZ – within the same co-culture based on differential blue-white plating over time. c, Plaque assay obtained after co-culturing pks+ or pks− E. coli with a lambda lysogen separated by a 0.4 µm membrane. Where indicated, MMC was added to the opposing side of the membrane from the lambda lysogen. d, Concentration of the colibactin prodrug motif N-myristoyl-D-asparagine obtained from pks− or pks+ E. coli in monoculture and in co-culture with lysogenic and non-lysogenic (phage-free) E. coli. e, PR-lux output in recA+ (black) and ∆recA (white) E. coli harbouring the reporter plasmid in the absence and presence of MMC. For e, RLU as in Fig. 1d. Data represented as mean of n = 3 biological replicates (a), as mean ± s.d. with n = 3 biological replicates (b, d, e), or n = 3 biological replicates from which a single representative image is shown (c).
Colibactin induces prophages
Multiple lines of evidence suggest that bacteria should be susceptible to colibactin-mediated DNA damage. For example, the final gene in the pks gene cluster, clbS, encodes a self-resistance protein that is reported to hydrolyse and destroy the reactive cyclopropane warheads of colibactin18,19, and another gene, clbP, encodes a periplasmic peptidase that converts an inactive late-stage biosynthetic intermediate (precolibactin) to the final genotoxic metabolite in the periplasm before export20,21. Both bacterially encoded self-resistance mechanisms suggest that, like many toxic bacterial natural products, colibactin is potentially deleterious to non-producing bacteria. We next considered alternative consequences of colibactin-mediated DNA damage beyond inhibition of bacterial growth. One possible response of interest is phage induction. Specifically, it is known that DNA damage induced by ultraviolet irradiation or by chemical treatment (for example, mitomycin C (MMC)) activates lytic replication of prophages (a latent form of phage infection) in bacteria, killing the cell and potentially nucleating a phage epidemic within the larger microbial community22. We therefore wondered whether colibactin could affect bacterial populations by activating resident prophages.
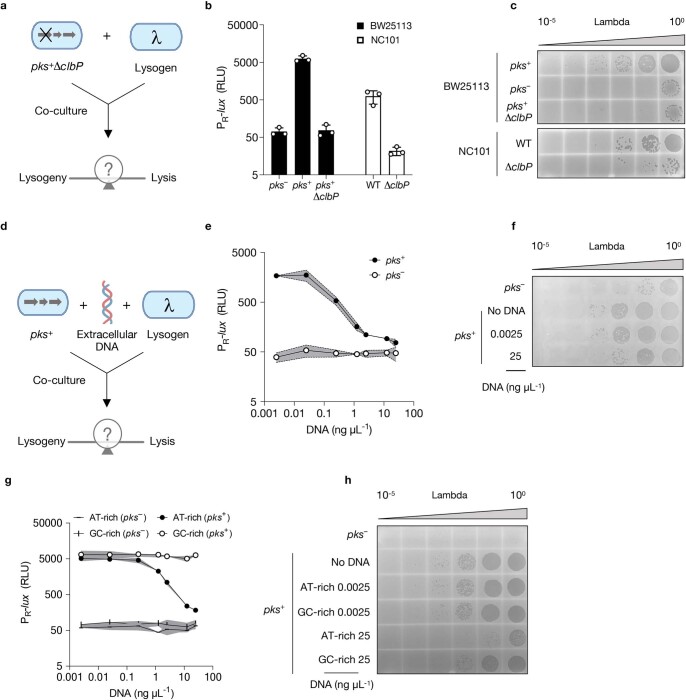
To test whether colibactin production alters the behaviour of prophages in neighbouring, non-colibactin-producing lysogens, we infected wild-type E. coli BW25113 with phage lambda and co-cultured this lysogen with pks+ or pks− E. coli. Twenty-four hours of co-culture with the pks+ strain increased phage titres by orders of magnitude above those obtained with the pks− strain (Fig. 1c). Physical separation of colibactin producers from the lysogen via a 0.4-µm filter ablated this effect (Extended Data Fig. 1c), suggesting that cell–cell contact is required1. Consistent with the resident prophage being the responsible agent, no plaques were observed for any condition on ∆lamB E. coli, which lacks the lambda phage receptor (Fig. 1c). Finally, we verified that levels of colibactin production were not markedly altered between co-culture and monoculture conditions and were unaffected by the presence of the prophage-containing strain (Extended Data Fig. 1d). These results suggest that colibactin production specifically affects prophage-carrying bacteria by inducing lytic development.
Regulation of lambda induction from the prophage state occurs via a repressor protein (cI) that is inactivated by the host-encoded SOS response, for which RecA is a master regulator23. To test whether prophage induction by colibactin follows a similar sequence of events, we engineered a transcriptional reporter to track the lambda lysis–lysogeny decision by fusing the lambda immunity region to the luciferase operon (lux) on a plasmid (hereafter called PR-lux). Light production by luciferase therefore reports the transcriptional de-repression of phage lambda lytic replication, which is induced by known DNA-damaging-agents, such as MMC (Extended Data Fig. 1e). To examine the effect of colibactin in this system, we co-cultured E. coli harbouring PR-lux with pks+ or pks− E. coli. The pks+ strain induced PR-lux in the reporter strain 40-fold compared to the pks− strain (Fig. 1d). Furthermore, the activating effect of both MMC and co-cultured pks+ cells was eliminated in ∆recA E. coli (Fig. 1d), showing that the transcriptional de-repression requires the canonical DNA-damage-inducible SOS response. Consistent with the active genotoxin being involved, deletion of the gene encoding the late-stage biosynthetic enzyme ClbP in the producing strain abolished the activation of PR-lux activity in co-cultured reporter cells and markedly reduced phage titres when co-cultured with the lambda lysogen (Extended Data Fig. 2a–c). Similar results were obtained using a native pks+ adherent-invasive colibactin-producing E. coli (NC101, which is used in mouse models of colorectal cancer carcinogenesis24) (Extended Data Fig. 2a–c, Supplementary Discussion). Finally, addition of extracellular DNA attenuated PR-lux activity and phage activation in a concentration-dependent, sequence-motif-specific manner (Extended Data Fig. 2d–h, Supplementary Discussion). Together, these data suggest that the ability to produce and transmit the final genotoxic product is important for the effect of colibactin on bacteria.
Extended Data Fig. 2. Prophage induction is dependent on colibactin-mediated DNA alkylation and addition of extracellular DNA ameliorates this effect.
a, Schematic of co-culture experiment with a colibactin biosynthesis-defective ∆(clbP) pks strain. b, PR-lux output from reporter cells co-cultured with E. coli BW25113 (pks+, pks−, and pks+∆clbP; black bars) or native-colibactin producing E. coli, NC101 (WT and ∆clbP; white bars). c, Plaque assays obtained from analogous incubations as in b but with a lambda lysogen used in place of the reporter strain. d, Schematic of co-culture experiment in which extracellular DNA is added to the medium. e, PR-lux output from reporter cells co-cultured with either pks+ or pks− E. coli and the indicated concentration of herring sperm DNA. f, Plaque assays of the analogous incubations as in e but with a lambda lysogen used in place of the reporter strain. g, PR-lux output from reporter cells co-cultured with pks+ E. coli in the presence of varying amounts of extracellular DNA (AT-rich and GC-rich DNA, black and white symbols, respectively). h, Plaque assays of the analogous incubations as in g but with a lambda lysogen used in place of the reporter strain. In b, e, g, RLU as in Fig. 1d. Data represented as mean ± s.d. with n = 3 biological replicates (b, e, g); or n = 3 biological replicates from which a single representative image is shown (c, f, h).
pks induces human-associated prophages
Given that bacteria frequently exist in polymicrobial communities, that prophages are pervasive in these communities and that the SOS response is highly conserved, we wondered whether the genotoxic effect of colibactin could extend to prophages that reside in phylogenetically distinct, Gram-negative and Gram-positive bacteria. To investigate this, we co-cultured pks+ or pks− E. coli with multiple isolates of prophage-carrying Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium) (one harbouring prophage P22 and another harbouring prophages BTP1 and Gifsy-1), multiple isolates of Staphylococcus aureus (one harbouring prophage phi11 and another harbouring phi80α), a Shiga toxin encoding isolate of Citrobacter rodentium (harbouring an stx2dact prophage), and a commensal isolate of Enterococcus faecium obtained from human faeces (harbouring a temperate phage phi1). Each phage–bacteria system resulted in a pks-dependent increase in prophage induction, as measured by enumerating plaque-forming units, antibody-based toxin detection and quantitative PCR (qPCR), showing that colibactin functions as a broad inducer (Fig. 2a–d). Notably, the increased Shiga toxin production from the stx2dact prophage that was observed upon co-culture of C. rodentium with pks+ E. coli reveals how the effects of colibactin on susceptible bacteria and prophage have functionally relevant consequences beyond microorganisms.
Fig. 2. Colibactin activates prophages in diverse bacteria and in complex communities.
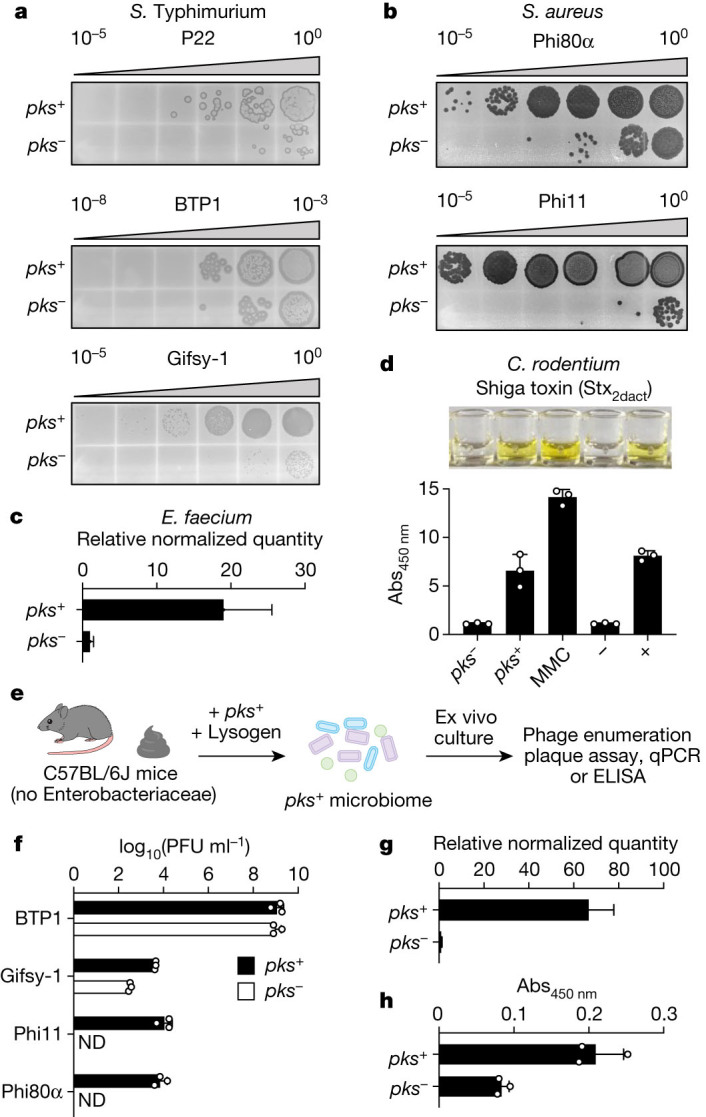
a, b, Plaque assays obtained from co-cultures of pks+ or pks− E. coli with a S. Typhimurium P22 lysogen (a, top), a S. Typhimurium BTP1 and Gifsy-1 polylysogen (a, bottom two panels) or two S. aureus lysogens (b). The indicator strains used for each plaque assay are specific to the different phages indicated. c, Relative quantities of E. faecium-specific host and phage DNA as measured by qPCR after co-culture with pks− or pks+ E. coli. d, Enzyme-linked immunosorbent assay (ELISA) of Shiga toxin (Stx2dact) in cultures of C. rodentium harbouring phage stx2dact co-cultured with pks− or pks+ E. coli or induced with 1.5 µg ml−1 MMC. Negative and positive conditions (bars designated – and +) indicate Stx-negative and Stx-positive standards included with the ELISA kit (Methods). Top, image of representative assay results from microtitre wells. Bottom, absorbance measurements from above samples (absorbance at 450 nm; Abs450 nm). e, Schematic of ex vivo community experiment. Faecal communities from C57BL/6J mice were cultivated anaerobically before the addition of pks− or pks+ E. coli along with each of the indicated phage-containing isolates indicated in f–h. Supernatants of the resulting samples were analysed for phage induction using plaque assays, qPCR and ELISA. f, Plaque forming units (PFU) of lysogens BTP1, Gifsy-1, phi80α and phi11 in ex vivo communities. ND, not detected. g, Relative quantities of E. faecium-specific host and phage DNA as measured by qPCR from ex vivo communities. h, Shiga toxin ELISA on C. rodentium harbouring phage stx2dact in ex vivo communities. In a, b, data are shown as a single representative image from n = 3 biological replicates. In d, f, h, data are mean ± s.d. with n = 3 biological replicates. In e, schematic created with BioRender.com. In c, g, data are mean ± s.e.m. with n = 3 biological replicates, each with 2 and 3 technical replicates (c and g, respectively).
Having experimentally demonstrated prophage induction in these bacteria under in vitro co-culture conditions, we next sought to test the action of colibactin in a setting that more closely resembles the complex multispecies environment of the gut. To achieve this, we anaerobically co-cultured pks+ or pks− E. coli ex vivo with complex faecal microbiomes from C57BL/6J mice, to which we added the individual gut-associated bacteria from our above panel25 (S. Typhimurium, S. aureus, E. faecium and C. rodentium) (Fig. 2e). In all cases except for prophage BTP1 from the S. Typhimurium polylysogen, we observed an increase in phage or toxin production within the pks+ communities relative to the pks− communities (Fig. 2f–h). The lack of pks-dependent induction for BTP1—which is possibly due to the high degree of spontaneous induction in anaerobic communities—is notable given the significant induction observed under the same conditions for Gifsy-1, which is co-harboured by the same host bacterium. Together, these results demonstrate that colibactin-producing bacteria induce prophages in human- and gut-relevant strains and in complex microbial communities.
pks− strains can be colibactin-resistant
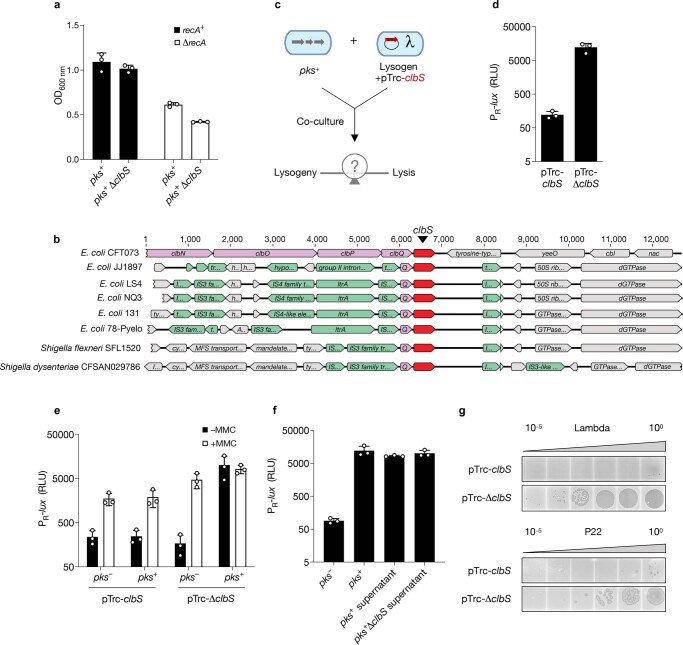
Our results predict that colibactin, like MMC, is a generally effective inducer of prophages. However, unlike MMC, in which self-protection to the producing organism is thought to require the combined action of multiple resistance proteins26,27, protection from colibactin exposure in pks+ organisms involves one 170-amino-acid resistance protein, ClbS18,19 (Fig. 1a). Studies using genetic deletions of clbS found that colibactin producers deficient for ClbS are viable, but that their growth depends on RecA, indicating that DNA repair mechanisms are needed for growth18 (Extended Data Fig. 3a). Acquisition of clbS would therefore be a potential strategy for susceptible community members to protect themselves against the effects of colibactin. We thus wondered whether clbS-like genes exist in closely related, non-colibactin-producing bacteria, and whether the function of these genes may alter phage–host dynamics in response to colibactin. To gain insight into its context, we performed a bioinformatic search (tBLASTn) for genes that encode proteins with amino acid sequences identical to that of E. coli ClbS. In 97% of the examined hits (230 total; Supplementary Table 1), the clbS homologue was found in a pks gene cluster with the same genetic organization as that of known colibactin-producing strains. In the seven cases (3%) in which clbS was not associated with an intact pks gene cluster, the gene normally encoded upstream of clbS—clbQ—was present but truncated, and both genes were surrounded by predicted transposase-associated genes (Extended Data Fig. 3b, Supplementary Table 1), indicating that the region may be subject to horizontal transfer. This search, consistent with a recent report28, reveals that the clbS gene found in pks+ E. coli also exists in isolates of the same species that lack a pks gene cluster.
Extended Data Fig. 3. ClbS provides intracellular protection from colibactin, and clbS-like genes are present in the genomes of diverse bacteria, including those that lack pks-biosynthetic genes.
a, 24 h growth of recA+ E. coli (BW25113, black bars) or ∆recA E. coli (DH10β, white bars), each harbouring either the full pks cluster (pks+) or the cluster with clbS removed (pks+∆clbS), as indicated. b, Genomic context of clbS found within the E. coli pks cluster encoded by a known colibactin-producing isolate (CFT073) as compared to pks− isolates of E. coli that lack the colibactin biosynthetic genes but contain an identical clbS coding sequence (red) and truncated clbQ (purple) in regions flanked with predicted transposase-associated genes (green-coloured genes). Numbering above genomes denotes prophage genome size in base pairs. c, Schematic of co-culture experiment with the gene encoding colibactin resistance, clbS, expressed in trans. d, PR-lux reporter output obtained from pks+ E. coli co-cultured with pks− E. coli harbouring the reporter plasmid and either pTrc-clbS or the same vector with clbS removed (pTrc-∆clbS) expressed in trans. e, PR-lux reporter output in the absence and presence of MMC in E. coli harbouring the PR-lux reporter plasmid, the indicated second plasmid (pTrc-clbS or pTrc-∆clbS), and co-cultured with pks− or pks+ E. coli. f, PR-lux reporter output obtained from culturing pks+ or pks− E. coli with pks− E. coli harbouring the reporter plasmid to which cell-free supernatants of cells expressing clbS or a vector control (∆clbS) were added (right two bars). g, Upper: Plaque assays obtained from analogous incubations as in d but with a lambda lysogen used in place of the reporter strain. Lower: Plaque assays obtained from co-culturing pks+ E. coli with S. Typhimurium harbouring P22 and either pTrc-clbS or pTrc-∆clbS expressed in trans. In d, e and f, RLU as in Fig. 1d. Data represented as mean ± s.d. with n = 3 biological replicates (a, d, e, f); or n = 3 biological replicates from which a single representative image is shown (g).
To test whether expression of ClbS in non-colibactin-producing E. coli can provide protection from colibactin exposure, we introduced and expressed plasmid-encoded clbS (from pks+ E. coli, pTrc-clbS) in a pks− strain harbouring either the PR-lux reporter or phage lambda (Extended Data Fig. 3c). When co-cultured with pks+ E. coli, the reporter strain harbouring the clbS expression vector prevented PR-lux reporter activity, whereas the same reporter strain transformed with pTrc-∆clbS did not (Extended Data Fig. 3d). ClbS did not inhibit PR-lux reporter activity when MMC was used as the inducing agent, suggesting that protection is specific to colibactin (Extended Data Fig. 3e). Moreover, supernatants from ClbS-expressing cells did not provide protection against colibactin, indicating that ClbS-based resistance is intracellular and not shared between cells (Extended Data Fig. 3f). Consistent with the reporter activity, the lambda lysogen harbouring pTrc-clbS yielded 1,000-fold fewer phage particles than the same lysogen carrying pTrc-∆clbS when co-cultured with pks+ E. coli (Extended Data Fig. 3g). The pTrc-clbS construct also repressed the induction of phage P22 when introduced into S. Typhimurium, indicating that this resistance mechanism is functional beyond E. coli (Extended Data Fig. 3g).
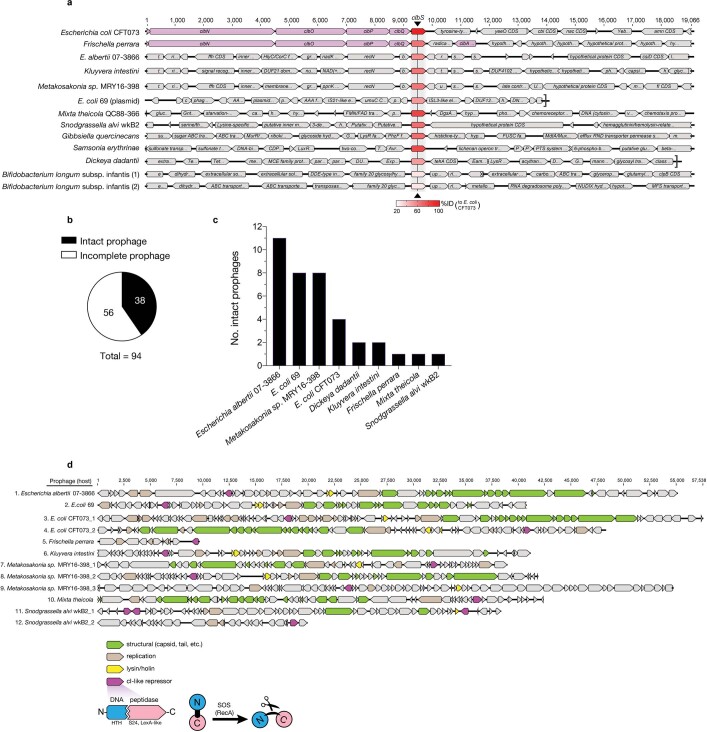
We next sought to examine whether clbS-like genes exist in more distantly related bacteria that lack the pks gene cluster; and, if so, whether they also have a protective function in this context. Hypothesizing that organisms that are found in close proximity with colibactin producers would benefit from colibactin resistance, we searched for more distant ClbS homologues (50% amino acid identity cut-off) in the genomes of bacteria from two specific niches in which pks encoders are reported to exist: the human gastrointestinal tract and the honey bee gut5,29. We found multiple clbS-like genes in pks− human-associated bacteria, including Escherichia albertii 07-3866, Kluyvera intestini and Metakosakonia sp. MRY16-398, the latter two of which were isolated from patients with gastric cancer and patients with sigmoid colon diverticulitis, respectively30,31. We also identified a clbS-like gene in Snodgrasella alvi, a pks− core member of the honey bee gut32 (Extended Data Fig. 4a).
Extended Data Fig. 4. Prophages with predicted DNA-damage-responsive repressors co-occur in clbS-encoding bacteria.
a, Genomic organization surrounding clbS-like genes encoded by diverse bacteria identified in this study. Purple-coloured genes denote the known pks biosynthetic genes. E. coli CFT073 and F. perrara were previously known to carry pks-associated clbS. Red-coloured genes denote clbS. The saturation of red for each clbS is proportional to the percent identity in amino acid sequence of that gene relative to pks+ E. coli (CFT073), as indicated in the key. b, Distribution of PHASTER-predicted prophage regions present in the 12 bacterial genomes that encode the clbS-like genes tested in Fig. 3a (genomic context for each shown in a). A total of 94 prophage regions were predicted, 38 of which are considered to be intact prophages. c, Number and distribution of intact prophages within each bacterial species from b. d, Organization of predicted intact prophages that encode prototypical DNA-damage-responsive repressors (12 from the 38 intact phages identified in a and b). Genes coloured according to predicted function, designated in the key. In a and d, numbering above genomes denotes size in base pairs. In d, domain analysis was used to predict the cI-like repressor (maroon genes) on the basis that it harbours a helix-turn-helix DNA-binding domain (blue, N-terminal domain) and a LexA-like, S24 peptidase domain (pink, C-terminal domain). The same two-domain architecture is found in the lambda cI repressor protein and confers an autoproteolytic mechanism in which the repressor is cleaved in the presence of a DNA-damage-induced, RecA-active protein complex, leading to phage lysis.
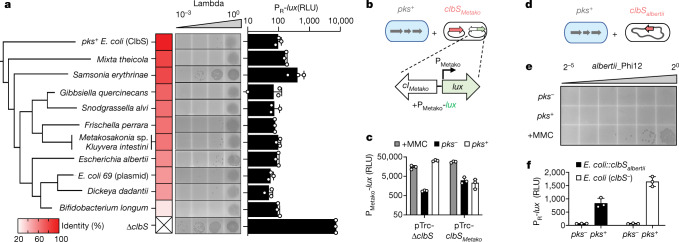
To assess whether these homologues could protect against colibactin-induced phage lysis, we heterologously expressed a subset of the identified ClbS-like proteins in the E. coli lambda lysogen or reporter strain and co-cultured these bacteria with pks+ E. coli. All four ClbS-like proteins attenuated DNA damage and prophage induction, both in terms of reporter output and plaques produced, suggesting the potential for the bacteria harbouring these genes to be protected from colibactin (Fig. 3a). Removing the niche-specific criteria and further lowering the cut-off in our search led us to uncover a wider range of ClbS-like proteins (25–80% amino acid identity relative to E. coli ClbS), an additional six of which we chose as a representative panel for heterologous expression and evaluation in our assays (Extended Data Fig. 4a). A summary of all ClbS-like proteins identified in our search is presented in Supplementary Table 1 (BLASTp 5,000 hits; Methods). Every ClbS-like protein tested in our panel provided protection against colibactin-induced DNA damage and prophage induction (Fig. 3a). Collectively, these results show that protection from colibactin can be gained through distantly related ClbS-like proteins that are found in bacteria that lack all other pks genes.
Fig. 3. ClbS and ClbS-like proteins from diverse bacteria provide protection against colibactin-activated prophage induction.
a, Plaque assay (images, left) and PR-lux reporter output (bar graph, right) obtained from pks+ E. coli co-cultured with pks− E. coli harbouring a vector encoding the clbS-like gene of the indicated organism or the pTrc-∆clbS vector. For plaque assays, the pks− E. coli harboured phage lambda; for the PR-lux reporter assay, the pks− E. coli harboured the reporter plasmid. The heat map and clustering of the ClbS-like proteins are based on amino acid identity to pks+ E. coli ClbS. Metakosakonia sp. and K. intestini share the same ClbS sequence. b, Schematic of co-culture experiment between pks+ E. coli with E. coli harbouring the Metakosakonia-derived prophage reporter (PMetako-lux) and a second vector containing the clbS-like gene from the same organism (pTrc-clbSMetako) or pTrc-∆clbS. c, PMetako-lux reporter output from co-cultures as described in b. Grey bars indicate the reporter response in monoculture to MMC. d, Schematic of co-culture experiment between pks+ E. coli with an isolate of E. albertii that natively encodes a clbS-like gene in its genome and harbours a prophage (albertii_phi12). e, Plaque assay obtained from co-cultures as described in d. The supernatants were serially diluted (twofold) and spotted on E. coli BW25113 to measure plaque formation. f, PR-lux reporter output obtained from culturing pks+ or pks− E. coli with pks− E. coli harbouring the reporter plasmid or pks− E. coli that was recombineered to encode clbSalbertii from the same chromosomal locus in E. coli as it occurs in E. albertii (designated E. coli::clbSalbertii). In a (plaque assay), e, data are shown as a single representative image from n = 3 biological replicates; for a (lux reporter), c, f, data are represented as mean ± s.d. with n = 3 biological replicates and RLU as in Fig. 1d.
Resistance as a phage-silencing strategy
To investigate the effect of colibactin resistance on prophage induction in non-colibactin-producing bacteria, we focused on two human-associated clbS+ organisms from our panel: Metakosakonia sp. MRY16-398 and E. albertii 07-3866, both of which harbour predicted DNA-damage-responsive prophages (Extended Data Fig. 4b–d, Supplementary Discussion). In Metakosakonia sp. MRY16-398, one of the predicted prophages corresponds to an uncharacterized 40-kb element. We synthesized a region of approximately 1 kb from this Metakosakonia prophage, encompassing the putative immunity region (containing a possible cI-like repressor; Supplementary Discussion), and fused the counter-oriented promoter to lux on a plasmid, called PMetako-lux (Fig. 3b). When introduced into recA+ E. coli, the activity of PMetako-lux was activated both by MMC and by co-culture with pks+ E. coli (Fig. 3c), suggesting its DNA damage inducibility. Next, to determine whether the clbS-like gene encoded by Metakosakonia sp. MRY16-398 (clbSMetako) affects induction of the Metakosakonia phage, we introduced plasmid-based clbSMetako into the reporter strain (resulting in PMetako-lux + pTrc-clbSMetako). We found that MMC continued to activate reporter expression, whereas co-culture with pks+ E. coli did not (Fig. 3c). Although we could not obtain an isolate of Metakosakonia MRY16-398 for these investigations, the results predict that if the ClbS protein is expressed in this Metakosakonia host, the organism will be resistant to the prophage-inducing effects of colibactin.
As another example, we turned to an available human-associated clbS+ isolate of E. albertii (Fig. 3d). Escherichia albertii 07-3866 was isolated from human faeces33, encodes a ClbS homologue that is unique from those of Metakosakonia sp. MRY16-398 and pks+ E. coli (Extended Data Fig. 4a), and harbours multiple predicted prophages (Extended Data Fig. 4c). When exposed to MMC, lysates of E. albertii 07-3866 cultures formed distinct plaques on E. coli, indicating that E. albertii 07-3866 harbours a DNA-damage-inducible prophage (Fig. 3e). In contrast to treatment with MMC, co-culture of E. albertii 07-3866 with pks+ E. coli did not lead to plaque formation (Fig. 3e). We investigated whether the failure of this strain to produce phages specifically during pks+ co-culture could be explained by the expression of E. albertii ClbS. Escherichia albertii is related to E. coli, and the region that surrounds clbS in the E. albertii 07-3866 genome is highly conserved in E. coli (around 90% nucleotide identity in an approximately 18-kb vicinity). We transferred the clbS locus from E. albertii to the same relative location in the pks− E. coli genome. As shown in Fig. 3f, when co-cultured with pks+ E. coli, pks− E. coli harbouring the chromosomally integrated E. albertii clbS exhibited a reduction of approximately 50% in PR-lux reporter activity relative to the unprotected, wild-type clbS− strain. The results of these experiments suggest that native clbS expression levels in E. albertii are sufficient to attenuate colibactin-specific prophage induction. More generally, our data from both Metakosakonia sp. and E. albertii lead us to propose that clbS+ organisms are protected from colibactin-mediated prophage induction. These results also imply that the acquisition of orphan clbS genes is an effective strategy for prophage-carrying bacteria to resist the production of colibactin by neighbouring community members.
Discussion
The knowledge that colibactin induces prophages in diverse bacteria, combined with the finding that non-colibactin-producing bacteria from distinct environmental origins have functional clbS-like genes, leads us to speculate that colibactin production is more widespread than currently recognized, and that this genotoxin is likely to have evolved to target bacteria rather than a mammalian host. So far, studies of colibactin have primarily focused on its role in carcinogenesis, but this activity raises important questions with regard to the evolutionary role of the toxin for the producing bacterium. Colibactin genes have also been implicated in siderophore biosynthesis and microcin export, suggesting that these factors may collectively be involved in bacterial competition34,35. Although other functions of colibactin may exist, our discovery that it induces prophages provides one mechanism by which production of and immunity to this natural product might confer a competitive advantage over other microorganisms. For example, because cell lysis is an irreversible consequence of prophage induction22, this mechanism could explain a previously reported observation of pks-dependent growth inhibition of a subset of S. aureus strains, a bacterium with an evolutionary history shaped by phage activity17,36 (Supplementary Discussion). Moreover, the broad-spectrum activity of colibactin in inducing prophages across distinct bacteria suggests that this natural product could have effects on many members of a community, potentially accounting for colibactin-associated changes in the composition of the gut microbiome that have previously been observed in animal models16. Beyond bacteria, our observation that exposure to colibactin increases the production of Stx in mixed communities hints at mechanisms by which this natural product could affect host health and highlights how inducing prophages may regulate other behaviours within the microbial community.
Our study underscores major gaps in our understanding of the molecular mechanisms that underlie prophage induction in microbiomes. MMC and ultraviolet light are the most common methods of activating prophage induction in the laboratory; however, the ecologically relevant triggers for prophages found in natural environments remain largely unidentified. Previous work has shown that the human gut commensal bacterium, Lactobacillus reuteri, harbours a prophage that undergoes induction during gastrointestinal transit in response to dietary fructose and short-chain fatty acids37. In the vaginal community, metabolism of benzo[a]pyrene—a constituent of tobacco smoke—and subsequent secretion in the vagina induces multiple Lactobacillus prophages38. In the nasal microbial community, hydrogen-peroxide-producing Streptococcus has been shown to selectively eliminate prophage-carrying S. aureus39. Unlike benzo[a]pyrene, which humans encounter through outside exposures, and hydrogen peroxide, which has a wide range of biological targets and proposed functions, colibactin is a complex natural product produced by human gut bacteria. By uncovering the phage-inducing activity of colibactin-producing bacteria, our findings reveal a previously unrecognized mechanism by which colibactin and potentially other DNA-damaging natural products may shape microbial communities. More generally, the modulation of phage behaviours represents a distinct and underappreciated ecological role for microbial natural products. Our findings add to this growing understanding14,40–43 and, notably, demonstrate phage induction by a natural product in co-culture. Finally, as links between the human gut virome and diseases continue to be established44, our findings set the stage for further investigations of how gut bacterial metabolite production modulates phage behaviours and may influence human disease.
Methods
Bacterial strains, plasmids and routine cultivation
Bacterial strains and plasmids used in these studies are listed in Supplementary Table 2 and Supplementary Table 3, respectively. Unless otherwise noted, E. coli DH10β (NEB) was used for all strain construction and propagated aerobically in Luria-Bertani (LB-Lennox, RPI) broth at 37 °C. All experiments involving faecal communities were performed in an anaerobic chamber (70% N2, 25% CO2, 5% H2). Oligonucleotides (Sigma) and dsDNA gene blocks (IDT) used in plasmid construction are listed in Supplementary Table 4. Plasmid construction steps and recombineering were performed using enzymes obtained from NEB (NEBuilder HiFi DNA assembly master mix, T4 DNA ligase and DpnI) and lambda red (pKD46 and pKD3), respectively. Sequencing of all inserts was performed using Sanger sequencing. Plasmid sequencing of PR-lux revealed that the vector consists of two copies of the DNA-damage-responsive element (cI and PR). Growth, reporter and lysis assays were all carried out in M9 medium supplemented with 0.4% casamino acids (M9-CAS, Quality Biological) unless otherwise specified. Antibiotics, inducers and indicators were used at the following concentrations: 100 μg ml−1 ampicillin (IBI Scientific), 50 μg ml−1 kanamycin (VWR), 25 μg ml−1 chloramphenicol (Sigma), 100 ng ml−1 MMC (Sigma), 40 μg ml−1 5-bromo-4-chloro-3-indolyl β-d-galactosidase (X-gal, Takara Bio), and 500 μM isopropyl β-d-1-thiogalactopyranoside (IPTG, Teknova), unless otherwise specified.
Growth and competition assays
For growth inhibition by cell-free fluids
Overnight cultures of wild-type E. coli BW25113 harbouring either BAC-pks or the empty BAC were centrifuged (16,100g and 1 min) and the supernatant was passed through a 0.22-μm filter (Corning Spin-X). Growth of non-colibactin-producing E. coli cultures was assayed in fresh LB in the presence of varying amounts of each supernatant (5%, 10%, 20%, 50% v/v). OD600 was measured at regular intervals using a BioTek Synergy HTX multi-mode plate-reader.
For testing ClbS protection from cell-free fluids
Overnight cultures of wild-type E. coli BW25113 harbouring either pTrc-clbS or pTrc-∆clbS were centrifuged (16,100g and 1 min) before addition at 10% v/v to co-cultures containing a 1:1 ratio of E. coli BW25113 harbouring the PR-lux reporter and E. coli BW25113 harbouring either BAC-pks or the empty BAC. Bioluminescence was measured after 24 h and quantified in a plate-reader as outlined below (see ‘E. coli-based reporter assay’).
For E. coli–E. coli competition assays
Overnight cultures of lacZ + E. coli MG1655 (KIlacZ, Addgene: 52696) harbouring BAC-pks were back-diluted 1:100 into fresh M9-CAS and mixed in a 1:1 ratio with a similarly back-diluted culture of lacZ − E. coli MG1655 (delta-Z, Addgene: 52706) harbouring the empty BAC. The co-cultures were incubated at 37 °C, and, at regular intervals, an aliquot was taken for differential plating on LB supplemented with X-gal and IPTG. Both BAC combinations (pks+ versus empty) and marker combinations (lacZ + versus lacZ −) were tested to rule out the influence of carrying the lacZ marker.
For assaying RecA-dependent growth of pks+∆clbS E. coli
Overnight cultures of wild-type E. coli BW25113 or wild-type E. coli DH10β, each individually harbouring BAC-pks or BAC-pks∆clbS, were back-diluted 1:100 into fresh M9-CAS. The monocultures were incubated at 37 °C and the OD600 nm readings were obtained after 24 h.
For E. coli–S. aureus competition assays
S. aureus RN450 lysogenic for phi80α and S. aureus RN450 lysogenic for phi11 were grown overnight at 37 °C in fresh brain heart infusion (BHI) medium, and E. coli BW25113 harbouring BAC-pks or empty BAC were grown overnight at 37 °C in fresh LB broth supplemented with chloramphenicol. The overnight cultures were back-diluted 1:100 into fresh BHI medium and mixed in a 1:1 ratio and incubated at 37 °C for 24 h. The cultures were plated on LB agar supplemented with Cm for E. coli colony-forming units (CFUs), and mannitol salt phenol-red agar (Sigma) for S. aureus CFUs.
For differential MMC susceptibility of phage-free S. aureus and E. coli
lacZ − E. coli MG1655 (delta-Z, Addgene: 52706) and S. aureus RN450were grown overnight at 37 °C in fresh LB and BHI media, respectively. The overnight cultures were back-diluted 1:100 into the same respective fresh medium and a twofold dilution series of MMC was added to achieve a final concentration ranging from 78 ng ml−1 to 5,000 ng ml−1. Cultures were subsequently incubated overnight at 37 °C and OD600 nm readings were obtained after 24 h. Normalized OD600 nm was calculated as the OD600 nm at a given MMC concentration relative to the OD600 nm of the same strain to which no MMC was added (defined as 100%).
Production and isolation of phage lambda by MMC induction
An overnight culture of the lambda lysogen was back-diluted 1:100 into fresh LB and incubated at 37 °C. After reaching an OD600 nm of 0.4–0.5, MMC (500 ng ml−1 final concentration) was added and the cultures were returned to 37 °C for an additional 3–5 h, over which time noticeable clearing occurred. After chloroform treatment and centrifugation (16,100g and 1 min), the clarified lysates were filter-sterilized and stored at 4 °C before use.
Quantification of phage induction by colibactin
E. coli-based reporter assay
Overnight cultures were back-diluted 1:100 into fresh M9-CAS medium with appropriate antibiotics before being dispensed (200 μl) into white-walled 96-well plates (Corning 3610). For co-culture experiments, the two cultures were mixed 1:1 immediately after back-dilution. Monoculture controls for each strain were prepared by adding 100 μl of the back-diluted cultures to an equivalent volume of M9-CAS. For DNA interference experiments, herring sperm DNA (Promega) was used. To test DNA with varying AT richness, complementary oligonucleotide pairs (JWO-1046 and JWO-1047) and (JWO-1044 and JWO-1045) were annealed in 10 mM aqueous Tris-HCl buffer, and the resulting duplexes were added to the wells at the indicated concentrations. Plates were shaken at 37 °C and the OD600 nm and bioluminescence readings were obtained after 24 h. Relative light units (RLU) were calculated by dividing the bioluminescence by the OD600 nm.
Phage quantification for phages of E. coli, S. Typhimurium, S. aureus, E. albertii 07-3866 and E. faecium E1007
Preparing and measuring viral titres from co-cultures with phage-infected isolates was carried out according to the identical conditions used for the reporter assays with the exception that the reporter strain was substituted for the relevant lysogen. Co-cultures with phage-infected E. coli, S. Typhimurium and E. albertii were conducted in M9-CAS, whereas co-cultures with phage-infected S. aureus and E. faecium were conducted in BHI as the growth medium. To prepare phage lysates, cultures were transferred after 24 h co-culture to microcentrifuge tubes and centrifuged at 16,100g for 1 min. The supernatant was removed and passed through a 0.22-µm filter. For phage quantification by plaque assays, supernatants were diluted logarithmically from 100 to 10−5, and 10 µl spotted on top agar (preparation below) containing the relevant indicator. For E. albertii 07-3866 phi12, 2-fold dilutions of the supernatants instead of 10-fold were used. In the case of quantifying S. Typhimurium phages from faecal communities, culture supernatants were concentrated approximately 40-fold from their starting volume in protein concentrators (Pierce, 100 kDa MWCO, spin columns) before use in plaque assays. For phage quantification by qPCR, supernatants were diluted 100-fold, treated with DNase (Promega) to remove residual DNA, then boiled to release encapsidated phage DNA. Host (JWO-1120 and JWO-1121) and phage (JWO-1116 and JWO-1117) specific primer pairs were used for PCR amplification using the Luna Universal qPCR kit (NEB) in a CFX96 real-time PCR detection system (Bio-Rad). Data were processed and analysed by comparing the relative amplification within samples of phage-specific primer pairs to host-specific primer pairs (Pfaffl method) using the Gene Expression calculator in the CFX Manager software (Bio-Rad).
Preparation of top agar
The indicators used to assay each phage-bacteria system were as follows: for lambda-E. coli and phi12-E. albertii (wild-type E. coli BW25113 or the lambda-resistant lamB::kan mutant); for P22-S. Typhimurium (S. Typhimurium D23580ΔΦ); for BTP1-S. Typhimurium (S. Typhimurium SNW22 D23580 ΔBTP1); for Gifsy-1-S. Typhimurium (S. Typhimurium D23580 ΔΦ ΔwaaG::aph); for phi80α and phi11 (S. aureus RN450). In each case, overnight cultures of the relevant indicator strains were back-diluted 1:100 into LB (for E. coli and S. Typhimurium) or BHI (for S. aureus) and incubated at 37 °C. At an OD600 nm of 0.3–0.5, E. coli and S. Typhimurium cultures were diluted 1:10 into molten LB-agar (0.6%) supplemented with 10 mM MgSO4 and 0.2% maltose and poured onto a LB-agar (1.5%) plate. For S. aureus, cultures were back-diluted 1:10 into molten tryptic soy agar (0.6%) supplemented with 10 mM CaCl2 and poured onto a denser layer (1.5%) of the same agar.
ELISA for Stx2dact detection
Detection of Stx2dact from both aerobic co-cultures and faecal communities was performed using the Premier EHEC test kit, which specifically detects Shiga toxins I and II (Meridian Biosciences), following the manufacturer’s instructions with the following modifications: for aerobic co-cultures, overnight monocultures of C. rodentium harbouring stx2dact were back-diluted 1:100 and co-cultured in M9-CAS at a 1:1 ratio with E. coli BW25113 harbouring either BAC-pks or the empty BAC. For faecal community experiments, anaerobic monocultures of C. rodentium harbouring stx2dact were mixed with faecal communities in BHI as described in the relevant section below. To verify toxin production in response to a known DNA-damaging agent under these conditions, MMC (1.5 µg ml−1 final concentration) was added to aerobic cultures of exponentially growing stx2dact-harbouring C. rodentium in M9-CAS. In all cases, samples collected after 24-h incubations (exact volumes detailed below) were diluted in 200 µl of diluent buffer provided by the manufacturer before addition to Stx-specific antibody-coated microwells. The use of kit-provided positive and negative controls as well as all wash and substrate addition steps were carried out exactly according to the manufacturer-supplied protocol. The stop reagent was added approximately 2–5 min after adding the final substrate to each well, at which time the images used in Fig. 3c were taken. Absorbance at 450 nm (Abs450 nm) was measured using a plate-reader. According to the manufacturer, Abs450 nm values ≥ 0.180 are considered a positive test result. For aerobic experiments including MMC controls, 2 µl of culture supernatants were used as the sample input. For faecal community experiments, culture supernatants were first concentrated approximately 20-fold from the initial volume in protein concentrators (Pierce, 30 kDa MWCO, spin columns). Forty microlitres of the concentrated retentate was used as the sample input.
Faecal sample processing
Faecal pellets from C57BL/6J mice from the Jackson Laboratory (which lack Enterobacteriaceae and do not contain colibactin-producing organisms) were suspended in pre-reduced PBS supplemented with 0.1% l-cysteine (5% w/v), then left to stand to allow insoluble particles to settle. The supernatant was carefully removed and mixed with an equal volume of 40% glycerol. Aliquots (50 µl) of this suspension were stored at –80 °C until required.
Ex vivo culture with faecal communities
An aliquot of the faecal suspension prepared above was thawed and inoculated into BHI (1:100) and incubated at 37 °C in an anaerobic chamber alongside the relevant human-associated phage-containing bacteria and E. coli BW25113 harbouring either BAC-pks or the empty BAC. After 24 h incubation, the overnight cultures were back-diluted (1:1,000) and mixed in equal proportions in fresh BHI, then incubated for a further 24 h. Phages and toxin produced from faecal communities were measured in the same assays used in two-way cultures, involving plaque assays for S. Typhimurium (BTP1 and Gifsy-1) and S. aureus (phi80α and phi11), qPCR for E. faecium (phi1), and Stx ELISA for C. rodentium (stx2dact).
Assaying protection by clbS-like open reading frames
For reporter assays, each of the clbS-like open reading frames (ORFs) (or the pTrc-∆clbS construct) were transformed into E. coli BW25113 harbouring the PR-lux reporter. After overnight growth of each strain in monoculture, strains were back-diluted 1:100 and co-cultured in M9-CAS at a 1:1 ratio with E. coli BW25113 harbouring BAC-pks. Bioluminescence was measured after 24 h incubation at 37 °C in a plate-reader as detailed for all other E. coli-based reporter assays above. For measuring protection by the clbS-like ORFs from phage induction, the same clbS-like ORF-encoding constructs from the reporter assay were individually transformed into a E. coli BW25113 lambda lysogen. An identical dilution and co-culture procedure to that of E. coli BW25113 harbouring BAC-pks was used, after which phage production was measured by plaque assay as described in the relevant section above. To measure protection provided by a chromosomal copy of E. albertii-encoded clbS (clbSalbertii), the locus surrounding clbSalbertii from the E. albertii 07-3866 genome was PCR-amplified and transferred using lambda-red recombineering into wild-type E. coli BW25113 (JSO-1966–1973; Supplementary Table 4). The PR-lux reporter plasmid was introduced into the resulting strain, E. coli::clbSalbertii, and measured for its ability to be induced by colibactin using the identical co-culture procedure as that used for E. coli-based reporter assays, as noted in the relevant section above.
Quantification of N-myristoyl-d-asparagine prodrug production by pks+E. coli
For culture conditions and sample preparation
Overnight cultures of E. coli BW25113 harbouring either BAC-pks or the empty BAC were back-diluted 1:100 and co-cultured in M9-CAS at a 1:1 ratio with phage-free E. coli BW25113 or lambda-infected BW25113. Cultures (1 ml) were dispensed into deep-well plates (VWR) and incubated with shaking at 37 °C. After 24 h, 10 µl deuterated (d27) N-myristoyl-d-asparagine (10 µM in DMSO stock solution) was added to each sample. Samples were flash-frozen in liquid nitrogen, lyophilized for 48 h, then reconstituted in methanol (1 ml) and vortexed for 1 min. Three hundred microlitres of the mixture was filtered through a 0.22 µm filter (Pall) before mass spectrometry analysis.
For prodrug quantification
Analysis of the N-myristoyl-d-asparagine prodrug in samples was performed using an ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) system model Xevo TQ-S (Waters). The mass spectrometer system consists of a triple quadrupole equipped with a dual-spray electrospray ionization (ESI) source. Samples were analysed using an Agilent Poroshell 120 EC-C18 column (2.7 mm, 4.6 mm × 50 mm) with the following elution conditions: isocratic hold at 90% solvent A in solvent B for 0.5 min: linear gradient from 90% to 5% solvent A in solvent B from 0.5–2 min; isocratic hold at 5% solvent A from 2–3 min, gradient from 5% to 98% solvent A in solvent B from 3–3.5 min; isocratic hold at 98% solvent A in solvent B from 3.5–4 min (solvent A: 95% water + 5% methanol + 0.03% ammonium hydroxide; solvent B: 80% isopropanol + 15% methanol + 5% water; flow rate = 0.75 ml min−1; injection volume = 5 µl). The mass spectrometer was run in negative-mode MRM with a Cone voltage of 50 V, monitoring transitions of m/z 341 -> m/z 114 (retention time (rt) = 2.2 min, collision energy (CE) = 24 V) for the prodrug scaffold and m/z 368 -> m/z 114 (rt = 2.2 min, CE = 28 V) for the deuterated internal standard (d27-N-myristoyl-d-asparagine). For all samples, peak areas for the m/z 341 -> m/z 114 were normalized to the m/z 368 -> m/z 114 transition for the same sample, and then normalized values compared to a standard curve of unlabelled N-myristoyl-d-asparagine containing 100 nM d27- N-myristoyl-d-asparagine, which was run in triplicate.
Bioinformatic analyses
NCBI tBLASTn (nr/nt database, expect threshold = 0.05, word size = 6, BLOSUM62 matrix) was used to identify clbS genes that match E. coli ClbS (WP_000290498) but that are found outside of pks clusters. The more distantly related ClbS-like proteins examined in this study (Fig. 3d) were compiled from BLASTp results using E. coli ClbS as the query (nr protein sequences database, expect threshold = 0.05, word size = 6, BLOSUM62 matrix, 5,000 entries). After excluding entries that occur in genomes with pks clusters, the isolation source of the remaining hits was considered in identifying bee gut and human-associated isolates. Other members in the representative panel selected for cloning and heterologous expression were chosen heuristically and to cover the range in per cent identities returned by the BLAST search (spanning Mixta theicola having 80% pairwise identity and Bifidobacterium longum with 26.8% pairwise identity to E. coli ClbS). The genomes encoding clbS-like genes in the representative panel were submitted to PHASTER for identification of prophage regions. Genes encoded by predicted intact prophages (score higher than 90) were further analysed by domain analysis (InterPro) for features matching the lambda repressor (DNA-binding and peptidase domains), as mentioned in the main text and Supplementary Discussion, and shown in Extended Data Fig. 4d.
Quantification and statistical analysis
Software used to collect and analyse data generated in this study consisted of: GraphPad Prism 9 for analysis of growth- and reporter-based experiments; Gen5 v.3 for collection of growth- and reporter-based experiments; Bio-Rad CFX Manager 3.0 for quantification and analysis of qPCR data; ImageJ 1.53c for colony counting in competition experiments; and Geneious Prime 2020 for analysis of publicly available data and primer design. Data are presented as mean ± s.d. unless otherwise indicated in the figure legends. The number of independent biological replicates for each experiment is indicated for each experiment and included in the legend.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-022-04444-3.
Supplementary information
This file contains additional discussion pertinent to study findings; the caption for Supplementary Table 1; Supplementary Tables 2–4; and the Supplementary References.
tBLASTn result of top 230 clbS-like genes encoding proteins matching E. coli ClbS, WP_000290498 (tab 1); BLASTp result of top 5,000 ClbS-like proteins using E. coli ClbS, WP_000290498 as query (tab 2, available as a separate Excel file).
PFU ml−1 for each plaque assay used in this study (available as a separate Excel file).
CFU ml−1 and OD600 nm data displayed in Supplementary Discussion Fig. 1 (available as a separate Excel file).
Acknowledgements
We thank all members of the E.P.B. laboratory for insightful discussions; K. Papenfort for feedback; the laboratory of J. R. Penadés (Imperial College London) for sharing S. aureus strains; the laboratory of J. M. Leong (Tufts University) for sharing C. rodentium; and the laboratory of W. Garrett (Harvard School of Public Health) for sharing of faecal pellets. This work was supported by the National Institutes of Health (NIH) grant R01 CA208834. J.W.H.W. was supported by the A*STAR NSS (PhD) predoctoral fellowship. S.V.O. and M.B. were partially supported by the NIH NIGMS award R35GM133700, the David and Lucile Packard Foundation, and the Pew Charitable Trusts. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Extended data figures and tables
Source data
Author contributions
J.E.S., J.W.H.W. and E.P.B. conceived the project. J.E.S. and J.W.H.W. contributed equally to this work and ordering of authorship was determined in no particular order. J.E.S. and J.W.H.W. constructed strains, conducted all bioinformatic analyses and performed all growth-based-, reporter-based- and plaque assays. S.V.O. assisted in constructing and acquiring S. Typhimurium and S. aureus strains. All authors interpreted data, provided critical feedback and wrote the paper.
Peer review
Peer review information
Nature thanks Breck Duerkop and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All unprocessed plaque assay images (Figs. 1c, 2a, b, 3a, Extended Data Figs. 1c, 2c, f, h, 3g) and source data (Figs. 1b, d, 2c, d, f–h, 3a, c, Extended Data Figs. 1a, b, d, e, 2b, e, g, 3a, d–f, 4b, c) generated in the course of this study are available without restriction and deposited on Zenodo (10.5281/zenodo.4683077). Total PFU data are available in Supplementary Table 5, and total CFU and growth data are available in Supplementary Table 6 (Supplementary Fig. 1 in the Supplementary Discussion). Identifiers for all entries in NCBI BLAST results are listed in Supplementary Table 1. Protein accession numbers for the relevant ClbS sequences tested in this study are as follows: E. coli CFT073 (WP_000290498), M. theicola (PNS10644), S. erythrinae (WP_132453050), Gibbsiella quercinecans (WP_095844971), S. alvi (WP_025331471), F. perrara (WP_039103908), Metakosakonia sp. (BBE76153), K. intestini (PWF54517), E. albertii (WP_000115842), E. coli 69 (QDM73539), Dickeya dadantii (WP_038909824) and B. longum (WP_193641739). Accession numbers used for the design of qPCR primers and reporter construction are: E. faecium E1007 (AHWP00000000), E. coli BW25113 and lambda (NZ_CP009273 and NC_001416.1), E. albertii 07-3866 (NZ_CP030781) and Metakosakonia sp. (AP018756). Accession and identifier information can be found at NCBI. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Justin E. Silpe, Joel W. H. Wong
Extended data
is available for this paper at 10.1038/s41586-022-04444-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-022-04444-3.
References
- 1.Nougayrède J-P, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 2.Auvray F, et al. Insights into the acquisition of the pks island and production of colibactin in the Escherichia coli population. Microb. Genomics. 2021;7:000579. doi: 10.1099/mgen.0.000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty MW, Jobin C. Shining a light on colibactin biology. Toxins. 2021;13:346. doi: 10.3390/toxins13050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondarev V, et al. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ. Microbiol. 2013;15:2095–2113. doi: 10.1111/1462-2920.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel P, Vizcaino MI, Crawford JM. Gut symbionts from distinct hosts exhibit genotoxic activity via divergent colibactin biosynthesis pathways. Appl. Environ. Microbiol. 2015;81:1502–1512. doi: 10.1128/AEM.03283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moretti C, et al. Erwinia oleae sp. nov., isolated from olive knots caused by Pseudomonas savastanoi pv. savastanoi. Int. J. Syst. Evol. Microbiol. 2011;61:2745–2752. doi: 10.1099/ijs.0.026336-0. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MR, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019;363:eaar7785. doi: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dziubańska-Kusibab PJ, et al. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat. Med. 2020;26:1063–1069. doi: 10.1038/s41591-020-0908-2. [DOI] [PubMed] [Google Scholar]
- 9.Pleguezuelos-Manzano C, et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature. 2020;580:269–273. doi: 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faïs T, Delmas J, Barnich N, Bonnet R, Dalmasso G. Colibactin: more than a new bacterial toxin. Toxins. 2018;10:151. doi: 10.3390/toxins10040151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams PC, Wernke KM, Tirla A, Herzon SB. Employing chemical synthesis to study the structure and function of colibactin, a “dark matter” metabolite. Nat. Prod. Rep. 2020;37:1532–1548. doi: 10.1039/d0np00072h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balskus EP. Colibactin: understanding an elusive gut bacterial genotoxin. Nat. Prod. Rep. 2015;32:1534–1540. doi: 10.1039/c5np00091b. [DOI] [PubMed] [Google Scholar]
- 13.Wernke KM, et al. Probing microbiome genotoxicity: a stable colibactin provides insight into structure–activity relationships and facilitates mechanism of action studies. J. Am. Chem. Soc. 2021;143:15824–15833. doi: 10.1021/jacs.1c07559. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, et al. Reactivity of an unusual amidase may explain colibactin’s DNA cross-linking activity. J. Am. Chem. Soc. 2019;141:11489–11496. doi: 10.1021/jacs.9b02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue M, et al. Structure elucidation of colibactin and its DNA cross-links. Science. 2019;365:eaax2685. doi: 10.1126/science.aax2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tronnet S, et al. The genotoxin colibactin shapes gut microbiota in mice. mSphere. 2020;5:e00589-20. doi: 10.1128/mSphere.00589-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faïs T, et al. Antibiotic activity of Escherichia coli against multiresistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2016;60:6986–6988. doi: 10.1128/AAC.00130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossuet‐Greif N, et al. Escherichia coli ClbS is a colibactin resistance protein. Mol. Microbiol. 2016;99:897–908. doi: 10.1111/mmi.13272. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi P, et al. ClbS is a cyclopropane hydrolase that confers colibactin resistance. J. Am. Chem. Soc. 2017;139:17719–17722. doi: 10.1021/jacs.7b09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois D, et al. ClbP is a prototype of a peptidase subgroup involved in biosynthesis of nonribosomal peptides. J. Biol. Chem. 2011;286:35562–35570. doi: 10.1074/jbc.M111.221960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brotherton CA, Balskus EP. A prodrug resistance mechanism is involved in colibactin biosynthesis and cytotoxicity. J. Am. Chem. Soc. 2013;135:3359–3362. doi: 10.1021/ja312154m. [DOI] [PubMed] [Google Scholar]
- 22.Ofir G, Sorek R. Contemporary phage biology: from classic models to new insights. Cell. 2018;172:1260–1270. doi: 10.1016/j.cell.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Gimble FS, Sauer RT. λ Repressor mutants that are better substrates for RecA-mediated cleavage. J. Mol. Biol. 1989;206:29–39. doi: 10.1016/0022-2836(89)90521-4. [DOI] [PubMed] [Google Scholar]
- 24.Arthur JC, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobel L, Cao YG, Fenn K, Glickman JN, Garrett WS. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science. 2020;369:1518–1524. doi: 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheldon PJ, Mao Y, He M, Sherman DH. Mitomycin resistance in Streptomyces lavendulae includes a novel drug-binding-protein-dependent export system. J. Bacteriol. 1999;181:2507–2512. doi: 10.1128/jb.181.8.2507-2512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin TW, et al. Molecular basis of mitomycin C resistance in Streptomyces: structure and function of the MRD protein. Structure. 2002;10:933–942. doi: 10.1016/s0969-2126(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 28.Tripathi P, Bruner SD. Structural basis for the interactions of the colibactin resistance gene product ClbS with DNA. Biochemistry. 2021;60:1619–1625. doi: 10.1021/acs.biochem.1c00201. [DOI] [PubMed] [Google Scholar]
- 29.Putze J, et al. genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 2009;77:4696–4703. doi: 10.1128/IAI.00522-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekizuka T, et al. Complete genome sequence of blaIMP–6-positive Metakosakonia sp. MRY16-398 isolate from the ascites of a diverticulitis patient. Front. Microbiol. 2018;9:2853. doi: 10.3389/fmicb.2018.02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tetz G, et al. Complete genome sequence of Kluyvera intestini sp. nov., isolated from the stomach of a patient with gastric cancer. Genome Announc. 2017;5:e01184-17. doi: 10.1128/genomeA.01184-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong WK, Moran NA. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016;14:374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsey RL, Rowe LA, Batra D, Smith P, Strockbine NA. PacBio genome sequences of eight Escherichia albertii strains isolated from humans in the United States. Microbiol. Resour. Announc. 2019;8:e01663-18. doi: 10.1128/MRA.01663-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin P, et al. Interplay between siderophores and colibactin genotoxin biosynthetic pathways in Escherichia coli. PLoS Pathog. 2013;9:e1003437. doi: 10.1371/journal.ppat.1003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massip C, et al. Deciphering the interplay between the genotoxic and probiotic activities of Escherichia coli Nissle 1917. PLoS Pathog. 2019;15:e1008029. doi: 10.1371/journal.ppat.1008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014;21:593–601. doi: 10.1016/j.meegid.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Oh J-H, et al. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe. 2019;25:273–284. doi: 10.1016/j.chom.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Pavlova SI, Kiliç AO, Mou SM, Tao L. Phage infection in vaginal lactobacilli: an in vitro study. Infect. Dis. Obstet. Gynecol. 1997;5:36–44. doi: 10.1155/S1064744997000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selva L, et al. Killing niche competitors by remote-control bacteriophage induction. Proc. Natl Acad. Sci. USA. 2009;106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronheim S, et al. A chemical defence against phage infection. Nature. 2018;564:283–286. doi: 10.1038/s41586-018-0767-x. [DOI] [PubMed] [Google Scholar]
- 41.Kever, L. et al. Aminoglycoside antibiotics inhibit phage infection by blocking an early step of the phage infection cycle. Preprint at 10.1101/2021.05.02.442312 (2021). [DOI] [PMC free article] [PubMed]
- 42.Jancheva M, Böttcher T. A metabolite of Pseudomonas triggers prophage-selective lysogenic to lytic conversion in Staphylococcus aureus. J. Am. Chem. Soc. 2021;143:8344–8351. doi: 10.1021/jacs.1c01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell. 2019;176:268–280. doi: 10.1016/j.cell.2018.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago-Rodriguez TM, Hollister EB. Human virome and disease: high-throughput sequencing for virus discovery, identification of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses. 2019;11:E656. doi: 10.3390/v11070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains additional discussion pertinent to study findings; the caption for Supplementary Table 1; Supplementary Tables 2–4; and the Supplementary References.
tBLASTn result of top 230 clbS-like genes encoding proteins matching E. coli ClbS, WP_000290498 (tab 1); BLASTp result of top 5,000 ClbS-like proteins using E. coli ClbS, WP_000290498 as query (tab 2, available as a separate Excel file).
PFU ml−1 for each plaque assay used in this study (available as a separate Excel file).
CFU ml−1 and OD600 nm data displayed in Supplementary Discussion Fig. 1 (available as a separate Excel file).
Data Availability Statement
All unprocessed plaque assay images (Figs. 1c, 2a, b, 3a, Extended Data Figs. 1c, 2c, f, h, 3g) and source data (Figs. 1b, d, 2c, d, f–h, 3a, c, Extended Data Figs. 1a, b, d, e, 2b, e, g, 3a, d–f, 4b, c) generated in the course of this study are available without restriction and deposited on Zenodo (10.5281/zenodo.4683077). Total PFU data are available in Supplementary Table 5, and total CFU and growth data are available in Supplementary Table 6 (Supplementary Fig. 1 in the Supplementary Discussion). Identifiers for all entries in NCBI BLAST results are listed in Supplementary Table 1. Protein accession numbers for the relevant ClbS sequences tested in this study are as follows: E. coli CFT073 (WP_000290498), M. theicola (PNS10644), S. erythrinae (WP_132453050), Gibbsiella quercinecans (WP_095844971), S. alvi (WP_025331471), F. perrara (WP_039103908), Metakosakonia sp. (BBE76153), K. intestini (PWF54517), E. albertii (WP_000115842), E. coli 69 (QDM73539), Dickeya dadantii (WP_038909824) and B. longum (WP_193641739). Accession numbers used for the design of qPCR primers and reporter construction are: E. faecium E1007 (AHWP00000000), E. coli BW25113 and lambda (NZ_CP009273 and NC_001416.1), E. albertii 07-3866 (NZ_CP030781) and Metakosakonia sp. (AP018756). Accession and identifier information can be found at NCBI. Source data are provided with this paper.